Abstract
Interfering with the endotoxin-mediated cytokine cascade is thought to be a promising approach to prevent septic complications in gram-negative infections. The synthetic lipid A analog SDZ MRL 953 has been shown to be protective against endotoxic shock and bacterial infection in preclinical in vivo models. As part of a trial of unspecific immunostimulation in cancer patients, we conducted a double-blind, randomized, vehicle-controlled phase I trial of SDZ MRL 953 to investigate, first, its biologic effects and safety of administration in humans and, second, its influence on reactions to a subsequent challenge of endotoxin (Salmonella abortus equi). Twenty patients were treated intravenously with escalating doses of SDZ MRL 953 or vehicle control, followed by an intravenous application of endotoxin (2 ng/kg of body weight [BW]). Administration of SDZ MRL 953 was safe and well-tolerated. SDZ MRL 953 itself increased granulocyte counts and serum levels of granulocyte colony-stimulating factor (G-CSF ) and interleukin-6 (IL-6), but not of the proinflammatory cytokines tumor necrosis factor-α (TNF-α), IL-1β, and IL-8. Compared with vehicle control, pretreatment with SDZ MRL 953 markedly reduced the release of TNF-α, IL-1β, IL-8, IL-6, and G-CSF, but augmented the increase in granulocyte counts to endotoxin. Induction of tolerance to the endotoxin-mediated cascade of proinflammatory cytokines by pretreatment with SDZ MRL 953 in patients at risk may help to prevent complications of gram-negative sepsis.
SEPSIS AND SEPTIC SHOCK are clinical syndromes reflecting a systemic response of an organism to an infection, which may lead to altered organ perfusion, hypotension, and, ultimately, multiorgan failure and death.1 Despite many therapeutical advances, including the use of antibiotics and intensive life-support procedures, the mortality rate from septic shock has not substantially improved in the last decades, still ranging from 25% to 75%.2 The predominant infectious agents associated with septic shock are gram-negative bacteria.3
It is now well appreciated that the fatal complications of gram-negative sepsis are the result of an overstimulation of the host's immune response, which is triggered by endotoxin (lipopolysaccharide [LPS]), a major component of the outer membrane of gram-negative organisms.4,5 By activating particularly monocytes/macrophages, neutrophils, and endothelial cells, endotoxin induces the production of a cascade of cytokines, lipids, and reactive oxygen intermediates, which, in turn, may cause local and systemic toxic effects.4-9
Among the proinflammatory cytokines found in endotoxemia, tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1) play a pivotal role in the pathogenesis of septic shock, because they can replace endotoxin in mediating its deleterious effects.10,11 Blocking TNF-α or IL-1 by natural inhibitors reduces the lethality of endotoxin-induced shock in animals.12-14 Serum levels of IL-6, a cytokine known to induce lymphocyte activation and hepatic acute-phase response15 but not shock,16 and IL-8, a chemoattractant for neutrophils and lymphocytes,17 were found to correlate with mortality in patients with sepsis.18,19 Furthermore, endotoxin induces the secretion of hematopoietic growth factors causing mobilization and activation of bone marrow-derived neutrophils and monocytes4,5,8 as well as the release of a variety of anti-inflammatory mediators,20 which counteract the initiated immune response. The balanced and coordinate expression of proinflammatory and anti-inflammatory cytokines is thought to be crucial for a physiologic, as opposed to an escalating, host response to gram-negative infection.21
Based on the growing insights into the pathophysiology of gram-negative sepsis, a number of efforts have been undertaken to improve therapeutic strategies by interfering with the endotoxin-induced cascade of proinflammatory mediators. Promising results in animal models and phase I/II clinical trials using IL-1 receptor antagonist (IL-1ra),22 monoclonal antibodies to endotoxin,23 or inhibitors of TNF13,24 have encouraged further studies. However, all of these agents failed to prove beneficial in placebo-controlled phase III studies in patients with established sepsis syndrome.25-27
An important feature of endotoxin is the induction of tolerance to its own effects. Repeated injections of endotoxin in humans cause a profound adaptation to subsequent challenges with respect to cytokine release and adverse events.28,29 Animals pretreated with low-dose injections of endotoxin survived an otherwise lethal challenge of the substance.30 To use this phenomenon as a therapeutic concept in patients at risk for gram-negative infections, derivatives of the lipid A moiety of endotoxin, which display less toxic but similar immunostimulatory effects as compared with endotoxin, have been isolated or synthesized.31-33
SDZ MRL 953, a synthetic monosaccharidic lipid A analog, has been developed, out of more than 200 chemical modifications of lipid A, by the specific cytokine profile it induced in animal and in vitro studies, which was characterized by high levels of granulocyte colony-stimulating factor (G-CSF ) but the almost complete absence of proinflammatory cytokines.33 In addition, the compound showed a strong stimulatory action on monocytes/macrophages and neutrophil granulocytes, but was shown to be less toxic than endotoxin (Salmonella abortus equi) in galactosamine-sensitized mice by a factor of at least 104.33 Furthermore, prophylactic injections of SDZ MRL 953 in neutropenic or immunocompetent mice proved to be highly protective in models of bacterial sepsis and endotoxemia.33 34 However, effects of SDZ MRL 953 in humans have not been tested so far.
Based on the data given above and the known antitumor activity of endotoxin due to its immunostimulatory property,8 29 we conducted a prospective, double-blind, randomized, vehicle-controlled phase I trial of SDZ MRL 953 in tumor patients first to evaluate its biologic response and safety of administration in humans. Second, we investigated the effects of pretreatment with SDZ MRL 953 on serum and blood levels of proinflammatory cytokines and neutrophils after a subsequent intravenous application of endotoxin (Salmonella abortus equi).
MATERIALS AND METHODS
Preparation of the test substances.SDZ MRL 953 was synthesized at the Sandoz Research Institute (Vienna, Austria) and purified to ≥98% by reversed phase high pressure liquid chromatography (RP-HPLC), as described elsewhere.33 The substance was formulated as a lyophilized liposomal preparation containing 15 mg/dL of synthetic palmitoyl-oleoyl-phosphatidylcholine and palmitoyl-oleoyl-phosphatidylglycerol in a 7/3 ratio and benzyl alcohol as a preservative (<0.2% after lyophilization). The mean particle diameter was 0.2 μm. Reconstitution with pyrogen-free water resulted in a stable isotonic suspension containing 2 mg of SDZ MRL 953 per milliliter. Endotoxin from Salmonella abortus equi was isolated from bacteria by the phenol-water method35 and purified further by the phenol-chloroform-petroleum ether extraction procedure.36 The resulting endotoxin was essentially free of protein (<0.08%) and free of nucleic acid. The preparation was electrodialyzed, converted to the uniform sodium salt,37 and lyophilized.
Patient selection.All patients entering this trial were treated at the Division of Hematology/Oncology, Department of Internal Medicine I, University Hospital of Freiburg (Freiburg, Germany). Patients with histopathologically proven advanced solid tumors (colorectal cancer, n = 13; stomach cancer, n = 2; others, n = 5), not amenable to conventional treatment, were included in the study. Eligibility criteria included age between 18 and 75 years, performance status ≥60% (Karnofsky scale),38 an estimated life expectancy of more than 3 months, and preserved hematologic (hemoglobin level, ≥10 g/dL; white blood cell count, ≥3,000/μL; platelet count, ≥100,000/μL), hepatic (bilirubin level, <2 mg/dL; aspartate transferase [AST] ≤2× normal values [N]; γ-glutamyl-trans-peptidase [γ-GT], ≤4× N), and renal (creatinine level, <1.5 mg/dL) function. Exclusion criteria included clinical or laboratory evidence or recent history of infection, severe additional medical disorders (cardiac or pulmonary failure, seizures, and autoimmune diseases), central nervous system metastases, and previous (2 months) or concomitant medication with nonsteroidal anti-inflammatory drugs, corticosteroids, or anticoagulants. Previous therapy with antineoplastic drugs, radiation, or modulators of the immune system had to be discontinued for at least 2 months. Informed, signed consent of each patient was obtained before the start of the trial. The study has been approved by the institutional review board.
All patients underwent a complete medical history and physical examination before entering the trial. The following diagnostic studies were performed: leukocyte, including differential, and platelet count; coagulation profile; biochemical screening profile (including electrolytes, glucose, creatinine, uric acid, total protein, albumin, bilirubin, cholesterol, triglycerides, AST, alanine transferase [ALT], alkaline phosphatase, lactate dehydrogenase, cholinesterase, γ-GT, C-reactive protein, and haptoglobin); an electrocardiogram, chest x-ray, and ultrasonography of the abdomen; a computed tomography scan of the tumor region; and, when necessary, special diagnostic studies.
Study design.The study, conducted as a prospective, double-blind, randomized, vehicle-controlled trial, was divided in two parts.
In the first part, the tolerability and biologic effects of SDZ MRL 953 in cancer patients were evaluated. Twenty patients were divided into five groups of 4 individuals receiving the same dose level of SDZ MRL 953. We selected as the starting dose level the minimal effective dose (MED) of the substance, defined as the lowest dose inducing biologic reactions in the patients (such as side effects, cytokine release, or laboratory changes). Because SDZ MRL 953 had not been tested in humans before, we established the MED first, before the main study, at 18.0 μg/kg of BW by applying low doses of the drug (starting from 1 ng/kg of BW) to patients, not being identical with those treated later. The first dose level of 18.0 μg/kg of BW was then increased by 5.2 μg/kg (corresponding to 30% of the MED) in the following dose levels, up to 39.6 μg/kg in the final dose level. If in a group of one dose level, at least 2 patients had shown toxic side effects according to World Health Organization grade III, the dose level below would have been defined as the maximal tolerable dose (MTD), and all following patients would have been treated with that dose. In each group, 1 of 4 patients randomly (double-blind) received sole liposomes as vehicle control instead of liposomes containing SDZ MRL 953.
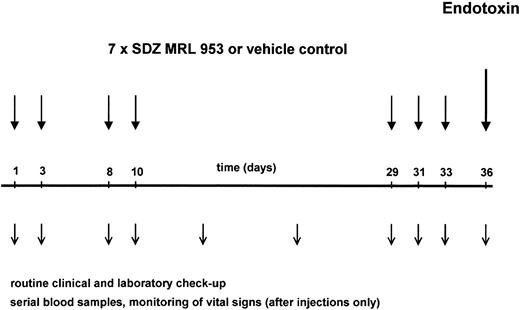
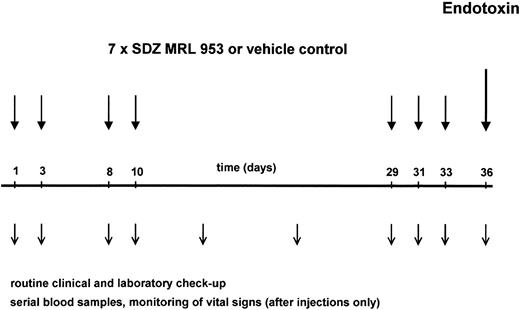
The application schedule is shown in Fig 1. Its development followed the consideration that toxicity and formation of tolerance to itself have been reported, for endotoxin in human8,28 and SDZ MRL 953 in animal39 studies, to be time-dependent phenomena. Therefore, to study short-term and long-term effects of SDZ MRL 953 with respect to those aspects, both being important for a prospective clinical use of the drug, we selected a study protocol with repeated injections, including time intervals from 2 to 19 days. Each patient received seven applications of SDZ MRL 953 or vehicle control as an intravenous bolus injection on days 1, 3, 8, 10, 29, 31, and 33. The patients were treated on an inpatient basis, allowing vital signs to be monitored hourly for 12 hours and at 24 hours after injection. Blood samples for complete blood cell count and cytokine measurements were drawn before and 1.5, 3, 6, 9, 12, 14, and 24 hours after injection. A serum chemistry profile (including electrolytes, renal and liver function tests, and C-reactive protein) and coagulation tests were obtained before and 12 and 24 hours after the injection of SDZ MRL 953. In addition, physical examination, vital signs, BW, control of performance status, and complete blood cell count, serum chemistry, and coagulation profile were performed at least once weekly. After 8 weeks, electrocardiography, ultrasonography of the abdomen, chest x-ray, and determination of the tumor size by computed tomography were repeated.
Schematic illustration of the study protocol. Twenty patients, divided in five dose subgroups (18.0 to 39.6 μg/kg of BW), were treated with seven intravenous bolus injections of SDZ MRL 953 at the indicated time points. One of four patients was randomized to receive double-blind vehicle control instead of SDZ MRL 953. Three days after the last injection of SDZ MRL 953 or vehicle control, each patient was challenged with an intravenous bolus injection of endotoxin (2 ng/kg of BW). Patients were monitored closely for toxic effects of the drugs, and serial blood samples were drawn for hematologic changes and systemic cytokine release, as described in the Materials and Methods.
Schematic illustration of the study protocol. Twenty patients, divided in five dose subgroups (18.0 to 39.6 μg/kg of BW), were treated with seven intravenous bolus injections of SDZ MRL 953 at the indicated time points. One of four patients was randomized to receive double-blind vehicle control instead of SDZ MRL 953. Three days after the last injection of SDZ MRL 953 or vehicle control, each patient was challenged with an intravenous bolus injection of endotoxin (2 ng/kg of BW). Patients were monitored closely for toxic effects of the drugs, and serial blood samples were drawn for hematologic changes and systemic cytokine release, as described in the Materials and Methods.
In the second part of the trial, the influence of pretreatment with SDZ MRL 953 on a subsequent challenge with endotoxin was evaluated. Seventy-two hours after the last injection of SDZ MRL 953 or vehicle control (day 36, see Fig 1), each patient received an intravenous bolus injection of endotoxin (Salmonella abortus equi) in a dosage of 2 ng/kg of BW. To prevent severe constitutional symptoms, each patient received two doses of 800 mg of ibuprofen (Hoechst, Frankfurt, Germany) orally, the first administered 90 minutes before and the second at the time of endotoxin injection. As shown previously by us7 and others,6 preceding applications of ibuprofen at these time points do not compromise cytokine production to endotoxin. Vital signs were monitored half-hourly for 6 hours after injection, and blood samples were drawn for complete blood cell count and cytokine measurements before and 1.5, 3, 6, and 24 hours after injection. For serum chemistry and coagulation profile, samples were collected before and 6 and 24 hours after the administration of endotoxin.
Cytokine measurements.For cytokine assays, the blood samples were centrifuged and the serum was decanted within 10 minutes and stored at −70°C. Serum concentrations of G-CSF, TNF-α, IL-1β, IL-6, and IL-8 were assayed by enzyme-linked immunosorbent assay kits (Quantikine; R&D Systems, Inc, Minneapolis, MN) following the manufacturer's instructions. The sensitivities for the assays were 10.9, 0.18, 0.1, 0.7, and 18.1 pg/mL for G-CSF, TNF-α, IL-1β, IL-6, and IL-8, respectively.
Statistical analysis.Where not stated otherwise, calculated values are expressed as the mean ± SEM. Statistical analysis (Student's t-test for unpaired samples) was performed using commercially available software (Fig. P, Biosoft, Cambridge, UK) and was reported in the text. P values of <.05 were considered to be significant and are marked in the figures. The data of adjacent dose subgroups were pooled only when differences between them did not reach statistical significance.
RESULTS
The study was conducted in two parts. First, SDZ MRL 953 was tested with respect to biologic response and safety of administration in humans. Second, the effect of pretreatment with SDZ MRL 953 on a subsequent endotoxin challenge was investigated.
Toxicity profile of SDZ MRL 953.The side effects in the patients, being treated in the trial with doses ranging from 18.0 to 39.6 μg/kg of BW, are specified in Table 1. The most frequent adverse event observed in reponse to SDZ MRL 953 was fever. Seven of 15 patients experienced an elevation of body temperature greater than 38°C, with 2 of them having a temperature greater than 40°C. The appearance of fever was transient, almost invariably peaking at 8 to 12 hours after injection and coming back to normal after 24 hours at the latest. In 3 patients, fever was accompanied by a single episode of chills. Hepatic toxicity was weak, consisting of an isolated transient increase in bilirubin level in 7 patients. This effect was most pronounced in 1 patient with extensive liver metastases, whose baseline bilirubin level of 1.2 mg/dL increased to 3.2 mg/dL 24 hours after injection and returned to normal 48 hours later. Elevation of serum levels or activities of AST/ALT, alkaline phosphatase, or γ-GT was not found in any patient. Renal function parameters, such as creatinine, were not affected by SDZ MRL 953. No case of hypotension was seen, whereas blood pressure was elevated temporarily in 3 patients. Besides fever or chills, some patients complained about mild fatigue. A clear correlation between dose and toxicity was not obvious in our patients. The MTD of SDZ MRL 953, as defined above, was not reached up to a dose level of 39.6 μg/kg of BW. In the 5 patients treated with vehicle control, no significant adverse events occured.
Biologic reactions to SDZ MRL 953.Cytokine serum concentrations and changes of white blood cells in response to SDZ MRL 953 are depicted in Figs 2 and 3.
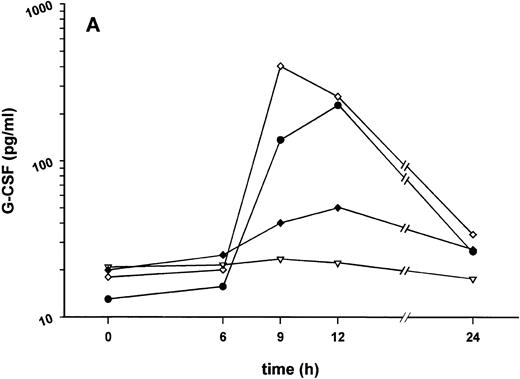
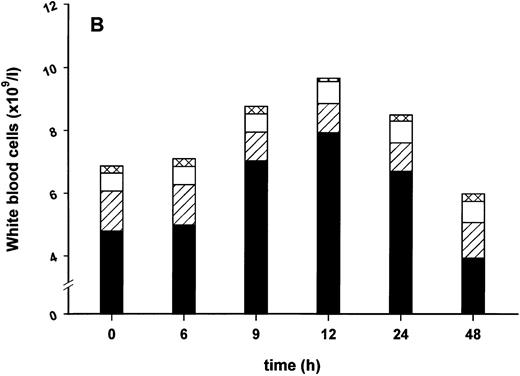
Effect of SDZ MRL 953 on serum levels of G-CSF and white blood cell counts in cancer patients. (A) Mean values of G-CSF serum concentrations in patients after the first of seven injections of (▿) vehicle control (n = 5) or SDZ MRL 953, subdivided into three dose groups: (♦) 18.0 μg/kg (n = 3), (⋄) 23.4 and 28.8 μg/kg (n = 6), and (•) 34.2 and 39.6 μg/kg (n = 6). Statistics of the highest dose group are included in the text. (B) Mean values of white blood cells, specified in neutrophil granulocytes (solid box), lymphocytes (single dashed box), monocytes (open box), and other leukocytes (double-dashed box), in patients after the first of seven injections of SDZ MRL 953 (34.2 and 39.6 μg/kg, n = 6).
Effect of SDZ MRL 953 on serum levels of G-CSF and white blood cell counts in cancer patients. (A) Mean values of G-CSF serum concentrations in patients after the first of seven injections of (▿) vehicle control (n = 5) or SDZ MRL 953, subdivided into three dose groups: (♦) 18.0 μg/kg (n = 3), (⋄) 23.4 and 28.8 μg/kg (n = 6), and (•) 34.2 and 39.6 μg/kg (n = 6). Statistics of the highest dose group are included in the text. (B) Mean values of white blood cells, specified in neutrophil granulocytes (solid box), lymphocytes (single dashed box), monocytes (open box), and other leukocytes (double-dashed box), in patients after the first of seven injections of SDZ MRL 953 (34.2 and 39.6 μg/kg, n = 6).
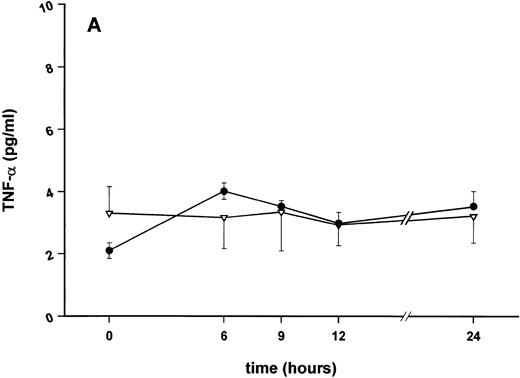
Effect of SDZ MRL 953 on serum levels of proinflammatory cytokines in cancer patients. Serum concentrations of (A) TNF-α, (B) IL-1β, (C) IL-8, and (D) IL-6 in patients after the first of seven injections of (•) SDZ MRL 953 (34.2 and 39.6 μg/kg, n = 6) or (▿) vehicle control (n = 5). Data are expressed as the mean values ± SEM.
Effect of SDZ MRL 953 on serum levels of proinflammatory cytokines in cancer patients. Serum concentrations of (A) TNF-α, (B) IL-1β, (C) IL-8, and (D) IL-6 in patients after the first of seven injections of (•) SDZ MRL 953 (34.2 and 39.6 μg/kg, n = 6) or (▿) vehicle control (n = 5). Data are expressed as the mean values ± SEM.
Intravenous bolus injections of SDZ MRL 953 induced, to a certain extent in a dose-dependent manner (Fig 2A), the release of measurable amounts of G-CSF, although statistical significance was not reached between dose subgroups and placebo. In the patient group comprising the two highest dose levels (34.2 and 39.6 μg/kg of BW), serum concentrations of G-CSF increased from baseline levels of less than 30 pg/mL to maximum levels of 227.4 ± 142.5 pg/mL (placebo, 22.2 ± 3.0 pg/mL; P = .209), peaking at 9 to 12 hours after injection and returning near to baseline after 24 hours (Fig 2A). Peak serum concentration showed marked interindividual differences, ranging from 24 to 929 pg/mL. Elevated concentrations of G-CSF were paralleled by a leukocytosis, predominantly caused by neutrophil granulocytes (Fig 2B), whereas changes in neither G-CSF serum levels (Fig 2A) nor white blood cell counts (data not shown) were found in response to vehicle control.
Serum concentrations of TNF-α, IL-1β, IL-8, and IL-6, obtained in response to SDZ MRL 953 in the patients receiving the highest doses (34.2 and 39.6 μg/kg) of the substance, are shown in Fig 3. SDZ MRL 953 caused only a slight, if any, elevation of serum concentrations of TNF-α, IL-1β, and IL-8. Peak levels were 4.0 ± 0.2 pg/mL at 6 to 9 hours for TNF-α (placebo, 3.3 ± 1.2 pg/mL; P = .452; Fig 3A), 0.8 ± 0.4 pg/mL at 9 hours for IL-1β (placebo, 0.5 ± 0.3 pg/mL; P = .429; Fig 3B), and 57.8 ± 7.2 pg/mL at 9 hours for IL-8 (placebo, 45.1 ± 9.9 pg/mL; P = .344; Fig 3C), thus being at the respective limit of detection. In contrast, considerable, although nonsignificant, amounts of circulating IL-6 could be found in the sera of SDZ MRL 953–treated patients. Serum levels of IL-6 before injection of SDZ MRL 953 were less than 10 pg/mL, increasing to peak levels of 74.3 ± 37.5 pg/mL (range, 9.7 to 276.8 pg/mL) at 9 to 12 hours (placebo, 9.5 ± 5.0 pg/mL; P = .202) and having almost decreased to baseline levels 24 hours after injection (Fig 3D).
None of the cytokines tested were found to be greater than baseline levels at 1.5 and 3 hours after injection of SDZ MRL 953, and it was confirmed that peak serum levels for each cytokine were reached at the latest 12 hours after injection (data not shown).
Repeated injections of SDZ MRL 953.The effect of repeated injections of SDZ MRL 953, administered in intervals of varying lengths (at days 1, 3, 8, 10, 29, 31, and 33), on the respective peak serum levels of G-CSF (reached at 9 to 12 hours after injection, as shown in Fig 2A) and the parallel increase in leukocyte counts is shown in Table 2.
The release of G-CSF in response to SDZ MRL 953 decreased with administration at short intervals (2 to 5 days), but recovered after an interval of about 3 weeks (day 29). Desensitization of G-CSF induction appeared to be incomplete, because no further reduction of peak G-CSF serum levels was seen from injections three to four (days 8 and 10).
The increase of white blood cells in response to SDZ MRL 953 did not exactly parallel the G-CSF pattern. However, the maximum leukocyte counts, induced by repeated injections of SDZ MRL 953, showed a certain degree of adaptation as well (Table 2).
Effect of pretreatment with SDZ MRL 953 on the endotoxin-mediated induction of cytokines and changes of white blood cells.In the second part of the study, the influence of pretreatment with SDZ MRL 953 on a subsequent intravenous bolus injection of endotoxin (Salmonella abortus equi) was evaluated. The time course of cytokine release in response to the endotoxin challenge, administered 72 hours after the last of seven applications of SDZ MRL 953 or vehicle control (day 36, see Fig 1), is shown in Figs 4 and 5A.
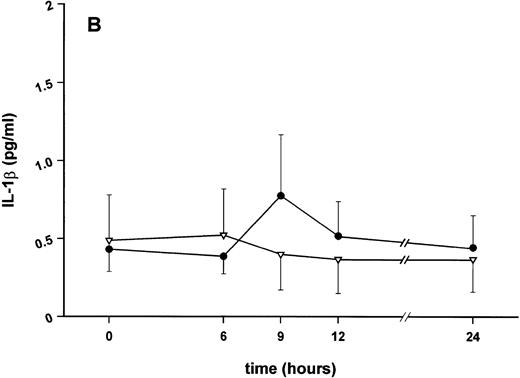
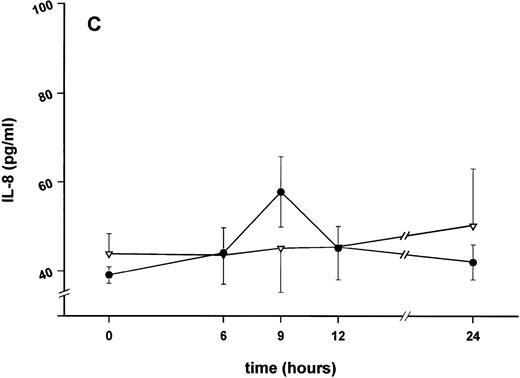
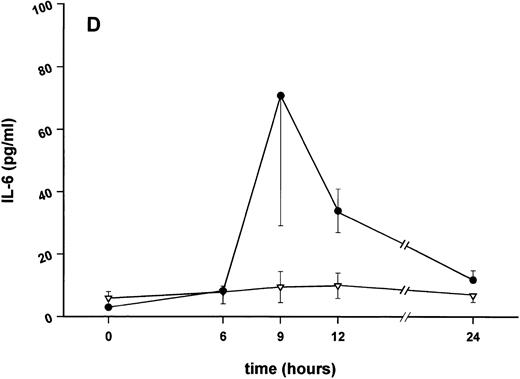
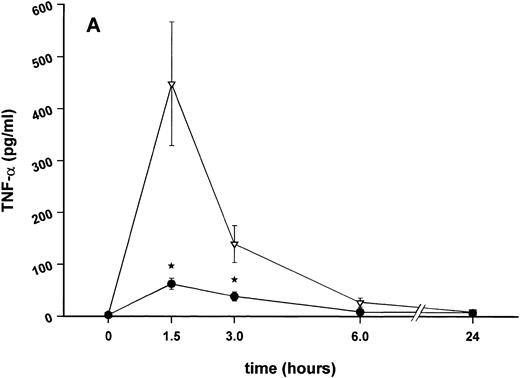
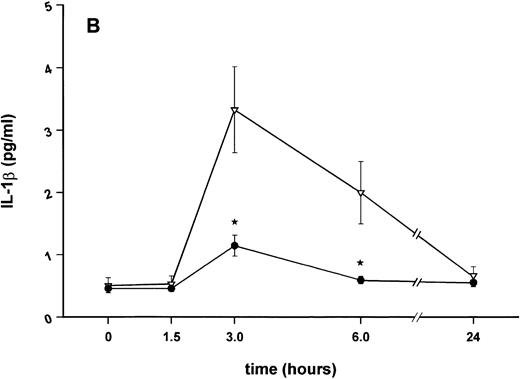
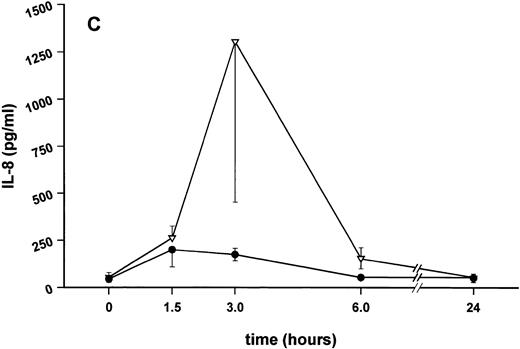
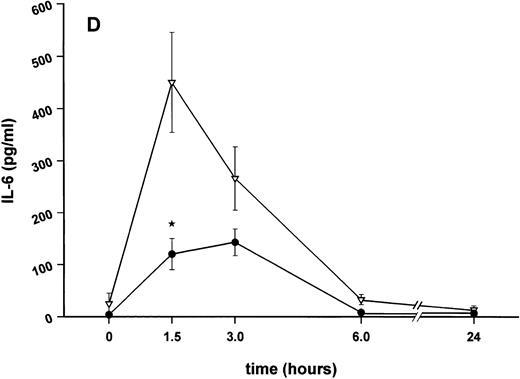
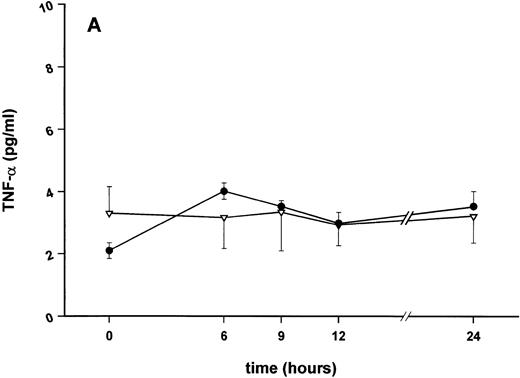
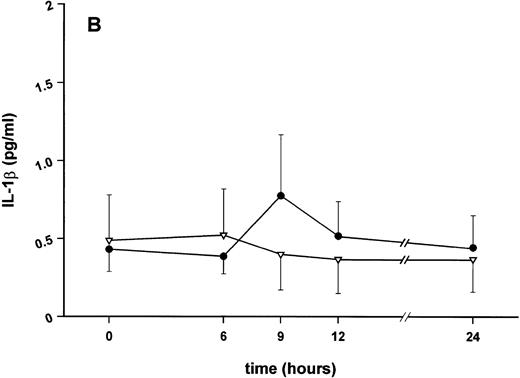
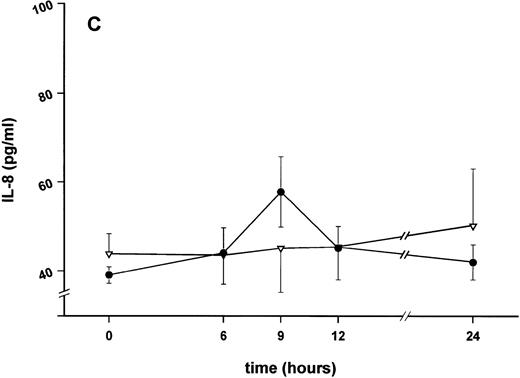
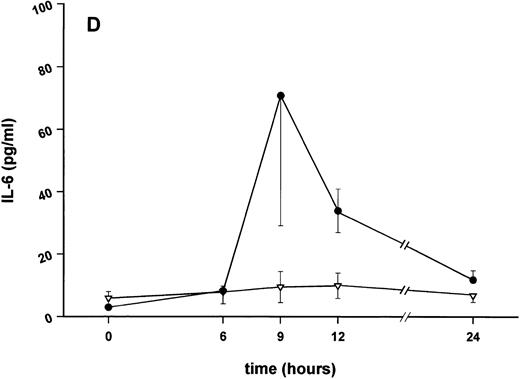
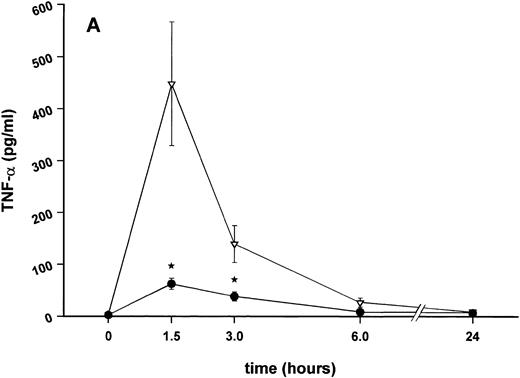
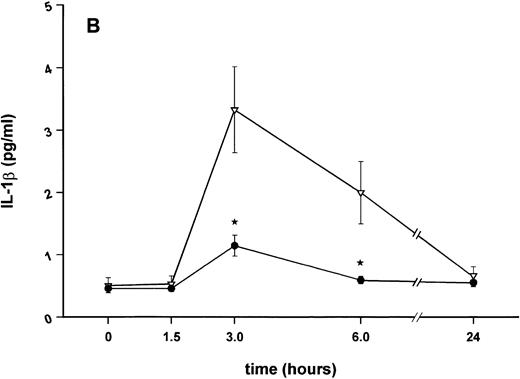
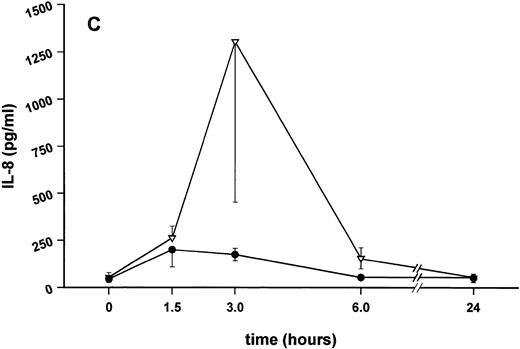
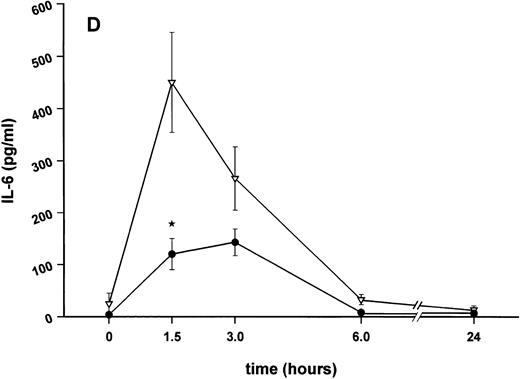
Influence of pretreatment with SDZ MRL 953 on the endotoxin-induced release of proinflammatory cytokines in cancer patients. Serum concentrations of (A) TNF-α, (B) IL-1β, (C) IL-8, and (D) IL-6 in patients after an intravenous bolus injection of LPS (2 ng/kg) administered 72 hours after the last of seven injections of (•) SDZ MRL 953 (18.0 to 39.6 μg/kg, n = 14) or (▿) vehicle control (n = 5). Data are expressed as the mean ± SEM. *P < .05 between groups.
Influence of pretreatment with SDZ MRL 953 on the endotoxin-induced release of proinflammatory cytokines in cancer patients. Serum concentrations of (A) TNF-α, (B) IL-1β, (C) IL-8, and (D) IL-6 in patients after an intravenous bolus injection of LPS (2 ng/kg) administered 72 hours after the last of seven injections of (•) SDZ MRL 953 (18.0 to 39.6 μg/kg, n = 14) or (▿) vehicle control (n = 5). Data are expressed as the mean ± SEM. *P < .05 between groups.
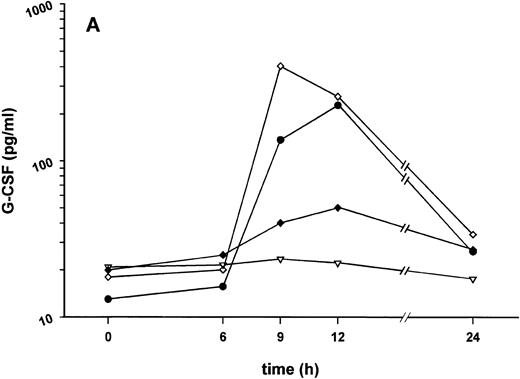
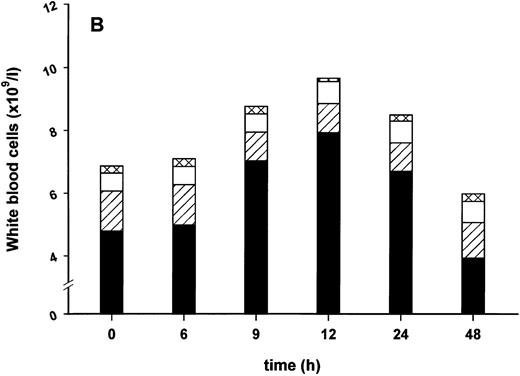
Influence of pretreatment with SDZ MRL 953 on the endotoxin-induced release of G-CSF and changes in neutrophil counts in cancer patients. (A) Serum levels of G-CSF and (B) percentage changes of neutrophil granulocytes in patients after an intravenous bolus injection of LPS (2 ng/kg) administered 72 hours after the last of seven injections of (•) SDZ MRL 953 (18.0 to 39.6 μg/kg, n = 14 [A] and n = 10 [B]) or (▿) vehicle control (n = 5). Data are expressed as the mean ± SEM. *P < .05, **P < .01 between groups.
Influence of pretreatment with SDZ MRL 953 on the endotoxin-induced release of G-CSF and changes in neutrophil counts in cancer patients. (A) Serum levels of G-CSF and (B) percentage changes of neutrophil granulocytes in patients after an intravenous bolus injection of LPS (2 ng/kg) administered 72 hours after the last of seven injections of (•) SDZ MRL 953 (18.0 to 39.6 μg/kg, n = 14 [A] and n = 10 [B]) or (▿) vehicle control (n = 5). Data are expressed as the mean ± SEM. *P < .05, **P < .01 between groups.
As compared with vehicle control, pretreatment with SDZ MRL 953 caused a marked and significant reduction in the endotoxin-mediated production of the proinflammatory cytokines TNF-α, IL-1β and IL-6 (Fig 4A, B, and D). This induction of tolerance to endotoxin appeared to be most pronounced for TNF-α. In SDZ MRL 953-pretreated subjects, endotoxin-induced peak serum levels of TNF-α were 62.4 ± 10.3 pg/mL, compared with 447.9 ± 106.0 pg/mL in vehicle control-pretreated patients (P < .05; Fig 4A). Cytokine peak serum levels to endotoxin did not differ significantly with increasing doses of SDZ MRL 953 (P = .407 for TNF-α, comparing the lowest and highest subgroups) in the range tested (18.0 to 39.6 μg/kg of BW).
Similar to TNF-α, endotoxin-induced maximum serum levels of IL-1β (1.14 ± 0.17 v 3.33 ± 0.69 pg/mL; P < .05; Fig 4B) and IL-6 (143.0 ± 25.9 v 449.9 ± 95.6 pg/mL; P < .05; Fig 4D) were substantially lower after previous treatment with SDZ MRL 953 in comparison to vehicle control, whereas alterations in IL-8 levels did not reach significance (200 ± 92 v 1,304 ± 853 pg/mL; P = .256; Fig 4C).
The desensitization of biologic reactions to endotoxin by pretreatment with SDZ MRL 953 appeared not to be restricted to the release of proinflammatory cytokines but also included the production of G-CSF, peaking at 186.6 ± 54.8 pg/mL in SDZ MRL 953-pretreated and at 698.3 ± 210.6 pg/mL in vehicle control-pretreated patients (P = .07; Fig 5A).
In contrast to this finding, the increase in neutrophil granulocytes after the endotoxin application was not downregulated by pretreatment with SDZ MRL 953 (Fig 5B). After the injection of endotoxin, neutrophil counts increased up to 201.8% ± 16.8% of baseline levels in SDZ MRL 953-pretreated patients (peak levels at 3 hours after injection), hence occurring earlier and being more pronounced (P < .05) than the granulocytosis reaction in the control group (peak values, 144.0% ± 16.2% of baseline; maximum at 6 hours after injection).
DISCUSSION
SDZ MRL 953 is a synthetic monosaccharidic lipid A analog, which, administered prophylactically, proved to be protective in preclinical in vivo models of sepsis, while being at least 104 times less toxic than endotoxin in galactosamine-sensitized mice.33 34 The present double-blind, randomized, controlled study confirms the low toxicity of SDZ MRL 953 in cancer patients, presumably due to the small amount of cytokines being released. Furthermore, the systemic release of proinflammatory cytokines after an intravenous injection of endotoxin was markedly downregulated by pretreatment with SDZ MRL 953.
Tolerability and biologic effects of SDZ MRL 953.Intravenous application of SDZ MRL 953 in our patients was safe and well-tolerated. The doses of SDZ MRL 953 used in our trial (18.0 to 39.6 μg/kg of BW) were chosen according to the results of the preceding pilot phase, in which very low doses (1 ng/kg) up to the dose causing a minimal biologic response (18 μg/kg) were used. The MTD for SDZ MRL 953, up to a dosage of 39.6 μg/kg, has not been reached. Because the MTD for endotoxin (Salmonella abortus equi) was previously found to be 4 ng/kg of BW in a comparable population,8 SDZ MRL 953 was at least 104 times less toxic as compared with endotoxin. This is consistent with findings in hypersensitized mice.33
Apparently, the improved tolerability of SDZ MRL 953, as compared with endotoxin, was related to its low activity in inducing the secretion of proinflammatory cytokines. Particularly, TNF-α and IL-1β, which are primarily involved in mediating sepsis-related symptoms such as hypotension and shock,10,11 were not found to be significantly elevated in SDZ MRL 953-treated patients. No patient experienced hypotensive reactions. The transient increase of body temperature in some patients may be attributed to the moderate induction of IL-6, which is known to be pyrogenic15,16 and essential for endotoxin-mediated fever reactions.40
The almost complete absence of proinflammatory cytokines but induction of considerable amounts of G-CSF implicates a cytokine profile being markedly different from the endotoxin-induced cytokine pattern. The difference may be demonstrated by calculating the ratios of the TNF-α to G-CSF peak levels, which are about 8/1 for endotoxin (Salmonella abortus equi, 4 ng/kg of BW)41 and 1/60 for SDZ MRL 953 (34.2 and 39.6 μg/kg). This finding is paralleled by in vitro studies showing SDZ MRL 953 to be the least potent of six synthetic lipid A analogs and lipid A itself in stimulating the release of TNF-α in peritoneal macrophages.32 Furthermore, SDZ MRL 953–mediated serum levels of TNF-α were 10-fold lower than those of G-CSF in rhesus monkeys.39
The mode of action of SDZ MRL 953 is still incompletely understood. In vitro studies suggest that SDZ MRL 953 may act independently of CD14,42 a glycosyl-phosphatidylinositol–anchored membrane protein known to be essential for endotoxin signaling.43 Unlike endotoxin, SDZ MRL 953 was not able to activate endothelial cells in the presence of soluble CD14, and its action on TNF-α release in human peripheral blood mononuclear cells could not be blocked by monoclonal antibodies to CD14.42 Taken together, these data point to cell receptors distinct from CD14 by which SDZ MRL 953 activates the target cells. Indeed, such receptors have also been identified for endotoxin44,45 and have been postulated to mediate endotoxin signaling cooperatively45 or independently44 of CD14. Although little is known about the functional significance of different membrane receptors in endotoxin and lipid A signaling, it may be an explanation for the substantially diverse cytokine profile induced by endotoxin and SDZ MRL 953. Additionally, distinct signaling pathways used by SDZ MRL 953 and endotoxin could account for the apparent different kinetics of cytokine release in response to either substance (compare Fig 2A with Fig 5A and Fig 3 with Fig 4), but might also be due to the liposomal formulation of SDZ MRL 953, thus causing delayed effects of the substance.
Recently, monophosphoryl lipid A (MLA), another derivative of lipid A, has been investigated in healthy volunteers.46 Although pretreatment with MLA downregulated the endotoxin-mediated release of proinflammatory cytokines, the compound itself (20 μg/kg) stimulated the systemic release of considerable amounts of TNF-α, IL-6, and IL-8, which may be partly responsible for the rather high percentage of adverse events (especially fever and chills) that has been reported. Thus, the principal difference of SDZ MRL 953 as compared with endotoxin, lipid A, and other related substances that have been studied clinically so far is the characteristic and, in terms of tolerability, apparently favorable cytokine pattern induced by SDZ MRL 953.
Effect of pretreatment with SDZ MRL 953 on reactions to endotoxin.Injection of endotoxin (Salmonella abortus equi, 2 ng/kg) in the control patient group, having been pretreated with liposome carriers without SDZ MRL 953, elicited the well-known cytokine response,4-9,29,41,46 47 consisting of high serum concentrations of TNF-α, IL-6, IL-8, and G-CSF, and a small, but consistent increase in serum levels of IL-1β.
Pretreatment with SDZ MRL 953 substantially downregulated the endotoxin-induced release of all the proinflammatory cytokines tested and of G-CSF as well. This is in contrast to in vitro findings in human peritoneal macrophages, in which SDZ MRL 953 downregulated the production of TNF-α and IL-6, but upregulated the endotoxin-induced production of IL-1β and G-CSF.48 This discrepancy is most likely due to the different settings, comparing isolated macrophages with the whole body system. Such a lack of correlation between different systems, with respect to the effects of endotoxin itself28 and G-CSF49,50 on the endotoxin-mediated cytokine release, has been shown before by us and others.
However, no discrepancy exists when comparing the three compounds MLA,46 SDZ MRL 953 (this study), and endotoxin itself28 by their influence on the endotoxin-induced cytokine production. Although it seems to be attractive to assume a common mode of action, the mechanism remains speculative. It has been shown that endotoxin tolerance is determined at the level of the monocytes/macrophages,30 and there is some evidence that it is mediated by other molecules rather than by the tolerance inducing agent itself. First, tolerance with respect to cytokine production can be induced by transferring the supernatant of endotoxin-treated macrophages to naive cells51 and, second, can be mimicked, at least partially, by cytokines in vitro52-54 and in vivo.55 In our trial, TNF-α or IL-1β most probably did not play a major role, because only little, if any, of these cytokines could be detected in response to SDZ MRL 953. However, G-CSF was found in considerable amounts in the sera of SDZ MRL 953–treated patients and has previously been shown to bias levels of cytokine production in whole blood stimulated ex vivo with endotoxin.49 However, in vivo, a recent study by Pollmächer et al50 in healthy volunteers showed that human recombinant G-CSF enhanced cytokine reactions to endotoxin. Therefore, other mediators, such as anti-inflammatory cytokines, are more likely to mediate endotoxin tolerance in vivo. IL-10 was found to decrease the endotoxin-induced release of monokines,56 and monoclonal antibodies to IL-10 and TGF-β were able to abolish the endotoxin-mediated induction of endotoxin tolerance in vitro.53 However, the in vivo significance of anti-inflammatory cytokines for mediating endotoxin tolerance has still to be elucidated.
On the cellular level, little is known about the mechanism that renders monocytes insensitive to endotoxin. It is unlikely that endotoxin receptors are altered in endotoxin tolerance, because several groups found endotoxin binding or expression of CD14 not to be downregulated in endotoxin-treated monocytes or macrophages.57,58 This result is in accordance with our findings of an unchanged expression of CD14 on monocytes in patients before and after treatment with SDZ MRL 953 (data not shown). The fact that the in vivo tolerance includes a whole array of cytokines may suggest a mechanism early in the endotoxin signaling pathway. Alternatively, the transcription of distinct cytokine genes may be affected at points at which their signaling pathways cross-talk, for instance at the transcriptional level, where a complex interaction of transcription factors is known to specifically regulate cytokine gene transcription.59 Indeed, as shown by studies of Ziegler-Heitbrock et al,58 modification of transcription factors may be involved in endotoxin tolerance in vitro.
Remarkably, the rebound increase of neutrophil granulocytes after the administration of endotoxin is enhanced rather than downregulated in patients being pretreated with SDZ MRL 953, although the amount of endotoxin-induced G-CSF is markedly reduced (Fig 5). An enhancement of the leukocytosis reaction has been described before by Liehl et al39 in granulocytopenic mice when the animals had been pretreated with SDZ MRL 953. This phenomenon has been discussed as being the result of a recruitment of mature neutrophils from the bone marrow, presumably prefilled by the preceding applications of SDZ MRL 953. This assumption may be supported by our findings that the endotoxin-induced granulocytosis was not merely more pronounced, but also started earlier after pretreatment with SDZ MRL 953. Another, even more speculative, explanation would be that another hematopoietic growth factor or hormone, such as granulocyte-macrophage colony-stimulating factor, IL-3, or cortisone, had been stimulated by SDZ MRL 953.
Notably, phagocytes from SDZ MRL 953-treated mice were primed for an increased respiratory burst in vitro,33,34,39 and this effect was even enhanced after repeated injections of the compound. Thus, whereas the proinflammatory cytokine response was substantially downregulated,39 the phagocytic cells were activated by the SDZ MRL 953 pretreatment, an effect that could be related to the animals' increased resistance to bacterial infections.33,34 39
Although the clinical significance for the latter has yet to be proven, SDZ MRL 953 meets important conditions for its prophylactic employment in patients at risk for gram-negative infections. First, its administration is safe and well-tolerated. Second, it effectively downregulates the proinflammatory cytokine response to an endotoxin challenge used as a model for gram-negative infection. Third, supported by its ability to induce the production of G-CSF as well as to enhance the subsequent granulocytosis reaction to endotoxin, it potentially stimulates the unspecific immune resistance expressed by an increased pool of primary defense cells. Although it may be tempting to speculate about an additional functional priming of those cells, as suggested by the above-mentioned animal studies, this still has to be confirmed in the clinical setting.
It may be advantageous that treatment with SDZ MRL 953 does not completely suppress the endotoxin-mediated cytokine response, because it has been shown that small amounts of TNF-α and IL-1β are necessary for the immune response to infection in animals60,61 and that mice deficient for the 55-kD TNF-receptor are resistant to endotoxin but are not capable of defying bacterial pathogens.62,63 In addition, a recent phase III study of a TNF receptor:Fc fusion protein in patients with septic shock showed that neutralization of circulating TNF-α might be associated with an even increased mortality.26 Thus, inflammatory mediators, although being deleterious when excessively produced, perform important functions in infection defense, and it should be favorable to inhibit them partially rather than completely to limit the harmful, yet preserve the necessary beneficial effects. Furthermore, because the development of sepsis is a multifactorial process, it may be more promising to downregulate the whole array of endotoxin-mediated reactions rather than to interfere with one specific factor. The time-point of therapeutical intervention may be crucial. There are many hints that, once the escalating immune response is initiated, it may hardly be controllable. Hence, it might be reasonable to treat patients at risk for severe infections prophylactically.
Although the phase I character of the present study limited the number of individuals in the respective dose subgroups, the findings indicate a prospective role for a prophylactic use of nontoxic lipid A derivatives in patients at risk for gram-negative infections. It was demonstrated that seven injections of SDZ MRL 953, applied over a period of 33 days, resulted in a significant, but relative, tolerance to endotoxin for at least 3 days after the last injection. Potential patient groups that could benefit from such a prophylactic treatment may include those undergoing myelosuppressive chemotherapy (eg, between subsequent therapy cycles) or major surgery. However, any schedule for clinical application will depend on the extended knowledge of the earliest start and the longest duration of the tolerance and the dose and number of injections of SDZ MRL 953 being necessary to induce this presumably protective effect in infection and sepsis.
ACKNOWLEDGMENT
The excellent technical assistance of Marlies Braun, who performed the cytokine measurements, is gratefully acknowledged. The authors thank Lilo Kuner for helping with the study documentation.
Supported by Sandoz Pharma Ltd (Nuremberg, Germany).
Address reprint requests to Rupert Engelhardt, MD, Division of Hematology/Oncology, Department of Internal Medicine I, University Hospital of Freiburg, Hugstetter Str. 55, D-79106 Freiburg, Germany.

![Fig. 5. Influence of pretreatment with SDZ MRL 953 on the endotoxin-induced release of G-CSF and changes in neutrophil counts in cancer patients. (A) Serum levels of G-CSF and (B) percentage changes of neutrophil granulocytes in patients after an intravenous bolus injection of LPS (2 ng/kg) administered 72 hours after the last of seven injections of (•) SDZ MRL 953 (18.0 to 39.6 μg/kg, n = 14 [A] and n = 10 [B]) or (▿) vehicle control (n = 5). Data are expressed as the mean ± SEM. *P < .05, **P < .01 between groups.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/4/10.1182_blood.v90.4.1673/3/m_bl_0035f5a.jpeg?Expires=1768178388&Signature=ymxS53Gm31R6L1pmoOv-7jyDzomHbnXmi8euHBqkrsas8-wnj5f~Gt0PPD~hOOaWgcPfJEFj-o4VulkrdL3Gq6EWy3T-KLOTctHcmud4ngIqvCT1mJddHQ1bJTi-pyFcOj0R4anAB5iEVc6WL9ckFk9Pa1eSly3LbFAVZQG~8ZmHbEOu1s2CoPFpN1GVhPgEflJtIGEcmssm5zI12AJ723zqEz2fGCS~kbhMI2ApKFyZ6Z8ylv4gE5Qhn2CbhDNNl02YWDvQg47SW1H0U9QwlxAVmKCdGDEF6Z0va50Xz50izPO0-ogx-1p1ZMVDAh7jZADrkF8Pfq3rrwcZdM2uSg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Influence of pretreatment with SDZ MRL 953 on the endotoxin-induced release of G-CSF and changes in neutrophil counts in cancer patients. (A) Serum levels of G-CSF and (B) percentage changes of neutrophil granulocytes in patients after an intravenous bolus injection of LPS (2 ng/kg) administered 72 hours after the last of seven injections of (•) SDZ MRL 953 (18.0 to 39.6 μg/kg, n = 14 [A] and n = 10 [B]) or (▿) vehicle control (n = 5). Data are expressed as the mean ± SEM. *P < .05, **P < .01 between groups.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/4/10.1182_blood.v90.4.1673/3/m_bl_0035f5b.jpeg?Expires=1768178388&Signature=Eky3a~RvkP9jSRhb2BTYPkF042AqCAQ-PnZGxRY2p60~iCyJ1hVryNyT74aVBgzx7bDTaM78jhyegedc4FQuxa34SwQpXM~-Y36B1Fekb7gAyX1ohmc7giYulbawl4jvKQybDviAHHz4GQ3eZxgP7zzdRzNhUZYpLX4nPjWGq0BqgU8Q3SfNsp7BO1mxOxCqAgNUCNykBJPIkrTejuIGCCG8lQYBtNSg6RYgpsWH4PLD7vyYwUqVr37lpF81cRx89jTvW3fdVCbpccVPZ0XGlQapz9RyBLUydb3aUZaR488tYy3Z8YATCabmzoUsR-7X1WEKRP45rluketgKHcndbA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 5. Influence of pretreatment with SDZ MRL 953 on the endotoxin-induced release of G-CSF and changes in neutrophil counts in cancer patients. (A) Serum levels of G-CSF and (B) percentage changes of neutrophil granulocytes in patients after an intravenous bolus injection of LPS (2 ng/kg) administered 72 hours after the last of seven injections of (•) SDZ MRL 953 (18.0 to 39.6 μg/kg, n = 14 [A] and n = 10 [B]) or (▿) vehicle control (n = 5). Data are expressed as the mean ± SEM. *P < .05, **P < .01 between groups.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/4/10.1182_blood.v90.4.1673/3/m_bl_0035f5a.jpeg?Expires=1768178389&Signature=VczGQ7WCszjCKkM9wYbfp62QfX0tFfpD3ANTI3NQ6gJnHFvzLMiPWemOhxcow4BXgXjltS8PAobj7tKOfuL2SPAVx9wBA82ejz2yDhg4bduA~fMvmRA002BC89oHvYCSAhjvQcunvfTXXtMU8GN4QZQV04fQwN7~Q9dEjs0i0cPvGlMFz8b93Ydtv3r~MfEzzQKtlyoy4fhu~FSAFQ5zTSGPDpVijSMX3oiyrKubD8rKHKO~wQqtsX-gFwH71OLXvcD0Gb4mFBVVC-3sav7bVrWCdNwMc6WQtXBnvwL7yXv~kZx1CE9SRATi8XI-QVpoXx3Iwfd6ymw6g~98vPrGqw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Influence of pretreatment with SDZ MRL 953 on the endotoxin-induced release of G-CSF and changes in neutrophil counts in cancer patients. (A) Serum levels of G-CSF and (B) percentage changes of neutrophil granulocytes in patients after an intravenous bolus injection of LPS (2 ng/kg) administered 72 hours after the last of seven injections of (•) SDZ MRL 953 (18.0 to 39.6 μg/kg, n = 14 [A] and n = 10 [B]) or (▿) vehicle control (n = 5). Data are expressed as the mean ± SEM. *P < .05, **P < .01 between groups.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/4/10.1182_blood.v90.4.1673/3/m_bl_0035f5b.jpeg?Expires=1768178389&Signature=Brw3fd7~Z-mxFDdKpP~tRqHoD022ucb6qjG96WVdQMC0uuEa1-TUIaSef5vXGE4R-bWchvlVRD87TYyTKcXBmXjPanfYVb2Ugg-RKIqAVVeILWYgJOUdoEPDXHlZmbHBIC4lsS0JFcmLcPrygSvOMO3EF2WGDA8ny3HXWWZwaMVOvJ3Dju4DSYrunnAY0wQE0GJwwwP7dNdIisZXaHCdnSSRd4AXOUtWycVerOy3MX~SaDQ1UVFv64xkxhC9ANgz8WShF80L~2ksXY1HXXnhEuszRshaFxcxaxnTYUjkzlURMlsGBygBJxdDPR0ljHT7WiVmBRz3E9aWAIuriUg4wg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)