Abstract
The interleukin-3 (IL-3)–dependent murine bone marrow–derived cell line FDC-P2/185-4 (185-4) undergoes apoptosis when IL-3 is withdrawn from culture medium. Previous results from our studies indicated that a high concentration of aggregated mouse IgG prevented apoptosis of 185-4 cells through FcγRIII by an autocrine mechanism, producing IL-3. But after 24 hours, 185-4 cells expressed CD95 (Fas/Apo-1) on their surfaces on stimulation via FcγRIII. In addition, this CD95 was functional and apoptosis was induced by anti-CD95 monoclonal antibody (MoAb). We investigated how these conflicting effects were induced by FcγRIII stimulation within the context of cell survival and death. The results showed that IL-3 was induced by calcium ionophore and that the IL-3 induced by FcγRIII stimulation was blocked by EGTA or FK506, but not by staurosporine (protein kinase C [PKC] inhibitor), indicating the important role of calcium-calcineurin in this system. On the other hand, the CD95 expression induced by FcγRIII stimulation was blocked by staurosporine, but not by EGTA or FK506, and phorbol myristate acetate (PMA) induced CD95 expression in the same manner as FcγRIII, indicating the involvement of PKC in the CD95 expression induced by FcγRIII stimulation. Thus, FcγRIII-mediated stimulation even while promoting immediate survival of the bone marrow cells, also triggers mechanisms that will facilitate their eventual deletion at the end of the response. These results suggest that a balance between cell survival and death is maintained to avoid unlimited cell growth caused by FcγRIII-ligand interaction in hematopoiesis during inflammation.
FCγR MEDIATES BOTH humoral and cellular immunity by immune complexes. Immune complexes are important as mediators of type II and III allergy. Knockout mice of FcγR γ chain or FcγRIII do not develop either antibody-dependent cellular cytotoxicity (ADCC) or Arthus reaction, indicating the key role of FcγRIII in both type II and type III allergy.1-3 FcγR-mediated stimulation induces many inflammatory cytokines such as interleukin-1 (IL-1), IL-6, IL-8, tumor necrosis factor (TNF )α, granulocyte colony-stimulating factor (G-CSF ), and granulocyte-macrophage colony-stimulating factor (GM-CSF ) in monocytes.4-8 We also reported that IL-3 was induced by cross-linking of FcγRIII in bone marrow-derived cell line.9 Thus, it is thought that the FcγR, especially FcγRIII plays an important role in the integration of immune responses such as acute and chronic inflammation. On the other hand, the FcγR mediates apoptosis in IL-2–stimulated human natural killer (NK) cells and in CD4 lymphocytes stimulated with chimaeric anti-CD4 monoclonal antibody (MoAb).10,11 Moreover, NK cells activated by anti-FcγRIII MoAb facilitate cell-mediated cytotoxicity and subsequent apoptosis by expressing Fas ligand (FasL).12 Thus, FcγR-mediated stimulation not only activates cells, but also depletes them thereafter. FDC-P2/185-4 (185-4) is an IL-3–dependent murine bone marrow-derived cell line that expresses FcγRIII on the surface. We reported that 185-4 cells, stimulated via FcγRIII produced IL-3 and proliferated by an autocrine mechanism.9 We postulated that, although 185-4 cells proliferated on FcγRIII stimulation, negative feedback avoids unlimited cell growth under physiologic hematopoiesis. Therefore, we investigated the experiments to determine whether negative regulation is a feature of our system by studying CD95 expression.
CD95 (Fas/Apo-1) is a cell-surface receptor, belonging to the family of nerve growth factor (NGF )/TNF receptors.13 It is expressed in various tissues, such as thymus, heart, liver, lungs, and ovaries.14 CD95 is also expressed in activated lymphocytes and in leukemia cell lines. Some anti-CD95 MoAb or FasL can induce apoptotic death in cells expressing CD95.15,16 Similarly, antibodies to CD3/T-cell receptor (TCR) complex induce the apoptosis after activation of immature thymic T cells in vitro and in vivo.17,18 This phenomenon is known as activation-induced cell death (AICD). The mechanisms of this type of programmed cell death have been extensively studied in T cells and CD95 plays a critical role in AICD.19-21 Here we examined the FcγRIII-mediated cell growth and induction of apoptosis through CD95 pathway in 185-4 cells. Our data provide evidence of the induction and the role of CD95 in regulating the balance between cell growth and death and shows the mechanisms of simultaneous signal transduction towards opposite direction.
MATERIALS AND METHODS
Cells and cell culture.FDC-P2/185-4 (185-4) is a murine IL-3–dependent bone marrow–derived cell line. It was established in our laboratory as the FcγRIII positive clone with the proliferative response to serum of MRL/lpr mouse22 from FDC-P2 cells originally established by Dexter et al.23 The lineage and the stage of differentiation of the FDC-P2/185-4 cells are not well known, but they are thought to be myeloid precursors as described previously.9 The cells were maintained in RPMI 1640 containing 10% heat-inactivated fetal calf serum (FCS) (GIBCO-BRL, Gaithersburg, MD), 2 mmol/L glutamine, 1 × 10−5 mol/L 2-Mercaptoethanol (2-ME), and 100 μg/mL kanamycin and maintained at 37°C in a 5% CO2 atmosphere. For the growth of 185-4 cells, the culture medium was supplemented with 10% WEHI-3 conditioned medium as a source of IL-3.
Antibodies and other reagents.Purified mouse IgG was purchased from Organon Teknika (West Chester, PA). Mouse IgG was aggregated by heating at 63°C for 20 minutes before use as described.9 A hamster MoAb recognizing mouse CD95 (Jo-2) was purchased from PharMingen (San Diego, CA). A rat MoAb 2.4G2, which recognizes mouse FcγRII/III, was a gift from Dr M. Inaba (Kansai Medical University, Osaka, Japan). Normal hamster IgG and normal rat IgG were purchased from Cedarlane (Ontario, Canada) and Organon Teknika (Durham, NC), respectively. Calcium ionophore (A23187), EGTA, PMA, and staurosporine were from Sigma Chemical Company (St Louis, MO). FK506 was a gift from Fujisawa Pharmaceutical Co, Ltd (Osaka, Japan).
IL-3 detection by bioassay and reverse transcriptase-polymerase chain reaction (RT-PCR).The 185-4 cells (5 × 106) were cultured in 1 mL of medium containing 50 μg of aggregated IgG and/or other reagents in 24-well culture plates. After 3 hours, culture supernatant was separated and used for the IL-3 assay. The culture supernatant was dialyzed against RPMI medium, then incubated with 1 × 104 of IL-3–dependent cells (FDC-P2) in 96-well flat-bottomed microtiter plates and cell growth was measured by the MTT assay. The specificity was cofirmed by using anti–IL-3 antibody (Ab). The rIL-3 concentration was assayed to generate a standard curve. The IL-3 concentration of each sample was then calculated by using Probit analysis.24 After the culture supernatant was separated, total RNA was extracted from 5 × 106 of stimulated 185-4 cells using guanidium isothiocyanate. Total RNA (5 μg) was reverse transcribed (RT) and the products obtained by RT were amplified by PCR using the sense primer, 5′ GGGAAGCTCCCAGAACCTGA3′ and the antisense primer, 5′ GTCATCCAGATCTCGAATGA3′, on a thermal cycler (Atto Corp, Tokyo, Japan). Amplification proceeded at 94°C for 1 minute, 55°C for 1 minute, and 72°C for 2 minutes. Each cDNA was amplified by 30 cycles.
CD95 expression by flow cytometry.The 185-4 cells were stimulated by reagents with or without PKC inhibitor for the indicated periods. Cell surface CD95 was stained by single color indirect immunofluorescence. The first Ab was a hamster anti-mouse CD95 MoAb or a normal hamster IgG as the negative control. Fluorescein isothiocyanate (FITC)-conjugated goat F(ab′)2 fragment anti-hamster IgG Ab was the second. The FITC-conjugated second Ab did not cross-react with mouse or rat IgG. We set the CD95 negative or positive region of stained cells and analyzed on an EPICS PROFILE flow cytometer (Coulter Electronics Inc, Hialeah, FL).
Measurement of apoptosis.Apoptotic cell death can be measured by propidium iodide (PI) uptake. Apoptotic cells are PI positive and their size of DNA is smaller than that of viable cells. We applied this procedure for 185-4 cells as described.9 We stimulated 185-4 cells with aggregated IgG or PMA for 24 hours, then added 1 μg/mL of anti-CD95 MoAb. Cells were incubated for the indicated period and washed twice with phosphate-buffered saline (PBS). Each pellet was dissolved in hypotonic fluorochrome solution (PI 50 μg/mL in 0.1% sodium citrate containing 0.1% Triton X-100). Samples were placed in the dark overnight before flow cytometry. PI fluorescence was measured using EPICS PROFILE flow cytometer. Debris was excluded from the analysis by electronic gating of the forward and side scatter measurements. The data were plotted on a logarithmic scale.
DNA fragmentation assay.To analyze the DNA fragmentation, 5 × 106 cells were collected by centrifugation at 300g and resuspended in 400 μL of 10 mmol/L Tris-HCl (pH 8.0), 0.1 mmol/L EDTA (pH 8.0), 1% sodium dodecyl sulfate (SDS), and 100 μg/mL DNase-free RNase. Proteinase K was added 1 hour later at a final concentration of 100 μg/mL. After an overnight incubation at 37°C, DNA was extracted with phenol/chloroform, ethanol precipitated, and resuspended in 10 mmol/L Tris-HCl containing 1 mmol/L EDTA. DNA (5 μg) was mixed with 3 μL of loading buffer containing 60% glycerol, 0.1% xylene cyanol, and 0.1% bromphenol blue, and the electrophoresis proceeded on 1% agarose gel in Tris/acetic acid/EDTA buffer.
RESULTS
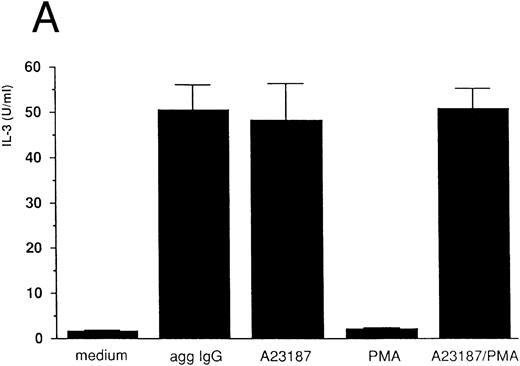
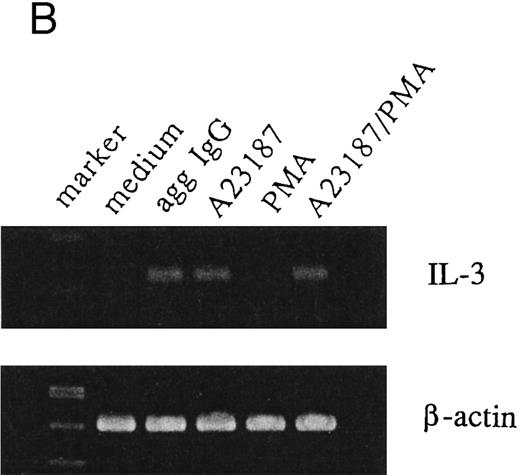
IL-3 induction by aggregated IgG or calcium ionophore in 185-4 cells.Previous results from our studies indicated that 185-4 cells underwent apoptosis when IL-3 was withdrawn from culture medium. In addition, a high concentration of aggregated IgG prevented the apoptosis of 185-4 cells through FcγRIII by an autocrine mechanism, producing IL-3.9 We performed experiments to determine the signal transduction of IL-3 production using stimulators of second messengers such as the calcium ionophore A23187, which induces intracellular calcium, PMA, which activates PKC, or a combination of both. As shown in Fig 1A, aggregated IgG induced IL-3 in 185-4 cells as described, and calcium ionophore also did so, but PMA did not. The same results were obtained at the mRNA level by RT-PCR as shown in Fig 1B. We synthesized specific primers for the cDNA of IL-3 and amplified 225-bp bands. Specific bands were evident on stimulation with aggregated IgG and calcium ionophore, but not by PMA. These results indicate that calcium, but not PKC, is important as the second messenger of IL-3 production induced by FcγRIII-ligand interaction. As shown in Fig 1A and B, the effects of calcium ionophore and PMA were not synergistic.
IL-3 production by stimulated 185-4 cells. (A) The 185-4 cells were cultured with medium alone, aggregated IgG (50 μg/mL), calcium ionophore A23187 (250 ng/mL), PMA (100 ng/mL), or a combination of A23187 and PMA. After 3 hours, culture supernatants were dialyzed. IL-3 was measured in each sample by a bioassay using IL-3–dependent cells as described in Materials and Methods. Data represent the means ± standard deviation (SD) of five replicates. (B) RT-PCR analysis of IL-3 production by 185-4 cells. The 185-4 cells were cultured with each stimulator for 3 hours. Total RNA (5 μg) from the cells was reverse transcribed and PCR amplified as described. PCR products were resolved in 2% agarose gel and visualized by staining with ethidium bromide.
IL-3 production by stimulated 185-4 cells. (A) The 185-4 cells were cultured with medium alone, aggregated IgG (50 μg/mL), calcium ionophore A23187 (250 ng/mL), PMA (100 ng/mL), or a combination of A23187 and PMA. After 3 hours, culture supernatants were dialyzed. IL-3 was measured in each sample by a bioassay using IL-3–dependent cells as described in Materials and Methods. Data represent the means ± standard deviation (SD) of five replicates. (B) RT-PCR analysis of IL-3 production by 185-4 cells. The 185-4 cells were cultured with each stimulator for 3 hours. Total RNA (5 μg) from the cells was reverse transcribed and PCR amplified as described. PCR products were resolved in 2% agarose gel and visualized by staining with ethidium bromide.
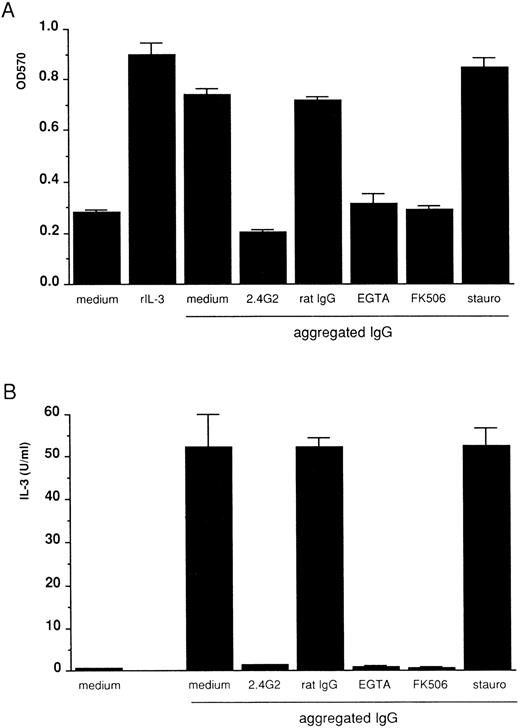
FcγRIII-ligand interaction induces IL-3 and the autocrine growth of 185-4 cells mediated by the calcium-calcineurin system, but not by PKC.To determine whether FcγRIIIs on 185-4 cells are involved in aggregated IgG-induced proliferation, 185-4 cells were cultured with aggregated IgG in the presence of anti-FcγRII/III MoAb (2.4G2). The MTT assay showed that when compared with control rat IgG, the cell growth was significantly reduced by 2.4G2 (Fig 2A). IL-3 production was the same manner as shown in Fig 2B. These results showed that aggregated IgG specifically induced IL-3 production and cell growth by binding to FcγRIII. We next studied the effects of various inhibitors of second messengers such as EGTA or FK506, which blocks calcium-calcineurin system or staurosporine, which inhibits PKC. Figure 2A shows growth of 185-4 cells measured by MTT assay. Aggregated IgG induced cell growth, as well as the rIL-3. However, the effects of aggregated IgG were completely blocked by EGTA or FK506 to the level of background, but not by staurosporine. As shown in Fig 2B, IL-3 production was affected in the same manner as cell growth. These results confirmed the results shown in Fig 1, indicating the indispensable role of the calcium-calcineurin system as the second messenger of IL-3 production in 185-4 cells induced by aggregated IgG. In addition, PKC was not apparently involved in IL-3 induction.
Inhibition of 185-4 cell growth induced by aggregated IgG. (A) The 185-4 cells were cultured with aggregated IgG (50 μg/mL) in the presence of medium alone, 10 μg of anti-FcγRII/III MoAb (2.4G2), 10 μg/mL of rat IgG as a control of 2.4G2, EGTA (1 mmol/L), FK506 (100 nmol/L), or staurosporine (10 μmol/L) for 24 hours, then viability was examined by the MTT assay. The results are means ± SD of the OD570 of triplicate cultures. Cultures with medium alone or with rIL-3 (50 U/mL) were the negative and positive controls of cell viability, respectively. (B) Inhibition of IL-3 production from 185-4 cells by aggregated IgG. The 185-4 cells were cultured with aggregated IgG in the presence of blocking reagents for 3 hours. Culture supernatants were harvested and dialyzed for the IL-3 assay as described in the legend to Fig 1. Data represent mean ± SD of five replicates.
Inhibition of 185-4 cell growth induced by aggregated IgG. (A) The 185-4 cells were cultured with aggregated IgG (50 μg/mL) in the presence of medium alone, 10 μg of anti-FcγRII/III MoAb (2.4G2), 10 μg/mL of rat IgG as a control of 2.4G2, EGTA (1 mmol/L), FK506 (100 nmol/L), or staurosporine (10 μmol/L) for 24 hours, then viability was examined by the MTT assay. The results are means ± SD of the OD570 of triplicate cultures. Cultures with medium alone or with rIL-3 (50 U/mL) were the negative and positive controls of cell viability, respectively. (B) Inhibition of IL-3 production from 185-4 cells by aggregated IgG. The 185-4 cells were cultured with aggregated IgG in the presence of blocking reagents for 3 hours. Culture supernatants were harvested and dialyzed for the IL-3 assay as described in the legend to Fig 1. Data represent mean ± SD of five replicates.
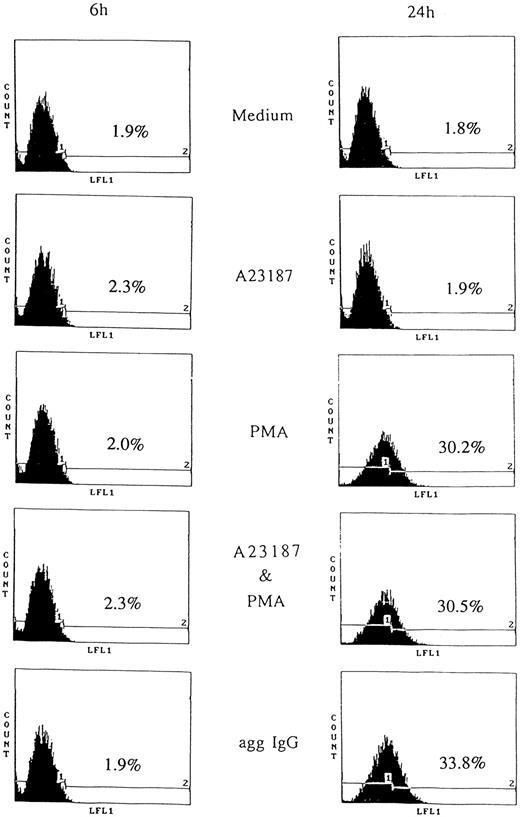
CD95 induction by aggregated IgG or PMA.Apoptosis caused by removing IL-3 was prevented by aggregated IgG through FcγRIII.9 However, this indicated unlimited cell growth by IgG, therefore, we postulated that negative feedback system. According to this notion, we studied the apoptosis mediated by CD95. First, we examined CD95 expression on 185-4 cells by flow cytometry. The 185-4 cells were stimulated by aggregated IgG or other reagents in the presence of IL-3 for the indicated periods, then stained with anti-CD95 MoAb. Figure 3 shows that CD95 was abundantly expressed on the surface of 185-4 cell after 24 hours of stimulation by aggregated IgG or PMA, but not after 6 hours. When stimulated by aggregated IgG, the FITC-conjugated second Ab (anti-hamster IgG) may have cross-reacted with mouse IgG bound to cell surface FcγR. Therefore, we used specific anti-hamster IgG Ab, which did not cross-react with mouse IgG (data not shown). These results showed that although aggregated IgG induced CD95 expression in addition to IL-3 production at the same time, PMA induced only CD95. In contrast, the calcium ionophore A23187 could not induce CD95 and no synergistic effects were apparent. Stimulation by IL-3 alone also could not induce CD95. These results suggested that the induction of CD95 caused by aggregated IgG was mediated by the activation of PKC in sharp contrast to IL-3 production.
CD95 induction on stimulated 185-4 cells. The 185-4 cells were cultured with medium alone, aggregated IgG (50 μg/mL), A23187 (250 ng/mL), PMA (100 ng/mL), or a combination of A23187 and PMA. After 6 and 24 hours, cells were stained using anti-CD95 MoAb (Jo-2) followed by FITC-conjugated goat F(ab′) of anti-hamster IgG Ab. As the negative control of Jo-2 MoAb, cells were stained with normal hamster IgG and the CD95 negative (1) or positive (2) region was set. The percentages of CD95+ cells were determined using an EPICS PROFILE flow cytometer. A representative of three experiments is shown.
CD95 induction on stimulated 185-4 cells. The 185-4 cells were cultured with medium alone, aggregated IgG (50 μg/mL), A23187 (250 ng/mL), PMA (100 ng/mL), or a combination of A23187 and PMA. After 6 and 24 hours, cells were stained using anti-CD95 MoAb (Jo-2) followed by FITC-conjugated goat F(ab′) of anti-hamster IgG Ab. As the negative control of Jo-2 MoAb, cells were stained with normal hamster IgG and the CD95 negative (1) or positive (2) region was set. The percentages of CD95+ cells were determined using an EPICS PROFILE flow cytometer. A representative of three experiments is shown.
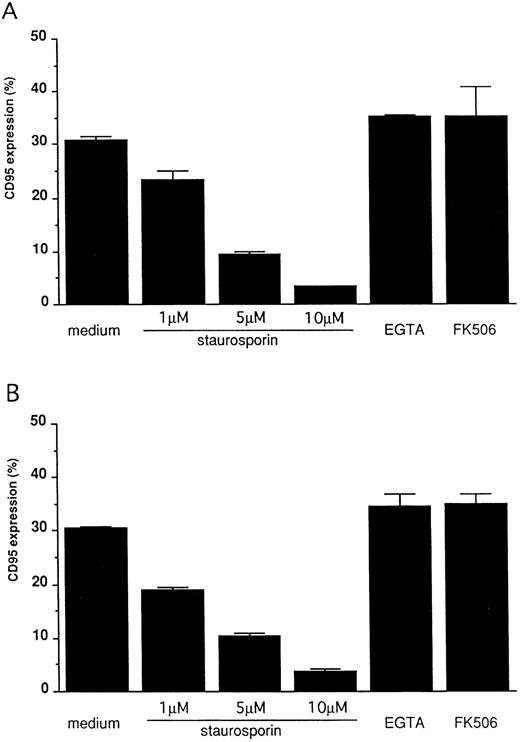
FcγRIII-ligand interaction induces CD95 expression on 185-4 cells mediated by PKC activation.To confirm that CD95 expression is induced by aggregated IgG, 185-4 cells were cultured with aggregated IgG in the presence of anti-FcγRII/III MoAb (2.4G2) or control rat IgG. The results of flow cytometry showed that when compared with control IgG, the CD95 expression was reduced in a dose-dependent manner by 2.4G2. (Fig 4). These results indicated that aggregated IgG specifically induced CD95 by binding to FcγRIII. We next studied the effects of various inhibitors of second messengers, as in the case of IL-3 production. As shown in Fig 5, CD95 expression was blocked in a dose-dependent manner by staurosporine, but not by EGTA or FK506 in the same manner as stimulation by PMA or aggregated IgG. This finding confirmed that PKC is involved in the induction of CD95 by FcγRIII-ligand interaction.
Blocking of FcγRIII-mediated CD95 expression by FcγRII/III MoAb. The 185-4 cells were cultured in the presence of aggregated IgG (50 μg/mL) with various concentration of anti-FcγRII/III (2.4G2) MoAb. Normal rat IgG (50 μg/mL) was used as the control of 2.4G2. After 24 hours, cells were stained using Jo-2 MoAb, followed by FITC-conjugated second Ab, then CD95+ cells were analyzed using an EPICS PROFILE flow cytometer. The results are mean ± SD of triplicate cultures.
Blocking of FcγRIII-mediated CD95 expression by FcγRII/III MoAb. The 185-4 cells were cultured in the presence of aggregated IgG (50 μg/mL) with various concentration of anti-FcγRII/III (2.4G2) MoAb. Normal rat IgG (50 μg/mL) was used as the control of 2.4G2. After 24 hours, cells were stained using Jo-2 MoAb, followed by FITC-conjugated second Ab, then CD95+ cells were analyzed using an EPICS PROFILE flow cytometer. The results are mean ± SD of triplicate cultures.
Blocking of CD95 expression on stimulated 185-4 cells. The 185-4 cells were cultured with either aggregated IgG (50 μg/mL) (A) or PMA (100 ng/mL) (B) in the presence of the indicated concentration of staurosporine, EGTA (1 mmol/L) or FK506 (100 nmol/L). After 24 hours, cells were stained using Jo-2 MoAb, followed by FITC-conjugated second Ab, then CD95+ cells were analyzed using an EPICS PROFILE flow cytometer. The results are mean ± SD of triplicate cultures.
Blocking of CD95 expression on stimulated 185-4 cells. The 185-4 cells were cultured with either aggregated IgG (50 μg/mL) (A) or PMA (100 ng/mL) (B) in the presence of the indicated concentration of staurosporine, EGTA (1 mmol/L) or FK506 (100 nmol/L). After 24 hours, cells were stained using Jo-2 MoAb, followed by FITC-conjugated second Ab, then CD95+ cells were analyzed using an EPICS PROFILE flow cytometer. The results are mean ± SD of triplicate cultures.
CD95 expressed on 185-4 cells is functional and anti-CD95 MoAb induces apoptosis.We determined whether this CD95 was functional and induced apoptosis. The anti-CD95 MoAb, Jo-2, induces apoptosis in murine cells expressing CD95.16 We stimulated 185-4 cells with rIL-3 alone, aggregated IgG, or PMA in the presence of IL-3 for 24 hours to induce CD95, then 1 μg/mL of Jo-2 MoAb was added. After various periods, we evaluated the apoptosis of 185-4 cells by measuring the amount of fragmented DNA after PI staining as described in Materials and Methods. As shown in Fig 6, PI fluorescence intensity was only high in cells incubated for 6 hours with Jo-2 MoAb. After 12 hours, however, the ratio (%) of low PI cells progressively increased in cells precultured with aggregated IgG or PMA. On the contrary, cells cultured with IL-3 alone, which did not express CD95, did not show low PI even after 48 hours. We next examined whether DNA fragmentation would occur by stimulation of anti-CD95 MoAb. We stimulated 185-4 cells with rIL-3 alone, aggregated IgG, or PMA with or without sturosporine for 24 hours, then 1 μg/mL of Jo-2 MoAb was added. After 24 hours, DNA was extracted and analyzed. As shown in Fig 7, cells precultured with rIL-3 alone contained only high molecular weight (m.w.) DNA, and DNA fragmentation was not observed. However, DNA fragmentation was observed in cells precultured with aggregated IgG or PMA. This confirms the results obtained in Fig 6. On the other hand, the cells precultured with either aggregated IgG or PMA in the presence of staurosporine showed no DNA fragmentation. This confirms the result that CD95 expression by aggregated IgG or PMA was inhibited by staurosporine as shown in Fig 5. These findings suggest that the CD95 is involved in the negative regulation by apoptosis of activated 185-4 cells by FcγRIII-ligand interaction.
Induction of apoptotic cell death in CD95 expressed 185-4 cells. The 185-4 cells were cultured with aggregated IgG (50 μg/mL), PMA (100 ng/mL), or rIL-3 (50 U/mL) as the negative control. After 24 hours, 1 × 106 cells were exposed to 1 μg/mL of anti-CD95 (Jo-2) MoAb and incubated for the indicated periods. Cells were then stained with PI. Percentages of apoptotic cells were determined using an EPICS PROFILE flow cytometer. A representative of three experiments is shown.
Induction of apoptotic cell death in CD95 expressed 185-4 cells. The 185-4 cells were cultured with aggregated IgG (50 μg/mL), PMA (100 ng/mL), or rIL-3 (50 U/mL) as the negative control. After 24 hours, 1 × 106 cells were exposed to 1 μg/mL of anti-CD95 (Jo-2) MoAb and incubated for the indicated periods. Cells were then stained with PI. Percentages of apoptotic cells were determined using an EPICS PROFILE flow cytometer. A representative of three experiments is shown.
Induction of oligonucleosomal DNA fragmentation. The 185-4 cells were cultured with aggregated IgG (50 μg/mL), PMA (100 ng/mL), or rIL-3 (50 U/mL) with or without staurosporine. After 24 hours, 5 × 106 cells were exposed to 1 μg/mL of Jo-2 MoAb. After a 24-hour incubation, cells were collected and DNA was extracted as described in Materials and Methods. DNA (5 μg) from each sample was resolved in 1% agarose gel and visualized by staining with ethidium bromide.
Induction of oligonucleosomal DNA fragmentation. The 185-4 cells were cultured with aggregated IgG (50 μg/mL), PMA (100 ng/mL), or rIL-3 (50 U/mL) with or without staurosporine. After 24 hours, 5 × 106 cells were exposed to 1 μg/mL of Jo-2 MoAb. After a 24-hour incubation, cells were collected and DNA was extracted as described in Materials and Methods. DNA (5 μg) from each sample was resolved in 1% agarose gel and visualized by staining with ethidium bromide.
DISCUSSION
In a recent study, FcγRIII knockout mice do not develop anaphylaxis, ADCC, and Arthus reaction. The identification of the FcγRIII mediating anaphylactic and inflammatory processes is of crucial importance for the control of immunopathological events. In contrast, despite the proposed role for FcγRIII in cell ontogeny, the development of neither lymphoid nor myeloid lineages was affected by the loss of FcγRIII of cells derived from any organ including bone marrow.3 Thus, although the defects of FcγRIII-mediated immunological reactions depend on the dysfunction of FcγRIII-bearing cells such as NK and mast cells in the FcγRIII knockout mice, our results enabled us to identify the possible physiologic importance of the FcγRIII, especially in transient hematopoiesis in emergency after ontogeny, such as infectious diseases and inflammatory states. The present study shows the role of FcγRIII of bone marrow cells in normal and pathologic immunologic events.
In this study, we showed the induction of contrary biological phenomena by FcγRIII-ligand binding: the induction of IL-3 and the induction of CD95. We reported that 185-4 cells undergo apoptosis on the withdrawal of IL-3.9 Thus, cytokine depletion and CD95 expression both led 185-4 cells to apoptosis. We questioned the need for this strict negative regulation. We described that immune complexes generally increased in many pathologic states such as chronic inflammation in autoimmune or infectious diseases and that the survival of inflammatory cells may be induced by FcγRIII-ligand interaction, which would prolong the inflammation in these diseases. Therefore, inflammatory cells must be deleted after inflammation ceases and avoid unlimited cell growth during hematopoiesis in emergency. We examined the blocking of these two types of apoptosis using IL-1β converting enzyme (ICE) inhibitor. The results showed that only the apoptosis induced by CD95 pathway was blocked by ICE inhibitor (data not shown). It also suggested that strict negative regulation was guaranteed by two different mechanisms of apoptosis in 185-4 cells.
Negative regulation of cell growth after activation has been identified in several systems. Antibodies to CD3/TCR complex induces AICD. The biologic importance of the CD95/FasL system has been extensively studied in T cells, where it plays a critical role in AICD.25-28 It is unclear at this moment whether AICD contributes to the regulation of immune responses in vivo. However, although CD4+ and CD8+ T cells from lpr/lpr mutant mice undergo normal primary activation, they exhibit a defect in the AICD pathway on subsequent antigenic challenge.29 This finding suggests that AICD is an important physiologic regulator of immune responses. Similarly, CD40 ligation induces cell activation and CD95 expression followed by apoptosis through the CD95 pathway in B lymphocytes.30,31 Thus, AICD may be a rather general phenomenon, not only limited to T and B cells, but bone marrow cells. FcγRIII was generally thought to enhance activation of NK cells. However, human NK cells activated by anti-FcγRIII MoAb facilitate cell-mediated cytotoxicity and subsequently induce apoptosis by expressing FasL.12 Although we also confirmed the negative regulation mediated by FcγRIII-ligand binding, we emphasize that it was accompanied by CD95 expression and differed from that identified in human NK cells. Thus, FcγRIII-mediated stimulation not only activates, but also depletes activated cells.
Under physiologic conditions, CD95 is expressed in various tissues including thymus, heart, liver, lungs, and ovaries.14 Several investigations have described the regulation of CD95 expression in some systems. For example, anti-CD3 Ab, superantigen, or phytohemagglutinin (PHA) stimulate peripheral resting T cells and induce CD95 after 1 or 2 days.32 However, the expressed CD95 was not necessarily functional and thus could not induce apoptosis. On the other hand, T cells cultured with IL-2 for a few days induce functional CD95.33 CD34+ hematopoietic progenitor cells express functional CD95 on stimulation by interferon (IFN)-γ or TNF-α.34,35 Thus, although we identified one of the mechanisms of CD95 expression in 185-4 cells, it is not necessarily applied in all systems. Furthermore, we used anti-CD95 MoAb to induce apoptosis mediated by CD95, but this MoAb does not exist under normal conditions in vivo. In addition, we could not detect FasL after any stimulation, even by aggregated IgG in 185-4 cells (data not shown by RT-PCR). This suggests 185-4 cells do not undergo apoptosis by themselves. In contrast to the tissue distribution of CD95, the constitutive expression of FasL is relatively limited. FasL is expressed at high levels in lungs, testis, and small intestine.36,37 CD3+ double negative NK1.1+ T cells express FasL in the thymus.38 Although the site of NK1.1+ T-cell differentiation is unknown, this type of cell is also found in bone marrow and liver.39 40 NK1.1+ T cells may function not only in thymus, but also in bone marrow and liver as regulators of hematopoiesis in vivo.
The signal transduction caused by the binding of ligands to the FcγRIII has been extensively studied in NK cells. The earliest biologic event detected in NK cells on FcγRIII-induced signal transduction is the activation of phospholipase C-γ1 (PLC-γ1), leading to phosphatidylinositol hydrolysis and production of diacylglycerol.41 Each event is followed by an increase in the intracellular calcium concentration and PKC activation, respectively, leading to more distal events including the synthesis of cytokines, as well as the expression of the IL-2 receptor and other surface activation antigens.42 43 In 185-4 cells, these two signals may have been induced by FcγRIII-ligand interaction; one being increased intracellular calcium and the other, PKC activation. However, the most significant issue was that each signal controlled two opposite biologic phenomena, namely cell survival through IL-3 production and simultaneous preparation for cell death by expressing CD95. However, the time courses of IL-3 and CD95 induction were different. IL-3 secretion started 2 to 3 hours after FcγRIII stimulation. It was mediated by the calcium-calcineurin system and cells survived for several days. On the other hand, CD95 was expressed after 24 hours mediated by PKC activation, and apoptosis was induced thereafter, even in the presence of IL-3. Although the details of this issue cannot be confirmed by this study, the most important finding was that the cell growth induced by IL-3 production proceeds faster than cell death induced by CD95. In addition, we emphasize that our results show the active induction of cell death via CD95 signaling even in the presence of IL-3. Thus these results indicate the physiologic order of cell growth and death in the regulation of hematopoiesis.
ACKNOWLEDGMENT
Y. Ohnuma is greatly acknowledged for her skillful secretarial work.
Address reprint requests to Kachio Tasaka, MD, Department of Parasitology and Immunology, Yamanashi Medical University, Tamaho-cho, Yamanashi 409-38, Japan.