Abstract
Interleukin-11 (IL-11) is a pleiotropic growth factor with a prominent effect on megakaryopoiesis and thrombopoiesis. The receptor for IL-11 is a heterodimer of the signal transduction unit gp130 and a specific receptor component, the α-chain (IL-11Rα). Two genes potentially encode the IL-11Rα: the IL11Ra and IL11Ra2 genes. The IL11Ra gene is widely expressed in hematopoietic and other organs, whereas the IL11Ra2 gene is restricted to only some strains of mice and its expression is confined to testis, lymph node, and thymus. To investigate the essential actions mediated by the IL-11Rα, we have generated mice with a null mutation of IL11Ra (IL11Ra−/−) by gene targeting. Analysis of IL11Ra expression by Northern blot and reverse transcriptase-polymerase chain reaction, as well as the absence of response of IL11Ra−/− bone marrow cells to IL-11 in hematopoietic assays, further confirmed the null mutation. Compensatory expression of the IL11Ra2 in bone marrow cells was not detected. IL11Ra−/− mice were healthy with normal numbers of peripheral blood white blood cells, hematocrit, and platelets. Bone marrow and spleen contained normal numbers of cells of all hematopoietic lineages, including megakaryocytes. Clonal cultures did not identify any perturbation of granulocyte-macrophage (GM), erythroid, or megakaryocyte progenitors. The number of day-12 colony-forming unit-spleen progenitors were similar in wild-type and IL11Ra−/− mice. The kinetics of recovery of peripheral blood white blood cells, platelets, and bone marrow GM progenitors after treatment with 5-flurouracil were the same in IL11Ra−/− and wild-type mice. Acute hemolytic stress was induced by phenylhydrazine and resulted in a 50% decrease in hematocrit. The recovery of hematocrit was comparable in IL11Ra−/− and wild-type mice. These observations indicate that IL-11 receptor signalling is dispensable for adult hematopoiesis.
THE ABILITY OF growth factors to influence cell growth and differentiation in immune and hematopoietic systems depends on their recognition and binding by specific cell surface receptor(s). There is growing evidence that different growth factors display a similar range of biologic actions.1 This overlapping function of growth factors is in part explained by shared receptor components.2-4 For example, the interleukin-6 (IL-6) family of cytokines, including leukemia inhibitory factor (LIF ), oncostatin-M (OSM), and ciliary neutrotrophic factor (CNTF ), display several common actions3,5 and share gp130 as a signal transduction unit.3,6 Several of these molecules also use the LIF receptor (LIFR) as a component of their receptor complex and, in addition, specific receptor chains exist for OSM, IL-6, and CNTF.3 6
IL-11 is a recent addition to the IL-6 family and was originally cloned from a bone marrow stromal cell line based on its proliferative activity on a murine plasmacytoma cell line7 and on its ability to inhibit adipogenesis in 3T3-L1 cells.8 IL-11 exhibits pleiotropic actions on the hematopoietic compartment. These include shortening of cell-cycle time of hematopoietic progenitor cells9-12 and, in synergy with stem cell factor (SCF ), support of the growth of multilineage progenitors.13-15 In addition, IL-11 can, in combination with other cytokines, cause in vivo and ex vivo expansion of primitive and committed hematopoietic progenitors.16-18 It stimulates myelopoiesis19-21 and multiple stages of erythropoiesis22-24 both in vivo and in vitro. There is also a large body of data on the influence of IL-11 on megakaryocyte growth and development. In vitro, most actions of IL-11 in supporting megakaryocyte progenitors have been observed in synergy with other growth factors such as IL-3 or mpl-ligand (also known as thrombopoietin [TPO] or megakaryocyte growth and development factor [MGDF ]).25-29 However, IL-11 on its own has been shown to promote maturation of megakaryocytes.26 In murine,30-34 canine,35 and nonhuman primate36 models and, more recently, in humans,37,38 in vivo administration of IL-11 has been shown to augment platelet levels and promote earlier hematopoietic recovery after toxic insults, an action with potential clinical importance. IL-11 also acts on other organ-systems: it stimulates acute-phase protein synthesis in the liver,39 regulates neuronal differentiation,40 and influences osteoclast development41,42 and B-lymphocyte maturation.43,44 IL-11 has also been shown to improve recovery of intestinal cells45,46 and spermatogenesis47 after cytotoxic therapy.
We, and others, have reported cloning of cDNAs encoding the murine and human IL-11–specific receptor subunit (α-chain).48-51 Expression of this receptor component alone confers low-affinity IL-11 binding, whereas coexpression with gp130 results in high-affinity binding of IL-11 and the capacity for signal transduction.48,51 We have previously described the presence of two loci for the murine IL-11 receptor α-chain, IL11Ra and IL11Ra2.52,53 In addition, we have shown that the IL11Ra2 locus is not present in all mouse strains. The cDNAs from the two loci exhibit 99% nucleotide identity in the coding exons, but contain different 5′ untranslated regions (5′UTR). This divergence permits discrimination between transcripts from the two loci using reverse-transcriptase polymerase chain reaction (RT-PCR). Whereas transcripts corresponding to the IL11Ra locus were expressed widely, those from the IL11Ra2 locus (when present) were detected only in testis, lymph node, and thymus.53
Despite the varied actions of IL-11 within the hematopoietic compartment, its principal physiologic role remains unclear. The study of murine models made genetically deficient in growth factors or their cognate receptors has helped define the precise in vivo role of some of these molecules. For example, analysis of phenotypes resulting from targeted mutation of TPO54 or its receptor c-mpl55,56 or the mutation of granulocyte-macrophage colony-stimulating factor (GM-CSF )57 and its signal transduction molecule, the common β subunit58 59 have provided complementary data regarding the role of these ligands and their receptors. To delineate the essential functions of IL-11 in vivo, we have generated mice with a targeted null-mutation of the IL11Ra locus (IL11Ra−/−).
MATERIALS AND METHODS
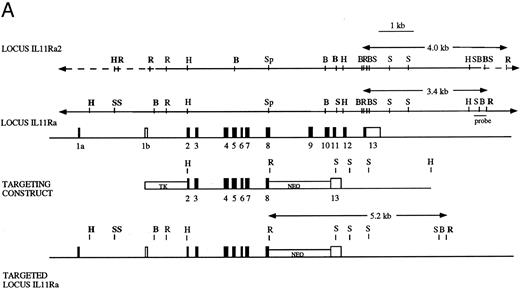
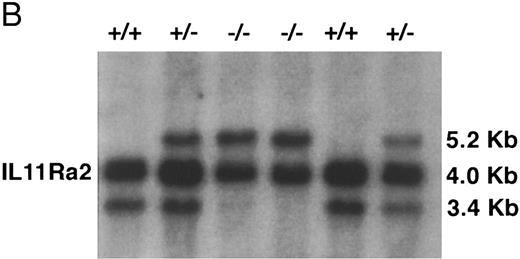
Disruption of IL-11Ra by homologous recombination in embryonic stem cells. The targeting construct (Fig 1A) was created using the pNTK vector (a kind gift of Dr Richard Mortensen, Brigham and Womens Hospital, Boston, MA) and contained neomycin phosphotransferase (pgkNEO) and thymidine kinase (pgkTK) cassettes for positive-negative selection. A 5′ 2.6-kb HindIII-Sph I fragment and a 3′ 2.9-kb BamHI-HindIII fragment (both derived from genomic clone l11.4)52 were blunt-ended and inserted at either side of the pgkNEO cassette at the BamHI and Cla I sites using BamHI and Cla I linkers, respectively. The neo cassette replaced parts of exons 8 and 13 and the intervening exons.9-12 The construct was linearized using Xho I and electroporated into W9.5 embryonic stem (ES) cells60 that were selected using gancyclovir and G418. Antibiotic-resistant ES cell clones were isolated, expanded, and screened by Southern blot hybridization.61 Genomic DNA was digested with EcoRI; transferred to nylon membrane (Gene Screen Plus; NEN Research Products, Boston, MA) by capillary blotting; and prehybridized and hybridized at 65°C in a solution containing 1 mol/L NaCl, 1% (wt/vol) sodium dodecyl sulfate (SDS), and 10% (wt/vol) dextran sulphate; and probed with a 600-bp HindIII-EcoRI fragment derived from genomic clone λ11.4.52 The probe was situated in the IL11Ra locus 3′ of the targeting construct and permitted distinction between the endogenous loci (3.4 kb for IL11Ra and 4.0 kb for IL11Ra2 loci) and the mutant IL11Ra locus (5.2 kb; Fig 1). The membranes were washed with 0.2× SSC (1× SSC is 0.15 mol/L NaCl, 0.015 mol/L trisodium citrate) and 0.1% (wt/vol) SDS at 65°C and exposed to radiographic film for 18 hours at −70°C using intensifying screens. ES cells targeted at the IL11Ra locus were confirmed to contain a single integration using a neo probe. These cells were used to derive chimeric mice that were mated with C57BL/6 female mice and the heterozygous offspring interbred to yield wild-type IL11Ra (+/+), heterozygous IL11Ra (+/−), and mutant IL11Ra (−/−) mice. Mice genotypes were determined by Southern blot analysis of genomic DNA obtained from tail biopsies.
(A) Disruption of the IL11Ra locus by homologous recombination. Genomic organization of the murine IL11Ra locus encoded by genomic phage clone λ11.452 is shown, with the exons indicated as boxes and numbered and the coding regions shown in black. Dashed lines indicate parts of locus IL11Ra2 that are not homologous with locus IL11Ra. Restriction enzyme sites for locus IL11Ra and IL11Ra252 are indicated. Sites unique to a particular locus are shown in bold. EcoRI (R), BamHI (B), Sac I (S), HindIII (H), and Sph I (Sp). Shown below is the targeting vector containing the 5′ and 3′ regions of homology and the cDNAs encoding neomycin transferase (NEO) and thymidine kinase (TK) and the recombinant IL11Ra locus. The probe used in Southern screening of embryonic stem cells and tail biopsies and the expected sizes of the endogenous IL11Ra (3.4 kb), targeted IL11Ra (5.2 kb), and endogenous IL11Ra2 (4.0 kb) loci after EcoRI restriction digest are indicated. (B) Southern analysis of EcoRI-digested genomic DNA extracted from a litter derived from a cross between heterozygous IL11Ra+/− mice showing heterozygous, homozygous IL11Ra−/−, and wild-type IL11Ra+/+ mice. Sizes of the endogenous IL11Ra (3.4 kb) and the targeted IL11Ra (5.2 kb) loci are indicated, as is the band for the IL11Ra2 locus (4.0 kb).
(A) Disruption of the IL11Ra locus by homologous recombination. Genomic organization of the murine IL11Ra locus encoded by genomic phage clone λ11.452 is shown, with the exons indicated as boxes and numbered and the coding regions shown in black. Dashed lines indicate parts of locus IL11Ra2 that are not homologous with locus IL11Ra. Restriction enzyme sites for locus IL11Ra and IL11Ra252 are indicated. Sites unique to a particular locus are shown in bold. EcoRI (R), BamHI (B), Sac I (S), HindIII (H), and Sph I (Sp). Shown below is the targeting vector containing the 5′ and 3′ regions of homology and the cDNAs encoding neomycin transferase (NEO) and thymidine kinase (TK) and the recombinant IL11Ra locus. The probe used in Southern screening of embryonic stem cells and tail biopsies and the expected sizes of the endogenous IL11Ra (3.4 kb), targeted IL11Ra (5.2 kb), and endogenous IL11Ra2 (4.0 kb) loci after EcoRI restriction digest are indicated. (B) Southern analysis of EcoRI-digested genomic DNA extracted from a litter derived from a cross between heterozygous IL11Ra+/− mice showing heterozygous, homozygous IL11Ra−/−, and wild-type IL11Ra+/+ mice. Sizes of the endogenous IL11Ra (3.4 kb) and the targeted IL11Ra (5.2 kb) loci are indicated, as is the band for the IL11Ra2 locus (4.0 kb).
Northern analysis. Northern analysis was performed as previously described.62 Washes were performed with 2.0× SSC, 0.1% (wt/vol) SDS at 65°C. Northern blots were first screened with a radiolabeled murine cDNA fragment, a 445-bp Sph I/Sac I fragment (nucleotides 714-1158 from the murine IL-11Ra clone 30.148 ) encoding the deleted exons 8-11. The filters were then stripped and reprobed with a radiolabeled 485-bp PCR-generated product encoding exons 2-6 and part of exon 7 of the murine IL-11Ra. PCR reactions were performed in 50 μL with 1× PCR buffer (Boehringer Mannheim, Castle Hill, New South Wales, Australia), 0.2 mmol/L of each dNTP, 0.5 U Taq Polymerase (Boehringer), and 200 ng of each primer: 5′-ATGAGCAGCAGCTGCTCAGGGCTG-3′ (within exon 2) and 5′-ACTTTCCCTCTGACTCTCAGCTCCTGG-3′ (within exon 7).48 Amplification conditions were initial denaturation of 96°C for 2 minutes followed by 25 cycles of 94°C for 30 seconds, 55°C for 30 seconds, and 72°C for 90 seconds. The PCR product was purified from an 2% agarose gel using Qiaex II Gel Extraction Kit (Qiagen, Hilden, Germany). mRNA loading was assessed by probing the filter with a 1.1-kb fragment from the rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene.
Examination of IL11Ra and IL11Ra2 transcripts by RT-PCR. Total RNA was isolated from testes and bone marrow cells of wild-type and IL11Ra−/− mice. RNA was also extracted using Trizol reagent (Life Technologies, Gaithersburg, MD) from pools of hematopoietic colonies grown from bone marrow cells in methyl cellulose cultures (minimum 120 colonies analyzed) stimulated for 7 days with 100 ng/mL SCF, 2 U/mL erythropoietin (EPO), and 10 ng/mL IL-11. All RNA samples were treated with RNase-free DNase1 (Boehringer Mannheim) and random primer and oligo(dT)-primed cDNA synthesis was performed using AMV-RT (Boehringer Mannheim) under conditions recommended by the manufacturer. cDNA (5 μL) was used for PCR amplification in a 50 μL volume reaction containing 1× PCR buffer (Boehringer Mannheim), 0.2 mmol/L of each dNTP, 0.5 U Taq Polymerase (Boehringer Mannheim), and 200 ng of each primer using the following amplification conditions: initial denaturation of 96°C for 2 minutes followed by 30 cycles of 94°C for 30 seconds, 55°C for 45 seconds, and 72°C for 60 seconds. The IL11Ra coding region was amplified using oligonucleotide primers in exon 10 (5′-CTGCAAGGCCTTCCTTGCAGCCGG-3′) and in exon 12 (5′-CGGTCCATCCTTCCCACTCCGTCT-3′).48 Because the exons encoding the 5′-UTR of IL11Ra and IL11Ra2 cDNAs differ, pairing of a sense-primer located in this region of divergence with a common antisense-primer located on coding exon-7 (identical in both cDNAs) enabled discrimination between transcripts from the two loci. Thus combination of sense primer 5′-CAGAGGGTGAGGGCGGAGGGCGCT-3′53 coupled with antisense primer 5′-CTCCAGAGGGTCCTGTGGACACGG-3′ was specific for IL11Ra transcript, whereas the combination of sense primer 5′CCTCAAAGGATTGTCCACTTCCGG-3′ with the same antisense primer was specific for IL11Ra2 transcript.53 RNA loading was assessed by amplifying 5 μL of cDNA from each of the above-mentioned samples for the hypoxanthine phosphoribosyltransferase (HPRT) transcript using primers described previously.63 Samples (15 μL) were electrophoresed through a 1.25% (wt/vol) agarose gel, transferred to nylon membranes, and probed with oligonucleotides internal to the amplified product.
Flow cytometry. Single-cell suspensions of bone marrow, spleen, lymph node, and thymus were incubated with an excess of 2.4G2 anti-Fcγ receptor antibody64 to reduce nonspecific binding and then with a panel of specific monoclonal antibodies to various murine antigens59,65: anti-CD4 and anti-CD8 (Becton Dickinson, Lincoln Park, NJ), anti-IgM (5.1), anti-Ly5-B220 (RA3-6B2), anti-Mac-1 (M1/70), F4/80, anti-Gr-1 (RB6-8C5), Ter-119, anti-Thy1.2 (30-H12), anti-TCRα/β (H57-697.1), and anti-TCRγ/δ (GL3-1A). The antibodies were directly coupled to fluorescein isothiocyanate or biotin, the latter being visualised using R-phycoerythrin-streptavidin (Caltag, San Francisco, CA). Analysis were performed on a FACScan analyzer (Becton Dickinson) and dead cells and erythrocytes were excluded by propidium iodide (1 μg/mL) staining and gating with forward and side light scattering.
Hematologic analysis. Orbital plexus blood was collected from anaesthetized mice and peripheral blood white blood cell count, hematocrit, and platelet counts were determined using either manual or automated (Sysmex K1000; TOA Medical Instruments, Kobe, Japan) counting techniques. Cell suspensions were made from bone marrow and spleen by standard techniques and total femoral and splenic cellularity was determined using a hemocytometer after eosin staining. Manual 100 to 400 cell differential counts were performed using May-Grünwald-Giemsa–stained blood smears and cytocentrifuge preparations of bone marrow and spleen. Bone marrow and spleen progenitor cells were assayed using semisolid agar and methyl cellulose cultures as previously described.59,66 For each sample, 50,000 nucleated cells were plated in triplicate in 1-mL cultures. Colony formation was stimulated by multiple combinations of purified recombinant growth factors at the following final concentrations: murine GM-CSF (Schering, Kenilworth, NJ) at 10 ng/mL; human granulocyte colony-stimulating factor (G-CSF; Amgen, Thousand Oaks, CA) at 10 ng/mL; murine IL-3 (PeproTech, Rocky Hill, NJ) at 10, 1, and 0.1 ng/mL; human IL-11 (R & D Systems, Minneapolis, MN) at 10 ng/mL; murine IL-6 (kind gift of Dr R. Simpson, Ludwig Institute for Cancer Research, Melbourne, Australia) at 100 ng/mL; human EPO (Amgen) at 2 U/mL; murine SCF (produced by expression in Pichia pastoris) at 100, 10, and 1.0 ng/mL; and pegylated-recombinant human MGDF (PEG-rhuMGDF; Amgen) at 200 ng/mL. After 7 days of incubation in a fully humidified atmosphere at 37°C supplemented with 10% or 5% CO2 , colonies were enumerated. The investigator was blinded to the genotype of the animals from which the cells were derived. Cultures were fixed with 2.5% glutaraldehyde in phosphate-buffered saline and stained using acetylcholine esterase, luxol fast blue, and hematoxylin, and the cellular composition of colonies was determined. Megakaryocyte colony assays were performed by stimulating 50,000 bone marrow cells with IL-3, SCF, and MGDF at above-noted concentrations. After 7 days of incubation, the plates were fixed with 2.5% glutaraldehyde and stained with acetylcholine esterase. Colonies were defined as three or more megakaryocyte cells. A minimum of 200 to 300 colonies were examined per mouse (n = 4 per genotype) and the results are expressed as a percentage. For assessment of the recloning potential (n = 6 IL11Ra+/+ and IL11Ra−/− mice), 25 to 50 colonies derived from bone marrow cells and grown in SCF (100 ng/mL) were isolated, and a single-cell suspension was prepared and replated in agar cultures stimulated by SCF (100 ng/mL). Secondary clones and colonies resulting from each primary colony were enumerated. The experiment was also performed (n = 3 mice of wild-type and IL11Ra−/− genotype) by specifically isolating colonies of blast morphology. Colony-forming unit-spleen (CFU-S) assays were performed using 75,000 bone marrow cells from 2 IL11Ra+/+ and 2 IL11Ra−/− mice in each experiment injected retro-orbitally into lethally irradiated (11 Gy, split dose) 129SV mice with 5 to 6 recipient mice per donor. Macroscopic colonies on the spleen surface were counted on day 12.67
Histologic analysis. Sections of sternum, spleen, liver, intestine, pancreas, kidney, lymph node, reproductive and genito-urinary organs, muscle, heart, aorta, lung, thymus, brain, and salivary glands from 6- to 8-week-old IL11Ra+/+ and IL11Ra−/− mice were stained with hematoxylin and eosin and examined by light microscopy.
5-Flurouracil (5-FU) and phenylhydrazine administration. Recovery of total white blood cells and platelets was assessed after a single dose of 5-FU of 150 mg/kg administered intraperitoneally (n = 6 IL11Ra+/+ mice; n = 5 IL11Ra−/− mice, with n = 4 for day 8 through 15 time points). Mice were bled retro-orbitally and the analysis was performed using the manual or automated techniques described above. Recovery of bone marrow progenitors was assessed in 3 IL11Ra+/+ and IL11Ra−/− mice killed on days 2, 4, and 6 after intravenous administration of 5-FU (150 mg/kg). Fifty thousand bone marrow cells from each mouse were plated in agar and methylcellulose cultures as described above. Cultures were stimulated with combination of GM-CSF, IL-3, IL-6, and SCF and with addition of either EPO or MGDF (final concentrations as listed above). In a second experiment, cultures were performed using 5,000 bone marrow cells on days 5, 7, and 10 after similar 5-FU treatment. Recovery of progenitors was also assessed on day 7 after more severe insult (5-FU at 150 mg/kg intravenously, followed 3 days later by irradiation of 6.0 Gy) using 50,000 bone marrow cells from 3 IL11Ra+/+ and IL11Ra−/− mice stimulated with GM-CSF, IL-3, IL-6, and SCF. Phenylhydrazine (60 mg/kg) was administered intraperitoneally to 5 IL11Ra+/+ and 4 IL11Ra −/− mice. Hematocrit and reticulocytes (analysis of 1,000 red blood cells after staining with New Methylene Blue) were enumerated manually from retro-orbital plexus blood samples.
Antiplatelet serum administration. Immune thrombocytopenia was induced by administration of rabbit antimouse platelet serum (Inter-Cell Technologies, Hopewell, NJ). One hundred microliters of a 1:20 dilution of the serum in phosphate-buffered saline was injected intraperitoneally as a single dose (n = 5 IL11Ra+/+ mice; n = 6 IL11Ra−/− mice). Platelet numbers were determined at the indicated time points.
Immune response. T-cell–dependent immunization was with the hapten 4(hydroxy-3-nitrophenyl)acetyl (NP) coupled to the protein-carrier bovine serum albumin (BSA) at an average conjugation ratio of 13 NP per 1 BSA. Mice were immunised by the intraperitoneal injection of 100 μg of alum-precipitated NP-BSA. Titers of NP-specific IgG1 in the sera of immunized mice were determined by enzyme-linked immunosorbant assay (ELISA) using NP coupled to human serum albumin (HSA) as a plate coat and isotype-specific revealing reagents. Affinity maturation of the response was followed by comparing the titer of IgG1 specific for NP13-HSA (measuring total anti-NP antibody) with that specific for NP2-HSA (measuring high-affinity antibody). Optical density values were converted to micrograms per milliliter using purified NP-specific monoclonal antibodies as standards. Quantitation of the specific antibodies was not extended beyond day 14, because the antibody titer usually decreases after this time. Absolute levels of Ig isotypes in preimmune sera were determined by ELISA, as previously described.68
Statistical analysis. Comparisons of mean values were made using the unpaired Student's t-test.
RESULTS
Disruption of the IL11Ra locus. The IL11Ra locus was disrupted by homologous recombination in ES cells. In the mutated locus, exons 9-12 and part of exons 8 and 13 were replaced by a neomycin expression cassette. This resulted in the deletion of part of the hematopoietin subdomain containing the WSxWS motif,48 the transmembrane region, and membrane-proximal portion of the cytoplasmic tail. The presence of two copies of the mutant allele therefore resulted in a null phenotype (Fig 1A). Neomycin-resistant ES cells were screened by Southern blot using a probe situated outside the 3′ limit of the targeting construct and a targeting frequency of 1:100 was noted. A successfully targeted ES clone heterozygous for disruption of the IL11Ra locus was injected into blastocysts to generate chimeric mice. Mice of the three expected genotypes (wild-type IL11Ra+/+, heterozygous IL11Ra+/−, and homozygous IL11Ra−/−) were obtained by interbreeding of the heterozygous offspring and screened by Southern blot of tail DNA with the above-mentioned probe (Fig 1B).
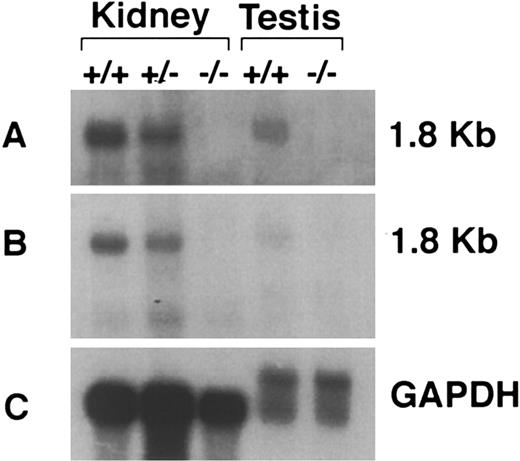
Confirmation of IL11Ra null mutation and absence of transcription from IL11Ra2 locus. Disruption of transcription from the IL11Ra locus was verified by Northern blot analysis, RT-PCR, and biologic assays. A Northern blot probed with a cDNA probe encoding the targeted exons 8-12 is shown in Fig 2A. This region is homologous in both IL11Ra and IL11Ra2 loci. The expected 1.8-kb full-length transcript was observed in the kidney of IL11Ra+/+ and IL11Ra+/− mice and testis of IL11Ra+/+ mice, whereas none was noted in either organ of IL11Ra−/− mice (Fig 2A). We have previously shown the presence of transcripts from the IL11Ra2 locus in testis using an RT-PCR assay.53 The absence of any transcript in the IL11Ra−/− testis in the above-noted Northern blot is probably a reflection of the lower abundance of the IL11Ra2 transcript relative to that from the IL11Ra locus. Examination of the above-noted Northern blot with a cDNA-derived probe encoding exon 2-7 (again common to transcripts from both loci and not deleted by the targeting strategy) did not show any normal or aberrant size transcripts in the IL11Ra−/− mice (Fig 2B).
Confirmation of null mutation by Northern analysis of poly(A)+ mRNA extracted from kidneys and testes of IL11Ra+/+, IL11Ra+/−, and IL11Ra−/− mice. The blot was initially examined with a probe encoding the deleted region of the IL11Ra locus (A) and then with a probe situated in the locus 5′ of the deleted region (B) and rat GAPDH to compare mRNA loading (C). The size of the transcript is indicated.
Confirmation of null mutation by Northern analysis of poly(A)+ mRNA extracted from kidneys and testes of IL11Ra+/+, IL11Ra+/−, and IL11Ra−/− mice. The blot was initially examined with a probe encoding the deleted region of the IL11Ra locus (A) and then with a probe situated in the locus 5′ of the deleted region (B) and rat GAPDH to compare mRNA loading (C). The size of the transcript is indicated.
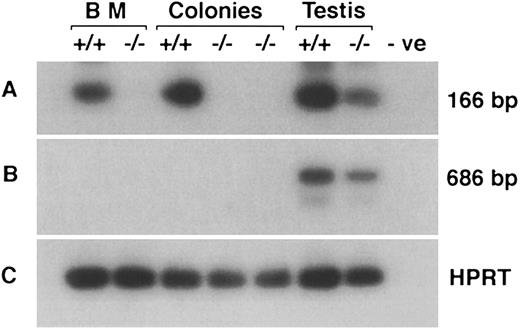
An RT-PCR strategy was used to confirm deletion of coding exons and absence of compensation by upregulation of the IL11Ra2 transcript. This was true for both bone marrow cells and hematopoietic colonies derived in vitro after stimulation of bone marrow cells with SCF, EPO, and IL-11. Amplification by oligonucleotide primers from exons 10 and 12 (deleted by the targeting construct) generated the appropriate 166-bp product from +/+ mice. As expected, there was no PCR product in either bone marrow cells or hematopoietic colonies from −/− mice (Fig 3A). The anticipated product was also noted in testis of IL11Ra−/− mice due to amplification of the IL11Ra2 transcript, because the nucleotide sequences of the IL11Ra and IL11Ra2 genes are identical in this region. The absence of an IL11Ra and IL11Ra2 RT-PCR product was also confirmed using mRNA derived from kidney of IL11Ra−/− mice (data not shown). Oligonucleotide primer pairs specific for transcripts from IL11Ra2 locus showed expression only in the testis (Fig 3B). There was no detectable IL11Ra2 transcript in IL11Ra+/+ or in IL11Ra−/− bone marrow, neither in the steady-state nor in hematopoietic colonies stimulated by IL-11. Although no IL11Ra transcripts were seen in the samples from IL11Ra−/− mice by Northern analysis and RNase protection assay (data not shown), RT-PCR using primers specific for exons 1-4 of the IL11Ra locus (not deleted by the targeting construct) showed a product. This was interpreted as representing an unstable RNA species. Additional confirmation of the null mutation was provided by the inability of hematopoietic cells from IL11Ra−/− mice to respond to IL-11 in culture (see below).
RT-PCR showing the absence of IL11Ra transcript and absence of expression of IL11Ra2 transcript in bone marrow (BM) cells and hematopoietic colonies from cultures stimulated with EPO, SCF, and IL-11. RT-PCR products encoding the deleted exons of IL11Ra locus (A), transcripts from IL11Ra2 locus (B), and HPRT gene (C) were examined by Southern hybridization with radiolabeled internal oligonucleotide probes. Testis that expresses both transcripts is included as a positive control. The product seen in IL11Ra−/− testis (A) is due to the 99% nucleotide identity displayed by transcripts from the two loci in the coding region. Negative (-ve; no cDNA) control is indicated.
RT-PCR showing the absence of IL11Ra transcript and absence of expression of IL11Ra2 transcript in bone marrow (BM) cells and hematopoietic colonies from cultures stimulated with EPO, SCF, and IL-11. RT-PCR products encoding the deleted exons of IL11Ra locus (A), transcripts from IL11Ra2 locus (B), and HPRT gene (C) were examined by Southern hybridization with radiolabeled internal oligonucleotide probes. Testis that expresses both transcripts is included as a positive control. The product seen in IL11Ra−/− testis (A) is due to the 99% nucleotide identity displayed by transcripts from the two loci in the coding region. Negative (-ve; no cDNA) control is indicated.
Phenotype of IL11Ra −/− mice. The genotype of pups from interbreeding of IL11Ra+/− mice displayed a normal Mendelian pattern of inheritance: 109 IL11Ra+/+, 263 IL11Ra+/−, and 128 IL11Ra−/− indicating no intrauterine fetal loss. However, unlike their IL11Ra+/− and IL11Ra+/+ littermates, IL11Ra−/− female mice appeared to be infertile. This observation is under further investigation.
At weaning, the weight of IL11Ra−/− pups was comparable with that of the littermate controls: 10.5 ± 1.3 g IL11Ra+/+ versus 10.8 ± 2.0 g IL11Ra+/− and 10.5 ± 1.2 g IL11Ra−/− (mean ± standard deviation [SD], n = 10 animals per group). The IL11Ra−/− mice appeared normal at birth and during adult life (oldest animals were 8 months of age). A histologic survey of hematopoietic and nonhematopoietic organs of 6- to 8-week-old animals showed no consistent morphologic abnormalities. Flow cytometric analysis of cells from bone marrow, spleen, thymus, lymph node, and peripheral blood using a panel of monoclonal antibodies for a range of lymphoid, myeloid, and erythroid antigens did not show any significant perturbations in the IL11Ra−/− animals (data not shown).
Hematologic analysis. The peripheral blood platelet count of the IL11Ra−/− mice was normal (901 ± 87 × 106/mL; Table 1). There were also no differences in the total white blood cell counts or differential counts between the three genotypes. The hematocrit and the steady-state red blood cell reticulocyte count were also normal (Table 1). Bone marrow cellularity was comparable between IL11Ra+/+ and IL11Ra−/− animals (10.7 ± 2.7 × 106 for IL11Ra+/+ and 13.0 ± 2.9 × 106 for IL11Ra−/−; mean cell count per femur ± SD, n = 6). The splenic mass of 7-week-old mice was similar between genotypes (90 ± 10 mg for IL11Ra+/+ and 89 ± 11 mg for IL11Ra−/−; mean ± SD, n = 6). As shown in Table 1, the cellular composition of bone marrow and spleen and the number of megakaryocytes were similar in IL11Ra+/+ and IL11Ra−/− mice.
The progenitor cell content of mutant and wild-type mice was assessed by in vitro clonal cultures using multiple cytokine combinations. The number of bone marrow granulocyte-macrophage (granulocyte-macrophage colony-forming cells [GM-CFC]), erythroid progenitors (burst-forming unit-erythroid [BFU-E]), and megakaryocyte progenitors (CFU-MEG) were comparable for IL11Ra+/+ and IL11Ra−/− mice (Fig 4). The less primitive erythroid progenitors, colony-forming unit-erythroid (CFU-E), were also normal (data not shown). There was also no significant difference in spleen GM-CFC and BFU-E between the wild-type and IL11Ra−/− animals (data not shown). Thus, the IL11Ra−/− mice showed no perturbation of progenitor cells.
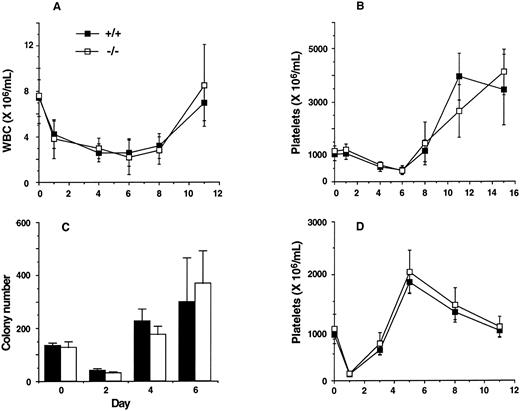
Granulocyte-macrophage (A), BFU-E (B), and megakaryocyte progenitors (C) from bone marrow of IL11Ra −/− mice. Mean of total colony numbers ± standard deviations (n = 7 for each genotype, for [A] and [B]) in replicate cultures containing 50,000 bone marrow cells stimulated with final concentrations of GM-CSF at 10 ng/mL, IL-3 at 10 ng/mL, SCF at 100 ng/mL, MGDF at 200 ng/mL, EPO at 2 U/mL, IL-6 at 100 ng/mL, and G-CSF at 10 ng/mL. Acetylcholine esterase-stained megakaryocyte progenitors (n = 4 for each genotype) are expressed as a percentage.
Granulocyte-macrophage (A), BFU-E (B), and megakaryocyte progenitors (C) from bone marrow of IL11Ra −/− mice. Mean of total colony numbers ± standard deviations (n = 7 for each genotype, for [A] and [B]) in replicate cultures containing 50,000 bone marrow cells stimulated with final concentrations of GM-CSF at 10 ng/mL, IL-3 at 10 ng/mL, SCF at 100 ng/mL, MGDF at 200 ng/mL, EPO at 2 U/mL, IL-6 at 100 ng/mL, and G-CSF at 10 ng/mL. Acetylcholine esterase-stained megakaryocyte progenitors (n = 4 for each genotype) are expressed as a percentage.
CFU-S day 12 are a population of stem cells with the capabilities of self-renewal and generation of multilineage committed precursors.67 Nucleated bone marrow cells (75,000) from IL11Ra+/+ and IL11Ra−/− mice were injected intravenously into lethally irradiated recipients (n = 5 to 6 recipients per donor). The recipients were sacrificed on day 12 and the colonies on the spleen surface were counted. Figure 5 represents data from two independent experiments and shows no differences in numbers of CFU-S day 12 from the IL11Ra+/+ and IL11Ra−/− mice.
CFU-S day 12 (CFU-S d12) progenitors in IL11Ra −/− mice. Mean ± SD of CFU-S d12 from two independent experiments. In each experiment, 75,000 bone marrow cells from each of 2 IL11Ra+/+ and IL11Ra−/− mice were administered intravenously to 5 or 6 lethally irradiated recipients. The recipients were killed on day 12 and macroscopic colonies on the spleen surface were counted.
CFU-S day 12 (CFU-S d12) progenitors in IL11Ra −/− mice. Mean ± SD of CFU-S d12 from two independent experiments. In each experiment, 75,000 bone marrow cells from each of 2 IL11Ra+/+ and IL11Ra−/− mice were administered intravenously to 5 or 6 lethally irradiated recipients. The recipients were killed on day 12 and macroscopic colonies on the spleen surface were counted.
Recloning studies were performed to determine whether the self-generation capacity of progenitor cells was altered in IL11Ra−/− mice. Individual colonies stimulated by SCF were resuspended and transferred to secondary agar cultures also stimulated by SCF. This provided an indirect analysis of the clonogenic potential of progenitor cells composing the original clone. Consistent with the above results, no significant difference was noted in the number of secondary clones generated from SCF-supported primary bone marrow colonies (of blast and multicentric morphology) from IL11Ra+/+ or IL11Ra−/− mice (data not shown).
Confirmation of the IL11Ra null phenotype. Whereas IL-11 has minimal effects in stimulating myeloid progenitor cells on its own, it is known to synergize with IL-3 and SCF in supporting the growth of these cells. We therefore studied the actions of both IL-3 and SCF alone and in combination with IL-11. As shown in Table 2, IL-11 alone at a final concentration of 10 ng/mL showed almost no effect on colony formation by IL11Ra+/+ bone marrow cells. IL-3 and SCF alone, at multiple concentrations, supported similar numbers of CFC in both IL11Ra+/+ and IL11Ra−/− mice. However, with the addition of IL-11, a clear enhancement of colony formation was seen in IL11Ra+/+ mice. For example, SCF (10 ng/mL) alone gave 50 ± 7 GM-CFC (mean number of colonies ± SD, n = 3) and the addition of IL-11 (10 ng/mL) increased this to 95 ± 6 (P < .05). Similarly, stimulation with IL-3 (0.1 ng/mL) generated 15 ± 4 GM-CFC per 50,000 bone marrow cells, whereas IL-3 together with IL-11 (10 ng/mL) resulted in 46 ± 12 GM-CFC (P < .05). Similar results were seen for erythroid cells: bone marrow cells from wild-type mice showed increased erythroid colony formation with IL-11 plus SCF and Epo when compared with SCF and Epo alone. No such increase in BFU-E was seen in IL-11–stimulated cultures of IL11Ra−/− bone marrow cells (data not shown). The absence of synergy between IL-11 and SCF or IL-3 in the IL11Ra−/− mice (Table 2) was additional evidence of an IL11Ra null phenotype. Moreover, these results further confirmed a lack of compensation from the IL11Ra2 locus.
Assessment of hematopoietic reserves. 5-FU selectively kills cycling cells.69 Recovery from such a stress involves recruitment of quiescent stem cells and induction of differentiation. IL-11, mostly in synergy with IL-3 or SCF, has been documented to support multilineage progenitors13,15,70 and stimulate entry of dormant stem cells into cell cycle.9-12 The recovery of IL-11Ra −/− mice was therefore assessed after administration of 5-FU. As shown in Fig 6, the kinetics of recovery of total white blood cells, platelets, and bone marrow progenitor cells in IL11Ra−/− mice were similar to those of IL11Ra+/+ mice. Also, no difference in recovery of bone marrow progenitors was noted in additional experiments performed on days 5, 7, and 10 after 5-FU administration and on day 7 after more severe hematopoietic insult (a single dose of 5-FU followed 3 days later with 6 Gy irradiation; data not shown). Platelet response was also examined in an immune model of thrombocytopenia induced by administration of antiplatelet serum. Recovery from such a stress depends in part on platelet production from existing megakaryocytes. After a 90% decrease in platelet levels 24 hours after administration, the rate of recovery was similar in IL11Ra+/+ and IL11Ra−/− mice.
Recovery of mature cells and progenitors after 5-FU–induced cytotoxicity and recovery of platelets after immune thrombocytopenia. Kinetics of recovery of white blood cells (WBC; A), platelets (B), and myeloid progenitors (C) after a single administration of 5-FU. The figure represents the mean ± SD of WBC and platelet numbers of IL11Ra+/+ (n = 6) and IL11Ra−/− (n = 5) mice after 150 mg/kg 5-FU (intraperitoneal). Granulocyte-macrophage colony number (mean ± SD) of replicate cultures from 3 mice of each genotype on days 2, 4, and 6 after 150 mg/kg 5-FU (intravenous) is also shown (C). Cultures contained 50,000 bone marrow cells stimulated with final concentrations of GM-CSF at 10 ng/mL, IL-3 at 10 ng/mL, IL-6 at 100 ng/mL, and SCF at 100 ng/mL. (D) Kinetics of recovery of platelets after a single intraperitoneal administration of antiplatelet serum. The figure represents the mean ± SD of platelet numbers (n = 5 IL11Ra+/+ mice; n = 6 IL11Ra−/− mice).
Recovery of mature cells and progenitors after 5-FU–induced cytotoxicity and recovery of platelets after immune thrombocytopenia. Kinetics of recovery of white blood cells (WBC; A), platelets (B), and myeloid progenitors (C) after a single administration of 5-FU. The figure represents the mean ± SD of WBC and platelet numbers of IL11Ra+/+ (n = 6) and IL11Ra−/− (n = 5) mice after 150 mg/kg 5-FU (intraperitoneal). Granulocyte-macrophage colony number (mean ± SD) of replicate cultures from 3 mice of each genotype on days 2, 4, and 6 after 150 mg/kg 5-FU (intravenous) is also shown (C). Cultures contained 50,000 bone marrow cells stimulated with final concentrations of GM-CSF at 10 ng/mL, IL-3 at 10 ng/mL, IL-6 at 100 ng/mL, and SCF at 100 ng/mL. (D) Kinetics of recovery of platelets after a single intraperitoneal administration of antiplatelet serum. The figure represents the mean ± SD of platelet numbers (n = 5 IL11Ra+/+ mice; n = 6 IL11Ra−/− mice).
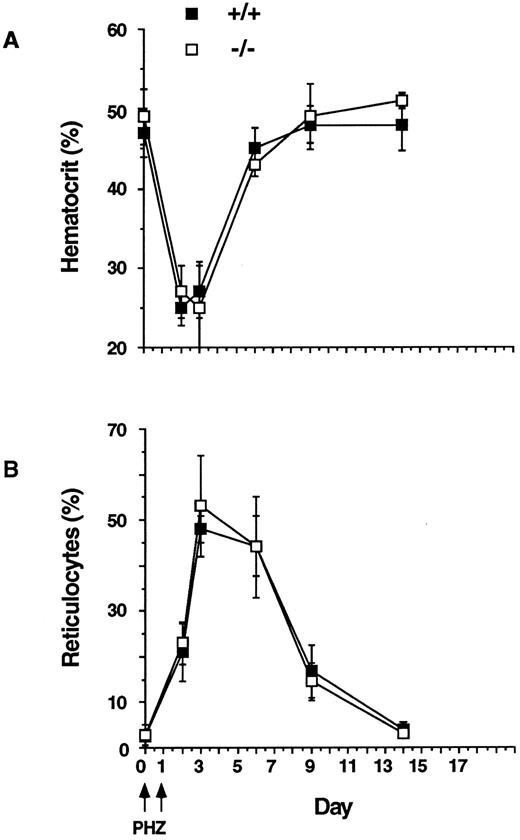
IL-11 is also known to influence erythropoiesis.22-24 We therefore examined the erythroid response to anemia by inducing acute hemolysis with phenylhydrazine.71 Recovery from such a stress is dependent on the progenitor reserve and the induction of terminal erythroid differentiation. Erythroid cells of IL11Ra+/+ and IL11Ra−/− mice were equally sensitive to phenylhydrazine (decrease in hematocrit to 50% of baseline by day 2; Fig 7) and the kinetics of erythroid recovery and the magnitude of this response were very similar in the IL11Ra+/+ mice and IL11Ra−/− mice (Fig 7).
Kinetics of recovery after acute hemolysis. Phenylhydrazine (PHZ; 60 mg/kg, intraperitoneal) was administered on days 0 and 1. Recovery was monitored by measurement of hematocrit and reticulocytes (mean % ± SD, n = 5, each genotype).
Kinetics of recovery after acute hemolysis. Phenylhydrazine (PHZ; 60 mg/kg, intraperitoneal) was administered on days 0 and 1. Recovery was monitored by measurement of hematocrit and reticulocytes (mean % ± SD, n = 5, each genotype).
Immunologic studies. Sera of IL11Ra+/+, IL11Ra+/−, and IL11Ra−/− mice were assessed for the concentration of the various Ig isotypes. The levels of IgM, IgG3, IgG1, IgG2a, and IgG2b were measured in mice of the three genotypes at the age of 4.5 to 5.0 weeks (Table 3). There were no consistent differences between the three groups, demonstrating that IL11Ra−/− mice were not deficient in the production of these Ig isotypes. It was formally possible that the levels of certain of the isotypes measured in these young mice were due to maternally transferred Ig and not endogenous production. We therefore measured the levels of the same isotypes in a limited number of 8.5-week-old animals and again observed no significant difference between control and IL11Ra−/− samples (data not shown).
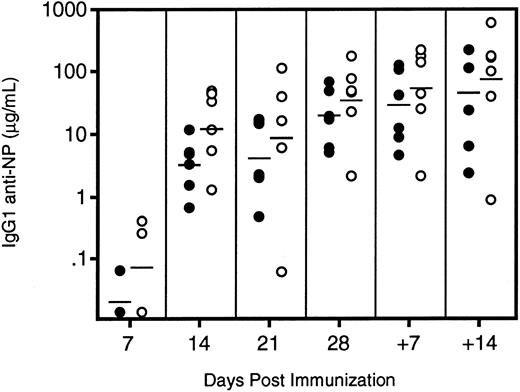
The antibody response of IL11Ra−/− mice to a T-cell–dependent antigen did not significantly differ from that of control animals (Fig 8). Only a fraction of mice showed antigen-specific IgG1 in their sera 7 days after primary immunization. At successive time points, all mice were positive and the average titer of antigen-specific antibody increased relative to the previous week. The IL11Ra −/− mice showed slightly higher levels of NP-specific IgG1 at each time point measured, but the difference never reached statistical significance. Five weeks after primary immunization, the mice were boosted with a soluble form of the immunizing antigen and serum samples were collected at weekly intervals. The modest increase in serum titers was attributed to the poor immunogenicity of NP-BSA, but again the degree of increase was comparable in both groups. At each time point, the proportion of the total anti-NP antibody that was of the high-affinity subtype was the same in both groups. Thus, affinity maturation occurred equally in both groups. Therefore, despite the evidence implicating IL-11 in B-cell maturation, there was no detectable abnormality in Ig responses in the IL11Ra−/− mice.
Levels of IgG1 anti-NP antibodies in immunized (○) IL11R−/− and (•) IL11Ra+/+ mice. Six mice of each genotype were immunized at day 0 and the response was followed by serum ELISA at weekly intervals. The mice were boosted with a soluble form of the antigen 35 days after primary immunization and the secondary response measured after 7 and 14 days. The geometric mean of each group at each time point is indicated.
Levels of IgG1 anti-NP antibodies in immunized (○) IL11R−/− and (•) IL11Ra+/+ mice. Six mice of each genotype were immunized at day 0 and the response was followed by serum ELISA at weekly intervals. The mice were boosted with a soluble form of the antigen 35 days after primary immunization and the secondary response measured after 7 and 14 days. The geometric mean of each group at each time point is indicated.
DISCUSSION
Several actions exhibited by IL-11 are shared with IL-6, LIF, CNTF, and OSM.3,72 To examine the unique actions of IL-11, we have generated mice with a targeted mutation of its known ligand-binding specific receptor component (α-chain). This receptor component, encoded by the locus IL11Ra, is normally expressed in a wide variety of adult and embryonic murine tissues, including those contributing to hematopoiesis.52 Using Northern analysis, RT-PCR, and biologic assays, we have confirmed that the targeting strategy led to a null mutation of the IL11Ra gene. In addition, there was no detectable expression of the product of the second IL-11 receptor gene, IL11Ra2.
Although IL-11 has been documented to enhance multilineage progenitors on its own and especially so in synergy with SCF or IL-3,13,15,70 its role in the regulation of very primitive murine and human stem cells is still unclear. Several investigators have reported that using in vitro assays IL-11 has little effect on this population.13,19,70 However, it has been shown that short-term incubation of 5-FU–treated and unmanipulated bone marrow cells with IL-11 and SCF led to significant expansion of multipotential progenitors73 and to an improved ability to reconstitute murine bone marrow transplant recipients.18 Also, retrovirus-mediated overexpression of IL-11 in bone marrow resulted in a 20- to 100-fold elevation in progenitor cells in spleen74 and to accelerated hematopoietic recovery in primary and secondary recipients.33,75 IL-6 and LIF, which share the same signal transduction molecule (gp130) with IL-11, also demonstrate similar actions on expansion of multi-lineage progenitors.5,76 Although the long-term repopulating stem cell compartment was not quantitated, multipotential stem cells (CFU-S day 12) and more committed progenitors (as assessed by in vitro clonal cultures) were normal in IL11Ra−/− mice. This observation was surprising, because IL-6−/− and LIF−/− mice show a reduction in CFU-S and, in IL-6−/− mice, a reduction in more primitive progenitors.71,77 We thus infer that, although IL-11 increases hematopoietic progenitors both in vitro and in vivo, its actions in the IL11Ra−/− mice are adequately compensated by other cytokines. Moreover, it is likely that IL-6 or LIF may play a greater role than IL-11 in maintaining the progenitor cell pool. Redundancy of function among cytokines is a common feature in hematopoiesis, but the degree of compensation may vary amongst cytokines and for the stages of hematopoiesis studied. For example, although LIF and IL-6 null mice have a deficiency of progenitor cells, in common with IL11Ra−/− mice, they maintain a normal peripheral blood white cell count.71,77 Because the predominant action of IL-11 on progenitor cell is in combination with SCF, an analysis of the IL11Ra−/− mutation in a SCF-deficient background (Steel or white spotting mutations78 ) may help in analyzing its role on this compartment.
We have shown here that the functional redundancy for IL-11 also extends to the erythroid compartment. IL-11 is known to influence multiple stages of erythropoiesis. For example, in vitro, in the presence of EPO, or in synergy with IL-3 or SCF, IL-11 can increase the primitive erythroid progenitors, BFU-E22,23; with EPO, it can also support the maturation of the more differentiated precursors, CFU-E.22 Similar effects also occur in vivo.24 However, the steady-state hematocrit, erythroid progenitor cell content, and also the recovery of hematocrit and reticulocyte count from hemolytic stress were normal in IL11Ra−/− mice. The normal kinetics of recovery from phenylhydrazine also highlights the potential differences within this family of cytokines despite their overall similarity of actions, because the IL-6−/− mice succumb to an identical hemolytic stress with 100% mortality.71
The search for the primary regulator of megakaryopoiesis has spanned several decades. Several growth factors, including IL-11, IL-6, LIF, and TPO, influence megakaryocyte growth and development and their administration leads to a significant platelet increment, although to a variable degree.76,79-82 Although preliminary clinical trials with IL-11 have produced encouraging results,37,38 it is possible that the effects seen with pharmacologic doses do not accurately reflect the normal physiologic role of IL-11. We have shown that IL11Ra−/− mice have normal baseline platelet counts and that they recover normally after cytotoxic stress. In addition, the number of megakaryocytes on histologic sections and their progenitors were similar to controls. In comparison, the IL-6−/− and LIF−/− mice also maintain normal platelet numbers but with reduced numbers of megakaryocyte progenitors,71,77 whereas mice deficient in TPO or its receptor c-mpl have markedly reduced platelets and multilineage progenitors.54-56 These results from gene targeting studies confirmed the observations reported by Kaushansky80 suggesting that TPO is the primary regulator of platelet production. However, because there is no overt hemostatic defect in these mice despite the severe platelet deficit, the non-TPO cytokines81 singly or in combination may provide a critically important back-up in the absence of TPO. An evaluation of the role of IL-11 in compensating for the platelet deficiency in TPO-deficient mice or c-mpl null mice can now be performed by genetic crosses.
In summary, our investigation of the physiologic role of IL-11R in the hematopoietic compartment has shown that, despite the manifold effects attributed to IL-11, the mutant adult mice displayed normal steady-state hematopoiesis. Also, when challenged with cytotoxic stress or hemolysis, the hematopoietic recovery paralleled that of wild-type mice. The absence of a response of IL11Ra−/− bone marrow cells to IL-11 in clonal cultures argues against compensation by a second receptor component. Our report highlights that, although the IL-6 group of cytokines shares several functions, the degree of compensation that can be achieved in the absence of individual cytokines is indeed variable. The hematopoietic defects resulting from IL-6 and LIF deficiencies imply that a subset of cells is critically dependent on these factors for optimal activity, whereas the role of IL-11 is fully compensated for by one or more of the above cytokines. However, studies of LIF deficiency have emphasised that the question of redundancy has to be interpreted in the context of the organ under study; IL-11 may be redundant in the hematopoietic compartment, but a survey of other organ systems may unveil its unique function(s).
ACKNOWLEDGMENT
The authors thank Dr Andreas Strasser for his generous gift of monoclonal antibodies, Dr Richard Simpson for murine IL-6, and Drs Tracy Willson and Jian Guo Zhang for murine SCF. The expertise of Dr Ruili Li in blastocyst injections is gratefully acknowledged. We also thank Rachel Mansfield and Dale Cary for expert technical assistance and Jodie Stanley for animal husbandry. We are indebted to Dr Douglas Hilton and Prof Nicos Nicola for valuable scientific discussion and critical examination of the manuscript.
Supported in part by the National Health and Medical Research Council of Australia, Australian Federal Governments Co-operative Research Centres Scheme, and the Amrad Corp.
Address reprint requests to Harshal H. Nandurkar, MD, The Walter and Eliza Hall Institute of Medical Research, Post Office, The Royal Melbourne Hospital, Victoria, 3050, Australia.

![Fig. 4. Granulocyte-macrophage (A), BFU-E (B), and megakaryocyte progenitors (C) from bone marrow of IL11Ra −/− mice. Mean of total colony numbers ± standard deviations (n = 7 for each genotype, for [A] and [B]) in replicate cultures containing 50,000 bone marrow cells stimulated with final concentrations of GM-CSF at 10 ng/mL, IL-3 at 10 ng/mL, SCF at 100 ng/mL, MGDF at 200 ng/mL, EPO at 2 U/mL, IL-6 at 100 ng/mL, and G-CSF at 10 ng/mL. Acetylcholine esterase-stained megakaryocyte progenitors (n = 4 for each genotype) are expressed as a percentage.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/6/10.1182_blood.v90.6.2148/3/m_bl_0036f4.jpeg?Expires=1768304163&Signature=Ah7FJKDqqBhRxMxwtznFOGaO7DBQrrG4FvzMaxAwYNgeXPlvyVdsfVgCuoK42TNGpkesv-ZCpPropXog8RMrLbauugXaCvBv8FKRLuMLWImshFmOADIsgkC43BIx1ol8Sv0nXbW-TwslZ~NLjrAexATb-RqqFRjS7qfRrw8tweIq4uyFtbyznfGN1A5~Ri0cOikEl0FBMeT-jgheBYTDnd91p~NjUxI5uNgpNMMOWFsDpzDvoyl9bsV1eoMTF5DSHfCwNFO3nHX05HYH7PqtXN-fv95E11nuyEy1UoygcG-tLz4FdwVht9cYMfgQRlXSSV8R6fJprE2WiTwUHYFxFQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)