Abstract
We infused six human immunodeficiency virus (HIV)-seropositive subjects with autologous CD8+ cytotoxic T cells (CTLs) enriched for HIV-specific cytotoxicity targeted against a diversity of HIV epitopes in gp120, gag p17 and p24, and nef. There was no toxicity and no subject deteriorated clinically. In the first 2 weeks, CD4 counts increased for all subjects and plasma viremia decreased in five of six subjects. Twenty-four weeks later, the mean values of all measures of viral burden and surrogate markers of HIV infection were either unchanged or improved, but none of the changes was statistically significant. Two subjects continued to have decreased cell-associated viral burden and another subject had more than doubled CD4 cell count. HIV-specific CTL activity increased in most subjects. The increase in CD4 T-cell counts in the first weeks after the infusion suggests that antiviral CTLs of diverse specificities do not play a significant role in CD4 T-cell decline. The lack of any acute toxicity or adverse effect on viral burden suggests that therapy with antiviral CTLs deserves further study.
VIRAL-SPECIFIC cytotoxic T lymphocytes (CTLs) suppress human immunodeficiency virus (HIV) replication in vitro by direct cytotoxicity and by secretion of soluble factors.1-5 The viremia of acute HIV-1 infection resolves coincident with the appearance of HIV-specific CTLs.6,7 Moreover, circulating antiviral CTLs decrease when opportunistic infections begin and the likelihood of progressing to acquired immunodeficiency syndrome (AIDS) increases in patients who lack gag-specific CTL.8-10 This evidence, together with the rapid course of HIV infection in neonates whose T-cell immunity is immature, suggests that CTLs may be important in controlling HIV infection.11
However, HIV-specific CTLs, which can lyse infected CD4 cells and even uninfected CD4 cells that bind serum gp120, could hasten the decline in CD4 cells during HIV infection. Antiviral CTLs might also play a role in the noninfectious pneumonitis or central nervous system manifestations of HIV infection.2,12,13 The pathogenic role of antiviral CTLs in lymphocytic choriomeningitis virus and hepatitis B infections has led some to suggest that HIV-specific CTLs may contribute to the immunodeficiency of HIV infection.14 This concern has been reinforced by the possible deterioration in a single subject with moderately advanced disease after infusions with a nef-specific CTL clone administered with interleukin-2 (IL-2).15
The efficacy of cellular immunotherapy by adoptive transfer of viral-specific CTLs has been shown in mice infected with leukemogenic retroviruses.16-18 In murine adenovirus, influenza, and lymphocytic choriomeningitis virus (LCMV) infections, the adoptive transfer of viral-specific CTLs also prevents fatal infection.19,20 In scid mouse models reconstituted with human peripheral blood mononuclear cells (PBMCs), the transfer of CD8 CTL has also been shown to be protective for HIV infection and for lymphoproliferative disease caused by Epstein-Barr virus (EBV).21-23
In humans, adoptive transfer of lymphokine-activated CTLs (LAK cells) or tumor-infiltrating lymphocytes (TILs) has been used to treat malignant melanoma and renal cell carcinoma.24,25 In at least one patient, the transfer of EBV-specific T-cell lines controlled an established lymphoproliferative disease.26 Infusions of cytomegalovirus (CMV)-specific CD8+ CTL clones to bone marrow transplantation patients at risk for CMV disease were well tolerated. CMV-specific cytotoxicity was present after the transfer of as few as 3.3 × 107 cells/m2 and CMV-specific CTLs persisted for up to 12 weeks. Moreover, treated patients did not develop posttransplantation CMV disease.27 28
To treat HIV infection, a pilot trial studied infusions of 108 to 1010 CD4-depleted LAK-like lymphocytes in patients with AIDS-related complex or AIDS. The antiviral activity of the infused cells was variable and not specifically enhanced. There was neither significant toxicity nor clear benefit in late-stage patients.29,30 In preliminary studies, we found that it is unnecessary to deplete cell lines of CD4+ T cells to eliminate the risk of transferring virus.31 Ex vivo-expanded HIV-specific CD8+ T-cell clones have more recently been used to treat HIV-infected individuals. When a patient with moderately advanced disease was treated with multiple infusions of a nef-specific CTL clone together with IL-2, the patient showed no benefit and his condition may have worsened. However, some of the virus isolated in this progressing patient escaped CTL recognition by deletion of the relevant nef coding region.15 A subsequent attempt to treat HIV-infected patients with genetically marked gag-specific clones was thwarted by the elimination of infused CTLs by a cellular immune response to the genetic marker.32
Our approach involves infusion of polyclonal T-cell lines enriched for HIV-specific CTLs. Circulating precursor CTLs from asymptomatic HIV-infected subjects recognize a small number of HIV peptides and occur at high frequency.8,31,33 It is thus possible to generate polyclonal T-cell lines with potent cytolytic activity against HIV-expressing autologous targets by selectively expanding CTLs against particular HIV peptides.31 A substantial fraction of these cells are specific for the HIV peptides to which they are exposed. From 1 mL of blood, the ex vivo expansion yields approximately 1 to 10 billion cells that lack detectable replicating HIV. The expanded cells are predominantly CD8 T cells (85% ± 10%) with a small admixture of CD3−CD16+ (14% ± 14%) and CD4+ (4.2% ± 4.4%) cells. The HIV-specific cytotoxicity of these cell lines is primarily major histocompatibility complex-restricted.31 34
We gave 6 HIV-infected subjects a single infusion of 1 billion autologous T cells enriched for HIV-specific cytolytic activity against a variety of HIV epitopes in gp120, gag p17 and 24, and nef. We found no acute toxicity associated with the infusion and followed changes in plasma and cell-associated virus and CD4+ T cells in the recipients for 6 months.
MATERIALS AND METHODS
Subjects.Adult HIV-seropositive individuals with CD4 counts of 100 to 400/μL and no history of AIDS-defining opportunistic infections or neurologic symptoms were enrolled in January and February 1993, after obtaining informed consent. The clinical characteristics of the six subjects at the time of treatment (3 to 12 months after enrollment) are shown in Table 1. All but one had a history of HIV-related symptoms and two met the 1993 Centers for Disease Control criteria for AIDS.35 Four subjects were taking antiretroviral drugs for at least 6 weeks before the infusion with no change in antiretroviral drug or dosage during the study.
HIV CTL epitope characterization.The HIV peptide epitopes contained in HIV LAI or SF2 env, gag, rev, tat, and nef recognized by the CTL of mitogen-stimulated T-cell lines generated from PBMCs were determined for each subject as in Lieberman et al.34 An epitope was defined if autologous B-lymphoblastoid cell lines (B-LCL) incubated with peptide were lysed at least 5% more than targets incubated with media at an effector:target (E:T) ratio of 25:1 in at least two assays if peptide-specific lysis was greater than 10% or in three independent assays if the specific lysis was 5% to 10%. The characterized epitopes were confirmed by the enrichment for epitope-specific CTLs after exposure of T-cell lines to peptide. Eight to 10 days after exposure to peptide-incubated PBMCs, T-cell lines were tested for cytotoxicity in a 4-hour 51Cr release assay against autologous peptide-incubated and media-control B-LCL as in Lieberman et al.31 The peptide-specific cytotoxicity of the peptide-exposed line was compared with the specific cytotoxicity of the T-cell line not exposed to peptide. The three peptides that best sensitized target cells for lysis were used for CTL expansion. For gag and env epitopes, the enhanced cytolysis was also verified against vaccinia-gag– and vaccinia-env–infected targets.
Peptide-selected CTLs for infusion.T cells for infusion were prepared by exposing mitogen and IL-2–stimulated T-cell lines, cultured for 3 to 4 weeks in T-cell medium (RPMI supplemented with 15% fetal calf serum [JRH Biosciences, Lenexa, KS] and 200 U/mL recombinant human IL-2 [Chiron Oncology, Emeryville, CA], 2 mmol/L glutamine, 2 mmol/L HEPES, and 50 μmol/L β-mercaptoethanol), to autologous PBMCs incubated with one to three HIV peptides as in Lieberman et al.31 Briefly, 1 to 3 × 108 PBMCs were incubated at 37°C for 2 hours in 100 mL of T-cell medium with 1 mg of HIV-1 GLP high-performance liquid chromatography-purified peptides (Bachem, Torrance, CA), consisting of an equal mixture of up to three peptides that the subject's T cells were determined to recognize. The peptide-incubated PBMCs were washed once to remove unbound peptide, resuspended in 100 mL of T-cell medium, and added to 1 to 4 × 109 cultured T cells at a concentration of 1 × 106/mL. Ten days later, T cells for therapy were separated by Ficoll-Hypaque density separation and washed before intravenous infusion over 1 hour. Before infusion, therapy T cells were assayed for contamination with bacteria by aerobic and anaerobic culture, for mycoplasma by T.C. Rapid Detection System test (Gen-Probe, San Diego, CA), and for endotoxin by Limulus amoeba lysate assay (Microbiological Associates, Rockville, MD). One to 2 days before infusion, T cells were assayed, using a 51Cr release assay as in Lieberman et al,34 for their ability to lyse autologous B-LCL incubated with the peptides to which they were exposed and to autologous B-LCL infected with HIVLAIenv-, pol-, gag-, and lacZ-expressing vaccinia recombinant virus. The sum of the HIV-specific cytotoxicity (defined as the sum of the specific lysis of nef peptide-incubated targets [corrected for background lysis in the absence of peptide] and the specific lysis of the 3 HIV-vaccinia infected targets [after the lysis of the lacZ-vaccinia infected target background was subtracted for each]) was required to be at least 25% at an E:T ratio of 20:1. T-cell lines were also tested for replicating HIV using a p24 Ag assay of supernatants after coculture with seronegative phytohemagglutinin (PHA) lymphoblasts.36
Subject evaluation.Subjects were evaluated at two baseline visits (within 10 days of infusion) and at 1, 2, 4, 8, and 24 weeks after therapy using history and physical examination, complete blood count with differential and platelet count, serum creatinine level, bilirubin and serum aspartate aminotransferase (SGOT), T-cell subsets, β2 -microglobulin, quantitative PBMC HIV titer,37 plasma p24 and acid dissociated p24 Ag,38 and plasma bDNA assay.39 When plasma bDNA assay was below the level of detection, plasma was assayed for HIV using quantitative reverse transcription-polymerase chain reaction (RT-PCR; Roche, Sommerville, NJ). In addition, electrolytes, lactate dehydrogenase, chest x-ray, and pulmonary function tests with DLCO were tested before and after infusion. At each time and at 2 and 18 hours after infusion, PBMCs were obtained to generate T-cell lines assayed after 21 to 35 days in culture for HIV-specific cytotoxicity as in Lieberman et al.34
Statistical analysis.Wherever possible, data obtained 9 to 10 days before and just before the infusion were averaged to obtain a more stable baseline value. The squared differences between these two values were averaged and halved to estimate biologic and assay variability for the CTL activity measures. Changes after 1 week and 24 weeks were tested for significance using paired t-tests.
RESULTS
Characteristics of the infused T cells.Peptide epitopes in HIV env, gag, nef, tat, or rev were recognized by the cell lines of six of the first eight subjects studied (Table 2).41 The two subjects for whom we could not identify peptide epitopes were not eligible for treatment and were not observed. Because the peptides available for this study derive from laboratory isolates and do not correspond to all HIV genes, our analysis may underestimate the prevalence of HIV-specific CTLs. Gag peptides were recognized by CTL from four of the six subjects and env and nef peptides each by three subjects. No subjects had significant CTL activity against rev or tat expressing targets. If overlapping peptides are counted as one epitope, the subjects' CTLs recognized between one and nine epitopes. The three peptides that best sensitized target cells for lysis were used for CTL expansion to treat two individuals whose CTLs recognized more than three epitopes.
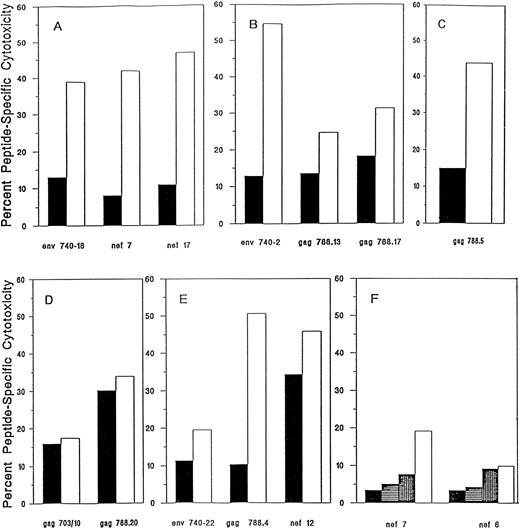
All cell lines met the infusion criteria (no evidence of microbial contamination and >25% HIV-specific CTL activity at an E:T ratio of 20:1, where HIV-specific cytotoxicity is defined as the sum of the percentage of specific lysis of gp160-, RT-, gag-, and nef-expressing targets after subtraction of the percentage of specific lysis of control targets), except for the cell line from subject 226, who had deteriorated clinically in the 6 months between enrollment and the time we obtained blood to initiate the T-cell line (Fig 1 and Table 2). His cell line no longer recognized the HIV nef epitopes determined 6 months earlier or any other HIV-expressing targets, but after repetitive stimulation with two previously recognized nef peptides, we generated a line that lysed the nef-presenting targets (20% lysis of 1 nef peptide, 8% lysis of another at an E:T ratio of 10:1; Fig 1F ). The sum of the HIV-specific cytotoxicity of the infused cells for the six subjects ranged from 42% to 103% at an E:T ratio of 10:1 (Table 2).
HIV peptide-specific cytotoxicity of T-cell lines infused into subjects after in vitro stimulation with HIV CTL epitope peptides from subjects 202 (A), 204 (B), 216 (C), 203 (D), 214 (E), and 226 (F ). Peptide-specific cytotoxicity was defined as the difference between the percentage of specific cytotoxicity in the presence or absence of peptide in a 4-hour 51Cr release assay at an E:T ratio of 20:1. T-cell line (▪) before and (□) after peptide exposure. For subject 226, the pretherapy T-cell line had lost HIV-specific cytotoxicity and the peptide selection was repeated three times to obtain sufficient antiviral activity to meet the infusion criteria (<25% HIV-specific cytotoxicity at an E:T ratio of 20:1). The background lysis of autologous B-LCL targets was less than 10%, except for T-cell line 202, for which it was 16%.
HIV peptide-specific cytotoxicity of T-cell lines infused into subjects after in vitro stimulation with HIV CTL epitope peptides from subjects 202 (A), 204 (B), 216 (C), 203 (D), 214 (E), and 226 (F ). Peptide-specific cytotoxicity was defined as the difference between the percentage of specific cytotoxicity in the presence or absence of peptide in a 4-hour 51Cr release assay at an E:T ratio of 20:1. T-cell line (▪) before and (□) after peptide exposure. For subject 226, the pretherapy T-cell line had lost HIV-specific cytotoxicity and the peptide selection was repeated three times to obtain sufficient antiviral activity to meet the infusion criteria (<25% HIV-specific cytotoxicity at an E:T ratio of 20:1). The background lysis of autologous B-LCL targets was less than 10%, except for T-cell line 202, for which it was 16%.
Because lysis of peptide-incubated targets may overestimate the lysis of infected targets where antigen processing and presentation may be limiting, we also calculated the HIV-specific cytotoxicity against targets infected with the three vaccinia-HIV recombinants, vPE16, vCF21, and vDK1. If lysis of only the vaccinia-HIV recombinant targets is taken into account for the T-cell lines infused into the five patients who recognized at least one non-nef epitope, the sum of the specific cytotoxicities ranged from 46% to 84% (mean, 62% ± 15%) at an E:T ratio of 10:1, well above the limits set by the protocol. The sum of the cytotoxicities for the T-cell line of the sixth patient (subject 226), who only recognized nef epitopes, was only 14%, which would have been below the cutoff for treatment.
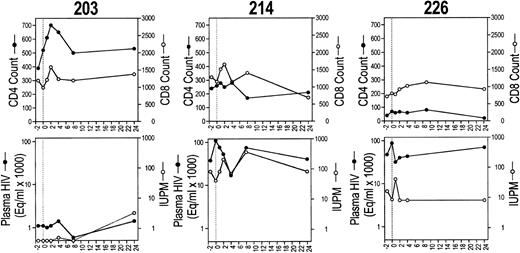
CD4 and CD8 T-cell counts (upper row) and plasma and cell-associated viremia (IUPM; lower row) for each of the six study subjects before and after CTL infusion.
CD4 and CD8 T-cell counts (upper row) and plasma and cell-associated viremia (IUPM; lower row) for each of the six study subjects before and after CTL infusion.
The peptide-selected cell lines infused into four of the five other subjects showed twofold to sixfold enhanced lysis of HIV peptide-bearing targets (Fig 1). The two gag peptides used to stimulate CTL from subject 203 were able to arm targets for lysis, but they did not appreciably augment cytotoxicity. However, even without enhancement, this subject's T-cell line had sufficient cytotoxicity to meet the criteria for infusion.
T-cell lines were predominantly CD8+, with a mean of 4.2% ± 4.4% CD4 cells admixed. Despite the presence of residual CD4 cells, none of the T-cell lines contained replicating HIV, assayed by p24 Ag in supernatants after coculture with seronegative PHA lymphoblasts.36 The CD8 subpopulation had the phenotype of antigen-specific activated cytotoxic cells, although it did not express the high-affinity IL-2 receptor: 86% ± 8% TcRαβ+, 4% ± 5% TcRγδ+, 14% ± 10% CD28+, 92% ± 14% CD38+, 70% ± 30% DR+, 1.1% ± 0.9% CD25+, 2% ± 2% CD16+, 18% ± 11% CD56+, 56% ± 20% CD57+.
Safety.No subject had an adverse reaction to the infusion. Blood chemistries, hematologic profile, chest x-ray, and pulmonary function tests were unchanged. No subject developed new symptoms of HIV-1 disease during 6 months of observation, although one subject (216) developed presumed Pneumocystis carinii pneumonia 9 months after the infusion. The plasma and cell-associated viral burden and T-cell counts for all time points for each subject are shown in Fig 2 and are discussed in more detail below.
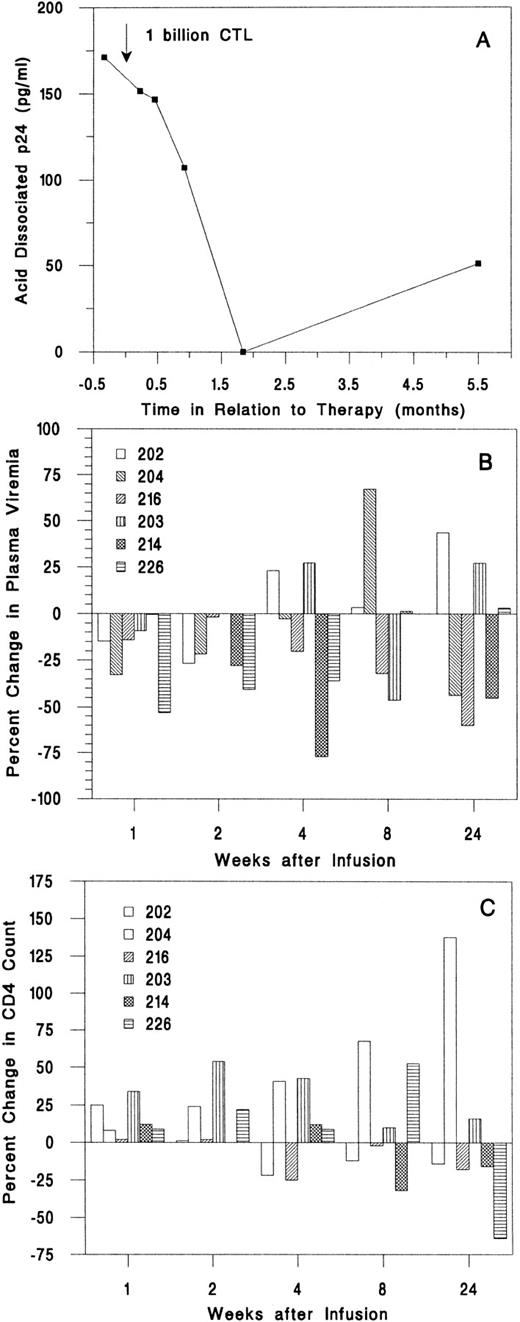
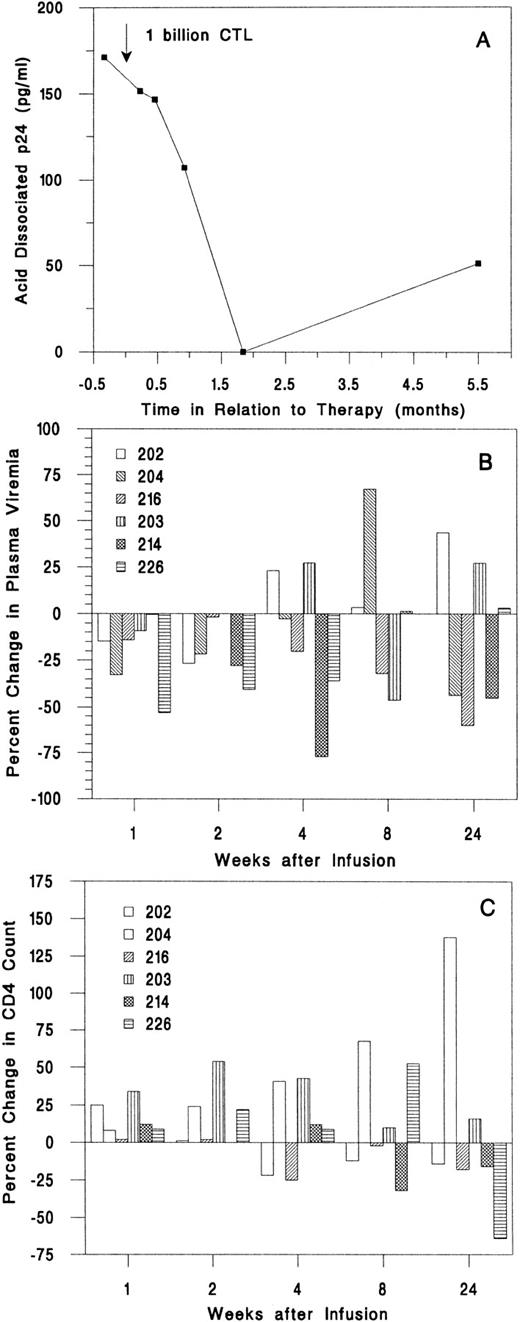
Effect on viral burden.In all samples, serum p24 antigen level was undetectable and the β2 -microglobulin level was not elevated. The acid dissociated serum p24 was detectable only in subject 216; it decreased from 171 pg/mL pretherapy to undetectable 8 weeks later to 51 pg/mL at 24 weeks (Fig 3A). Plasma virus was detectable in four subjects by branched DNA-signal amplification (bDNA) assay and by quantitative RT-PCR assay in the other two subjects. The plasma viral load decreased below the pretherapy mean at weeks 1 and 2 in five of six subjects (Fig 3B). However, none of the plasma viral changes was greater than threefold. For this assay and other assays of viral burden, threefold changes or greater are considered significant. At all times after the infusion, the mean values for plasma viremia were modestly reduced compared with the baseline means, but the changes were not significant (Table 3 and data not shown).
Change in plasma viral burden and CD4 T-cell count after infusion. (A) Acid-dissociated p24 Ag was detectable only in subject 216. (B) Plasma bDNA levels decreased in the first 2 weeks for five of six subjects, but only by small amounts. (C) The absolute CD4 count increased for the first 2 weeks after infusion for all subjects.
Change in plasma viral burden and CD4 T-cell count after infusion. (A) Acid-dissociated p24 Ag was detectable only in subject 216. (B) Plasma bDNA levels decreased in the first 2 weeks for five of six subjects, but only by small amounts. (C) The absolute CD4 count increased for the first 2 weeks after infusion for all subjects.
Viral burden in circulating mononuclear cells was assayed by limiting dilution cultures of PBMCs (Table 3). The infectious units per million cells (IUPM) of two baseline values for each subject agreed to within threefold. Five of six subjects had detectable virus before infusion; in two of these (subjects 202 and 216), there were substantial decreases in IUPM, which were sustained for at least 6 months (Fig 2). In subject 202, the IUPM declined from a mean of 30 before treatment to below the detection threshold (<0.5 IUPM) at 6 months. In subject 216, who had the highest plasma and cell-associated viral burden, the IUPM decreased to 1% of the pretherapy a mean time of 1 week after the infusion and remained fourfold lower than the pretreatment value at 6 months. Only one subject (204) had a significant increase in IUPM at the end of the study, but the increase was detected only at the last visit. The mean IUPM for all subjects at the end of the study was 57% of the baseline mean (47 ± 66 at baseline and 27 ± 31 at 24 weeks), but the change was not statistically significant (Table 3).
Effect on T-cell counts.One and 2 weeks after infusion, the absolute CD4 count of all subjects was equal to or greater than the mean baseline value (Fig 3C). By 6 months after the infusion, the CD4 count had decreased for 4 subjects to below the baseline value. The CD4 count of subject 204 increased from a low of 180/μL at infusion to 440/μL 6 months later. One week after the infusion, the mean CD4 and CD8 counts for all subjects had increased by 11% and 9%, respectively, and the CD4/CD8 ratio had increased by 10%. By the end of the study, the mean CD4 count was little changed (up 4%), but the mean CD8 count had increased 11% (Table 3).
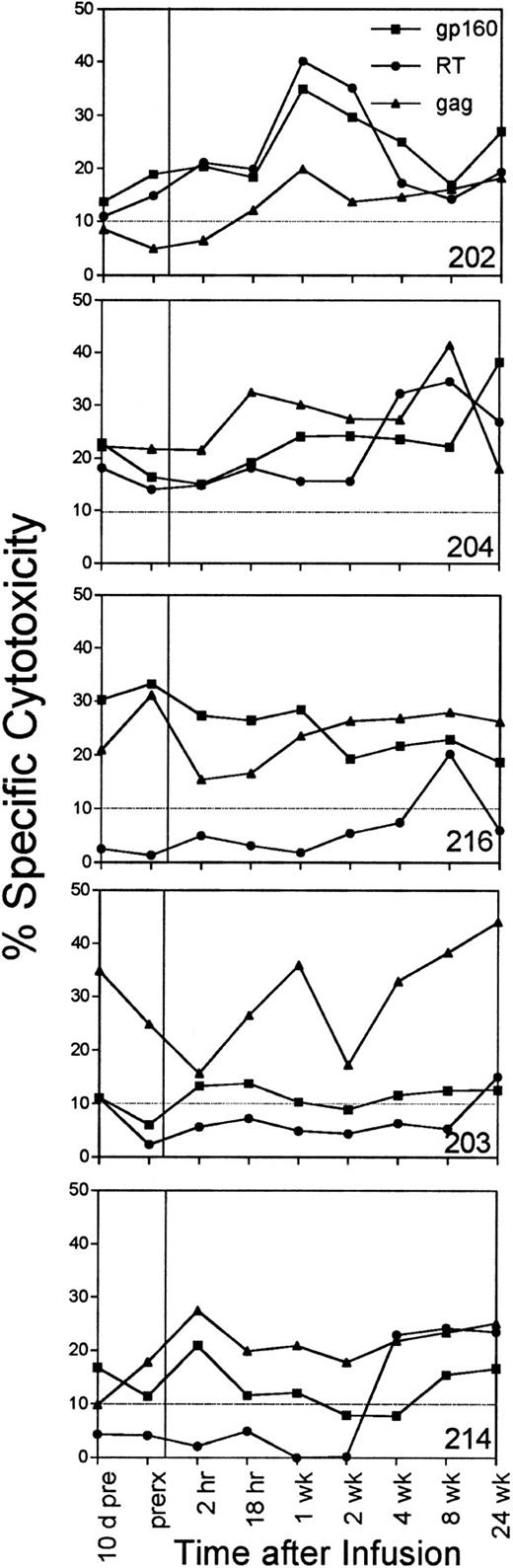
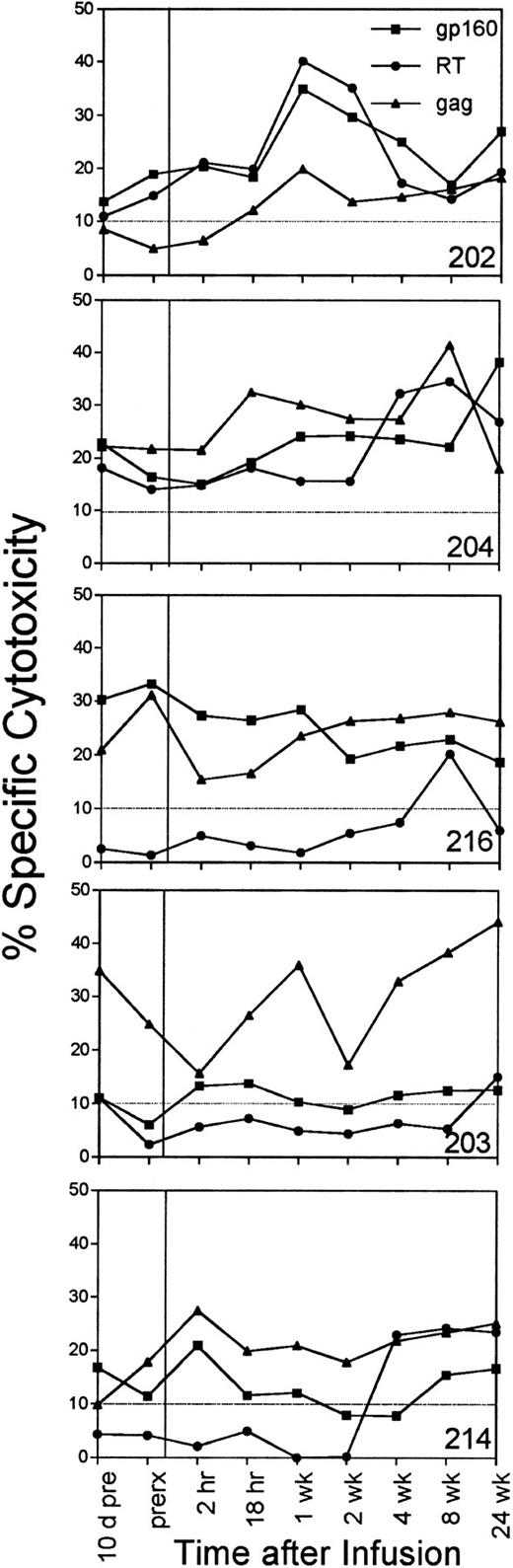
Effect on circulating HIV-specific CTLs.In four of six subjects, an increase in circulating CTL reactivity against HIV env-, gag-, and pol-expressing targets occurred within 1 week after the infusion and was evident 6 months later (Fig 4). The four subjects (202, 203, 204, and 216) with increased HIV-specific CTLs included the subjects (202 and 216) who had a long-term decrease in cell-associated viral burden and the subject (204) with a sustained increase in his CD4 count. The two subjects with reductions in cell-associated viral burden (202 and 216) also began with twice as many circulating CD8+ T cells. Their mean baseline CD8 counts were 2,339/μL and 2,170/μL, respectively; CD8 counts for the other subjects ranged from 765 to 1,225/μL.
The percentage of specific cytotoxicity of PBMC-derived T-cell lines against HIV env-, RT-, and gag-expressing autologous target cells measured just before the infusion and at 2 and 18 hours after the infusion and at later time points. No data are shown for subject 226, whose specific cytotoxicity against these targets was below background at all times. The background lysis of control targets expressing the lacZ gene was subtracted to determine the HIV-specific cytotoxicity, depicted at an E:T ratio of 25:1.
The percentage of specific cytotoxicity of PBMC-derived T-cell lines against HIV env-, RT-, and gag-expressing autologous target cells measured just before the infusion and at 2 and 18 hours after the infusion and at later time points. No data are shown for subject 226, whose specific cytotoxicity against these targets was below background at all times. The background lysis of control targets expressing the lacZ gene was subtracted to determine the HIV-specific cytotoxicity, depicted at an E:T ratio of 25:1.
The mean anti-gp160, RT, and gag CTL activity for all subjects increased slightly at 1 week. These increases were above the estimated biologic and assay variability (3.6%, 3.3%, and 4.7%, respectively) calculated by comparing the assay values obtained just before and 10 days before the infusion, but the changes were not statistically significant (Table 4). The mean changes for the whole group were comparable to the mean changes in anti-gp160 or anti-gag cytotoxicity of the subset of subjects treated with T cells targeted to epitopes in those proteins. The baseline mean sum of the percentage of specific cytotoxicity against env-, gag-, and RT-expressing autologous targets was 42% ± 15%. The mean was 60% ± 23% 1 week after the infusion and was 58% ± 20% 6 months later. The mean was also above the baseline at all time points in the study. At 6 months, the increase in RT-specific cytotoxicity was statistically significant (P = .05), but the overall increase in the sum of HIV-specific activity was not (P = .06).
The four subjects who had a quantitative increase in antiviral cytotoxicity also recognized a broader range of HIV proteins and peptide epitopes. Bulk CTL cultures from subject 202 recognized HIV gag-expressing targets with more than 10% specific cytotoxicity within 1 week of the infusion and for the remainder of the study. Subject 214 (and to a lesser extent subjects 203 and 216) developed RT-specific CTL for the first time at later time points. When the peptide epitopes in env, gag, and nef were mapped 24 weeks after the initial infusion, five new peptide epitopes were recognized by the six subjects (Lieberman, manuscript in preparation).
DISCUSSION
The results of this pilot trial suggest that it is possible to infuse HIV-infected subjects with ex vivo-expanded HIV-specific CTLs safely. No toxicity was observed after the infusion of T cells targeted against a variety of HIV epitopes contained in gp120, gag p17 and p24, and nef. No subject deteriorated clinically during our follow-up of 6 months. Our results suggest that antiviral CTLs of whatever specificity do not aggravate HIV infection. Although almost no CD4 T cells were infused, the CD4 counts of all subjects increased during the first few weeks after the CTL infusion. Three subjects had improvements at 6 months in at least one of the principal study end points: cell-associated virus (subjects 202 and 216), plasma viral load (subject 216), and CD4 count (subject 204). A single infusion of 1 billion T cells enhanced for anti-HIV cytolytic activity without cytokine support increased circulating viral-specific cytotoxicity in most subjects. Because the infused cells did not bear an identifying marker, we could not assess whether the in vivo rise in viral-specific CTL activity was due directly to the infused cells. The finding of CTLs against HIV proteins that were not recognized above background before treatment suggests that the infusion may have broadened the CTL response to the virus. This effect may protect against viral escape mutants.
We developed polyclonal cell lines that recognize several viral peptides because a highly mutable HIV might readily escape a single or a few CTL clones. Possible HIV CTL escape mutants have been reported in infected patients42 and in a patient who was infused with a nef-specific CTL clone.15 Sequencing of viral isolates obtained before and after T-cell infusion in a follow-up study are in progress to determine the extent of escape mutants to polyclonal T-cell therapy.
Viral-specific CTL lines are simpler to prepare than CTL clones, but have some disadvantages for pilot studies compared with clones. The antiviral cytolytic activity/cell of T-cell lines is less than that of T-cell clones. Interpretation of results is less clear because of the heterogeneity of cell lines that include nonspecific CTLs and natural killer cells as well as antiviral CTLs. It is also difficult to determine the half-life of infused polyclonal cells without a marker gene. Marked CMV-specific T cells persisted in allograft recipients for up to 12 weeks.28 However, a recent study of infusion of genetically marked gag-specific CTL clones was foiled by the development of a CTL response to the marker gene, which resulted in loss of the infused cells.32
The infused T cells, unlike most activated CTLs, did not express CD25, the IL-2 receptor, although they had other cell surface receptors associated with activated CTLs. This suggests that, at least at the time of infusion, the cells did not depend on IL-2 for function or survival, which may have allowed them to survive in subjects with defects in CD4 T-cell function.
An HIV-seropositive individual has a high frequency of circulating HIV-specific CTLs estimated to be as much as 1 in 10,000 lymphocytes.8 The frequency of potential viral-specific precursor CTLs has been estimated to be much higher and may be great enough to be measured in percentages.31,33 If there are approximately 103 CD8 T cells/μL of blood or 5 billion in circulation, then the pool of circulating antiviral CTL may be anywhere from 5 × 105 up to 108 potential precursors. The total body lymphoid compartment of spleen, thymus, and lymph nodes is difficult to estimate but is approximately 100-fold larger than the circulating compartment to yield a crude estimate of approximately 5 × 107 mature anti-HIV CTLs and up to 1010 anti-HIV CTL precursors. These calculations are rough guidelines to compare with our infusion of 1 billion CD8 T cells. A frequency analysis of a representative ex vivo peptide-expanded T-cell line suggests that about one-half to one-eighth of the infused cells are specific for a particular HIV peptide used to selectively expand antiviral CTLs.31 Therefore, we estimate that this infusion represents a substantial increase in the number of activated anti-HIV CTLs present in the subject at the time of the infusion, but possibly a small number compared with the potential number of anti-HIV precursor CTLs. Although a large number of specific antiviral precursors exist, they may not function efficiently in vivo because of the excessive amounts of viral antigen or because of other derangements of immune function in the HIV-infected host. By expanding precursor CTLs ex vivo under conditions favorable for their activation, the effect of an infusion may be greater than might be predicted based on the numbers of HIV-specific precursor CTLs present.
There is at present no consensus about the elements of protective immunity in HIV infection. Circumstantial evidence suggests that HIV-specific CTLs are important mediators of protection. This evidence includes the presence of HIV-specific CTLs in exposed but uninfected individuals,43-46 the increase in antiviral CTLs coincident with the resolution of the viremia of acute HIV infection,6,7 the more rapid course of neonatal infection (a time of immaturity of T-cell immunity),11 the increased anti-HIV CTL precursors and lower viral burden in long-term nonprogressors,47,48 and the loss, with progression to clinical disease, of HIV-specific CTL activity.9,10 Supporting this indirect evidence is the protective role of CTLs in animals infected with other pathogenic viruses.16-20 However, there are legitimate concerns that HIV-specific CTLs may contribute to immunopathology, as has been shown for nonpathogenic viruses, including LCMV and hepatitis B.14 This pilot study, which involves the infusion of large numbers of CD8 T cells enriched for antiviral activity of varied specificities, allows us to show that the infusion of antiviral CTLs is not harmful.
Whether antiviral CTL therapy will prove to be an effective treatment for HIV infection remains to be shown. This pilot trial did not have the statistical power to demonstrate that CTL therapy is beneficial. Not only was the number of subjects small, but also they were a heterogeneous group in regards to extent of disease, treatment, and the antiviral specificities and potency of the infused cells. If infusion of HIV-specific T cells shows a benefit, further studies will be necessary to determine an optimal number of T cells per infusion, how frequently infusions will need to be administered, and which patient population will benefit.
This pilot trial suggests that infusion of autologous T-cell lines containing CD8+ CTLs against a diversity of HIV peptides is safe and merits further investigation. Multiple infusions or treatment with more or more potent CTLs or together with cytokines might have advantages over the single infusion we administered.
ACKNOWLEDGMENT
The authors thank Malcolm Gefter, Robert Schwartz, Fred Rosen, and the late Sheldon M. Wolff for advice and support; Ann Marie Brown, Marie Carten, Joan O'Neil, and the New England Medical Center Clinical Study Unit staff for patient care; Bernard Moss and the NIH AIDS Research and Reference Reagent Program for vaccinia recombinants; the MRC AIDS Reagent Project for gp160 and gag peptides; and the EVA Programme for tat, rev, and nef peptides.
APPENDIX
The following persons participated in the DATRI 006 study team: Lead Investigator: Judy Lieberman (Boston, MA); Investigators: Paul R. Skolnik, Michael B. Atkins (Boston, MA), Jonathan Kagan (NIH Division of AIDS, Rockville, MD); Clinical Trials Specialist: Bernard Landry (Rockville, MD); Medical Officers: Jeremy Gradon, Daniel Stein, Nzeera Virani-Ketter (NIH Division of AIDS); Study Managers: Mary Banach, Marcia Scott, Jackie Meyers (Rockville, MD); Statistician: James Bethel (Rockville, MD); Scientific Coordinators: Edward Lee, Hal Standiford (Baltimore, MD); Research Associates: Jessica A. Fabry, Donna M. Fong, Alicia Wang, Devra Beyer (Boston, MA).
Supported by the National Institute for Allergy and Infectious Diseases Division of AIDS Treatment Research Initiative (N01-AI15123), by a Pew Scholar Award in the Biomedical Sciences, and by National Institutes of Health Grants No. K08-CA01449 and U19-AI36611 (J.L.), K08-AI01046 and R01-AI33290 (P.R.S.), T32-AI07438 (G.R.P.), and M01-RR00054 (New England Medical Center General Clinical Research Center).
Address reprint requests to Judy Lieberman, MD, PhD, Center for Blood Research, 800 Huntington Ave, Boston, MA 02115.