Abstract
Purified primitive progenitor/stem cells from bone marrow represent likely target populations for ex vivo expansion of stem cells to be used in high-dose chemotherapy or gene therapy. Whereas such primitive progenitor cells require combined stimulation by multiple cytokines for growth, some cytokines selectively promote viability rather than growth when acting individually. We investigated here for the first time the direct effects of cytokines on survival of primitive CD34+CD38− human bone marrow progenitor cells at the single-cell level. Interleukin-3 (IL-3) and the ligands for c-kit (KL) and flt3 (FL) had direct and selective viability-promoting effects on a small fraction of CD34+CD38− but not CD34+CD38+ progenitor cells. Interestingly, the recently cloned thrombopoietin (Tpo), although stimulating little growth, kept most CD34+CD38− progenitors viable after prolonged culture, maintaining twofold and fourfold more progenitors viable than KL and IL-3, respectively. A high fraction of these progenitors had a combined myeloid and erythroid differentiation potential, as well as capacity for prolonged production of progenitor cells under stroma-independent conditions. In addition, Tpo promoted viability of CD34+CD38− long-term culture-initiating cells, further supporting the idea that Tpo promotes viability of primitive human progenitor cells. Finally, Tpo suppressed apoptosis of CD34+CD38− cells in culture. Thus, the present studies show a novel effect of Tpo, implicating a potential role of this cytokine in maintaining quiescent primitive human progenitor cells viable.
IT HAS BECOME increasingly clear that hematopoiesis is governed not only by signals affecting proliferation and differentiation,1-3 but also that the regulation of apoptosis is likely to play an important role.4
Stimulatory hematopoietic growth factors, such as the colony-stimulating factors (CSFs), multiple interleukins (ILs), erythropoietin (Epo), c-kit ligand (KL), and flt3 ligand (FL), have all been shown to stimulate the growth and differentiation of murine as well as human hematopoietic progenitor cells in vitro.1-3,5 The optimal clonogenic growth of primitive hematopoietic progenitors requires in general simultaneous activation through multiple cytokine receptors.1-3 However, a number of studies on candidate murine stem cells have shown that the same cytokines, when acting individually, although inducing little or no growth, can in a direct manner promote viability of subpopulations of primitive progenitor/stem cells.6-14 Although less is known about putative viability factors for human stem cells, IL-3, granulocyte-macrophage colony-stimulating factor (GM-CSF ), KL, and FL have been shown to enhance viability of enriched primitive human progenitor cell populations in culture.15-17 However, at variance with the murine studies, the effects of putative viability factors for primitive human progenitor cells have not yet been investigated at the single-cell level, thus allowing limited conclusions to be made with regard to what degree the observed effects are in fact mediated directly on the progenitor cells.
The most primitive human bone marrow (BM) progenitor cells have been shown to express CD34 but little or no CD38.18-20 The CD34+CD38− progenitor cell population represents only 0.05% to 0.1% of human BM mononuclear cells and has been shown to be highly enriched in multipotent progenitor cells, long-term culture-initiating cells (LTC-IC), and long-term reconstituting stem cells.18-21 Whereas previous studies implicated that CD34+CD38− progenitor cells might require direct interaction with stroma to be able to proliferate, recent studies have clearly shown that such growth can be obtained under stroma-free conditions in the presence of early acting cytokines.19,20 However, and for unknown reasons, effective growth of CD34+CD38− cells occurs only in liquid medium and not in semisolid medium such as methylcellulose, which more selectively promotes growth of more committed progenitor cells.19,20,22 In addition to being used extensively for in vitro studies of primitive hematopoietic progenitor cells, CD34+CD38− BM cells are also currently being investigated for their potential clinical utility, in particular for ex vivo expansion and gene therapy.19,20,23,24 Whereas much is known about the ability of different cytokines to stimulate growth and differentiation of the CD34+CD38− BM progenitor cell population when acting in combination,1-3,19 20 no studies have yet investigated to what degree individual cytokines might maintain these progenitor cells viable.
Multiple previous studies have suggested that KL is a potent and by far the most efficient viability factor for primitive murine progenitor cells, including pluripotent long-term reconstituting stem cells, and that it can maintain the majority of such progenitor cells viable after prolonged culture.11-13 In contrast, studies of human CD34+DR− BM progenitor cells as well as CD34+CD38− fetal liver cells have reported more variable and less extensive effects on viability by KL.15-17 This could be a consequence of culture conditions either not being optimized for allowing cytokine-induced survival of human progenitor cells or could alternatively implicate that other not yet investigated cytokines might prove more efficient than KL at promoting survival. Thrombopoietin (Tpo), the recently cloned ligand for c-mpl, appears to be a candidate for promoting survival of primitive human progenitor cells, because recent studies have suggested that Tpo, in addition to its established role as the primary regulator of platelet production,25 also might play an important role in early hematopoiesis. Although most evidence supporting this has been made on murine stem cells or in c-mpl–deficient mice,26-30 there are also data supporting that Tpo might play a role in early human hematopoiesis. c-mpl has been shown to be expressed on candidate human stem cell populations, including CD34+CD38− BM cells,31,32 and two recent studies found Tpo to synergistically enhance the growth of primitive human progenitor cells,33,34 although having little or no proliferative effect when acting alone.33 Thus, the main objective of the present study was to investigate whether Tpo might directly promote viability of primitive CD34+CD38− human BM progenitor cells and, if so, to what degree surviving progenitors had committed to specific cell lineages or remained multipotent. In comparison, Epo, KL, IL-3, and FL were investigated for their direct effects on viability of human CD34+CD38− progenitor cells. We show here for the first time that Tpo potently and more efficiently than KL, FL, or IL-3 promotes viability of multipotent CD34+CD38− BM progenitor cells, thus implicating a novel effect of Tpo in early human hematopoiesis.
MATERIALS AND METHODS
Hematopoietic growth factors.Recombinant human (rh) Tpo was a generous gift from Dr Si Lok (ZymoGenetics Corp, Seattle, WA) and rhEpo was kindly supplied by Boehringer Mannheim Corp (Mannheim, Germany), whereas rh granulocyte colony-stimulating factor (G-CSF ), rhKL, and rhIL-3 were generously provided by Dr Ian K. McNiece (Amgen Corp, Thousand Oaks, CA). rhFL, rhGM-CSF, and rhIL-1α were kindly supplied by Immunex (Seattle, WA), rhIL-6 was a generous gift from Genetics Institute (Cambridge, MA), and rhCSF-1 was kindly provided by Dr Michael Geier (Cetus Corp, Emeryville, CA). Unless otherwise indicated, all growth factors were used at the following predetermined optimal concentrations: 50 ng/mL rhTpo; 5 U/mL rhEpo; 50 ng/mL rhG-CSF; 25 ng/mL rhGM-CSF; 50 ng/mL rhCSF-1; 50 ng/mL rhKL; 50 ng/mL rhFL; 20 ng/mL rhIL-1α; 50 ng/mL rhIL-3; and 50 ng/mL rhIL-6. When a multifactor combination consisting of all of these cytokines was used to stimulate clonal growth, 50% of the above listed cytokine concentrations were found to stimulate optimal clonal formation and size and were therefore used throughout this study and termed multifactor combination. The biologic activity of rhTpo was completely blocked by a neutralizing antihuman Tpo antibody (goat IgG) from R&D Systems (Minneapolis, MN) (O.J.B. and S.E.W.J., unpublished observation).
Enrichment and purification of CD34+CD38− and CD34+CD38+ human BM cells.After informed consent was given by the patient, BM cells were obtained from the hip or knee of otherwise healthy patients undergoing hip or knee surgery. BM cells were collected in syringes containing preservative-free heparin (final concentration, 100 IU/mL; Fujisawa USA Inc, Deerfield, IL). The BM was diluted 1:1 with 20% fetal calf serum (FCS; BioWhittaker, Walkersville, MD) in Iscove's Modified Dulbecco's Medium (IMDM; BioWhittaker) and filtered through a 60-μm mesh before BM mononuclear cells were isolated by Ficoll-Hypaque (Lymphoprep; Nycomed, Oslo, Norway) gradient centrifugation. Positive selection of CD34+ cells and subsequent separation of CD34+CD38− cells were performed according to previously described procedures.35,36 Briefly, BM mononuclear cells were rosetted with Dynabeads M-450 (Dynal, Oslo, Norway) directly coated with a mouse antihuman CD34 monoclonal antibody (MoAb) BI-3C5 for 45 minutes at 4°C with a cell:bead ratio of 2:1. Rosetted cells were attracted to a magnetic particle concentrator (MPC-6; Dynal), nonrosetting cells (CD34−) were removed by pipetting, and rosetted cells were washed seven times (to remove unspecifically bound CD34− cells). The detachment of beads from positively selected CD34+ cells was next performed by incubating cells with anti-Fab antiserum (DETACHaBEAD; Dynal) for 1 hour at room temperature according to the manufacturer's instructions. Isolated cells, free of beads, were washed and counted, and the purity of the CD34+ cells was reproducibly greater than 90%, as determined by flow cytometry. The antigen profile of CD34+ cells selected by this method has previously been shown to be intact.35
CD34+CD38− and CD34+CD38+ cells were obtained by incubating enriched CD34+ cells with a phycoerythrin (PE)-conjugated antihuman CD38 MoAb (5 μg/mL) and a fluorescein isothiocyanate (FITC)-conjugated mouse antihuman CD34 MoAb (6 μg/mL) (or isotype-matched irrelevant control antibodies; all from Becton Dickinson, San Jose, CA) for 30 minutes at 4°C. Subsequently, CD34+ cells with (92% to 98% of CD34+ cells; CD34+CD38+) or without detectable expression of CD38 (2% to 8% of CD34+ cells; CD34+CD38−) were sorted on a Coulter Epics Elite Cell Sorter (Coulter Electronics, Hialeah, FL). In some experiments, CD34+CD38− cells were isolated by negative bead selection as previously described.36 Briefly, CD34+ cells were incubated with mouse antihuman CD38 MoAb (Serotec, Oxford, UK; 5 μg antibody/1 × 106 cells in a total volume of 100 μL) for 30 minutes at 4°C. Cells were washed once and incubated with Dynabeads M-450 (Dynal) conjugated with sheep antimouse IgG for 30 minutes on a mixing wheel (Robbins Scientific Corp, Sunnyvale, CA) at room temperature with a cell:bead ratio of 1:25. Rosetted cells (CD34+CD38+ cells) were attracted to a magnet and nonrosetting cells were collected. This cycle was repeated once with the same absolute amount of magnetic beads and processed as for the first bead separation. The remaining CD34+CD38− cells were collected, counted, and kept in IMDM supplemented with 20% FCS until use (<6 hours). The purity of CD34+CD38− cells isolated by this method was reproducibly 85% to 90%. The same patterns of cytokine responses were observed with cells isolated by both methods.
Single-cell clonogenic assay.CD34+, CD34+CD38+, or CD34+CD38− cells were seeded in Terasaki plates (Nunc, Kamstrup, Denmark) at a density of 1 cell per well in 20 μL serum-depleted medium (X-vivo 15; BioWhittaker) supplemented with 1% detoxified bovine serum albumin (BSA; StemCell Technologies Inc, Vancouver, British Columbia, Canada) containing 100 U/mL penicillin (BioWhittaker), 100 U/mL streptomycin (BioWhittaker), 2 mmol/L L-glutamine (BioWhittaker), and 10−4 mol/L 2-mercaptoethanol (Sigma, St Louis, MO). In some experiments, wells were carefully visualized by microscopy 2 to 10 hours after plating and only wells containing 1 cell per well were included in these experiments. No difference in results were observed whether or not such microscopy identification was performed. Wells were scored for cell growth (≥5 cells) after 14 days of incubation at 37°C and 5% CO2 in air.
Single-cell indirect viability assay: delayed addition studies.At initiation of culture, CD34+, CD34+CD38+, or CD34+CD38− BM cells were plated individually in 60-well Terasaki plates, as previously described.13,37,38 Cells were plated in 10 μL serum-depleted medium alone or medium supplemented with a multifactor cytokine combination (containing IL-1, IL-3, IL-6, G-CSF, GM-CSF, CSF-1, FL, KL, Epo, and Tpo) capable of stimulating most if not all in vitro clonogenic progenitors to proliferate, or in the presence of a putative viability factor (ie, Tpo, IL-3, KL, FL, or Epo). Cells were preincubated for different periods of time at 37°C and 5% CO2 in air before an additional 10 μL of serum-depleted medium supplemented with the multifactor combination was added to all groups to determine the number of cells that could still be recruited into proliferation, such that cells not proliferating after preincubation in the absence of cytokines or in the presence of a putative viability factor were presumed to be dead. Wells were scored for cell growth (≥5 cells) after an additional 14 days of incubation at 37°C and 5% CO2 in air. If a higher number of clones was observed after preincubation in the presence of the putative viability factor than medium alone, the factor was considered to promote viability of the investigated progenitor cell population. The delayed addition study used here as well as by others,10,13,15,16 37 although being indirect, is considered the most reliable viability assay for primary hematopoietic progenitor cells, because it specifically assays progenitor cells rather than other cells present in enriched progenitor cell populations. In some experiments, single cells were identified immediately after plating (2 to 10 hours to allow cells to sediment) as well as after 116 hours to exclude cells that had proliferated in response to Tpo (ie, contained >1 cell) during the preincubation period. In these experiments, the multifactor combination was added only to wells identified to contain a single cell at initiation of culture and less than 2 cells after 116 hours.
Multilineage potential of progenitor cells surviving in response to early acting cytokines.CD34+CD38− BM cells were seeded at a density of 1 cell per well in 10 μL serum-depleted medium in the presence of Tpo and preincubated for 116 hours at 37°C and 5% CO2 in air before an additional 10 μL of medium supplemented with IL-1, IL-3, IL-6, G-CSF, GM-CSF, CSF-1, FL, KL, Epo, and Tpo were added to each well, to provide a potent proliferative stimulation. After another 10 days of culture at 37°C and 5% CO2 in air, clones containing 10 or more cells (an average of 21 clones per group per experiment) were plated in 1 mL serum-depleted medium supplemented with 1% detoxified BSA, 1.2% methylcellulose (Methocel; Fluka Chemie AB, Buchs, Switzerland), 100 U/mL penicillin, 100 U/mL streptomycin, 2 mmol/L L-glutamine, 10−4 mol/L 2-mercaptoethanol, and the same multifactor combination in 35-mm Petri dishes (Nunc). Cultures were examined for granulocyte/macrophage colonies (>40 cells; GM), burst-forming units-erythroid (BFU-E), and mixed GM/E colonies after an additional 12 to 14 days of incubation at 37°C, 5% O2 , 10% CO2 , and 85% N2 . To confirm the colony phenotype, individual colonies were picked, transferred to a glass slide with a jet air stream, dried, fixed, Giemsa-stained, and examined by microscopy.
Viability of LTC-IC.Long-term cultures were established and maintained according to previously described methods.36 39 Briefly, stroma layers were initiated with human BM mononuclear cells (isolated as described above) by seeding 25 × 106 cells in 15 mL myeloid long-term culture medium (MyeloCult H5100; StemCell Technologies) containing 12.5% horse serum, 12.5% FCS, 0.2 mmol/L i-Inositol, 20 mmol/L folic acid, 10−4 mol/L 2-mercaptoethanol, and 2 mmol/L L-glutamine in α-minimun essential medium (α-MEM) supplemented with fresh 10−6 mol/L hydrocortisone (Sigma) in a 80-cm2 culture flask (Nunc). Cultures were incubated for the first 3 to 5 days at 37°C and thereafter at 33°C, both in 5% CO2 in humidified air. Half the medium was carefully replaced with fresh long-term culture medium every week until the stroma layer was confluent. At confluence, usually after 4 to 5 weeks, the cells were irradiated in the flask to an absorbed dose of 15 Gy with 6 MV x-rays from a medical linear accelerator (Phillips SL25). To assure a homogenous dose distribution, the flask was filled with phosphate-buffered saline (PBS) and positioned with the cell layer at 5-cm depth in a polystyrene phantom. The cells were then trypzinated (BioWhittaker) and transferred to 24-well microtiter plates (each flask sufficient for 35 wells). CD34+CD38− cells were added to the irradiated stroma layer 1 to 7 days after irradiation. The cocultures were maintained by half medium change weekly using the long-term culture medium without hydrocortisone.
Five hundred and twenty-five CD34+CD38− BM cells were seeded for each group at a density of 1 cell per well in 10 μL serum-depleted medium in stroma-free cultures. As a control for content of LTC-IC in freshly isolated CD34+CD38− cells, cells were harvested directly after seeding, pooled, and split between 5 wells with preestablished irradiated human stroma. Single-cell cultures incubated for 116 hours in the presence of Tpo, KL, or medium alone were examined microscopically and wells containing 2 or more cells were excluded. The remaining cells were for each group pooled and divided into 5 wells with preestablished irradiated human stroma. After 5 weeks on stroma, nonadherent and adherent cells were transferred to methylcellulose cultures containing predetermined optimal concentrations of Tpo, Epo, G-CSF, KL, FL, and IL-3. Colony-forming cells (CFC) were scored after an additional 12 to 14 days in culture. Stroma cells without added CD34+CD38− cells were used as a control for efficient irradiation of stroma layers and produced no CFC.
Apoptosis assay.Apoptotic cells were detected using a modification of a previously described method.40 Briefly, 50,000 CD34+CD38− cells were incubated in serum-depleted medium alone or supplemented with Tpo for 44 hours. The cells were pelleted in a microcentrifuge (3000g for 5 minutes at room temperature) and fixed in 1% methanol-free formaldehyde (Polyscience Inc, Warrington, PA) for 15 minutes on ice, pelleted (as above), resuspended in 70% EtOH, and stored at −20°C until further developed (minimum 2 hours). Before labeling, the cells were washed once in PBS (BioWhittaker) containing 1% FCS. A terminal deoxynucleotidyl transferase (TdT) kit (In Situ Death Detection Kit, Fluorescein) from Boehringer Mannheim Corp was used. After washing, the cells were resuspended in 45 μL label solution (containing fluorescein-dUTP and optimized buffer concentrations), and 5 μL enzyme solution (containing TdT) was added. After 60 minutes of incubation at 37°C, the cells were resuspended in 300 μL PBS containing 1% FCS and analyzed on a flow cytometer (FACSort; Becton Dickinson).
Statistical analysis.All results were expressed as means (±SEM) of data obtained from three or more separate experiments, unless otherwise specified. The Student's t-test was used for statistical analysis.
RESULTS
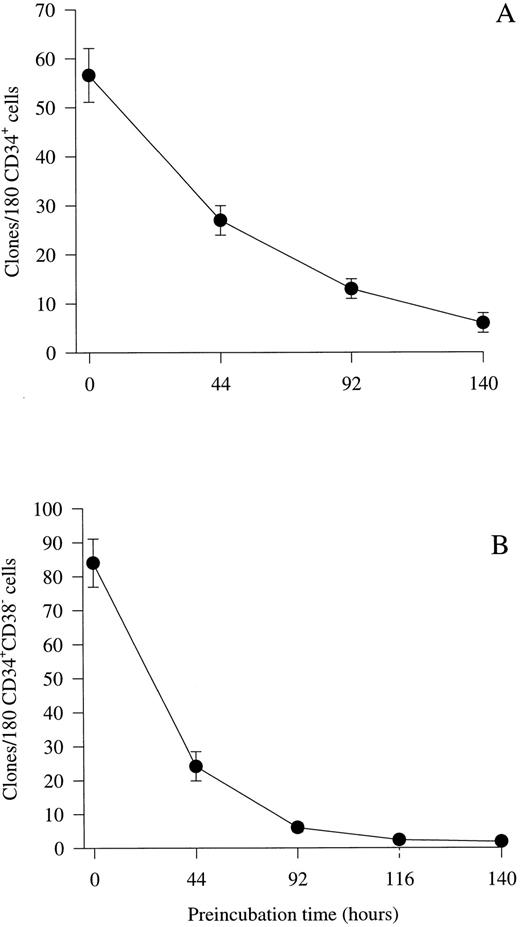
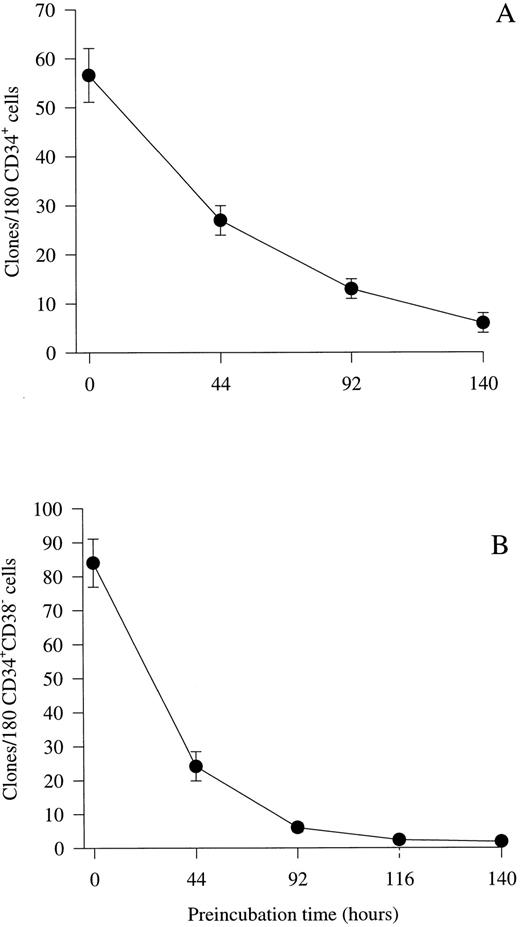
Optimizing culture conditions for viability studies of CD34+CD38− BM progenitor cells.Previous studies by others15,16 as well as initial experiments in our laboratory (O.J.B. and S.E.W.J., unpublished observations) showed that human hematopoietic progenitor cells lose viability slowly in FCS-containing medium, as compared with murine progenitor cells.41 Therefore, we evaluated whether a serum-depleted medium (X-vivo 15 supplemented with 1% detoxified BSA) might efficiently support cytokine-stimulated clonogenic growth of human progenitor cells, while allowing progenitors to die when cultured in medium in the absence of cytokines. First, experiments were performed by plating unfractionated CD34+ BM progenitor cells at a density of 1 cell per well in 10 μL serum-depleted medium in the absence of cytokines. Subsequently, a strong proliferative cytokine combination (IL-1, IL-3, IL-6, G-CSF, GM-CSF, CSF-1, FL, KL, Epo, and Tpo) was added after incubation for various periods of time. If CD34+ cells were cultured individually with the combination of all 10 cytokines from initiation of culture, a high cloning frequency was observed (32%; 57 clones from 180 cells; Fig 1A). However, if addition of the multifactor combination was delayed for 44 hours, only 47% (27 of 57 clones) of these progenitors could be recovered in response to the same cytokines, which decreased to 11% by 140 hours. Because the primitive CD38− fraction of CD34+ progenitor cells represents only a low fraction of the total CD34+ population, a similar series of experiments was performed to examine the effect of delayed cytokine addition on the survival of CD34+CD38− progenitor cells. As recently shown by others,19 20 the cloning frequency of CD34+CD38− BM cells was high (47%; 84 clones from 180 cells) when the cytokine cocktail was added at initiation of culture (Fig 1B). A slightly different pattern of progenitor survival was observed for CD34+CD38− progenitor cells than for unfractionated CD34+ progenitors in that less than 29% (24 clones of 84) of the progenitors were recovered when the addition of cytokines was delayed for 44 hours. In addition, almost no clonogenic progenitor cells could be recovered in response to the cytokine cocktail after 116 to 140 hours of incubation. Importantly, there was little or no difference in the cloning frequency or proliferative potential if cells were cultured in 20 μL of the cytokine combination from the initiation of culture or in 10 μL of cytokine-containing medium for the first 116 hours and then supplemented with another 10 μL for the remaining of the culture time (O.J.B. and S.E.W.J., unpublished observations), showing that the low incubation volume does not affect the survival and cytokine responsiveness of CD34+CD38− progenitors. Based on these results, subsequent delayed addition studies were performed by preincubating cells for 116 hours in serum-depleted medium in the presence or absence of putative viability factors, before a strong proliferative cytokine cocktail was added to allow the clonogenic growth of viable progenitor cells.
Kinetic study of the viability of CD34+ and CD34+CD38− progenitor cells in serum-depleted medium. CD34+ (A) and CD34+CD38− (B) cells were cultured at a density of 1 cell per well in 10 μL serum-depleted medium alone. After 44, 92, 116, or 140 hours of preincubation, 10 μL serum-depleted medium supplemented with a multifactor combination (IL-1, IL-3, IL-6, G-CSF, GM-CSF, CSF-1, FL, KL, Epo, and Tpo) was added to each well to yield predetermined optimal concentrations. Clones (≥5 cells) were scored after an additional 14 days of culture at 37°C and 5% CO2 in air. The number of colonies observed at time 0 represents colony formation when the cytokine cocktail was added at the initiation of culture and thus is a control for optimal clonogenic growth in response to this cytokine combination. Results represent the means (±SEM) of the total number of clones formed per 180 CD34+ BM progenitor cells from two experiments (A) or 180 CD34+CD38− progenitor cells from five individual experiments (B), with 180 wells scored per time point in each experiment.
Kinetic study of the viability of CD34+ and CD34+CD38− progenitor cells in serum-depleted medium. CD34+ (A) and CD34+CD38− (B) cells were cultured at a density of 1 cell per well in 10 μL serum-depleted medium alone. After 44, 92, 116, or 140 hours of preincubation, 10 μL serum-depleted medium supplemented with a multifactor combination (IL-1, IL-3, IL-6, G-CSF, GM-CSF, CSF-1, FL, KL, Epo, and Tpo) was added to each well to yield predetermined optimal concentrations. Clones (≥5 cells) were scored after an additional 14 days of culture at 37°C and 5% CO2 in air. The number of colonies observed at time 0 represents colony formation when the cytokine cocktail was added at the initiation of culture and thus is a control for optimal clonogenic growth in response to this cytokine combination. Results represent the means (±SEM) of the total number of clones formed per 180 CD34+ BM progenitor cells from two experiments (A) or 180 CD34+CD38− progenitor cells from five individual experiments (B), with 180 wells scored per time point in each experiment.
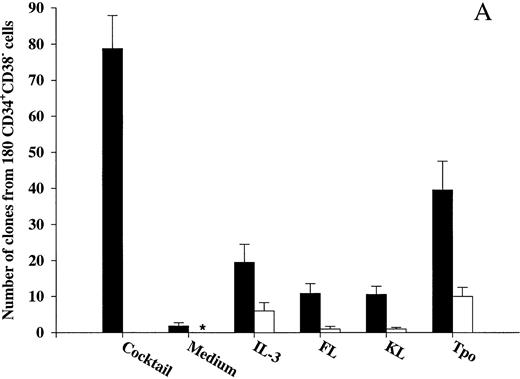
Effects of early acting cytokines on viability of CD34+CD38− and CD34+CD38+ BM progenitor cells.To determine whether observed effects on viability in the present study were in fact mediated directly on the progenitors, CD34+CD38− and CD34+CD38+ BM cells were cultured at 1 cell per culture well. As previously shown by others on CD34+CD38− fetal liver cells,17 IL-3, FL, and KL individually only stimulated the clonogenic growth of a very low number of CD34+CD38− progenitor cells (Fig 2A). In addition, only limited proliferation was obtained by these clones, in that they all consisted of less than 50 cells after 12 to 14 days of culture. In contrast, 44% of CD34+CD38− cells were induced to proliferate in response to a potent combination of 10 cytokines (IL-1, IL-3, IL-6, G-CSF, GM-CSF, CSF-1, FL, KL, Epo, and Tpo; Fig 2A), and many of these clones formed large colonies (Table 1). Also, Tpo stimulated the growth of a low fraction of CD34+CD38− progenitor cells; without exception, all of these clones produced less than 50 cells and thus were representative of clusters rather than colonies.
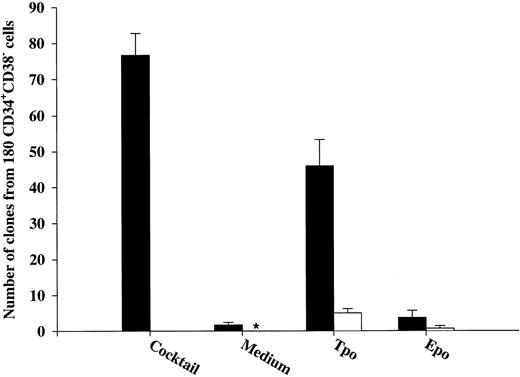
The ability of Tpo and other putative viability factors to promote viability of CD34+CD38− and CD34+CD38+ progenitor cells. CD34+CD38− (A) or CD34+CD38+ (B) BM cells were cultured at a density of 1 cell per well in 10 μL serum-depleted medium and predetermined optimal concentrations of the indicated cytokines. After 116 hours of preincubation, 10 μL of medium containing IL-1, IL-3, IL-6, G-CSF, GM-CSF, CSF-1, FL, KL, Epo, and Tpo was added to each well to yield predetermined optimal concentrations. Clones (≥5 cells) were scored after an additional 14 days of incubation at 37°C and 5% CO2 in air (▪). A total of 180 CD34+CD38− (A) or CD34+CD38+ (B) cells were also cultured at a density of 1 cell per well in 20 μL serum-depleted medium and incubated with each of the putative viability factors alone and scored for clonal growth (≥5 cells) after 14 days of culture at 37°C and 5% CO2 in air (□). The results represent the means (+SEM) of total number of clones per 180 cells of five and three individual experiments (A and B, respectively), with 180 wells scored per group in each experiment. *No clones were formed by cells incubated for 14 days in medium alone.
The ability of Tpo and other putative viability factors to promote viability of CD34+CD38− and CD34+CD38+ progenitor cells. CD34+CD38− (A) or CD34+CD38+ (B) BM cells were cultured at a density of 1 cell per well in 10 μL serum-depleted medium and predetermined optimal concentrations of the indicated cytokines. After 116 hours of preincubation, 10 μL of medium containing IL-1, IL-3, IL-6, G-CSF, GM-CSF, CSF-1, FL, KL, Epo, and Tpo was added to each well to yield predetermined optimal concentrations. Clones (≥5 cells) were scored after an additional 14 days of incubation at 37°C and 5% CO2 in air (▪). A total of 180 CD34+CD38− (A) or CD34+CD38+ (B) cells were also cultured at a density of 1 cell per well in 20 μL serum-depleted medium and incubated with each of the putative viability factors alone and scored for clonal growth (≥5 cells) after 14 days of culture at 37°C and 5% CO2 in air (□). The results represent the means (+SEM) of total number of clones per 180 cells of five and three individual experiments (A and B, respectively), with 180 wells scored per group in each experiment. *No clones were formed by cells incubated for 14 days in medium alone.
After 116 hours of incubation in the presence of IL-3, KL, or FL, a low fraction of CD34+CD38− progenitor cells remained viable (25%, 14%, and 14%, respectively), which for all three cytokines was significantly (P < .05) higher than their ability to induce clonal growth (Fig 2A). Interestingly, after 116 hours of incubation in the presence of Tpo, 51% of multifactor-responsive CD34+CD38− progenitor cells remained viable, which was significantly higher than for IL-3, FL, or KL (P < ,05). It is also noteworthy that most of the progenitors surviving in response to Tpo did so in the absence of detectable cell growth (75%; Fig 2A) and that their proliferative capacity was very high when compared with the growth of CD34+CD38− progenitors in response to Tpo alone (Table 1). The differences in the abilities of FL, KL, IL-3, and Tpo to promote viability of CD34+CD38− progenitor cells were not a consequence of using suboptimal concentrations of cytokines, because a 4 times increase in the concentration of each of the cytokines did not further increase their effect on viability (O.J.B. and S.E.W.J., unpublished observations).
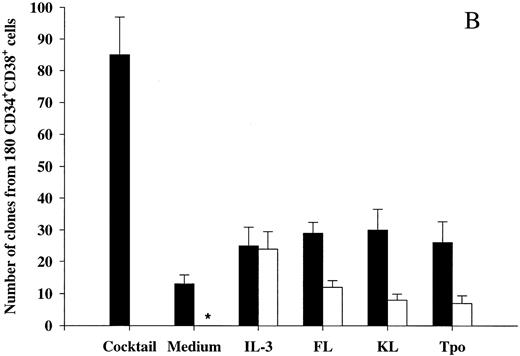
We also investigated the ability of Tpo as well as IL-3, FL, and KL to promote viability of CD34+CD38+ BM cells (Fig 2B), representing most of the progenitor cells in the BM and shown to consist predominantly of more committed progenitor cells.18 These studies were performed with cells from the same donors as the experiments with CD34+CD38− cells (Fig 2A), and their results were therefore directly comparable. IL-3, FL, and KL promoted growth of CD34+CD38+ progenitor cells (28%, 14%, and 9%, respectively) when compared with the clonogenic growth observed in response to the multifactor combination, which was significantly higher than for CD34+CD38− progenitor cells (P < .05 for all 3 cytokines). As suggested in our initial experiments with CD34+ cells (Fig 1A), a higher (although low; 15%) fraction of CD34+CD38+ progenitor cells remained viable after 116 hours of incubation in medium alone as compared with the more primitive CD34+CD38− progenitor cells (3%; Fig 2A). The number of CD34+CD38+ progenitor cells growing in response to the multifactor cytokine combination after 116 hours of incubation in the presence of IL-3 was similar to the clonogenic growth in response to IL-3 alone, suggesting that IL-3 does not have a selective viability-promoting effect on CD34+CD38+ progenitor cells. FL- and KL-preincubated cultures contained a higher number of multifactor-responsive CD34+CD38+ progenitor cells than the number of clones formed in response to FL or KL alone (Fig 2B). However, when correcting for the number of progenitor cells surviving in medium alone, no significant and specific viability-promoting effect of either FL or KL could be observed. Unlike IL-3, FL, and KL, Tpo rather induced growth of a lower fraction of CD34+CD38+ than CD34+CD38− progenitors, and, in contrast to its potent ability to enhance viability of CD34+CD38− progenitor cells, Tpo had no selective viability-promoting effect on CD34+CD38+ progenitor cells.
An additional set of experiments was next performed to even more carefully and stringently investigate whether Tpo promotes viability of CD34+CD38− progenitor cells in a direct manner and in the absence of cell division. Thus, culture wells were visualized at initiation of culture by microscopy to ensure that only wells containing 1 cell were included in the study (Table 2). After 116 hours of preincubation in the presence of Tpo, wells that initially contained 1 cell were examined carefully again and wells in which cells had proliferated (2 or more cells; 7% of single cells) were scored and excluded from the study. Of the wells that contained Tpo-preincubated cells that had not proliferated at this time, 19% formed clones in response to the multifactor combination, which was comparable to the data obtained in the experiments described in Fig 2A (22%; 40 clones from 180 cells). Thus, Tpo appears unique in its ability to directly and selectively promote viability of CD34+CD38− BM progenitor cells.
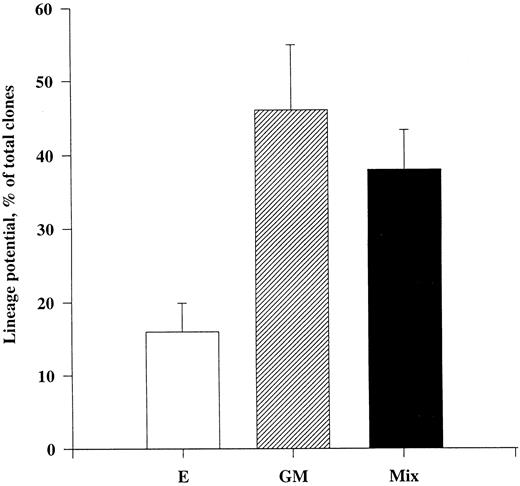
Tpo promotes viability of CD34+CD38− progenitor cells with multilineage potential.We next sought to establish to what degree CD34+CD38− progenitor cells surviving in response to Tpo were committed to either the myeloid or erythroid cell lineages or whether they were multipotent. This was of particular importance, because two recent studies showing a growth-promoting activity of Tpo on primitive human progenitor cells found only a low fraction of the targeted progenitor cells to be multipotent.33 34 To better uncover the differentiation potential of CD34+CD38− progenitor cells, we used a three-step method (Fig 3). Cells were plated individually and cultured in the presence of only Tpo for 116 hours, at which time cultures were pulsed with a combination of 10 cytokines (IL-1, IL-3, IL-6, G-CSF, GM-CSF, CSF-1, FL, KL, Epo, and Tpo) and incubated for an additional 10 days. Clones were subsequently transferred to a methylcellulose culture containing the same cytokines and incubated for 12 to 14 days, at which time cultures were scored for myeloid (GM), erythroid (E), or mixed (GM/E) colonies. Through this 25- to 27-day assay we were able to show that as much as 38% of CD34+CD38− progenitor cells surviving in response to Tpo were multipotent, whereas 16% and 49% of the surviving progenitors generated only GM and E colonies, respectively. Thus, at least a large fraction of CD34+CD38− BM progenitor cells surviving in response to Tpo are multipotent.
Multilineage potential of CD34+CD38− progenitor cells surviving in response to Tpo. CD34+CD38− BM cells were seeded at a density of 1 cell per well in Terasaki plates and incubated in serum-depleted medium supplemented with Tpo for 116 hours before a strong proliferative cocktail of cytokines was added (containing IL-1, IL-3, IL-6, G-CSF, GM-CSF, CSF-1, FL, KL, Epo, and Tpo). After an additional 10 days of incubation at 37°C and 5% CO2 in air, clones containing 10 or more cells were picked and transferred to methylcellulose cultures containing the same cytokine cocktail and incubated for a final 12 to 14 days. Cultures were scored for the presence or absence of GM, E, or mixed (GM/E) colonies. Cultures containing both GM and E colonies or mixed (GM/E) colonies were collectively grouped as Mix. To confirm colony phenotypes, individual colonies were picked, transferred to glass slides with a jet air stream, dried, fixed, Giemsa-stained, and examined by microscopy. An average of 21 wells were picked per group and replated in methylcellulose, of which an average of 13 formed colonies. The results represent the means (+SEM) of a total of five individual experiments.
Multilineage potential of CD34+CD38− progenitor cells surviving in response to Tpo. CD34+CD38− BM cells were seeded at a density of 1 cell per well in Terasaki plates and incubated in serum-depleted medium supplemented with Tpo for 116 hours before a strong proliferative cocktail of cytokines was added (containing IL-1, IL-3, IL-6, G-CSF, GM-CSF, CSF-1, FL, KL, Epo, and Tpo). After an additional 10 days of incubation at 37°C and 5% CO2 in air, clones containing 10 or more cells were picked and transferred to methylcellulose cultures containing the same cytokine cocktail and incubated for a final 12 to 14 days. Cultures were scored for the presence or absence of GM, E, or mixed (GM/E) colonies. Cultures containing both GM and E colonies or mixed (GM/E) colonies were collectively grouped as Mix. To confirm colony phenotypes, individual colonies were picked, transferred to glass slides with a jet air stream, dried, fixed, Giemsa-stained, and examined by microscopy. An average of 21 wells were picked per group and replated in methylcellulose, of which an average of 13 formed colonies. The results represent the means (+SEM) of a total of five individual experiments.
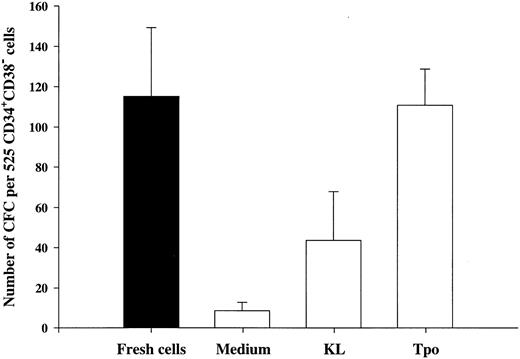
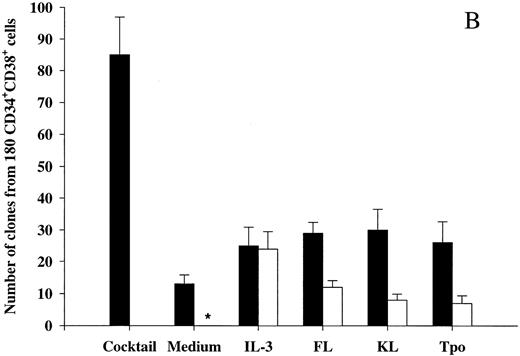
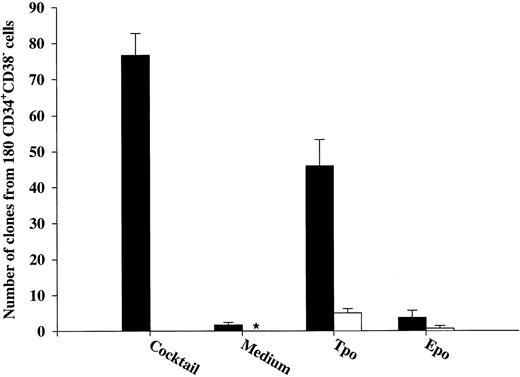
Tpo and KL promote viability of LTC-IC.The LTC-IC assay has been shown to detect very primitive hematopoietic progenitor cells and probably represents the best in vitro assay for evaluating candidate human stem cells.20,39,42 Thus, we investigated the ability of Tpo to promote viability of CD34+CD38− LTC-IC. This set of experiments had the following main objectives. (1) To compare the number of LTC-IC surviving in response to Tpo and KL, because KL has been shown to be most efficient at promoting viability of murine long-term reconstituting stem cells12,13 and also because the most primitive human progenitor stem cells have been suggested to express low levels of c-kit.43,44 (2) Because Petzer et al20 recently showed that Tpo alone (Fig 1A) could slightly expand LTC-IC and because we found that some CD34+CD38− progenitor cells proliferate in response to Tpo alone, it was important to exclude cells that had proliferated within 116 hours of incubation in Tpo or KL alone.
To achieve these objectives, single CD34+CD38− cells were incubated in medium alone, KL, or Tpo for 116 hours, at which time wells containing 2 or more cells were identified and excluded. Both Tpo and KL promoted viability of 5-week CD34+CD38− LTC-IC (Fig 4). However, more LTC-IC survived in response to Tpo than to KL (P < .05). It was noteworthy and somewhat surprising that most if not all LTC-IC survived in the presence of Tpo.
The ability of Tpo and KL to promote viability of LTC-IC. A total of 525 CD34+CD38− BM cells were seeded for each group at a density of 1 cell per well in 10 μL serum-depleted medium in stroma-free cultures. As a control of the initial content of LTC-IC, cells were harvested directly after seeding and transferred to irradiated preestablished human BM stroma. After 116 hours in the presence of Tpo, KL, or medium alone, cells were examined microscopically and wells containing 2 or more were excluded. The remaining wells were harvested, pooled, and divided into 5 wells containing irradiated stroma. The cultures were maintained as described in the Materials and Methods and after 5 weeks the content (adherent and nonadherent cells) in each well was transferred to methylcellulose cultures and the number of CFC was evaluated after an additional 12 to 14 days in culture. The results represent the means (+SEM) of total number of CFC formed by 525 seeded CD34+CD38− cells per group in each of four individual experiments.
The ability of Tpo and KL to promote viability of LTC-IC. A total of 525 CD34+CD38− BM cells were seeded for each group at a density of 1 cell per well in 10 μL serum-depleted medium in stroma-free cultures. As a control of the initial content of LTC-IC, cells were harvested directly after seeding and transferred to irradiated preestablished human BM stroma. After 116 hours in the presence of Tpo, KL, or medium alone, cells were examined microscopically and wells containing 2 or more were excluded. The remaining wells were harvested, pooled, and divided into 5 wells containing irradiated stroma. The cultures were maintained as described in the Materials and Methods and after 5 weeks the content (adherent and nonadherent cells) in each well was transferred to methylcellulose cultures and the number of CFC was evaluated after an additional 12 to 14 days in culture. The results represent the means (+SEM) of total number of CFC formed by 525 seeded CD34+CD38− cells per group in each of four individual experiments.
Distinction between the ability of Tpo and Epo to promote viability of CD34+CD38− BM progenitor cells.Epo and its receptor show high homology with Tpo and c-mpl,45 respectively, and Epo is the principal regulator of erythropoiesis as Tpo is now believed to be for thrombocytopoiesis.25,46,47 In addition, Epo is a viability factor for committed erythroid progenitor cells.48 However, to our knowledge, it has not been investigated whether Epo might promote viability of primitive human progenitor cells. Accordingly, it was of interest to address whether Tpo and Epo had similar effects on the viability of CD34+CD38− progenitor cells. Interestingly, and unlike the potent viability-promoting activity of Tpo, Epo had no viability-promoting effect on CD34+CD38− progenitor cells in serum-depleted medium (Fig 5).
Comparison between the ability of Tpo and Epo to promote viability of CD34+CD38− progenitor cells. CD34+CD38− BM cells were cultured at a density of 1 cell per well in 10 μL serum-depleted medium and predetermined optimal concentrations of the indicated cytokines. After 116 hours of preincubation, 10 μL medium containing IL-1, IL-3, IL-6, G-CSF, GM-CSF, CSF-1, FL, KL, Epo, and Tpo was added to yield predetermined optimal concentrations. Clones (≥5 cells) were scored after an additional 14 days of incubation at 37°C and 5% CO2 in air (▪). A total of 180 CD34+CD38− cells were also cultured at a density of 1 cell per well in 20 μL serum-depleted medium alone or supplemented with Tpo or Epo and scored for clonal growth (≥5 cells) after 14 days in culture at 37°C and 5% CO2 in air (□). The results represent the means (+SEM) of total number of clones per 180 cells of three individual experiments, with 180 wells scored per group in each experiment. *No clones were formed by cells incubated for 14 days in medium alone.
Comparison between the ability of Tpo and Epo to promote viability of CD34+CD38− progenitor cells. CD34+CD38− BM cells were cultured at a density of 1 cell per well in 10 μL serum-depleted medium and predetermined optimal concentrations of the indicated cytokines. After 116 hours of preincubation, 10 μL medium containing IL-1, IL-3, IL-6, G-CSF, GM-CSF, CSF-1, FL, KL, Epo, and Tpo was added to yield predetermined optimal concentrations. Clones (≥5 cells) were scored after an additional 14 days of incubation at 37°C and 5% CO2 in air (▪). A total of 180 CD34+CD38− cells were also cultured at a density of 1 cell per well in 20 μL serum-depleted medium alone or supplemented with Tpo or Epo and scored for clonal growth (≥5 cells) after 14 days in culture at 37°C and 5% CO2 in air (□). The results represent the means (+SEM) of total number of clones per 180 cells of three individual experiments, with 180 wells scored per group in each experiment. *No clones were formed by cells incubated for 14 days in medium alone.
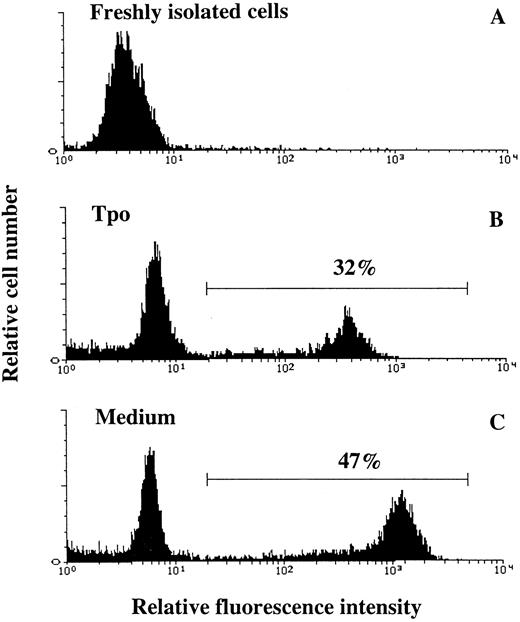
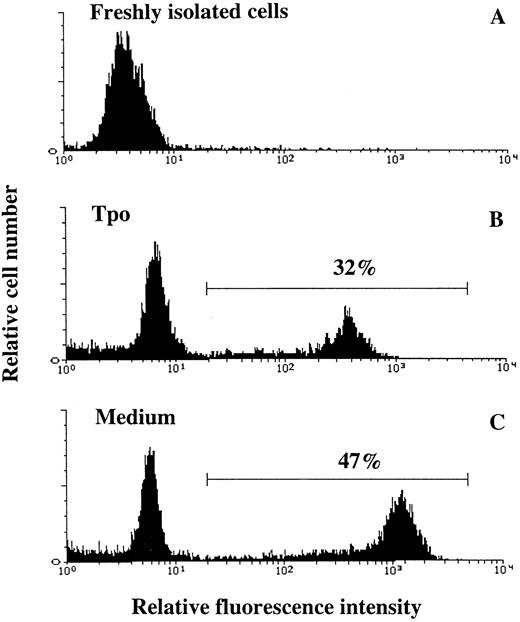
Tpo suppresses apoptosis of CD34+CD38− BM cells.IL-3, KL, and Epo have been suggested to support the viability of hematopoietic progenitor cells by preventing apoptosis.16,48,49 To examine whether the increased viability of CD34+CD38− BM progenitor cells observed in response to Tpo correlated with suppression of apoptosis, cells were cultured in serum-depleted medium in the absence or presence of Tpo and examined for apoptosis. Because apoptotic hematopoietic cells rapidly undergo secondary necrosis and disintegrate in culture,37,50,51 we chose the time-point for most pronounced decrease in viability (44 hours; Fig 1B) rather than 116 hours for studying apoptosis. Apoptosis was detected by enzymatic incorporation of fluorescein-dUTP using free 3′-OH DNA-ends as templates, which is symptomatic for DNA degradation found in cells undergoing apoptosis40 (Fig 6). The percentage of cells incorporating fluorescein-dUTP (apoptotic cells) decreased from 50% ±2% when cells were incubated in serum-depleted medium alone to 35% ± 2% when incubated in the presence of Tpo (mean of 3 experiments; P < .05). Thus, Tpo inhibits apoptosis of CD34+CD38− BM cells.
Tpo counteracts apoptosis of CD34+CD38− BM cells. Thirty thousand CD34+CD38− BM cells were fixed directly after isolation (A), whereas 50,000 CD34+CD38− BM cells were incubated in serum-depleted medium for 44 hours in the presence of Tpo (B) or in the absence of cytokines (C). A viable cell scatter gate was set to include greater than 90% of freshly isolated CD34+CD38− BM cells (A). Cells falling within this gate were analyzed for the presence of fluorescein-dUTP labeling by flow cytometry as described in the Materials and Methods. Results are from one of three experiments, with similar results.
Tpo counteracts apoptosis of CD34+CD38− BM cells. Thirty thousand CD34+CD38− BM cells were fixed directly after isolation (A), whereas 50,000 CD34+CD38− BM cells were incubated in serum-depleted medium for 44 hours in the presence of Tpo (B) or in the absence of cytokines (C). A viable cell scatter gate was set to include greater than 90% of freshly isolated CD34+CD38− BM cells (A). Cells falling within this gate were analyzed for the presence of fluorescein-dUTP labeling by flow cytometry as described in the Materials and Methods. Results are from one of three experiments, with similar results.
DISCUSSION
Whereas a number of studies in recent years have dissected the ability of different cytokines to promote viability and suppress apoptosis of primitive murine hematopoietic progenitor cells,4,6-14,37,49 50 less is known about the ability of cytokines to promote viability of populations of candidate human stem cells.
The present study is the first dissecting the ability of cytokines to promote viability of human CD34+CD38− BM progenitor cells. CD34+CD38− cells represent a minor fraction of BM cells, but are highly enriched in primitive progenitor cells.18-20 Thus, in addition to being used extensively for in vitro studies of primitive human hematopoietic progenitor cells, this population represents a likely target population for gene therapy protocols as well as for ex vivo expansion of stem cells to be used for autologous transplantation in conjunction with high-dose chemotherapy.23 24
Two previous studies had examined the ability of KL and IL-3 to promote viability of CD34+DR− BM cells,15,16 which also have been shown to be enriched in primitive progenitor cells.52,53 Leary et al15 showed that IL-3 (and GM-CSF ) but not KL promoted the viability of CD34+DR− blast cell progenitor cells. In contrast, Brandt et al16 found that KL but not IL-3 promoted survival of CD34+DR− c-kit+ progenitor cells. The different conclusions reached in these two studies might be a result of somewhat different methods used for cell isolation as well as culture conditions. A more recent study found that both IL-3 and KL individually promoted survival of CD34+CD38− fetal liver progenitor cells.17 In addition, FL was found to enhance viability, although less efficiently than KL and IL-3.17 It is noteworthy that all of these previous studies were performed in cultures containing high numbers of cells, and thus effects observed could (at least in part) be mediated indirectly rather than directly on the progenitor cells. Thus, an important objective of the present studies was to use single-cell cultures to establish to what degree these cytokines could directly promote progenitor cell survival. Through such experiments, we were able to show that IL-3 and to a slightly lesser degree FL and KL were able to directly promote viability of CD34+CD38− progenitor cells, similar to what had previously been shown for CD34+CD38− fetal liver progenitors.17 By careful mapping of individual cells, we also showed that most of the CD34+CD38− progenitor cells surviving in response to IL-3, FL, or KL do so without undergoing cell division. Although the viability-promoting effect of these cytokines might still involve movement in the cell cycle, these experiments clearly show that these cytokines, when acting individually, have a selective viability-promoting effect on primitive progenitor cells. In contrast, most if not all of the viability-promoting effect of the same cytokines on the more committed CD34+CD38+ progenitor cells was associated with cell proliferation. These observations are in agreement with the notion that proliferation of more mature progenitor cells can occur in response to single cytokines, whereas combined signaling through multiple cytokine receptors are required to stimulate growth of more primitive progenitor cells.1-3 Accordingly, the CD34+CD38− cells that proliferate in response to individual cytokines are likely to represent different and probably more mature progenitors than those surviving in the absence of cell division and subsequent proliferate in response to the cocktail.
The present findings on CD34+CD38− BM progenitor cells were in agreement with those previously made on candidate murine stem cell populations,8-14 in that IL-3, KL, and FL can directly promote viability of primitive progenitor cells. However, whereas KL has been found to be the most potent viability-promoting cytokine for primitive murine progenitor cells and to maintain most progenitors viable through prolonged culture,11-13 37 KL (as well as IL-3 and FL) were only capable of maintaining a low fraction of CD34+CD38− progenitor cells viable.
The present studies suggest that Tpo might have a unique role in promoting the viability of primitive human hematopoietic progenitor cells, in that it directly and through prolonged incubation maintained most CD34+CD38− BM progenitor cells viable. The number of CD34+CD38− progenitor cells surviving in response to Tpo was twofold and fourfold higher than in response to IL-3 and KL, respectively. Also the viability-promoting ability of Tpo appears to be directly mediated, as determined by single-cell experiments. Furthermore, when acting alone, Tpo appears to be selectively enhancing viability rather than proliferation of CD34+CD38− progenitor cells. In contrast, Tpo had a slight growth-enhancing but not viability-promoting effect on the more committed CD34+CD38+ progenitor cells. Thus, Tpo selectively promotes viability of CD34+CD38− progenitor cells.
It is of interest that Tpo is much more efficient than KL in promoting viability of primitive human progenitor cells, because most if not all hematopoietic progenitors express c-kit.33,54,55 One reason for this could be that the most primitive human progenitor cells have been suggested to express rather low levels of c-kit.33 Alternatively, c-mpl might be more efficient than c-kit in delivering a viability-enhancing signal.
The ability of Tpo to promote viability of CD34+CD38− progenitor cells is likely to be a consequence of suppression of apoptosis occurring in the absence of viability-promoting signals.4 This idea was supported by the observation that Tpo suppressed apoptosis of CD34+CD38− BM cells, as has previously been shown for IL-3 and KL on CD34+DR− cells.16 The ability of Tpo to suppress apoptosis of hematopoietic cells was also supported by a recent study on a human progenitor cell line expressing c-mpl.56
Because CD34+CD38− BM cells, like any other enriched progenitor cell population, are somewhat heterogeneous, it remains possible that the apoptotic cells do not necessarily represent progenitor cells. However, the delayed addition studies specifically investigating the viability of clonogenic progenitor cells support the idea that Tpo suppresses apoptosis of CD34+CD38− progenitor cells.
It was important to establish whether CD34+CD38− progenitor cells surviving in response to Tpo were multipotent, in particular because a recent study had only uncovered multipotentiality of a low fraction of CD34+CD38− progenitor cells stimulated to grow by Tpo in combination with KL and IL-3.33 In the present studies, we found that as much as 38% of the CD34+CD38− progenitor cells surviving for 116 hours in the presence of Tpo had a combined myeloid/erythroid potential, even after an additional 10 days of incubation in the presence of potent combinations of 10 cytokines. This finding importantly demonstrates that Tpo acts on a primitive population of human BM progenitor cells with multilineage potentiality and with the ability to produce CFC of myeloid, erythroid, and mixed lineage potential after prolonged and extensive cellular expansion. We only observed a few colonies with megakaryocytes in the present studies (O.J.B. and S.E.W.J., unpublished observation). This most likely reflects a methodological limitation, because Kobayashi et al33 observed the same lack of megakaryocyte production from CD34+CD38− progenitor cells, whereas megakaryocytes were formed from unfractionated CD34+ BM cells.
Interestingly, and to our surprise, Tpo promoted viability of practically all (96%) CD34+CD38− LTC-IC. Although all wells containing 2 or more cells were excluded before transfer to the LTC-IC assay, we cannot exclude that the viability-promoting effect of Tpo might in part be result of cell cycle activation, potentially affecting the clonogenic output of LTC-IC.
KL, which has been implicated to be the best viability-promoting cytokine for murine stem cells,12,13,38 also promoted viability of LTC-IC in the present study. However, these studies clearly establish the superior activity of Tpo to promote viability of 5-week LTC-IC. However, because the 5-week LTC-IC are heterogeneous, we cannot exclude the possibility that the progenitors surviving in response to KL might be more (or less) primitive than those responding to Tpo. The recently described extended LTC-IC assay,42 although not an ultimate human stem cell assay, might prove useful in further addressing the different roles of these and other cytokines in maintaining candidate human stem cells in culture.
Collectively, the present as well as previous studies on primitive murine progenitor cells12,13,37 suggest that KL is more efficient in maintaining viability of murine than of human stem cells. Furthermore, because we have recently found that Tpo promotes the viability of primitive murine BM progenitor cells less efficiently than KL,38 Tpo might play a more prominent role in promoting viability of human than murine progenitor cells. This difference might be important, although we cannot exclude that the observed differences between the two species might be due to different and heterogeneous progenitor cell populations having been investigated.
In conclusion, the present studies show that Tpo is considerably more potent than IL-3, KL, and FL in promoting viability of CD34+CD38− BM progenitor cells in culture. This observation might have important clinical implications, because it suggests that Tpo, in addition to its potential role in promoting platelet recovery,57 58 might prove important to include in protocols for ex vivo expansion of stem cells as well as in gene-targeted therapy with stem cells.
ACKNOWLEDGMENT
The authors thank Kenneth Pohl, MD, and the Department of Hematology (Lund University Hospital) for generous help with providing human BM; Per Nilsson, PhD, for help with irradiation of human stroma layer; Ole P. Veiby, O. Joseph Trask, Jr, and Per Anders Bertilsson for help with cell sorting; Marcia Siefring and David Kuhlman for technical assistance; and Gösta Bergh, MD, for critically reviewing this manuscript.
Supported by grants from the Swedish Cancer Society, the Georg Danielssons research trust, and the Kamprad Crafoord and Tobias Foundations.
Address reprint requests to Ole J. Borge, MSc, Stem Cell Laboratory, Department of Internal Medicine, University Hospital of Lund, 221 85 Lund, Sweden.