Abstract
Hematopoietic stem cells purified from mouse bone marrow are quiescent with less than 2% of Lin− Hoechstlow/Rhodaminelow (Lin− Holow/Rholow) and 10% to 15% of Lin−/Sca+ cells in S phase. These cells enter proliferative cycle and progress through G1 and into S phase in the presence of cytokines and 5% heat-inactivated fetal calf serum (HI-FCS). Cytokine-stimulated Lin− Holow/Rholow cells took 36 to 40 hours to complete first division and only 12 hours to complete each of 5 subsequent divisions. These cells require 16 to 18 hours to transit through G0 /G1 period and 28 to 30 hours to enter into mid-S phase during the first cycle. Up to 56% of Lin− Rholow/Holow cells are high-proliferative potential (7 factor-responsive) colony-forming cells (HPP-CFC). At isolation, HPP-CFC are quiescent, but after 28 to 30 hours of culture, greater than 60% are in S phase. Isoleucine-deprivation of Lin−Holow/Rholow cells in S phase of first cycle reversibly blocked them from entering into second cycle. After the release from isoleucine-block, these cells exhibited a G1 period of less than 2 hours and entered into mid-S phase by 12 hours. Thus, the duration of G1 phase of the cells in second cycle is 4 to 5 times shorter than that observed in their first cycle. Similar cell cycle kinetics are observed with Lin−/Sca+ population of bone marrow cells. Stem cell factor (SCF ) alone, in the presence of HI-FCS, is as effective as a cocktail of 2 to 7 cytokines in inducing quiescent Lin−/Sca+ cells to enter into proliferative cycle. Aphidicolin treatment reversibly blocked cytokine-stimulated Lin−/Sca+ cells at G1 /S boundary, allowing their tight synchrony as they progress through first S phase and enter into second G1 . For these cells also, SCF alone is sufficient for their progression through S phase. These studies indicate a very short G1 phase for stem cells induced to proliferate and offer experimental approaches to synchronize murine hematopoietic stem cells.
MURINE PRIMITIVE long-term repopulating lymphohematopoietic stem cells have been characterized by the absence of differentiated lineage markers (Lin−), the presence of cell surface Ly-6 antigen (Sca+) and c-Kit, and relatively low levels of staining with the supravital dyes rhodamine-123 and hoechst-33342. Lin− cells expressing Sca or c-Kit or showing low staining with rhodamine (Rholow) and hoechst (Holow) are relatively quiescent and enriched for multifactor-responsive high proliferative potential colony-forming cells (HPP-CFC), although using continuous bromo-deoxyuridine (BrdU) labeling, Bradford et al1 have shown that most of the Lin− Rholow/Holow cells have entered cell cycle over a 12-week time interval.
The primitive long-term repopulating cells can be induced to proliferate after in vivo exposure to cytotoxic agents, such as 5-fluorouracil (5-FU) or in vitro exposure to cytokines. Particular attention has focused on the possibility of using such proliferative stimuli to expand stem cells or to facilitate retroviral integration in gene therapies.2-7 These manipulations can result in expansion and cycle activation of primitive surrogate stem cells, HPP-CFCs, but with a concomitant loss of long-term, but not short-term, in vivo engraftment potential,8,9 and through in vivo hydroxyurea suicide experiments, it was shown that the cells engrafting into nonmyeloablated marrow, spleen and thymus were insensitive to hydroxyurea and, therefore, noncycling.10 Spleen colony-forming ability of cycling cells was reported to be 50% less than that of normal marrow or velocity sedimented noncycling cells.11 Studies using purified populations of stem cells showed that 100 G0 /G1 cells are capable of protecting 90% of lethally irradiated recipients, whereas the 100 S/G2 /M phase cells protect only 22% of recipients.12
Although these observations show an engraftment defect in mitotically active hematopoietic progenitor/stem cells, they fail to fully establish a cause and effect relationship between the engraftment defect and the cell cycle status of stem cells induced to proliferate. To address this issue more critically, it is important that the same population of purified pluripotent hematopoietic stem cells (PHSCs) in their quiescent state be followed for their ability to engraft at specific phases as they traverse the cell cycle after proliferative stimulation. This would require not only a fuller understanding of the kinetics of cell cycle entry and progression of cytokine-stimulated quiescent PHSCs, but also developing procedures that allow synchronization and enrichment of PHSCs to represent specific phases of cell cycle after proliferative stimulation. In the present studies, we have characterized the cell cycle kinetics of Lin− Rholow/Holow and Lin−/Sca+ stem cells after exposure to a 4-cytokine cocktail consisting of interleukin-3 (IL-3), IL-6, IL-11, and stem cell factor (SCF ) or SCF alone and developed experimental conditions that allow their synchronization in early S phase of the first cycle.
MATERIALS AND METHODS
Purification of Lin−/Sca+and Lin−Rholow/Holowcells from mouse bone marrow.Sorting of the Lin−/Sca+ cells involved recovering marrow from tibiae and femora of 6- to 8-week old Balb/c or C57BLK6 mice under sterile conditions and enriching low-density cells by equilibrium density centrifugation is as described by Wolf et al.13 Low-density cells were subjected to immunomagnetic bead depletion of lineage-committed cells using a panel of antibodies against Ter 119 (erythroid), B220 (B and pre-B lymphocytes), MAC-1 (macrophages), GR-1 (granulocytes), Lyt-2 (T-cells), L3T4 (T-helper cells), and YW25.12.17 (erythroid). Antibodies against B220, MAC-1, and GR-1 were also biotin-conjugated. Lineage-depleted cells were then incubated with fluorescein isothiocyanate (FITC)-conjugated anti–SCA-1 antibodies (PharMingen, San Diego, CA) and phycoerythrin (PE) streptavidin (Biomeda, Foster City, CA) and sorted on FACS-STAR (Becton Dickinson, San Francisco, CA) to recover FITC-positive and PE-negative cells.
The overall procedure for sorting of Lin−, Rholow/Holow cells from murine bone marrow is essentially as described by Wolf et al.13 Briefly, this procedure involved recovering lineage-depleted bone marrow cells as described above and labeling them with Rhodamine-123 and Hoechst-33342. The labeled cells were then sorted on FACS-STAR (Becton Dickinson) or on MoFlo (Cytomation, Fort Collins, CO) cell sorter to recover lowest 10% Rhodamine-123 staining and lowest 3% Hoechst-33342 staining cells.
In vitro culture and synchronization of hematopoietic progenitor/stem cells.Immediately after their purification, Lin−/Sca+ or Lin−, Rholow/Holow cells were incubated at a density of 10 to 20 × 103 cells/mL in Dulbecco's Modified Essential Medium (DMEM; GIBCO-BRL, Grand Island, NY) containing 5% heat-inactivated fetal calf serum (HI-FCS; HyClone Laboratories, Logan, UT), glutamine (2.5 mmol/L), penicillin (100 U/mL), streptomycin (100 μg/mL), and appropriate cytokines at 37°C in a 5% CO2 containing humidified incubator. Unless otherwise indicated, the concentration and the source of individual cytokines added in different combinations to the media in these studies were recombinant murine SCF (mSCF) at 100 ng/mL (R&D Systems, Minneapolis, MN), mIL-3 at 100 U/mL (Collaborative), mIL-6 at 50 U/mL (R&D), recombinant human IL-11 (hIL-11) at 50 ng/mL (Genetics Institute, Cambridge, MA), IL-1α at 250 U/mL (Hoffmann La Roche, Nutley, NJ), granulocyte-macrophage colony-stimulating factor (GM-CSF ) at 2.5 ng/mL (R&D), granulocyte colony-stimulating factor (G-CSF ) at 5 ng/mL (Collaborative), basic fibroblast growth factor (bFGF ) at 5 ng/mL (R&D), and CSF-1 at 5,000 U/mL (Chiron, Emeryville, CA).
For synchronization of the cells at G1 /S boundary, hematopoietic stem cells exposed to the medium containing a cocktail of 7 cytokines (IL-3, IL-1α, G-CSF, GM-CSF, SCF, CSF-1, and bFGF ) described above for 8 hours were treated with 2 μg/mL of aphidicolin (Sigma Chemical Co, St Louis, MO) for next 24 hours. To release the cells from aphidicolin-block, they were washed once with complete medium and placed in medium containing either the cocktail of 7 cytokines or mSCF (200 ng/mL) alone.
For synchronization of S phase Lin−, Rholow/Holow cells in their subsequent G0 /G1 phase, the cells were first incubated in the presence of medium containing a cocktail of 4 cytokines (mIL-3, mIL-6, hIL-11, and mSCF ) for 30 hours and then washed once with isoleucine-free DMEM and incubated with isoleucine-free DMEM (GIBCO-BRL) containing dialyzed HI-FCS (HyClone Laboratories), glutamine, penicillin, streptomycin, and a 4-cytokine cocktail at the concentrations indicated above. Thirty-six hours of isoleucine deprivation was found to be sufficient for reversibly blocking Lin− Rholow/Holow cells in G0 /G1 phase. Cells were released from isoleucine-block by replacing isoleucine-free medium with complete medium containing the appropriate cytokines.
Autoradiographic detection of 3H-thymidine–labeled nuclei.The number of cells in S phase were determined by autoradiography of the cells after pulse labeling with 3H-thymidine as described elsewhere.14 Briefly, duplicate aliquots of cells (500 to 1,000) at regular intervals as they are progressing through cell cycle were incubated with 2.5 μCi/mL of 3H-thymidine at 37°C in 5% CO2 containing humidified incubator for 30 minutes. The incorporation of 3H-thymidine was terminated by cytospin centrifugation and immediately fixing with methanol/acetic acid (2:1 vol/vol), followed by three washes in methanol. Slides were allowed to air dry overnight and a thin film of Kodak nuclear track NTB3 emulsion (Eastman Kodak, Rochester, NY) was applied. Slides were then incubated in the dark for 4 days, developed, and fixed with Kodak Decktol Developer and Kodak Fixer, respectively. Slides were washed extensively and stained with Giemsa. The percentage of cells with labeled nuclei (representing cells in S phase) was assessed by counting 150 to 200 cells on each slide.
Hematopoietic progenitor cell assay.The cloning efficiency of Lin− Rholow/ Holow cells was determined by analysis of HPP-CFC using a double-layer nutrient agar culture system essentially as described.15 One thousand cells obtained immediately after sorting or at indicated intervals after cytokine stimulation were withdrawn from the in vitro liquid culture and plated in 35-mm petri dishes containing double-layer nutrient agar at a density of 100 cells per plate. One milliliter of underlayer in each plate contained α-minimum essential medium (α-MEM) with 20% fetal calf serum supplemented with vitamins, glutamine, and 7 cytokines (IL-1, IL-3, CSF-1, GM-CSF, G-CSF, SCF, and bFGF ), along with a final agar concentration of 0.5%. Cells were included in 0.5 mL overlayer with a final agar concentration of 0.3%. Culture dishes with the cells were then incubated at 37°C for 14 days in the presence of 5% O2 , 10% CO2 , and 85% N2 and scored for HPP-CFC using a dissecting microscope. HPP-CFC typically generated colonies greater than 0.5 mm in diameter, consisting of tightly packed cells. Functionally, these are defined as primitive cells by their relative resistance to 5-FU, their synergistic growth factor requirements, and their copurification with long-term reconstituting cells in vivo.11 16
3H-thymidine suicide assay.This assay was performed essentially as described by Quesenberry and Stanley.17 The number of cells in S phase were detected in this assay by subjecting an aliquot of cells in culture to a high specific activity 3H-thymidine (200 μCi/mL) for 20 minutes at 37°C and determining their ability to form HPP-CFC in a double-layer agar system described above.
RESULTS
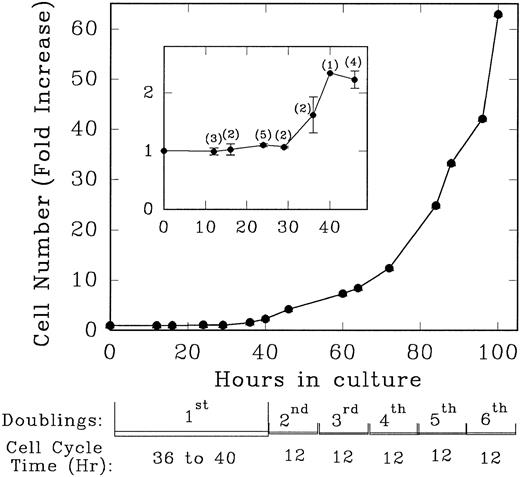
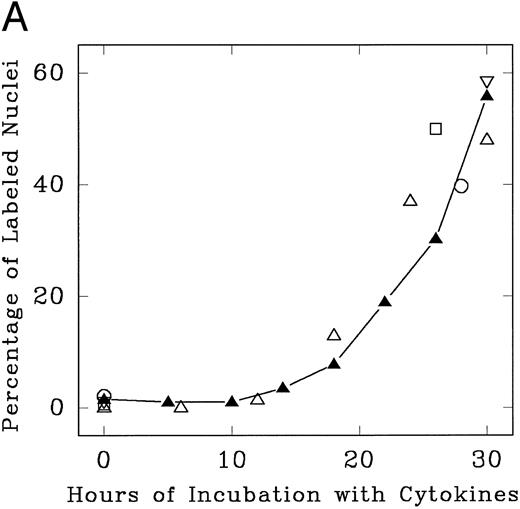
Lin− Rholow/Holow cells in culture with IL-3, IL-6, IL-11, and SCF evidence their first population doubling at between 36 to 40 hours, whereas subsequent population doublings occurred every 12 hours up to 100 hours (Fig 1). Details of the first cycle were determined by pulse labeling with 3H-thymidine. As shown in Fig 2, replicating and nonreplicating cells exhibit a distinct pattern of staining after autoradiography and Giemsa staining of pluripotent hematopoietic stem cells. Lin− Rholow/Holow cells in IL-3, IL-6, IL-11, and SCF (Fig 3A) or in a 7-cytokine cocktail consisting of IL-3, IL-1α, G-CSF, GM-CSF, SCF, CSF-1, and bFGF (Fig 3B) begin to enter S phase at approximately 16 to 18 hours, and the S phase peak is somewhere around 30 hours. In these experiments, about 60% of the cells were in S at 30 hours of culture using a 30-minute 3H-thymidine pulse label, but, when the cells were labeled over 4 hours (from 26 to 30 hours of culture), the nuclei of more than 90% of the cells were labeled with 3H-thymidine (data not shown), indicating that virtually all of the cells transit through the S phase of the first cycle during 30 hours of incubation in cytokines. Transition of all the cells incubated with cytokines through the first cycle is also indicated by a twofold increase in cell numbers (one doubling) by 36 to 40 hours (Fig 1). These data indicate that primitive Lin− Rholow/Holow cells, when stimulated to enter and transit cell cycle, have a G1 phase of about 18 hours and a total cell cycle length of 36 to 40 hours. Subsequent cycles are very rapid.
Growth rate and the doubling time of Lin− Rholow/Holow cells incubated in the presence of medium containing IL-3, IL-6, IL-11, and SCF. Immediately after their purification, Lin− Rholow/Holow cells were suspended at 10 × 103 cells/mL in medium containing 4 cytokines and incubated at 37°C in a 5% CO2 containing humidified incubator as described in the Materials and Methods. At indicated intervals, duplicate aliquots of cells from growing culture were counted using a hemocytometer. The numbers in the parentheses of the insert indicate the number of experiments from which the values for that particular time point were obtained.
Growth rate and the doubling time of Lin− Rholow/Holow cells incubated in the presence of medium containing IL-3, IL-6, IL-11, and SCF. Immediately after their purification, Lin− Rholow/Holow cells were suspended at 10 × 103 cells/mL in medium containing 4 cytokines and incubated at 37°C in a 5% CO2 containing humidified incubator as described in the Materials and Methods. At indicated intervals, duplicate aliquots of cells from growing culture were counted using a hemocytometer. The numbers in the parentheses of the insert indicate the number of experiments from which the values for that particular time point were obtained.
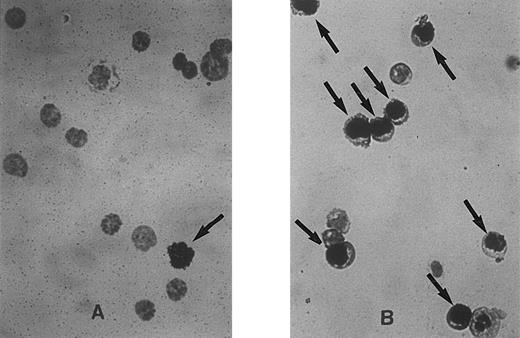
Representative fields of cells showing unlabeled and labeled nuclei at 0 hours (A) and 30 hours (B) after cytokine stimulation of Lin− Rholow/Holow cells. Experimental procedure for the sorting of the stem cells, their labeling with 3H-thymidine, autoradiography, and Giemsa staining are as described in the Materials and Methods. Cells with labeled nuclei are indicated by arrows.
Representative fields of cells showing unlabeled and labeled nuclei at 0 hours (A) and 30 hours (B) after cytokine stimulation of Lin− Rholow/Holow cells. Experimental procedure for the sorting of the stem cells, their labeling with 3H-thymidine, autoradiography, and Giemsa staining are as described in the Materials and Methods. Cells with labeled nuclei are indicated by arrows.
Progression of cytokine-stimulated Lin− Rholow/Holow cells through G0 /G1 and into S phase. Lin− Rholow/Holow were incubated in medium containing a cocktail of either 4 cytokines (IL-3, IL-6, IL-11, and SCF; A) or 7 cytokines (IL-3, IL-1α, GM-CSF, G-CSF, SCF, FGF, and CSF-1; B), and at indicated intervals duplicate aliquots of cells were analyzed for 3H-thymidine incorporation followed by autoradiography to assess the percentage of cells with labeled nuclei as described in the Materials and Methods. Each set of symbols in (A) represents observations from separate individual experiments.
Progression of cytokine-stimulated Lin− Rholow/Holow cells through G0 /G1 and into S phase. Lin− Rholow/Holow were incubated in medium containing a cocktail of either 4 cytokines (IL-3, IL-6, IL-11, and SCF; A) or 7 cytokines (IL-3, IL-1α, GM-CSF, G-CSF, SCF, FGF, and CSF-1; B), and at indicated intervals duplicate aliquots of cells were analyzed for 3H-thymidine incorporation followed by autoradiography to assess the percentage of cells with labeled nuclei as described in the Materials and Methods. Each set of symbols in (A) represents observations from separate individual experiments.
Seven-factor responsive HPP-CFC represents a primitive surrogate stem cell assayed in vitro. We determined the number and cell cycle status of HPP-CFC in the Lin− Rholow/Holow population both before and at 28 to 30 hours after liquid culture in IL-3, IL-6, IL-11, and SCF (Table 1). On isolation, 7-factor HPP-CFC, representing 28% of Lin− Rholow/Holow cells, showed essentially no kill with 3H-TdR (3% ± 3%) and were dormant. After 28 to 30 hours of culture in IL-3, IL-6, IL-11, and SCF, cloning efficiency was unchanged (31% ± 7%), but the majority of HPP-CFC were in S phase, as shown by a 60% ± 10% killing effect with 3H-TdR (Table 1).
Cell Cycle Status of Lin− Rholow/Holow-Derived HPP-CFC Cultured in Cytokines
| Time in Culture . | HPP-CFC/100 Cells* . | % Killed by 3H-TdR† . |
|---|---|---|
| 0 h | 28 ± 13 | 3 ± 3 |
| 28-30 h | 31 ± 7 | 60 ± 10‡ |
| Time in Culture . | HPP-CFC/100 Cells* . | % Killed by 3H-TdR† . |
|---|---|---|
| 0 h | 28 ± 13 | 3 ± 3 |
| 28-30 h | 31 ± 7 | 60 ± 10‡ |
Seven-factor–responsive HPP-CFC per 100 cells from initially isolated Lin− Rholow Holow cells and after 28 to 30 hours in culture with IL-3, IL-11, IL-6, and SCF (see the Materials and Methods).
Cell cycle status of HPP-CFC as determined by 3H-TdR suicide. These data are from four separate experiments with 4 to 10 culture plates per group in each experiment.
Significantly different from 0 hours value (Wilcoxon rank-sum test; P = .0209).
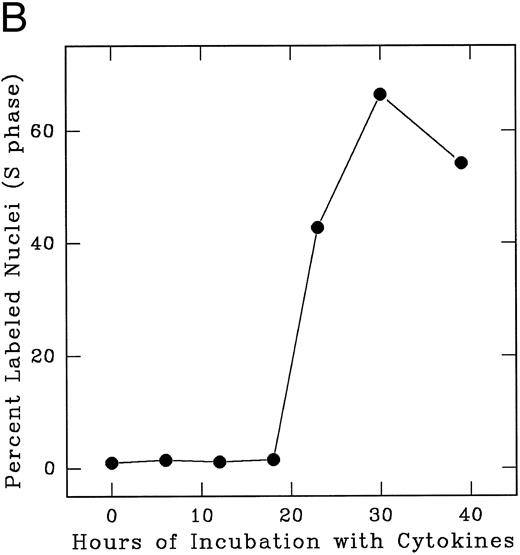
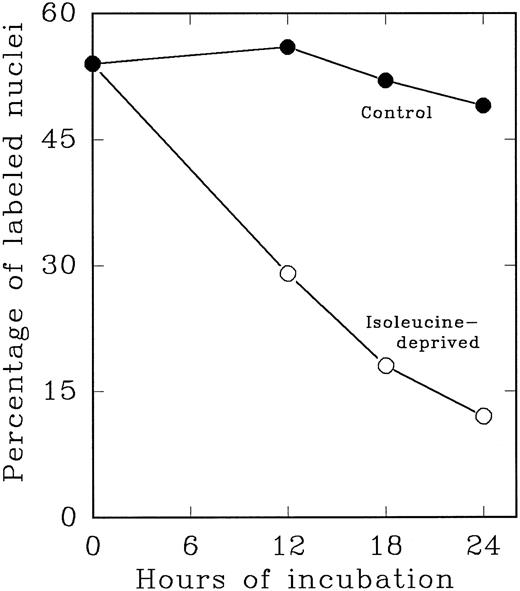
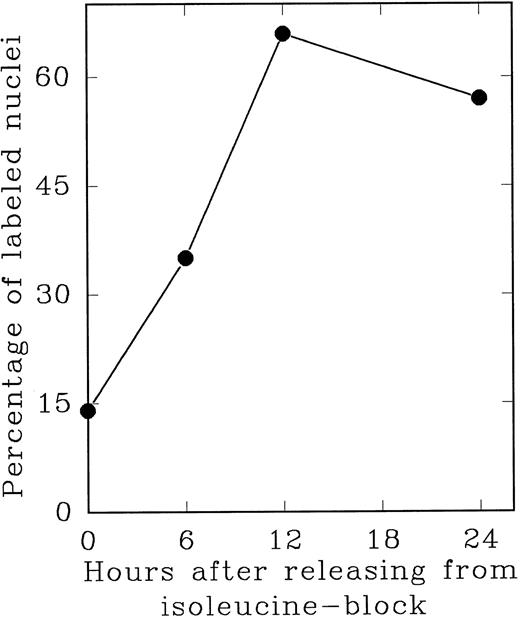
In an alternate approach to studying the G1 period of the cells in second cycle, Lin− Rholow/Holow cells stimulated for 36 hours with IL-3, IL-6, IL-11, and SCF were subjected to isoleucine-deprivation to arrest them in early-G1 of subsequent cycle. We observed that isoleucine-deprivation for 26 to 30 hours effectively limited stem cells completing first S phase from progressing through the subsequent G1 and entering into the second S phase (Fig 4). During this entire period, the cell number doubled once, indicating their arrest immediately after the first cycle, and their viability remained unchanged as determined by their ability to exclude Trypan-Blue. When isoleucine-deprived cells were refed with complete medium, they immediately entered into S phase (Fig 5), exhibiting a short (1 to 2 hours) G1 for cells that have completed first cycle.
Cytokine-stimulated Lin− Rholow/Holow cells in first S phase are blocked in subsequent G0 /G1 phase by isoleucine-deprivation. Lin− Rholow/Holow cells were incubated in medium containing a cocktail of 4 cytokines (IL-3, IL-6, IL-11, and SCF ) at 37°C in a humidified incubator with 5% CO2 . Thirty hours later, when about 60% of the cells are in S phase, the total culture was divided into two groups of equal cell number. One group of the cells was maintained in the same media as described above and the other group of cells was subjected to isoleucine-deprivation as described in the Materials and Methods. At indicated intervals, 200-μL aliquots of cells from each group were analyzed for the percentage of labeled nuclei as described in the Materials and Methods.
Cytokine-stimulated Lin− Rholow/Holow cells in first S phase are blocked in subsequent G0 /G1 phase by isoleucine-deprivation. Lin− Rholow/Holow cells were incubated in medium containing a cocktail of 4 cytokines (IL-3, IL-6, IL-11, and SCF ) at 37°C in a humidified incubator with 5% CO2 . Thirty hours later, when about 60% of the cells are in S phase, the total culture was divided into two groups of equal cell number. One group of the cells was maintained in the same media as described above and the other group of cells was subjected to isoleucine-deprivation as described in the Materials and Methods. At indicated intervals, 200-μL aliquots of cells from each group were analyzed for the percentage of labeled nuclei as described in the Materials and Methods.
S phase-enriched Lin− Rholow/Holow cells arrested in subsequent G0 /G1 phase by isoleucine-deprivation re-enter proliferative cycle after the release from isoleucine-block. Thirty hours after cytokine stimulation, Lin− Rholow/Holow cells were subjected to isoleucine-deprivation for 36 hours as described in the Materials and Methods. The cells were then released from isoleucine-block and at indicated intervals duplicate aliquots of cells were labeled with 3H-thymidine and processed for autoradiography to assess the percentage of cells with labeled nuclei as described in the Materials and Methods.
S phase-enriched Lin− Rholow/Holow cells arrested in subsequent G0 /G1 phase by isoleucine-deprivation re-enter proliferative cycle after the release from isoleucine-block. Thirty hours after cytokine stimulation, Lin− Rholow/Holow cells were subjected to isoleucine-deprivation for 36 hours as described in the Materials and Methods. The cells were then released from isoleucine-block and at indicated intervals duplicate aliquots of cells were labeled with 3H-thymidine and processed for autoradiography to assess the percentage of cells with labeled nuclei as described in the Materials and Methods.
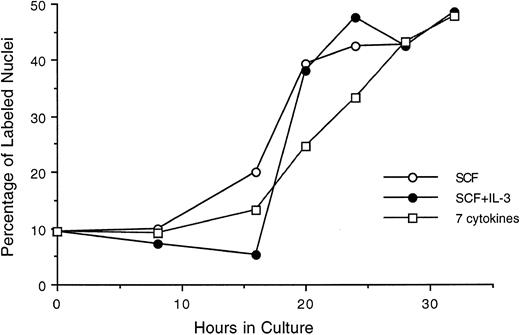
We also evaluated an alternate population of purified hematopoietic stem cells, Lin−/Sca+ cells, stimulated by SCF, SCF plus IL-3, or a 7-cytokine cocktail (Fig 6). Here, approximately 10% of the purified cells were labeled with 3H-thymidine at time 0 in culture, indicating a less quiescent population than that represented by Lin− Rholow/Holow. The general profile and the temporal order of their progression through G0 /G1 and into S phase is found to be essentially similar to that seen with Lin− Rholow/Holow cells. Furthermore, the lag period of 16 to 18 hours and the fraction of cells entering into S phase at 28 to 30 hours observed in the presence of SCF alone remained unchanged when SCF was combined with IL-3 (2-cytokine combination) or with IL-3, GM-CSF, IL-1α, bFGF, CSF-1, and G-CSF (7-cytokine combination). Thus, SCF alone in the presence of 5% HI-FCS is as effective as multiple cytokine combinations for inducing the progression of Lin−/Sca+ cells from a quiescent state into proliferative cycle and into S phase. However, supplementing SCF containing media with HI-FCS was found to be essential for cell viability, because, in its absence, a progressive cell death was observed during the first 24-hour incubation period (data not shown). Similarly, HI-FCS alone in the medium, without SCF, was not sufficient for the proliferative stimulation of the cells (data not shown). Thus, these cells require a minimum of serum factors and SCF for their entry into and subsequent progression through the cell cycle.
Progression of cytokine-stimulated Lin−/Sca+ cells through G1 and into S phase of cell cycle. Lin−/Sca+ cells were incubated in medium containing SCF alone, SCF plus IL-3, or a cocktail of 7 cytokines (IL-3, IL-1α, GM-CSF, G-CSF, SCF, FGF, and CSF-1), and at indicated intervals duplicate aliquots of cells from each group were analyzed for 3H-thymidine incorporation followed by autoradiography to assess the percentage of cells with labeled nuclei as described in the Materials and Methods.
Progression of cytokine-stimulated Lin−/Sca+ cells through G1 and into S phase of cell cycle. Lin−/Sca+ cells were incubated in medium containing SCF alone, SCF plus IL-3, or a cocktail of 7 cytokines (IL-3, IL-1α, GM-CSF, G-CSF, SCF, FGF, and CSF-1), and at indicated intervals duplicate aliquots of cells from each group were analyzed for 3H-thymidine incorporation followed by autoradiography to assess the percentage of cells with labeled nuclei as described in the Materials and Methods.
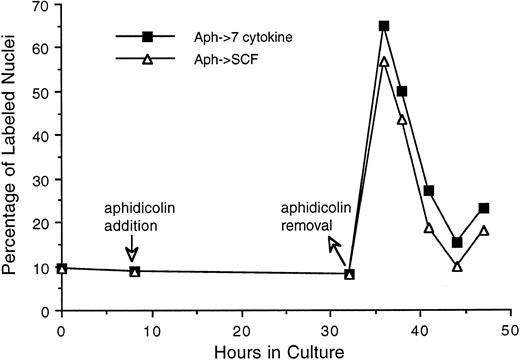
As can be seen in Fig 6, 50% to 60% of Lin−/Sca+ cells entered synchronously into S phase by 30 hours after cytokine stimulation. To achieve more synchronous entry of cells into first S phase and to establish procedures that allow the maintenance of stem cell synchrony as they complete first S phase and enter into subsequent cycle, we examined the ability of aphidicolin, an inhibitor of DNA polymerase α,18 to reversibly block cytokine stimulated Lin−/Sca+ cells at G1 /S boundary. As shown in Fig 7, Lin−/Sca+ cells exposed to a combination of 7 cytokines were limited from entering into S phase after aphidicolin treatment from 8 to 32 hours after cytokine stimulation. This is in contrast to more than 50% of the cells being in S phase in the absence of aphidicolin during the same incubation period (Fig 6). When the cells were released from aphidicolin block, they entered into S phase without any detectable lag period, indicating their arrest at G1 /S boundary or in early-S phase in response to aphidicolin. These cells retained a tight synchrony as they completed S phase and traversed through the subsequent phases of cell cycle (Fig 7). Furthermore, as in cytokine-stimulated cells, SCF alone was as effective as the 7-cytokine cocktail in allowing the progression of aphidicolin-synchronized Lin−/Sca+ cells through S phase.
Progression of Lin−/Sca+ cells synchronized at G1 /S boundary by aphidicolin treatment through S phase. Freshly isolated Lin−/Sca+ cells were incubated in medium containing 7 cytokines. From 8 to 32 hours thereafter, cells were treated with aphidicolin as indicated by the arrows. Cells were then washed once with cytokine-free medium and incubated with medium containing either 7 cytokines or SCF alone. At indicated intervals, duplicate aliquots of cells from each group were analyzed for 3H-thymidine incorporation followed by autoradiography to assess the percentage of cells with labeled nuclei as described in the Materials and Methods.
Progression of Lin−/Sca+ cells synchronized at G1 /S boundary by aphidicolin treatment through S phase. Freshly isolated Lin−/Sca+ cells were incubated in medium containing 7 cytokines. From 8 to 32 hours thereafter, cells were treated with aphidicolin as indicated by the arrows. Cells were then washed once with cytokine-free medium and incubated with medium containing either 7 cytokines or SCF alone. At indicated intervals, duplicate aliquots of cells from each group were analyzed for 3H-thymidine incorporation followed by autoradiography to assess the percentage of cells with labeled nuclei as described in the Materials and Methods.
DISCUSSION
The present data indicated that highly purified Lin− Rholow/Holow murine lymphohematopoietic stem cells are quiescent at isolation, but with exposure to a cytokine combination of IL-3, IL-6, IL-11, and SCF, enters and progresses through cell cycle with an initial cycle length of 36 to 40 hours. Subsequent cycles appear to be about 12 hours in length. These data were based on cell numbers and 3H-thymidine labeling and their functional significance was confirmed by induction of quiescent 7-factor HPP-CFC into S phase. Given the length of S phase, these data indicate that essentially all cells traversed S phase. In other studies using whole unseparated marrow exposed to the same cytokine combinations, we have shown that, at 48 hours of culture, the cells show very poor long-term engraftment in either irradiated or normal hosts, but more recent studies indicate that this engraftment defect is reversible over time, although it is virtually impossible to rule out some degree of differentiation within the stem progenitor classes in these experiments. The data cited above indicate that the population retains stem cell-like properties through multiple cell cycle transits. A detailed analysis of the first cell cycle transit indicates a G1 phase of about 18 hours and a total cycle length of 36 to 40 hours. Given the relative fixed minimum of 7 to 8 hours for S, 2 hours for G2 , and 1 hour for M, the G1 in the subsequent cycle must be very short, in the range of 1 to 2 hours. The isoleucine deprivation studies (see below) add further evidence for a very short G1 in cytokine-stimulated Lin− Rholow/Holow cell cycles after the first cell cycle transit.
Dogma suggests that Lin− Rholow/Holow cells are dormant and not easily stimulated to divide, but our data suggest that all of these cells are easily induced to enter cell cycle. Studies by Hendrikx et al19 on transplantation of highly purified murine marrow stem cells and using PKMH-26 fluorescence labeling indicate that the majority of engrafted cells have divided over a 24-hour period after intravenous infusion. These studies suggest cycle times for primitive stem cells stimulated from dormancy of 24 to 48 hours. This is to be contrasted by recent studies by Bradford et al,1 using prolonged continuous labeling with BrdU, indicating that the average turnover time of Lin− Rholow/Holow cells in vivo was 4.3 weeks with a time to 50% cycle of 2.75 weeks. These investigators also reported that, over a 12-week labeling period, 89% of Lin− Rholow/Holow cells incorporated BrdU. This is, of course, a steady-state situation, but these data do not distinguish between a prolonged cell cycle for most cells or an intermittent entrance of dormant cells into cell cycle with a shorter cycle length.
Studies of 7-factor–responsive HPP-CFC indicate that this surrogate stem cell was dormant in freshly isolated Lin− Rholow/Holow cells but was clearly transiting cell cycle by 28 to 30 hours of culture in IL-3, IL-6, IL-11, and SCF. The percentage of HPP-CFC in cycle (Table 1) closely approximated the percentage of Lin− Rholow/Holow cells that labeled with 3H-TdR (Fig 3).
As shown in Fig 3, cytokine-stimulated cells entered synchronously into S phase, with 55% to 60% of them in S phase at 30 hours. During 90 to 100 hours of incubation with cytokines after the first cycle, about 60% of cells continued to be in S phase as determined by autoradiography analysis of the cells pulse-labeled with 3H-thymidine at 12- to 14-hour intervals (Fig 4, data not shown). This indicates either that a partial synchrony of the cells persisted as they traversed through the subsequent cycles with maximum number of cells in S phase at every 12 to 14 hours examined or that the cells after first cycle grew exponentially, with 60% of the cells in S phase through the remainder of the proliferative period. The latter possibility implies that the S phase of exponentially growing cells constitutes greater than 60% of total cell cycle time during each division after the first cycle. The doubling time of 12 hours with a S period of 7 to 8 hours after the first division observed in the present studies (Fig 1) is consistent with such a possibility.
Alternate populations representing Lin−/Sca+ stem cells stimulated with SCF alone, SCF plus IL-3, or a cocktail of 7 cytokines also exhibited cell cycle kinetics similar to those observed with Lin− Rholow/Holow cells. However, unlike Lin− Rholow/Holow cells, Lin−/Sca+ cells were heterogeneous, with 10% to 15% of them in S phase immediately after their sorting (Fig 6). Their entry into, and progression through, the first cycle essentially remained the same whether they were stimulated with SCF alone or with 7 cytokines. A partial synchrony of these cells was maintained as they traversed through G1 into S phase (Fig 6).
To enrich PHSCs in first S and to acquire the synchrony that can be sustained as the cells transit through the first G2 and M phases, we tested the ability of aphidicolin to reversibly block Lin−/Sca+ cells at their first G1 /S boundary. Selective antimetabolites, including aphidicolin (inhibitor of DNA polymerase α), hydroxyurea (inhibitor of ribonucleotide reductase), and methotrexate (inhibitor of dihydrofolate reductase), that inhibit DNA replication and DNA precursor synthesis have been used to synchronize a variety of mammalian cells at G1 /S boundary.20,21 Our observations show that Lin−/Sca+ cells, like other cell systems, respond to a secondary synchronization at G1 /S boundary after aphidicolin treatment (Fig 7). Considering its selective inhibitory effect on DNA polymerase α17 and its ineffectiveness in altering G1 -associated erythroid differentiation of MELC cells in vitro,22 aphidicolin may not show secondary effects that are independent of cell cycle status that it induces.
We tested both the serum-deprivation and the isoleucine-deprivation methods that are commonly used to achieve G0 /G1 phase arrest of a variety of cells, including hematopoietic progenitor cells,23-25 for their ability to block PHSCs completing first S phase from entering into second proliferative cycle. Whereas serum (HI-FCS)-deprived PHSCs experienced a progressive death with time (data not shown), isoleucine-deprivation reversibly blocked them in a nonproliferative state after first division (see Figs 4 and 5). As compared with PHSCs in their first cycle, those released from isoleucine-block entered into S phase with a shorter lag (G1 ) period.
Thus, these studies describe essential cytokine and serum conditions required for the synchronous progression of purified PHSCs from their initial G0 /G1 phase into S phase and identify for the first time two specific approaches to synchronize PHSCs at G1 /S boundary of their first cycle (aphidicolin-induced synchrony) and at G0 /G1 phase of their second cycle (isoleucine-deprivation–induced synchronization of the cells completing first S phase). They also show a relatively long cycle time from dormancy of primitive Lin−Rholow Holow hematopoietic stem cells with a subsequent rapid cycle transit and short G1 . Given the apparent fluctuation of engraftment with cell cycle status, these results have important implications for clinical marrow transplantation and gene therapy strategies.
ACKNOWLEDGMENT
The authors are thankful to Christina Grimaldi, Judy Reilly, and Dr Ruud Hulspas for their help in sorting bone marrow cells.
Supported by National Institutes of Health Grants No. PO1HL-56920 and PO1DK-50222-01.
Address reprint requests to G. Prem Veer Reddy, PhD, Cancer Center, University of Massachusetts Medical Center, 373 Plantation St, Suite 202, Worcester, MA 01605.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal