Abstract
CD34 is a cell surface glycoprotein that is selectively expressed within the human hematopoietic system on stem and progenitor cells, and in early blood vessels. To elucidate its functions during early blood vessel formation and hematopoiesis, we analyzed the expression patterns, in day 8 to day 10 mouse embryos, of CD34 RNA by in situ hybridization and protein by immunohistochemistry using the monclonal antibody RAM 34. Levels of expression in embryonic blood vessels were correlated with the mode of vessel formation, being high in pre-endothelial cells and in vessels forming by vasculogenesis (particularly the dorsal aortae) or angiogenesis, but low in vessels forming by coalescence (the cardinal veins). CD34+ erythroid cells, presumably of yolk sac origin, were present in the liver of day 10 embryos; at the same stage, putative definitive hematopoietic cells, strongly CD34+, were present in the para-aortic mesenchyme. Possible sites of hemangioblastic differentiation were detected in the form of CD34+ endothelium-attached hematopoietic cells in the dorsal aorta and in two previously unreported locations, the proximal umbilical and vitelline arteries. These observations suggest functions for CD34 in relation to specific modes of blood vessel formation, and a hemangioblastic role in both embryonic and extraembryonic sites.
CD34 IS A 105- to 120-kD transmembrane cell surface glycoprotein which is selectively expressed within the human1-3 and murine4-6 hematopoietic systems on stem and progenitor cells; expression of CD34 is lost as cells mature. It is also expressed in vascular endothelial cells7 and in certain classes of fibroblasts.4 8
Despite the widespread clinical use of CD34 antibodies for the enumeration and purification of hematopoietic stem cells, the function of CD34 in these cells has only recently been addressed. The mucin-like structure of CD34 suggests a role in adhesion,9 and there is good evidence for this in both homotypic10,11 and heterotypic (hematopoietic cells to bone marrow stromal layers)12 adhesion during hematopoiesis. There is also evidence for an adhesion-related role for CD34 between endothelial cells.13 It may also play roles in hematopoietic cell proliferation14 and in differentiation control.15
Two laboratories have recently created targetted mutations at the CD34 locus in mice.16 17 Both studies show only minor abnormalities of hematopoiesis, and although cardiovascular development is not described, it is assumed to be normal since the mutation is viable. These observations suggest that CD34 is one of a number of cooperatively acting factors in hematopoiesis and vascular development, whose full roles are not revealed by its absence.
During development of the murine vascular and hematopoietic systems, CD34 has been detected by means of a polyclonal antibody, on newly forming blood vessels including the growth cone-like processes of capillary sprouts, and on a subset of hematopoietic progenitor cells18; it was proposed as a useful marker for analysing early blood system development. It has been detected in fetal liver between days 11.5 and 14 of development, corresponding to the period during which this organ is active in hematopoiesis.8 19
In this study of early mouse embryos, we analyze the localization of CD34 mRNA by in situ hybridization, and CD34 protein by means of a monoclonal antibody, during the period in which the basic vascular pattern is established and intraembryonic hematopoiesis begins (day 8 to day 10). The results confirm that CD34 is only present on a subset of hematopoietic cells, and additionally show that it is not expressed on all blood vessels during their formation. Our analysis of the sequence of appearance of RNA transcripts and protein in different blood vessels reveals correlations between the timing and level of CD34 expression and the mode of blood vessel formation. We also identify previously unreported sites of hematopoietic activity in the proximal extraembryonic arteries and show CD34+ cells in the liver at earlier stages than previously reported.
MATERIALS AND METHODS
Alkaline phoshatase-conjugated avidin staining using RAM34 antibody.The anti-CD34 monoclonal antibody (MoAb) (RAM34) was kindly provided by Pharmingen (San Diego, CA) for testing and validation before release and is now commercially available. We initially verified the specifity of this antibody by staining cell lines characterized as CD34-positive or -negative on the basis of Northern blot analysis. RAM34 reactivity was only observed in cells that expressed CD34 messenger RNA. We further showed that the reactivity of the RAM34 MoAb is indeed directed against the mouse CD34 antigen by immunostaining of COS cells transfected with a murine CD34 expression vector. Air-dried cytospins of COS cells20 untransfected or transfected with mouse CD34 cDNA4 and air-dried multichambered tissue culture slides (Nunc, Naperville, IL) of CMT64/61 fibroblasts21 and Swiss 3T6 fibroblasts22 were fixed in acetone for 7.5 minutes at room temperature. After fixation the slides were air-dried before rehydration in Tris-buffered saline (TBS) for 15 minutes. The wet slides were then placed in a humidified staining box and incubated for 5 minutes with 10% heat-inactivated goat serum (Sigma, Poole, Dorset, UK). After this the serum was tapped off the slide and the excess wiped away. The cells were then stained with 1 μg of rat antimouse CD34 antibody (RAM34, isotype IgG2a). An isotype matched negative control was included. Cells were incubated at room temperature for 45 minutes before washing for 15 minutes with TBS. Next, 1 μg of biotinylated goat antirat Igs (Southern Biotechnology, Birmingham, AL) was added to each slide and the slides incubated at room temperature for 45 minutes. After washing in TBS for 15 minutes, the cells were incubated with streptABComplex/AP (Dakopatts, Denmark, Sweden) for 45 minutes at room temperature. Slides were then washed in TBS for 15 minutes before the addition of an alkaline phosphate substrate solution (described on the Dakopatts data sheet), containing Fast-red TR salt (Sigma) and incubated at room temperature for 20 minutes. Slides were washed in TBS and counter-stained with Mayer's Hematoxylin (Sigma) for 5 minutes followed by washing in distilled water. They were then air-dried, mounted in Dako-Glycergel (Dakopatts) and examined for staining by light microscopy.
Flow cytometric analysis using RAM34 antibody.The following cell lines were analyzed: 416B,23 M1(ATCC TIB 192), PA6,24 WEHI-3B,25 EL4,26 18.8,27 MS5,28 CMT64/61, Swiss 3T6. Cells from each cell line were washed twice in phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin (BSA) and resuspended at 1 × 107 cells/mL. Aliquots of 50 μL of cell suspension were placed in polypropylene tubes and 1 μL of RAM34 (Pharmingen) was added to each tube and the tubes placed on ice for 30 minutes. An isotype specific control (rat IgG2a) was included. Cells were washed twice in 2 mL of wash buffer (PBS/0.1% sodium azide [Sigma]/0.1% BSA [Sigma]) and resuspended in 50 μL wash buffer. Cells were then incubated with 1 μg goat antirat Igs directly conjugated to fluorescein isothiocyanate (Southern Biotechnology) for 30 minutes. After washing in wash buffer, cells were resuspended in PBS and flow cytometric analyses were performed using a FACScan (Becton Dickinson, Oxford, UK).
Pretreatment of cells with glycosidases.A 416B cell line transfected with human CD34 cDNA12 expressing all three epitope subclasses of human CD34 (as defined by differences in glycosylation pattern) as well as endogenous mouse CD34 protein, was used to assess the glycoprotein specificity of the RAM34 antibody. Cells were treated separately with the enzymes O-sialoglycoprotease (Pasteurella haemolytica; Cedarlane laboratories Ltd, Ontario, Canada) and neuraminidase (Vibrio cholerae; Sigma), which have been used to classify the epitope specificity of human anti-CD34 antibodies29 using the human cell line KG1.30 For O-sialoglycoprotease treatment, 5 × 106 cells were treated with 10 μL of the enzyme (2.5 mg/mL) in RPMI (GIBCO-BRL Life Technologies, Paisley, Scotland) containing 5% fetal calf serum (FCS; PAA, Linz, Austria) for 30 minutes at 37°C. In the case of neuraminidase, 1 × 106 cell were treated with 10 μL (l0 mU/μL) of enzyme in PBS/0.1% BSA for 30 minutes at 37°C. Cells were then washed twice in RPMI/FCS and stained with RAM34 for flow cytometry. To confirm that the enzymes had worked both KG1 and the human CD34 expressing 416B cells were checked for the expression of the 3 human epitope subclasses using specific antibodies (Immu-133 [class I]; Immunotech, Marseilles, France; QBend 10 [class II]; Quantum Biosciences, Cambridge, UK; HPCA-2 [class III]; Beckton Dickinson).
Dissection of embryos from the uterus and preparation for in situ hybridization or immunohistochemistry.Embryos were derived from mice of the C57/Bl/6 strain; the morning of the day on which a vaginal plug was detected was designated day 0 of pregnancy. On day 8.0 to day 10.0 of pegnancy the pregnant uterus was placed in Tyrode saline, and the decidual swellings removed intact. The trophoblast, including the ectoplacental cone, was discarded; the yolk sac and amnion were opened to expose the embryo, but retained attached throughout the procedure. For whole mount in situ hybridization, embryos were fixed in 4% paraformaldehyde in PBS at 4°C for 4 to 5 hours (or overnight for embryos at day 9 or older), then washed twice in PBS containing 0.1% Tween-20 (PBT) and dehydrated through 25%, 50%, and 75% methanol in PBT to 100% methanol. Embryos were stored in methanol at −20°C. For immunohistochemistry, embryos were fixed in St Maries fixative (1 part glacial acetic acid to 99 parts 95% ethanol) overnight at −20°C. They were then either stored in 70% ethanol (room temperature) or dehydrated in 100% ethanol (3 × 1 hour washes), cleared in xylene and embedded in paraffin wax. Transverse sections (with respect to the head) were cut at 10 μm and mounted onto gelatinized slides. The sections were dried overnight at 37°C before use.
Whole mount in situ hybridization.A murine CD34 cDNA clone containing the entire coding region was inserted into pBS (SK−) (Stratagene, La Jolla, CA) and the plasmid linearized to produce either antisense or sense templates. Digoxigenin-labeled riboprobes were prepared by transcription using T3 and T7 RNA polymerases, respectively. Whole mount in situ hybridization was performed according to the method of Wilkinson.31 For embryos younger than day 9 postcoitum, the bleaching and proteinase K steps were omitted from the pretreatment protocol, with embryos simply being rehydrated through graded methanols to PBT then proceeding straight to the prehybridization step. When the color reaction was complete, embryos were dehydrated through graded methanols to 100% methanol (10 minute wash) to intensify the stain. They were then rehydrated and cleared through 50% and 80% glycerol in PBT. The embryos were flat-mounted in 80% glycerol under coverslips and photographed using bright-field illumination, through a blue filter.
Immunoperoxidase staining using RAM34 MoAb.The slides were dewaxed in xylene (3 to 5 minutes) then rehydrated through 2 × 100%, 95%, and 70% ethanol to distilled water. Endogenous peroxidase activity was blocked by incubating in 0.3% hydrogen peroxide in methanol for 30 minutes. The slides were washed in PBS for 20 minutes. The sections were then incubated in diluted normal rabbit serum (1 in 5 in PBS) to block nonspecific binding sites for secondary antibody. Excess serum was wiped from the slides and the primary antibody, diluted 1 in 50 in PBS, was applied. The slides were incubated overnight at room temperature in a humidified box. The following day, the slides were washed in PBS (10 minutes) then incubated for 30 minutes with biotinylated rabbit anti-rat antibody diluted 1 in 50 in PBS. The slides were washed in PBS (10 minutes) then incubated in preformed peroxidase-conjugated avidin-biotin complex (from a Vectastain kit; Vector Laboratories, Burlingame, CA) for 30 minutes. The slides were washed in PBS (10 minutes) then incubated in diaminobenzidine containing hydrogen peroxide (from a Sigma kit) until the color had developed to the desired extent (around 30 minutes). The slides were washed in tap water (5 minutes), counterstained in 0.1% Alcian Blue pH 2.5 (BDH, Poole UK), washed in tap water (5 minutes), dehydrated through graded methanols to xylene, and mounted in synthetic mounting medium.
RESULTS
Reactivity of the RAM34 antimurine CD34 antibody.Although polyclonal antimouse CD34 antisera have been raised within the last few years,5,6 a candidate monoclonal reagent, RAM34, has only recently become available; it has been used to enrich hematopoietic cells.32-34 We first confirmed the CD34 specificity of RAM34 by immunostaining of CD34-transfected and untransfected cells, and by flow cytometric analysis (data not shown; see Materials and Methods for details). Human CD34 is highly glycosylated with O- and N-linked sugars, including malic acid9; therefore, we examined to what extent epitope recognition by RAM34 was dependent on posttranslational modification of the CD34 molecule. We analyzed the neuraminidase and glycoprotease sensitivity of epitope recognition, using the murine CD34-expressing hematopoietic cell line 416B (Table 1). To control for the success of the enzyme treatments we transfected 416B with a human CD34 expression vector. The resultant cells (416B-hu-CD34) express human and mouse CD34 at similar levels and the human molecule reacts with class I, II, and III anti-hu-CD34 MoAbs. Treatment of these cells with neuraminidase or glycoprotease results in loss of reactivity to class I and class II anti-hu-CD34 antibodies, respectively. Reactivity to RAM34 was unaffected by these treatments. We conclude that RAM34 is of the class III type and consequently is expected to react with murine CD34 molecules regardless of their glycosylation state. Therefore, RAM34 is an ideal reagent for analyzing the distribution of murine CD34 antigen expression during murine development.
Class Analysis of Murine CD34 Using Human CD34 Epitope Subclass Criteria29 Indicates That RAM34 Recognizes a Class III Epitope
| . | Class I . | . | Class II . | . | Class III . | |||
|---|---|---|---|---|---|---|---|---|
| . | Neuraminidase Sensitive . | . | . | Glycoprotease Sensitive/Neuraminidase Resistant . | . | . | Glycoprotease Resistant/Neuraminidase Resistant . | . |
| Cell Line | Human CD34 (IMMU 133) | Mouse CD34 (RAM34) | Human CD34 (QBEND 10) | Mouse CD34 (RAM34) | Human CD34 (HPCA2) | Mouse CD34 (RAM34) | ||
| KG1A (human progenitor cell line) | + | N/A | + | N/A | + | N/A | ||
| 416B (mouse progenitor cell line) | N/A | − | N/A | − | N/A | + | ||
| 416B/HU CD34 (mouse line transfected with human CD34) | + | − | + | − | + | + | ||
| . | Class I . | . | Class II . | . | Class III . | |||
|---|---|---|---|---|---|---|---|---|
| . | Neuraminidase Sensitive . | . | . | Glycoprotease Sensitive/Neuraminidase Resistant . | . | . | Glycoprotease Resistant/Neuraminidase Resistant . | . |
| Cell Line | Human CD34 (IMMU 133) | Mouse CD34 (RAM34) | Human CD34 (QBEND 10) | Mouse CD34 (RAM34) | Human CD34 (HPCA2) | Mouse CD34 (RAM34) | ||
| KG1A (human progenitor cell line) | + | N/A | + | N/A | + | N/A | ||
| 416B (mouse progenitor cell line) | N/A | − | N/A | − | N/A | + | ||
| 416B/HU CD34 (mouse line transfected with human CD34) | + | − | + | − | + | + | ||
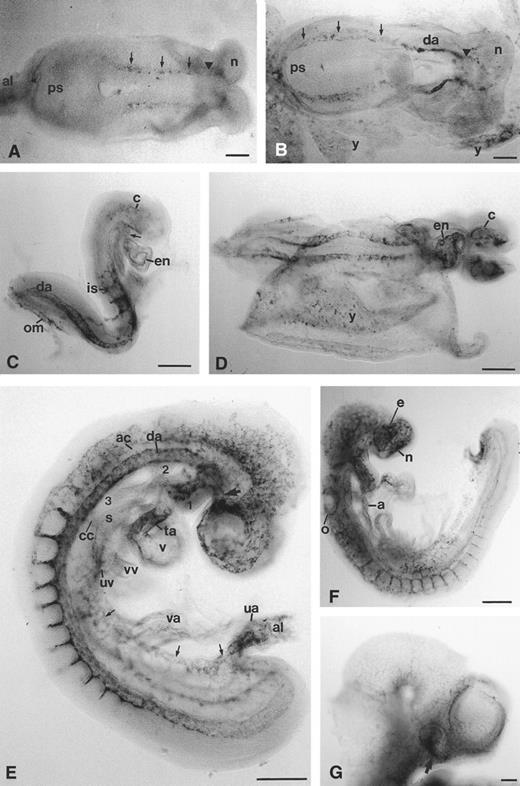
Expression of the CD34 gene in the developing embryo.CD34 transcripts were first detectable in the embryo early on day 8 of development, just before the onset of somitogenesis. At this stage, transcripts were detected in the pre-endothelial cells of the left and right dorsal aortae, which are continuous with the left and right endocardial tubes of the heart at their cranial ends (Fig 1A). By the 3-somite stage, CD34 expression in the developing dorsal aortae extended along the entire length of the embryo, meeting at the base of the allantois, and were now recognizable as definitive tubular structures in the cranial (anterior) half of the embryo (Fig 1B). From the 4-5–somite stage, CD34 mRNA was detectable in the endocardial layer of the now fused heart tubes (Fig 1C and D); staining was also prominent in the truncus arteriosus (Fig 1C). Transcripts were detected in cranial pre-endothelial cells that later coalesced to form a network of vessels, and in intersomitic vessels that branched from the dorsal aortae; these vessels were formed in a cranio-caudal sequence, lagging behind somite formation by a distance of around 2-3–somite boundaries.
Expression of CD34 mRNA during early cardiovascular development in the mouse embryo. Cranial/anterior is to the right in (A) and (B) (n, cranial neural fold). (A) 1-2–somite headfold stage: transcripts are detectable in two diffuse lines (arrowed), corresponding to the two presumptive dorsal aortae, continuous cranially with the presumptive paired endocardial heart tubes (arrowhead). (B) 3-somite stage: the paired dorsal aortae (da) are now clearly defined in the anterior half of the embryo, and are continuous with diffuse lines of stained cells, presumably pre-endothelial cells (arrowed), which join at the junction of the primitive streak (ps) and allantois; there is diffuse staining in the allantois and yolk sac (y). (C) 7-somite stage: caudally, the two dorsal aortae (da) are continuous with the omphalomesenteric artery (om) which is also stained; intersomitic arteries (is) have begun to branch from each dorsal aorta; endocardial (en) staining is clear in the ventricle and truncus arteriosus, which is continuous with the first aortic arch (arrow); there are also scattered stained cells within the cranial mesenchyme (c). (D) 9-somite stage: CD34 transcripts are now more abundant in the cranial mesenchyme (c) and in the endothelial lining (en) of the ventricle and truncus arteriosus of the heart; there are clear patches of stained cells in the yolk sac (y). (E) 14-somite stage: CD34 transcripts are abundant in the umbilical artery (ua), allantoic mesenchyme (al), umbilical vein (arrows) and proximal segment of the vitelline arteries (va); in the head, cranial mesenchymal cells expressing CD34 are beginning to condense around the developing eye close to the internal carotid artery (arrow); the endothelium of the first aortic arch artery (1) is stained, but transcript levels are low in the second aortic arch (2) and undetectable in the third aortic arch (3) or in the aortic sac that joins the second and third aortic arches; transcript levels are lower in the anterior cardinal vein (ac) and common cardinal vein (cc) than in the dorsal aorta (da) and umbilical vein (uv), and undetectable in the vitelline vein (vv); they are low in the ventricle (v), atrium (not visible) and sinus venosus (s), but high in the truncus arteriosus (ta). (F ) Day 9, 18-somite stage: transcripts are now present in the aortic sac (a) and all aortic arches, but in general are more abundant in capillary networks around the developing sense organs than in the larger vessels. (G) Day 10 embryo head: the hyaloid artery (arrow) is stained, but in general transcript levels are decreased in the head, being confined to capillary networks around the eye and brain and within the facial mesenchyme. e, eye; n, nasal pit; o, otic pit. Scale bars = 100 μm
Expression of CD34 mRNA during early cardiovascular development in the mouse embryo. Cranial/anterior is to the right in (A) and (B) (n, cranial neural fold). (A) 1-2–somite headfold stage: transcripts are detectable in two diffuse lines (arrowed), corresponding to the two presumptive dorsal aortae, continuous cranially with the presumptive paired endocardial heart tubes (arrowhead). (B) 3-somite stage: the paired dorsal aortae (da) are now clearly defined in the anterior half of the embryo, and are continuous with diffuse lines of stained cells, presumably pre-endothelial cells (arrowed), which join at the junction of the primitive streak (ps) and allantois; there is diffuse staining in the allantois and yolk sac (y). (C) 7-somite stage: caudally, the two dorsal aortae (da) are continuous with the omphalomesenteric artery (om) which is also stained; intersomitic arteries (is) have begun to branch from each dorsal aorta; endocardial (en) staining is clear in the ventricle and truncus arteriosus, which is continuous with the first aortic arch (arrow); there are also scattered stained cells within the cranial mesenchyme (c). (D) 9-somite stage: CD34 transcripts are now more abundant in the cranial mesenchyme (c) and in the endothelial lining (en) of the ventricle and truncus arteriosus of the heart; there are clear patches of stained cells in the yolk sac (y). (E) 14-somite stage: CD34 transcripts are abundant in the umbilical artery (ua), allantoic mesenchyme (al), umbilical vein (arrows) and proximal segment of the vitelline arteries (va); in the head, cranial mesenchymal cells expressing CD34 are beginning to condense around the developing eye close to the internal carotid artery (arrow); the endothelium of the first aortic arch artery (1) is stained, but transcript levels are low in the second aortic arch (2) and undetectable in the third aortic arch (3) or in the aortic sac that joins the second and third aortic arches; transcript levels are lower in the anterior cardinal vein (ac) and common cardinal vein (cc) than in the dorsal aorta (da) and umbilical vein (uv), and undetectable in the vitelline vein (vv); they are low in the ventricle (v), atrium (not visible) and sinus venosus (s), but high in the truncus arteriosus (ta). (F ) Day 9, 18-somite stage: transcripts are now present in the aortic sac (a) and all aortic arches, but in general are more abundant in capillary networks around the developing sense organs than in the larger vessels. (G) Day 10 embryo head: the hyaloid artery (arrow) is stained, but in general transcript levels are decreased in the head, being confined to capillary networks around the eye and brain and within the facial mesenchyme. e, eye; n, nasal pit; o, otic pit. Scale bars = 100 μm
Early on day 9 of development (12-somite stage), the CD34 gene was expressed in the umbilical and vitelline arteries and veins (Fig 1E). The cranial mesenchyme contained many stained pre-endothelial cells forming a capillary network, especially around the developing brain and eye. In contrast, the aortic sac and all aortic arches other than the first, formed as definitive vessels before CD34 RNA transcripts were observed in their walls (compare Fig 1E and F ); the same was true of the sinus venosus. The anterior cardinal vein showed a much lower level of transcripts than the dorsal aorta (Fig 1E). By the 18-somite stage, vessels showing CD34 expression were forming around the otic and nasal pits (Fig 1F ). At day 10 of development (30-somite stage), staining was observed in the hyaloid artery and in the capillary meshwork around the brain and eye, but was not detectable in larger vessels in the cranial region (Fig 1G). Strong CD34 expression was observed in caudal structures and in the dorsal aortae (see below).
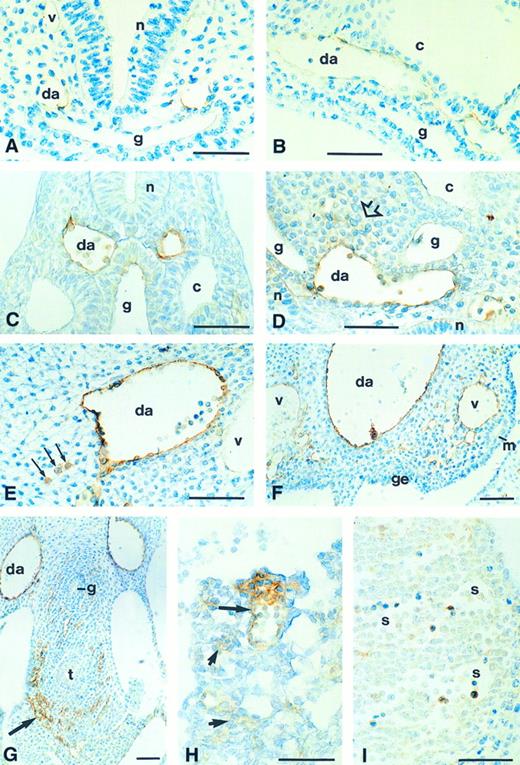
Localization of CD34 protein in the embryo.Expression of CD34 protein was first observed in the dorsal aortae at the 4-somite stage (Fig 2A). In 6-somite (Fig 2B) and 8-somite stage embryos, the intensity progressively increased, and extended into the first aortic arch and the outflow tract of the heart. In late day 8.5 and early day 9 embryos (10-12–somite stage), the CD34 protein pattern was consistent with that of mRNA expression, except that the protein was not detected in pre-endothelial cells. The greatest staining intensity was in the dorsal aortae, with strong staining also seen in the intersomitic and vertebral arteries (Fig 2C and D and data not shown). The developing cardinal veins are at this stage represented by a number of small vessels which later coalesce; none of these was stained (Fig 2C and data not shown). Staining was also present in mesenchyme adjacent to the dorsal aorta (Fig 2D); it is not clear whether this was related to hematopoiesis or vasculogenesis.
Immunohistochemical detection of CD34 glycoprotein in the developing embryo. (A) Transverse section of the cranial region of an 8-somite stage embryo, showing staining in the two dorsal aortae (da); staining is barely detectable in the primary head vein (v). (B) Dorsal aorta in the trunk region of an 8-somite embryo in longitudinal section: the endothelium expresses CD34. (C) Transverse section of a 10-somite stage embryo, showing CD34 expression in the endothelium of the dorsal aorta and in blood cells in the lumen; staining is not present in the posterior cardinal vein (v); slight staining of dorsal gut (g) epithelium. (D) Longitudinal section through the trunk of a 12-somite stage embryo: staining of the endothelium and blood cells of the dorsal aorta (da), in adjacent mesenchymal cells (open arrow), and in the neuroepithelium (n). (E) Transverse section (dorsal/ventral is left/right, medial/lateral is top/bottom) of one of the dorsal aortae (da) of a day 10 embryo, showing CD34 expression on the endothelial surface and in discrete rounded cells in the adjacent mesenchyme (arrows); these are probably hematopoietic cells. The posterior cardinal vein (v) is unstained. (F ) Fused dorsal aorta (da) of a day 10 embryo, showing staining on the endothelial surface and also on large (probably hematopoietic) cells attached to the vessel walls. There is also staining in many small vessels within the nephrogenic mesenchyme, and of the posterior cardinal veins (v), which appear to be generating the capillary sprouts. (G) Day 10 embryo, showing CD34+ precapillary sprouts (arrow) surrounding the tracheal diverticulum (t) and, to a lesser extent, the oesophagus (g). (H) Allantois of a 10-somite embryo, showing CD34 expression in the walls and blood cells of a formed vessel (large arrow) and in prevascular mesenchyme (small arrows). (I) Day 10 liver, showing staining in hematopoietic cells but not on the endothelium of the sinusoids (s). c, coelom; g, gut; ge, gonadal epithelium; m, mesonephric duct; n, neural epithelium. Scale bars = 50 μm
Immunohistochemical detection of CD34 glycoprotein in the developing embryo. (A) Transverse section of the cranial region of an 8-somite stage embryo, showing staining in the two dorsal aortae (da); staining is barely detectable in the primary head vein (v). (B) Dorsal aorta in the trunk region of an 8-somite embryo in longitudinal section: the endothelium expresses CD34. (C) Transverse section of a 10-somite stage embryo, showing CD34 expression in the endothelium of the dorsal aorta and in blood cells in the lumen; staining is not present in the posterior cardinal vein (v); slight staining of dorsal gut (g) epithelium. (D) Longitudinal section through the trunk of a 12-somite stage embryo: staining of the endothelium and blood cells of the dorsal aorta (da), in adjacent mesenchymal cells (open arrow), and in the neuroepithelium (n). (E) Transverse section (dorsal/ventral is left/right, medial/lateral is top/bottom) of one of the dorsal aortae (da) of a day 10 embryo, showing CD34 expression on the endothelial surface and in discrete rounded cells in the adjacent mesenchyme (arrows); these are probably hematopoietic cells. The posterior cardinal vein (v) is unstained. (F ) Fused dorsal aorta (da) of a day 10 embryo, showing staining on the endothelial surface and also on large (probably hematopoietic) cells attached to the vessel walls. There is also staining in many small vessels within the nephrogenic mesenchyme, and of the posterior cardinal veins (v), which appear to be generating the capillary sprouts. (G) Day 10 embryo, showing CD34+ precapillary sprouts (arrow) surrounding the tracheal diverticulum (t) and, to a lesser extent, the oesophagus (g). (H) Allantois of a 10-somite embryo, showing CD34 expression in the walls and blood cells of a formed vessel (large arrow) and in prevascular mesenchyme (small arrows). (I) Day 10 liver, showing staining in hematopoietic cells but not on the endothelium of the sinusoids (s). c, coelom; g, gut; ge, gonadal epithelium; m, mesonephric duct; n, neural epithelium. Scale bars = 50 μm
CD34 staining suggestive of intraembryonic hematopoiesis was clearly seen in 16-18–somite stage embryos. Strongly stained cells, presumed to be hematopoietic progenitor cells, were seen in the dorsal mesentery (Fig 2E) and attached to the aortic endothelium at the level of the mesonephros (Fig 2F ). Since primordial germ cells (PGCs) are migrating at the mouse embryonic stages investigated here, the possibility that the CD34+ mesenchymal cells were PGCs was considered. However, no other CD34+ cells were observed in the PGC migration pathway from the allantois, along the gut mesoderm and into the genital ridges at any stage. There was no evidence of specialized hemopoietic tissue in the floor of the dorsal aortae, as observed in avian embryos.35 It was remarkable that the whole of the aortic wall showed a different morphology from that of other intraembryonic vessels, having many rounded, strongly immunopositive cells (eg, compare the dorsal aorta and posterior cardinal vein in Fig 2E). This cellular morphology was observed by the 10-somite stage (Fig 2C and D), and was not biased in distribution around the circumference of the vessel.
CD34 protein was detected on many small blood vessels in the embryonic mesenchyme, especially in the developing mesonephric mesenchyme (Fig 2F ), around the gut (Fig 2G) and in capillary sprouts invading the neuroepithelium of the brain and spinal cord (not shown); growth cone-like structures were observed at the leading edges of these capillary sprouts, as shown previously using a polyclonal antibody.18 An exceptionally dense area of stained precapillaries was observed around the developing trachea, just above the level of the lung buds (Fig 2G). Slight CD34 immunostaining of the gut epithelium and neural tube was present before the formation of these capillaries (Fig 2C and D). Staining of posterior cardinal veins was not observed until day 10, where it was detected in sites associated with capillary sprouting (Fig 2F ). Staining was present in the allantoic mesenchyme from the onset of blood vessel formation there, before chorioallantoic fusion (Fig 2H). It was associated with developing blood vessel walls, with no evidence of hematopoietic cell staining. Many CD34+ hematopoietic cells were observed within the developing liver of day 10 embryos (Fig 2I); these had a proerythroid morphology. CD34 has been detected in liver sinusoidal endothelial cells at day 11.519; in our day 10 embryos, these cells were unstained.
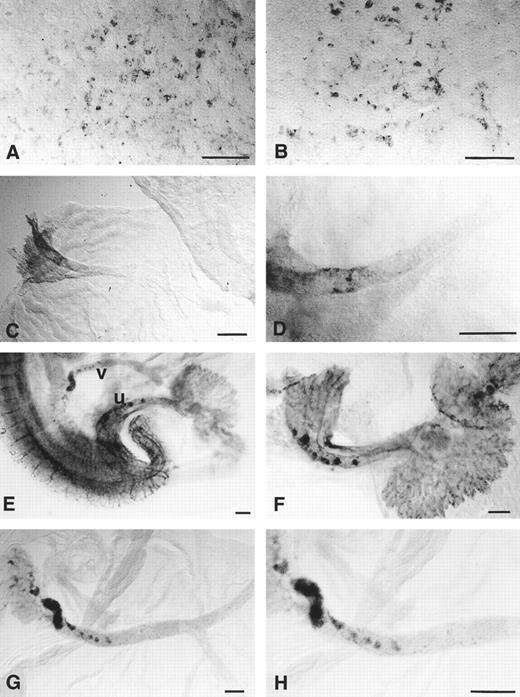
CD34 RNA transcripts in the yolk sac and extraembryonic blood vessels.CD34 transcripts could be detected in the yolk sac from the early headfold stage, around the time of onset of somitogenesis (Fig 3A). The gene was expressed in irregular patches that appeared to represent small numbers of hematopoietic cells and a meshwork of pre-endothelial cells; the meshwork pattern resembled that observed by endothelium-specific immunohistochemical staining of the presumptive endothelial cells in the area vasculosa of quail embryos.36 Examination of the cut edges of yolk sac membrane fragments (not shown) confirmed that the staining was exclusively mesenchymal. By day 9, when the yolk sac circulation was fully formed, CD34 was downregulated in the yolk sac vessels (Fig 3B) except in the vitelline artery close to the embryo, where patches of stained cells were observed (Fig 3C and D). By day 10 of development, foci of stained cells could be seen close to the embryo in the walls of the vitelline and umbilical arteries (Fig 3E and H). CD34 transcripts were also present throughout the capillary plexus of the chorioallantoic placenta (Fig 3F ).
Expression of CD34 mRNA in the developing mouse yolk sac and allantois. (A) 4-5–somite stage: many of the stained cells are elongated and surround unstained tissue, suggesting that they are endothelial cells lying around the periphery of the blood islands. (B) 12-somite stage: endothelial staining is weaker but the rounded haematopoietic cells retain CD34 expression. (C) Day 9, 20-somite stage: the only CD34 expression in the yolk sac is in the vitelline artery at the point that it emerges from the embryo. (D) Vitelline artery at the 20-somite stage at a higher magnification: the stained endothelial cells are discontinuous. (E) Day 10 embryo (around 30 somites), showing the points of emergence of the umbilical (u) and vitelline (v) vessels from the embryo. (F ) Day 10 umbilical vessels dissected away from the embryo, showing foci of CD34+ staining; the allantoic capillaries within the chorioallantoic placenta are strongly stained. (G and H) Day 10 vitelline artery dissected away from the embryo, showing foci of CD34+ cells at the proximal end (left) only. Scale bars = 100 μm
Expression of CD34 mRNA in the developing mouse yolk sac and allantois. (A) 4-5–somite stage: many of the stained cells are elongated and surround unstained tissue, suggesting that they are endothelial cells lying around the periphery of the blood islands. (B) 12-somite stage: endothelial staining is weaker but the rounded haematopoietic cells retain CD34 expression. (C) Day 9, 20-somite stage: the only CD34 expression in the yolk sac is in the vitelline artery at the point that it emerges from the embryo. (D) Vitelline artery at the 20-somite stage at a higher magnification: the stained endothelial cells are discontinuous. (E) Day 10 embryo (around 30 somites), showing the points of emergence of the umbilical (u) and vitelline (v) vessels from the embryo. (F ) Day 10 umbilical vessels dissected away from the embryo, showing foci of CD34+ staining; the allantoic capillaries within the chorioallantoic placenta are strongly stained. (G and H) Day 10 vitelline artery dissected away from the embryo, showing foci of CD34+ cells at the proximal end (left) only. Scale bars = 100 μm
Localization of CD34 protein in the yolk sac and extraembryonic blood vessels.CD34 protein was detected in the yolk sac blood islands before formation of the vitelline circulation, largely confined to flattened cells around the periphery which were most likely to be vascular endothelium (arrows on Fig 4A through C). Very few of the cells within the blood islands, which are predominantly erythroblasts at this stage, were stained. In the same sections there was staining of the endodermal layer of the yolk sac. This endodermal staining was unexpected, since CD34 mRNA was not detected in the yolk sac endoderm at any of the stages studied. It was absent from control sections not exposed to secondary antibody so is unlikely to be artefactual; it apeared to be within the cytoplasm, including the lysosomes close to the apical surface, and possibly also extracellular, associated with the basement membrane.
Expression of CD34 glycoprotein in the developing yolk sac and extraembryonic blood vessels. (A) Yolk sac from a 4-somite stage embryo: staining appears to be confined mainly to the endodermal layer (e); light staining (arrow) is present between two blood islands in the mesodermal layer. (B) Yolk sac from a 6-somite stage embryo: there is staining in the endodermal layer and in scattered cells around the periphery of the blood islands (arrows). (C) Yolk sac and allantois of an 8-somite embryo: CD34 is expressed in the endoderm (e) and around the periphery of the blood islands (arrows), but not in the hematopoietic cells nor in the allantoic mesenchyme (al) except for small blood vessels (open arrow). (D) Yolk sac from a 10-somite embryo: staining of the endothelium of the vitelline blood vessels (v) is comparable with that of the dorsal aorta (da); large cells (arrowed) within the vessels are also stained. (E) Yolk sac of an 18-somite stage day 9 embryo, showing less prominent staining in the vascular endothelium and a subset of strongly stained cells (arrows) within the lumina of the vessels. (F ) Day 10 yolk sac, showing CD34 expression on the endothelium of flattened vessels; large stained cells are absent. (G) Proximal portion of the vitelline artery, showing CD34+ sites of possible haematopoietic activity. (H) Umbilical artery, showing CD34+ hematopoietic cells (arrow) and a thickened, possibly haematopoietic region. Scale bars (A through F on F ) = 50 μm.
Expression of CD34 glycoprotein in the developing yolk sac and extraembryonic blood vessels. (A) Yolk sac from a 4-somite stage embryo: staining appears to be confined mainly to the endodermal layer (e); light staining (arrow) is present between two blood islands in the mesodermal layer. (B) Yolk sac from a 6-somite stage embryo: there is staining in the endodermal layer and in scattered cells around the periphery of the blood islands (arrows). (C) Yolk sac and allantois of an 8-somite embryo: CD34 is expressed in the endoderm (e) and around the periphery of the blood islands (arrows), but not in the hematopoietic cells nor in the allantoic mesenchyme (al) except for small blood vessels (open arrow). (D) Yolk sac from a 10-somite embryo: staining of the endothelium of the vitelline blood vessels (v) is comparable with that of the dorsal aorta (da); large cells (arrowed) within the vessels are also stained. (E) Yolk sac of an 18-somite stage day 9 embryo, showing less prominent staining in the vascular endothelium and a subset of strongly stained cells (arrows) within the lumina of the vessels. (F ) Day 10 yolk sac, showing CD34 expression on the endothelium of flattened vessels; large stained cells are absent. (G) Proximal portion of the vitelline artery, showing CD34+ sites of possible haematopoietic activity. (H) Umbilical artery, showing CD34+ hematopoietic cells (arrow) and a thickened, possibly haematopoietic region. Scale bars (A through F on F ) = 50 μm.
From around the 10-somite stage (day 9), nests of CD34 protein-positive hematopietic cells could be detected within the vessels of the yolk sac (Fig 4D and E). Endothelial staining of the vitelline capillaries was still present at the 10-somite stage, although little or no mRNA was detectable at this stage (see above: Fig 3B). By day 10, the yolk sac vessels either lie under endodermal loops, which begin to form on day 9 (Fig 4E), or they are regressing (Fig 4F ). CD34 immunostaining was weak in the functional vessels, but strong in the flattened, regressing vessels. Close to the embryo, but not elsewhere, the umbilical and vitelline arteries were strongly CD34+ (Fig 4G and H). In these regions, the histology of the endothelium resembled that of the dorsal aorta, the cells being rounded rather than squamous, and having attached CD34+ hematopoietic cells suggesting hemangioblast activity. These are the same sites as those in which patches of CD34 gene expression were observed (Fig 3).
DISCUSSION
Our results confirm that CD34 is expressed in pre-endothelial cells and in hematopoietic progenitor cells. In early intraembryonic blood vessels, expression is correlated with the manner in which particular vessels are formed, being high in vessels formed by vasculogenesis and angiogenesis, and low or undetectable in those forming by coalescence. Sites of possible hemangioblast activity were detected in the walls of the aorta, as previously reported,35 and in two new sites in the proximal segments of the extraembryonic blood vessels. Characterization of the RAM34 MoAb showed that it recognizes CD34 epitopes regardless of their glycosylation state. In general, CD34 glycoprotein was detected shortly after CD34 gene expression, although yolk sac endoderm showed CD34 glycoprotein staining where RNA was not detected.
CD34 and endothelial precursors.CD34 transcripts are detectable in presumptive vascular endothelial cells, before the formation of definitive blood vessels, within both the yolk sac and the embryo. This early pattern of expression is similar to the reported early expression patterns of the genes of the receptor tyrosine kinases of the vascular endothelial cell growth factor family, TIE and TEK,37 which are expressed sequentially in angioblasts and mature endothelial cells38 and have been implicated in the process of vasculogenesis. The presumptive endothelial cell expression also resembles the pattern observed in the prevascular quail embryo using a MoAb to quail endothelial cells.36 The observation that CD34 is first expressed in these diffusely scattered cells, which subsequently coalesce into definitive vessels, provides support for the suggestion that CD34 could form part of a mechanism by which pre-endothelial cells recognise one another, facilitating aggregation to form vessels.
CD34 in blood vessel endothelium.The formation of blood vessels by condensation of pre-endothelial cells is termed vasculogenesis.39 Vasculogenesis is the mode of establishment of the early vascular channels in the embryo, before the formation of links with the vitelline vessels at the 7-8–somite stage. These channels consist of the paired endocardial heart tubes, the left and right first aortic arches, the dorsal aortae and the omphalomesenteric arteries. Umbilical vessels are added in a similar manner on day 9. They show CD34 gene expression at the pre-endothelial cell stage, and are positive for CD34 protein soon after their formation. The dorsal aortae are exceptional in showing increasingly strong CD34 immunoreactivity from the time of their formation to the latest period studied (day 10). Other vessels showed their maximal staining during or soon after formation.
The first embryonic veins are small blood vessels which coalesce to form the paired anterior and posterior cardinal veins, draining into the sinus venosus of the heart via the common cardinal veins. None of these vessels showed strong staining for CD34, suggesting that CD34 is not significantly involved in their formation, and has no function in the process of coalescence to form larger channels. This is consistent with the pattern observed in the yolk sac, in which CD34 is downregulated by the time the blood islands have coalesced to form the vitelline vessels. High levels of CD34 protein were observed in vitelline vessels undergoing flattening and regression on day 10; in these, the adhesive properties of CD34 may play a role in closure of the channels.
A third method of blood vessel formation is angiogenesis, the sprouting of new capillaries from existing vessels.39 This pattern is characteristic of the intersomitic branches of the dorsal aortae and of the cranial mesenchymal capillary sprouts invading the neural epithelium. In general, angiogenesis was associated with a high level of CD34 transcripts and protein. The second and subsequent aortic arches and the aortic sac were exceptions to this rule: CD34 was not detected in these vessels until after their formation, although they form as outgrowths from the junction of the truncus arteriosus and first aortic arch, which were themselves highly CD34+. The relationship between CD34 expression and the method of blood vessel formation is summarized in Fig 5.
Diagrammatic summary indicating the intensity of staining for CD34 RNA transcripts and protein in developing blood vessels, indicating the relationship between staining intensity (as indicated by depth of shading) and the mode of blood vessel formation. In general, CD34 expression is strong in pre-endothelial cells, in vessels formed by condensation of pre-endothelial cells (ie, by vasculogenesis), particularly the dorsal aortae. Equally strong staining is observed in vessels forming by capillary sprouting, but the larger vessels of the aortic arch region are not strongly stained during their formation even though their origin can be described as angiogenetic. Vessels formed by the coalescence of networks of smaller vessels showed low or undetectable levels of CD34 staining.
Diagrammatic summary indicating the intensity of staining for CD34 RNA transcripts and protein in developing blood vessels, indicating the relationship between staining intensity (as indicated by depth of shading) and the mode of blood vessel formation. In general, CD34 expression is strong in pre-endothelial cells, in vessels formed by condensation of pre-endothelial cells (ie, by vasculogenesis), particularly the dorsal aortae. Equally strong staining is observed in vessels forming by capillary sprouting, but the larger vessels of the aortic arch region are not strongly stained during their formation even though their origin can be described as angiogenetic. Vessels formed by the coalescence of networks of smaller vessels showed low or undetectable levels of CD34 staining.
Sites of detection of CD34 protein without RNA.An intriguing feature of CD34 expression in the early (day 8.0) visceral yolk sac is the detection of CD34 protein, but not RNA, in the endodermal layer. Unless RNA was present below the level of detectability by the method used, this protein must be derived from translation of message within the mesodermal layer, suggesting involvement of CD34 in a mesenchymal-epithelial interaction, possibly related to the mechanism by which the blood islands coalesce to form vessels. This interpretation is supported by two recent studies on visceral yolk sac hematopoiesis and vasculogenesis in vitro, which indicate that endoderm is not required for the differentiation of primitive erythrocytes or endothelial cells, but is essential for the formation and organization of yolk sac blood vessels.40 41
CD34 protein was also detected in parts of the gut endoderm and in the neuroepithelium, neither of which showed expression at the RNA level. The significance of these observations is not known, but it may be remarked that the gut staining was confined to the midline region in contact with the strongly CD34+ dorsal aortae, and that the neuroepithelial staining precedes invasion by the CD34+ capillaries.
CD34 protein was detected in the vitelline vessels 24 hours after downregulation of gene expression. This may indicate that low levels of transcription were still occurring, or it may reflect the differential stability of CD34 mRNA and protein; the presence of an AT-rich sequence, known to be associated with unstable mRNAs, within the 3′ untranslated region of the murine CD34 transcript may be relevant in this context.4
CD34 and hematopoiesis in the yolk sac and embryo.CD34 mRNA and protein were detectable in a small minority of hematopoietic precursor cells of the yolk sac blood islands and vessels both before and after formation of the vitelline circulation. At these early stages, the yolk sac mainly forms erythrocytes, but by day 10.5, the CD34+ hematopoietic precursor cells are multipotent: coculture with CD34+ yolk sac endothelium resulted in their proliferation and differentiation into both erythroid and myeloid (especially monocyte and macrophage) cell lineages.14
Although the yolk sac blood islands are intensely active in the process of erythropoiesis, they only provide a temporary source of embryonic blood cells. In avian embryos, the definitive blood cells have been shown to be exclusively embryonic in origin, and the yolk sac becomes secondarily colonised by embryonic hematopoietic cells.42 The presence of CD34 protein in the yolk sac vessels may play a role in the adhesion of embryo-derived hematopoietic cells in this secondary recolonization process.
The sites of the earliest hematopoietic activity in avian embryos have been identified as the ventral walls of the dorsal aortae.35 CD34+ hematogenous cells have also been detected in the ventral wall of the dorsal aorta in a 5-week human embryo.43 It should be noted that a 5-week human embryo is approximately developmentally equivalent to a day 12 mouse embryo, ie, more advanced than the embryos examined in our study. This may explain why we did not detect an equivalent site in our material. In the mouse, multipotent hematopoietic cells have been detected in the para-aortic splanchnopleure,44,45 including that of presomite stage embryos,46 although these early cells are incapable of restoring the hematopoietic system of adult irradiated recipients. The explants used in these studies include the dorsal aortae and, at presomite stages, their pre-endothelial precursor cells, both of which we show here to be CD34+. The aorta/gonad/mesonephros (AGM) region of day 10 embryos (not earlier) appears to be the exclusive source of the definitive adult hematopoietic system, including the cells that colonize the liver.47,48 In day 10 embryos, but not at earlier stages, we observed CD34+ hematopoietic cells in the mesenchyme between the dorsal aorta and the gut mesentery; they were not close to any blood vessel, so are most likely to have differentiated in situ, and are good candidates for the AGM-derived cells defined by culture studies to be adult-type hematopoietic precursors.48
In contrast to the small proportion of the yolk sac hematopoietic cells that were CD34+ at any stage from day 8 to day 10, many CD34+ cells were detected in the day 10 embryonic liver. These are likely to be of extraembryonic origin, since hematopoietic precursors from the AGM region are unable to colonize other regions on day 10, nor are definitive (ie, embryo-derived) hematopoietic precursors present in the liver at that stage.48 (Incidentally, the timing established in that study48 argues against the possibility that the minority of yolk sac cells that we observed to be CD34+ are embryo-derived). We suggest that CD34 may play a role in the “homing” of yolk sac-derived cells to the liver, which may themselves serve to condition the liver environment as a future site of definitive hematopoiesis. This interpretation is supported by the observation that blood cells derived from the yolk sac of day 9 mouse embryos, when injected into the placenta of day 11 or older fetuses, can populate the liver and survive long term.49
The relationship between endothelial and hematopoietic lineages.The expression of CD34 on both endothelial and hematopoietic precursors has led to the suggestion that these two lineages may be derived from a common CD34+ precursor, analogous to the hemangioblast proposed on the basis of studies in avian embryos.50 Our observations indicate that from the earliest stages at which CD34 can be detected, most CD34+ endothelial and hematopoietic cells are morphologically and spatially distinct, indicating independent origin of CD34 expression in the two cell lineages. The endothelial cells of the dorsal aortae and the proximal parts of the umbilical and vitelline arteries are exceptions to this rule: on day 10 they show a characteristic rounded morphology with some attached hemopoietic precursor cells on their luminal surfaces; both cell types are strongly CD34+. Slight thickening of the CD34+ endothelia of the umbilical and vitelline arteries was also observed, somewhat similar to the aortic hematopoietic sites described in avian embryos.35
There is good evidence that a close morphological relationship between CD34+ endothelial and hematopoietic cells is functionally significant. Isolated CD34+ endothelial cells can stimulate proliferation of CD34+ hematopoietic cells,14 indicating a functional interaction between the two cell types. Dual potential of CD34+ cells has recently been demonstrated in CD34-purified cells from human cord blood: after 4 to 6 weeks in culture they formed a confluent monolayer which expressed endothelial cell markers and supported hematopoiesis after irradiation and addition of new CD34+ cells from fresh cord blood (Mie Nieda and Jill Hows, personal communcation, September, 1996).
There are three possible interpretations of our observation of CD34+ cells attached to the CD34+ endothelial lining of the dorsal aorta and the juxta-embryonic parts of the umbilical and vitelline arteries: (1) they are sites of hemangioblastic activity; (2) the endothelium could be supporting hematopoiesis in cells derived from the yolk sac; (3) hematopoietic progenitors originating in the para-aortic mesenchyme may traverse the aortic endothelium to enter the circulation, becoming attached where the endothelium is CD34+. It is clear that whichever possibility is correct, these arterial sites play a significant role in early haematopoiesis in mouse embryos, and deserve further investigation.
ACKNOWLEDGMENT
We thank Prof M.F.Greaves for helpful discussions, Pharmingen for making the RAM34 MoAb available before commercial release, and Youichirou Ninomiya for assistance in the preparation of Fig 5.
Supported by grants from the Human Frontier Science Program Organisation, Strasbourg, France, and the Leukaemia Research Fund, London, UK, and the National Institutes of Health, Bethesda, MD.
Address reprint requests to Prof G.M. Morriss-Kay, Department of Human Anatomy, South Parks Rd, Oxford OX1 3QX, UK.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal