Abstract
Plasminogen activators play a role in the response of the vessel wall to injury, presumably by mediating the degradation of extracellular matrix (ECM) by vascular smooth muscle cells (VSMCs) that is necessary for their migration and proliferation. We have therefore investigated the ability of VSMCs to assemble specific cell surface plasminogen-activating systems. Urokinase-type plasminogen activator (uPA) bound to a single class of site on VSMCs (kd, 2 nmol/L), binding of pro-uPA resulted in a large potentiation of plasmin generation and both were competed by antibodies to the uPA receptor (uPAR). Tissue-type plasminogen activator (tPA) also bound to VSMCs as determined by functional assay, with the binding isotherms showing two classes of binding site with apparent kds of 25 and 300 nmol/L. tPA binding to the higher affinity site caused a greater than 90-fold enhancement of the activation of cell bound plasminogen, whereas the lower affinity binding, mediated primarily by the ECM, had little effect on tPA activity. The high-affinity binding of tPA to VSMCs resulted in an eightfold greater potential for plasmin generation than the binding of uPA, with this difference increasing to 15-fold after thrombin stimulation of the cells due to a 1.8-fold increase in tPA binding. These data show a novel specific tPA receptor on VSMCs that may be important for the regulation of plasminogen activation in various vascular pathologies.
VASCULAR SMOOTH muscle cells (VSMCs) play a principal role in the formation and development of atherosclerotic lesions1 and in the fibrocellular intimal hyperplasia associated with restenosis after balloon angioplasty.2 The migration and proliferation of these normally quiescent cells involves the controlled and limited degradation of the extracellular matrix that surrounds and supports them. The degradation of the vascular extracellular matrix (ECM) also contributes to the profound arterial remodelling associated with compensatory enlargement, aneurysm formation, and plaque rupture in coronary atheroma due to weakening of the fibrous cap of the plaque.3 4 The ability of VSMCs to regulate the activity of the proteolytic enzymes needed to influence their surrounding ECM is therefore an important feature of the response of these cells to vascular injury.
Controlled ECM degradation involves an array of proteolytic enzyme systems that can act both independently and in a concerted manner. Two major systems that have been directly implicated in vessel wall pathology are the plasminogen activation system5-7 and the matrix metalloproteinases (MMPs).8,9 The plasminogen activation system has particular significance due to the broad substrate specificity of plasmin. In addition to directly degrading many components of the ECM, including fibronectin, laminin, and proteoglycans, plasmin can also propagate the pericellular proteolytic cascade initiated by the plasminogen activators due to activation of the latent MMPs, interstitial collagenase (MMP-1), 92-kD type-IV collagenase (MMP-9), and stromelysin-1 (MMP-3).10 These MMPs, which are representative of the three main subclasses of MMPs, are all expressed by VSMCs6 and may themselves further activate other MMPs.10 Plasmin can also modulate the activity of cytokines through activation of latent transforming growth factor-β (TGF-β) and the release of extracellar matrix-associated TGF-β and basic fibroblastic growth factor,11,12 both important autocrine regulators of cell growth in the vessel wall.13 14 Therefore, the generation of plasmin in the vessel wall has the potential to modulate a variety of events contributing to the development and progression of vascular lesions.
Both of the mammalian plasminogen activators, urokinase-type plasminogen activator (uPA) and tissue-type plasminogen activator (tPA), are expressed by VSMCs in the intima of human atherosclerotic lesions.6,7 A functional involvement of the plasminogen activators in these situations is suggested by experiments in animal models of vascular injury. Intimal thickening in response to carotid artery injury is markedly reduced in transgenic mice with targeted disruption of the uPA gene and is increased in mice with disruption of the plasminogen activator inhibitor type-1 (PAI-1) gene.15 In a rat carotid balloon injury model, both uPA and tPA expression are induced, with tPA expression correlating to the migratory phase of the response.5 Systemic administration of the lysine analog tranexamic acid, a general inhibitor of the plasminogen activation system, inhibits intimal thickening in this model,16 as does heparin, which has been shown to downregulate tPA, but not uPA, expression.17
Although both of the plasminogen activators are implicated in VSMC plasmin generation, it is primarily uPA that has been considered to be involved in pericellular proteolysis due to its binding to a specific cellular receptor (uPAR) that both potentiates and localizes its activity.18-20 This suggests that tPA may also function by a receptor-mediated mechanism on these cells, and binding of tPA,21 as well as uPA,22 to VSMCs has previously been observed. However, this binding of tPA cannot enable proteolysis, because it has been characterized as being mediated by PAI-1 with subsequent internalization via low-density lipoprotein receptor-related protein (LRP).21 This has led us to study the interaction of both uPA and tPA with human VSMCs by radioligand binding and functional assays, the latter showing the presence of a novel high-affinity binding site for tPA on these cells. Thus, in addition to uPAR-mediated potentiation of uPA-catalyzed plasmin generation, VSMCs can also assemble a tPA-catalyzed cell-surface plasminogen activation system, and both of these may endow the cells with the potential to degrade their surrounding ECM in a regulated manner.
MATERIALS AND METHODS
Purified proteins.Pro-uPA, plasminogen, and plasmin were all purified as described previously.18,20 Single-chain tPA was obtained from Boehringer Ingelheim (Ingelheim, Germany) and Ser478 → Ala tPA was a gift of Prof Roger Lijnen (Center for Molecular and Vascular Biology, Leuven, Belgium). The tPA domain deletion mutant consisting of kringle 2 and protease domains (K2P) expressed in Escherichia coli was a gift of Dr Jan Verheijen (Gaubius Institute, Leiden, The Netherlands). The amino-terminal fragment of uPA (ATF ), comprising residues 6 through 135, was a gift of Dr Jack Henkin (Abbott Laboratories, Chicago, IL). Forty-kilodalton receptor-associated protein (RAP) expressed in E coli as a fusion protein with glutathione S-transferase was a gift of Dr Dudley Strickland (American Red Cross, Rockville, MD). Phosphatidylinositol-specific phospholipase C (PI-PLC) was obtained from Boehringer Mannheim (Mannheim, Germany). Heparinase I and human α-thrombin were obtained from Sigma (St Louis, MO). Anti–PAI-1 polyclonal antibody (395R) was from American Diagnostica (Greenwich, CT) and monoclonal antibody (MoAb) 7F5 was a gift of Dr Paul deClerck (Laboratory for Pharmaceutical Biology, Leuven, Belgium). Anti-uPAR MoAb R3 was as previously described.23 Anti–α-actin MoAb was from Boehringer Mannheim and anticaveolin and anti-annexin II MoAbs were from Transduction Laboratories (Lexington, KY). A polyclonal antibody to annexin II was a gift from Prof Katherine Hajjar (Department of Pediatrics, Cornell University Medical College, New York, NY). ATF and tPA were labeled with 125I using Iodobeads (Pierce, Rockford, IL) to an activity of 100 to 200 mBq/nmol. uPA was inactivated with (4-amidinophenyl)-methanesulfonyl fluoride (Boehringer Mannheim) as previously described.24
VSMC culture.VSMCs were prepared from saphenous vein and aorta of patients undergoing cardiac bypass surgery. The tissue was processed by surgical removal of the adventitia and endothelium before fine mincing of the rinsed tissue with a scalpel. VSMCs were then isolated using the explant technique grown in Dulbecco's Modified Eagle's Medium (DMEM) with 15 mmol/L HEPES, pH 7.4, 10% heat-inactivated fetal calf serum, penicillin-streptomycin, and fungizone (all obtained from Sigma) and harvested by trypsinization after 1 to 2 weeks. Cells were subcultured at a split ratio of 1:4 into the same medium and used between passages 2 and 6. The cells were characterized as VSMCs by their morphologic appearance and positive immunostaining with an antihuman smooth muscle cell α-actin antibody.25 Cells for use in experiments were subcultured into 24-well tissue culture plates (50,000 cells/well), grown to confluence for 2 days, and washed three times in DMEM-HEPES before use. Alternatively, cells were scraped from the culture vessel in the absence of trypsin, washed three times in DMEM-HEPES, counted in a hemocytometer, and used as a cell suspension. All data shown are for cells isolated from saphenous vein, but no significant or systematic differences were observed with cells isolated from aorta.
Thrombin stimulation of VSMCs.Before stimulation, VSMCs were growth-arrested for 48 hours in serum-free DME. The cells were then incubated with varying concentrations of thrombin for 18 hours in the same medium.
Radioligand binding studies.The binding of uPA to VSMCs was determined using previously described methodology.26 In brief, a fixed concentration of 125I-labeled ATF (15 μBq/mL, approximately equal to 0.1 nmol/L) was incubated with VSMCs (0.5 × 106/mL) in phosphate-buffered saline (PBS) containing 2% bovine serum albumin (BSA) in the presence of varying concentrations of unlabeled pro-uPA. After equilibration at 4°C for 2 hours, bound and free ligand were separated by centrifugation through an oil cushion (80:20, vol/vol of dimethyldiphenylpolysiloxane and ρ = 0.88 g/mL mineral oil) in polypropylene microcentrifuge tubes. The binding of tPA to VSMCs was determined by incubation of the cells with varying concentrations of 125I-labeled tPA using the conditions described above. Nonspecific binding was assessed in the presence of at least a 50-fold excess of unlabeled tPA. Dissociation constants for both uPA and tPA were determined by fitting of the specific binding isotherms by nonlinear regression, and the specific binding sites per cell was calculated on the basis of ligand specific activity.
Chemical cross-linking.Chemical cross-linking of 125I-labeled ATF to VSMC uPAR was performed on Triton X-114 lysates. Cells (5 × 106) were lysed by incubation in 100 μL 3% Triton X-114 containing 1 mmol/L phenylmethylsulfonyl fluoride for 10 minutes at 0°C. Phase separation was induced by incubation at 37°C. Detergent phases were washed in 0.1 mol/L Tris-HCl, pH 8.1, at 0°C, followed by a second phase separation and the addition of 3-[(3-cholamidopropyl) dimethylammonio]-1-propane sulfonate (0.25% final concentration). Two-microliter aliquots of lysates were incubated with an equal volume of 125I-labeled ATF in a total of 10 μL PBS containing 0.1% Tween 80 for 60 minutes at 4°C. The homobifunctional cross-linker N,N′-disuccinimidyl suberate (2 mmol/L final concentration) was then added and, after 15 minutes at 25°C, the reaction was quenched by the addition of ammonium acetate (10 mmol/L final concentration). Samples were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and analyzed by fluorography. A similar procedure was also used to attempt to detect 125I-labeled tPA binding.
Determination of plasmin generation by VSMC-associated plasminogen activators.Plasmin generation by plasminogen activators exogenously bound to VSMCs was determined using both cells in suspension and in confluent monolayers. In both cases, cells were prepared as described above and further washed in 0.05 mol/L glycine, pH 3.0, 0.1 mol/L NaCl for 3 minutes followed by neutralization with an equal volume of 0.5 mol/L HEPES, pH 7.5, 0.1 mol/L NaCl to dissociate potential endogenously bound ligands.
Cells in suspension were assayed essentially as previously described for monocytoid cells.20 27 Briefly, VSMCs were incubated with pro-uPA (3 nmol/L) for 20 minutes at 37°C in PBS containing 0.2% BSA. After washing three times in the same buffer, the cells were resuspended at a density of 0.5 × 106/mL in 0.05 mol/L Tris-HCl, pH 7.4, 0.1 mol/L NaCl, 0.01% Tween 80 containing varying concentrations of plasminogen and the plasmin-specific fluorogenic peptide substrate H-D-Val-Leu-Lys-7-amido-4-methylcoumarin (AMC; 0.2 mmol/L). Fluorescence intensity was measured continuously in semimicro polystyrene cuvettes in a Perkin-Elmer LS-5B luminescence spectrometer at an excitation λ of 370 nm and emission λ between 470 and 510 nm. δF/δt was converted to plasmin concentration by reference to standard curves constructed using active-site titrated plasmin. In some experiments, the cells were preincubated with either anti-uPAR MoAb (R3, 20 μg/mL) or, to release glycosyl phosphatidylinositol (GPI)-anchored proteins, the enzyme PI-PLC (10 μg/mL). Assay of bound tPA was performed in the same way, with the exception that the cells were preincubated with 10 nmol/L tPA and assayed at a 10-fold lower density.
Confluent monolayers of VSMCs in 24-well tissue culture plates were assayed by preincubation of the cells with either pro-uPA (3 nmol/L) or tPA (10 nmol/L) for 20 minutes at 37°C in PBS containing 2% BSA. Cells were washed three times in the same buffer before the addition of 200 μL of 0.05 mol/L Tris-HCl, pH 7.4, 0.1 mol/L NaCl, 0.01% Tween 80 containing varying concentrations of plasminogen and H-D-Val-Leu-Lys-AMC (0.2 mmol/L). Plasmin generation was then determined as previously described.27 Briefly, an aliquot (150 μL) was removed from single wells at timed intervals and added to an equal volume of buffer and fluorescence was measured. Plasmin generation curves were constructed from δF/δt as described above, and in the case of tPA also by plots of F versus t2 (this being possible due to the linear rates of plasmin generation observed).
To determine the role of plasminogen binding to VSMCs, these assays were also performed by including 6-aminohexanoic acid (6AHA; 1 mmol/L) in the final reaction mixture as an antagonist of this binding.18 27 The determination of plasminogen activator activity under these conditions is complicated by the direct effect of 6AHA on Glu-plasminogen activation due to conversion of plasminogen into a more readily activatable substrate, thus leading to an underestimation of the effect of competing the cellular binding of plasminogen. In control experiments, 6AHA increased tPA-catalyzed solution phase plasminogen activation by 4.5-fold, and data were corrected for this effect when appropriate.
VSMC binding of plasminogen activators determined by functional assay.In addition to radioligand binding, the interaction of uPA and tPA with VSMCs was also determined by a functional, ie, plasminogen activation, assay. VSMCs in suspension (0.5 × 106/mL) were incubated with varying concentrations of pro-uPA (0 to 100 nmol/L) and washed and plasmin generation was determined as described above at a plasminogen concentration of 0.2 μmol/L. Maximum rates of plasmin generation were calculated and plotted against pro-uPA concentration. The pro-uPA concentration required to achieve half-maximal plasmin generation, approximately equivalent to the kd under these conditions, was determined by nonlinear regression.
In the case of tPA, VSMCs in 24-well tissue culture plates were incubated with varying concentrations of tPA (0 to 2 μmol/L) or K2P (0 to 1 μmol/L) for 30 minutes at 37°C in 0.05 mol/L Tris-HCl, pH 7.4, 0.1 mol/L NaCl containing 2% BSA. The plates were washed three times and incubated with plasminogen (0.2 μmol/L) and H-D-Val-Leu-Lys-AMC (0.2 mmol/L). Because plasmin generation was shown to be essentially linear with time under these experimental conditions, the cell supernatant was removed at a single time point (routinely 20 minutes), its fluorescence was measured, and plasmin generation was calculated by reference to standard curves. The data obtained at varying activator concentrations were fitted to one- and two-site binding models by unweighted nonlinear regression using the Marquardt algorithm (SigmaPlot; Jandel Scientific, Erkrath, Germany). Goodness of fit to the models was determined by analysis of parameter dependencies, least-squares analysis, and analysis of runs of residuals.
In some experiments the contribution of VSMC ECM to the functional binding of tPA was determined. For these experiments, cells were removed from the culture plates either by lysis in 30 mmol/L NH4OH, 0.5% Triton X-100 or with 1 mmol/L EDTA.
RESULTS
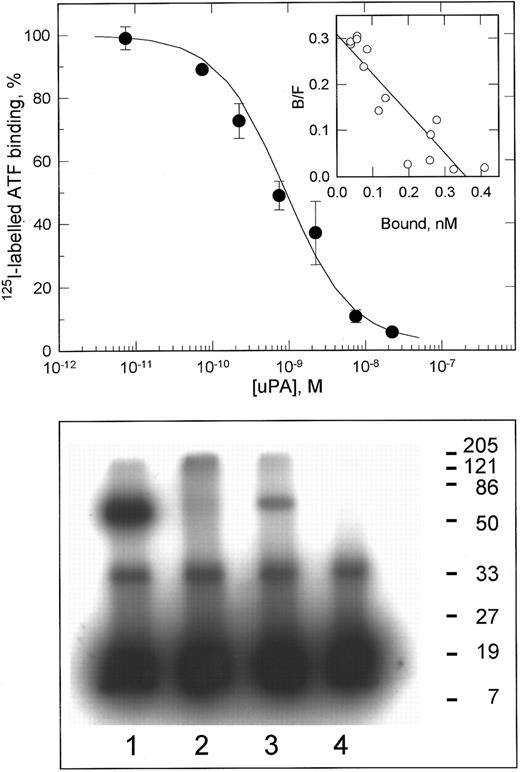
uPA binding to VSMCs.The characteristics of the interaction of uPA with VSMCs were determined by radioligand binding. As shown in Fig 1 (upper panel), uPA bound saturably to a single class of binding sites with a kd of 1.2 ± 0.4 nmol/L and 60,000 ± 16,000 (±SD, n = 3) binding sites per cell. This kd is within the range determined for uPA binding to uPAR on a variety of cell types.28 This binding was inhibited greater than 90% by the anti-uPAR MoAb R3. Chemical cross-linking of 125I-labeled ATF to lysates of VSMCs showed a complex of 70 kD, consistent with that of the complex between ATF and recombinant uPAR (Fig 1, lower panel). The physical interaction of uPA with VSMCs appeared therefore to be mediated by uPAR, and interactions with other uPA binding proteins such as LRP29 were not detected under these experimental conditions (although it should be noted that the conditions used favor the detection of uPAR).
125I-labeled ATF binding to VSMCs. The upper panel shows the competitive displacement curve for the specific binding of 125I-labeled amino-terminal fragment of uPA to VSMCs, after subtraction of nonspecific binding (<15% of total binding). Data shown are the mean ± SD of duplicate determinations. The inset shows a Scatchard transformation of these data. In three independent experiments, the binding constants were determined as kd = 1.2 ± 0.4 nmol/L, Bmax = 60,000 ± 16,000 sites/cell (mean ± SD). The lower panel shows chemical cross-linking of 125I-labeled ATF to Triton X-114 lysates of VSMCs. Lane 1, recombinant soluble uPAR; lane 2, aqueous phase of X-114 lysate; lane 3, detergent phase of X-114 lysate; lane 4, 125I-labeled ATF cross-linked alone. The ATF:uPAR complex in lane 3 migrates with an apparent molecular weight of 70 kD. The band of 35 kD present in all lanes represents cross-linked dimers of ATF.
125I-labeled ATF binding to VSMCs. The upper panel shows the competitive displacement curve for the specific binding of 125I-labeled amino-terminal fragment of uPA to VSMCs, after subtraction of nonspecific binding (<15% of total binding). Data shown are the mean ± SD of duplicate determinations. The inset shows a Scatchard transformation of these data. In three independent experiments, the binding constants were determined as kd = 1.2 ± 0.4 nmol/L, Bmax = 60,000 ± 16,000 sites/cell (mean ± SD). The lower panel shows chemical cross-linking of 125I-labeled ATF to Triton X-114 lysates of VSMCs. Lane 1, recombinant soluble uPAR; lane 2, aqueous phase of X-114 lysate; lane 3, detergent phase of X-114 lysate; lane 4, 125I-labeled ATF cross-linked alone. The ATF:uPAR complex in lane 3 migrates with an apparent molecular weight of 70 kD. The band of 35 kD present in all lanes represents cross-linked dimers of ATF.
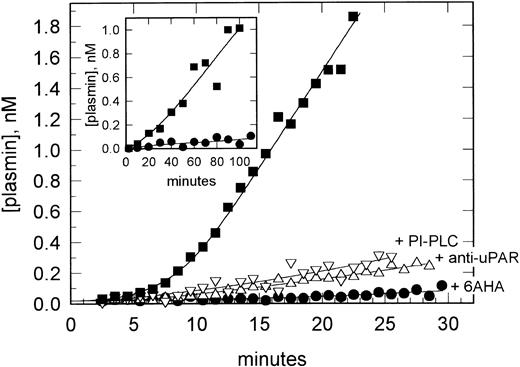
uPAR-mediated plasminogen activation on VSMCs. VSMCs in suspension were preincubated with pro-uPA and washed, and plasmin generation was determined after the addition of plasminogen (200 nmol/L) and H-;D-Val-Leu-Lys-AMC (0.2 mmol/L). Data are shown for plasmin generation assayed in the absence (▪) and presence (•) of 6AHA (1 mmol/L) as an antagonist of plasminogen binding and after preincubation of the cells with PI-PLC (▿) or the anti-uPAR MoAb R3 (▵). The inset shows a similar experiment performed with VSMC monolayers in a 24-well tissue culture plate. Data are shown in the absence (▪) and presence (•) of 6AHA.
uPAR-mediated plasminogen activation on VSMCs. VSMCs in suspension were preincubated with pro-uPA and washed, and plasmin generation was determined after the addition of plasminogen (200 nmol/L) and H-;D-Val-Leu-Lys-AMC (0.2 mmol/L). Data are shown for plasmin generation assayed in the absence (▪) and presence (•) of 6AHA (1 mmol/L) as an antagonist of plasminogen binding and after preincubation of the cells with PI-PLC (▿) or the anti-uPAR MoAb R3 (▵). The inset shows a similar experiment performed with VSMC monolayers in a 24-well tissue culture plate. Data are shown in the absence (▪) and presence (•) of 6AHA.
Effect of Disruption of VSMC Caveolae on uPAR-Mediated Plasminogen Activation
| Untreated | 247 ± 31 (100%) |
| Nystatin (50 μg/mL) | 293 ± 23 (118%) |
| Filipin (20 μg/mL) | 255 ± 26 (103%) |
| Untreated | 247 ± 31 (100%) |
| Nystatin (50 μg/mL) | 293 ± 23 (118%) |
| Filipin (20 μg/mL) | 255 ± 26 (103%) |
Confluent VSMCs in 24-well tissue culture plates were incubated with either nystatin or filipin for 30 minutes, incubated with pro-uPA, washed, and assayed for plasmin generation. Data are expressed as fluorescence intensity at 20 minutes after the addition of plasminogen (±SD of triplicate determinations) and the percentage of plasmin generation compared with untreated cells. These treatments were shown to disrupt caveolae as determined by a redistribution of caveolin immunofluorescence (data not shown).
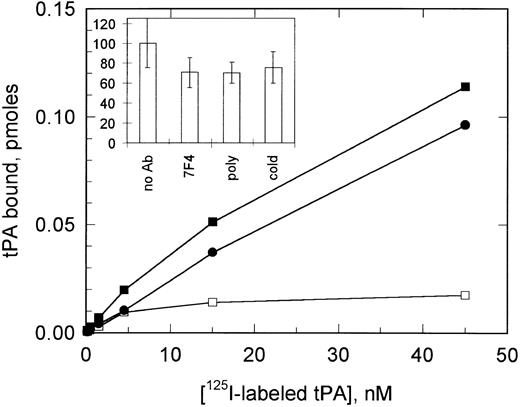
125I-labeled tPA binding to VSMCs. The binding of 125I-labeled tPA to VSMCs is shown in the absence (▪) and presence (•) of 1 μmol/L cold tPA, together with specific binding calculated from these data (□). The inset shows a comparison of the competition of total 125I-labeled tPA binding (0.5 nmol/L) by both cold tPA and by anti–PAI-1 MoAb (7F4) and polyclonal antibody at a concentration of 50 μg/mL. All data shown are the mean ± SD of triplicate determinations.
125I-labeled tPA binding to VSMCs. The binding of 125I-labeled tPA to VSMCs is shown in the absence (▪) and presence (•) of 1 μmol/L cold tPA, together with specific binding calculated from these data (□). The inset shows a comparison of the competition of total 125I-labeled tPA binding (0.5 nmol/L) by both cold tPA and by anti–PAI-1 MoAb (7F4) and polyclonal antibody at a concentration of 50 μg/mL. All data shown are the mean ± SD of triplicate determinations.
Binding of uPA to VSMCs was also determined using a functional assay in which varying concentrations of pro-uPA were preincubated with the cells and plasmin generation by bound enzyme was subsequently measured. Plotting of maximum plasmin generation rates against pro-uPA concentration gave an apparent kd of 1.4 ± 0.4 nmol/L (±SD, n = 3), equivalent to that determined by 125I-labeled ATF binding and validating this as a method to determine plasminogen activator binding.
Functional activity of VSMC-bound uPA.We have previously described that the presence of uPAR on monocytes, macrophages, and neutrophils enables the assembly of a ternary complex with cell surface-associated plasminogen, leading to a large overall potentiation of plasmin generation initiated by pro-uPA.18,20,30,31 To determine whether uPAR on VSMCs was also able to mediate efficient cell surface plasminogen activation, the cells were preincubated with pro-uPA and assayed for plasmin generation in the presence and absence of the lysine analog 6AHA, an antagonist of plasminogen binding. Figure 2 shows that plasminogen binding to the cells, ie, in the absence of 6AHA, led to at least a 40-fold increase in plasmin generation. These experiments were performed using VSMCs in suspension to minimize possible interference from ECM proteins, which have been shown to affect plasminogen activation in the absence of cells.32 However, when repeated using confluent VSMC monolayers, essentially the same effects were observed (Fig 2, inset).
Effect of disruption of caveolae on uPAR-mediated plasminogen activation.In certain cell types, including VSMCs and melanoma cells, uPAR has been shown to be intimately associated with the plasmalemmal vesicles known as caveolae.33,34 This localization, a consequence of the GPI-anchorage of uPAR, has been proposed to be involved in promoting efficient cell surface plasminogen activation.34 The uPAR staining seen by indirect immunofluorescence on fixed VSMCs was consistent with caveolar localization on the cells used in this study (data not shown). However, when the cells were treated with either of the cholesterol-binding drugs nystatin or filipin to disrupt these structures,35 no effect on plasmin generation was observed (Table 1). Brief treatment of the cells with phorbol myristate acetate (PMA), which has been shown to disrupt caveolae by an alternate mechanism,36 was also without effect.
125I-labeled tPA binding to VSMCs.125I-labeled tPA was found to bind saturably to VSMCs (Fig 3). High levels of apparently nonspecific or low-affinity binding made the determination of binding constants imprecise; however, the concentration of 125I-labeled tPA needed to reach half-maximal saturation was approximately 4 nmol/L. This specific tPA binding could be abolished by preincubation of the cells with neutralizing anti–PAI-1 MoAbs (Fig 3, inset).
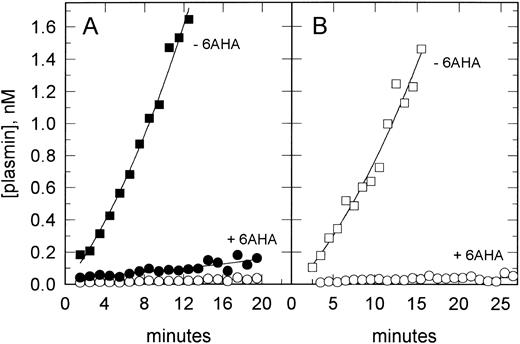
Functional binding of tPA to VSMCs.We therefore sought to detect the binding of tPA to VSMCs at sites other than PAI-1 using functional assays. Figure 4 shows that tPA could be bound to the cells in a form that retained plasminogen activator activity, ie, not bound to PAI-1, and furthermore that a greater than 20-fold reduction in plasmin generation was observed when assayed in the presence of 6AHA. In addition to its antagonism of the cellular binding of plasminogen, 6AHA converts native Glu-plasminogen in solution into a more readily activated conformation, thus leading to an underestimation of the competitive effect of 6AHA. Taking this effect into consideration shows that the real difference in plasmin generation by VSMC-bound tPA in the presence and absence of the cellular binding of plasminogen is approximately 100-fold (Fig 4A, open symbols). Measuring the activation of Lys-plasminogen, which does not undergo this conformational change, confirmed this effect as there was an approximately 90-fold reduction in plasmin generation in the presence of 6AHA.
tPA-catalyzed plasminogen activation on VSMCs. VSMCs in suspension were preincubated with tPA (10 nmol/L) and washed, and plasmin generation was determined after the addition of either Glu-plasminogen (200 nmol/L) or Lys-plasminogen (20 nmol/L) and H-D-Val-Leu-Lys-AMC (0.2 mmol/L). In (A), data are shown for Glu-plasminogen activation assayed in the absence (▪) and presence (•) of 6AHA (1 mmol/L) as an antagonist of plasminogen binding. Also shown (○) are the data in the presence of 6AHA corrected for its direct stimulatory effect on plasminogen activation (see the Materials and Methods). (B) shows data for Lys-plasminogen activation in the absence (□) and presence (○) of 6AHA.
tPA-catalyzed plasminogen activation on VSMCs. VSMCs in suspension were preincubated with tPA (10 nmol/L) and washed, and plasmin generation was determined after the addition of either Glu-plasminogen (200 nmol/L) or Lys-plasminogen (20 nmol/L) and H-D-Val-Leu-Lys-AMC (0.2 mmol/L). In (A), data are shown for Glu-plasminogen activation assayed in the absence (▪) and presence (•) of 6AHA (1 mmol/L) as an antagonist of plasminogen binding. Also shown (○) are the data in the presence of 6AHA corrected for its direct stimulatory effect on plasminogen activation (see the Materials and Methods). (B) shows data for Lys-plasminogen activation in the absence (□) and presence (○) of 6AHA.
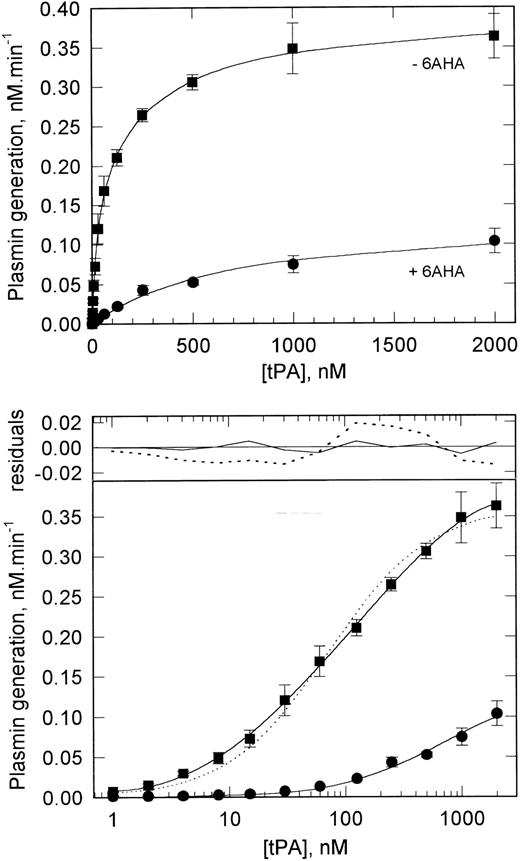
Characterization of the functional binding of tPA to VSMCs.Because these experiments showed the functionally favorable binding of tPA to VSMCs, the characteristics of this binding were investigated by plasminogen activation assay as validated above for pro-uPA binding. Figure 5 shows that, when assessed in this way, tPA shows a saturable binding to VSMCs. Fitting of these data to various binding models by nonlinear regression analysis showed that the best fit of the data was to a two binding-site model, with the higher affinity site having a kd of 25 nmol/L and the lower affinity site a kd of 300 nmol/L. The maximum binding of tPA to these two sites at saturation represents maximum rates of plasmin generation, rather than maximum number or concentration of binding sites, and is approximately equivalent for the two sites (190 pmol/L⋅min−1 for the high-affinity site v 210 pmol/L⋅min−1 for the low-affinity site). tPA was also found to bind specifically to rat and rabbit VSMCs, with analysis again showing two classes of binding site on each. The binding of tPA to the high-affinity site on rat cells was similar to that on human cells (kd, 20 nmol/L; 160 pmol/L⋅min−1), but rabbit cells displayed a somewhat higher affinity (kd, 2.8 nmol/L; 56 pmol/L⋅min−1).
Binding of tPA to VSMCs determined by functional assay. VSMCs grown to confluency in 24-well tissue culture plates were preincubated with varying concentrations of tPA. The cells were than washed and plasmin generation was determined after the addition of plasminogen (200 nmol/L) and H-D-Val-Leu-Lys-AMC (0.2 mmol/L). Data are shown for plasmin generation assayed in the absence (▪) and presence (•) of 6AHA (1 mmol/L) and represent the mean ± SD (n = 4). The solid lines in both the upper and lower panels show the best fit of binding models to the data. In the presence of 6AHA, the best fit is to a single-site model; in the absence of 6AHA, the best fit is to a two-site model. The latter is highlighted in the lower panel, in which the data are plotted semilogarithmically. The single-site (- - - - -) and two-site (─) fits for data in the absence of 6AHA are shown; in the upper part of the panel, the residuals, ie, the differences between the measured and theoretical values, for each model are shown. Analysis of parameter dependencies, sum of squared residuals, and extended runs of positive and negative residuals all indicate the goodness of fit of the two-site model. In the presence of 6AHA, both models give identical binding constants and the fitted curves are therefore superimposed. The binding constants derived from these data are shown in Table 2.
Binding of tPA to VSMCs determined by functional assay. VSMCs grown to confluency in 24-well tissue culture plates were preincubated with varying concentrations of tPA. The cells were than washed and plasmin generation was determined after the addition of plasminogen (200 nmol/L) and H-D-Val-Leu-Lys-AMC (0.2 mmol/L). Data are shown for plasmin generation assayed in the absence (▪) and presence (•) of 6AHA (1 mmol/L) and represent the mean ± SD (n = 4). The solid lines in both the upper and lower panels show the best fit of binding models to the data. In the presence of 6AHA, the best fit is to a single-site model; in the absence of 6AHA, the best fit is to a two-site model. The latter is highlighted in the lower panel, in which the data are plotted semilogarithmically. The single-site (- - - - -) and two-site (─) fits for data in the absence of 6AHA are shown; in the upper part of the panel, the residuals, ie, the differences between the measured and theoretical values, for each model are shown. Analysis of parameter dependencies, sum of squared residuals, and extended runs of positive and negative residuals all indicate the goodness of fit of the two-site model. In the presence of 6AHA, both models give identical binding constants and the fitted curves are therefore superimposed. The binding constants derived from these data are shown in Table 2.
These experiments were also performed by assaying the activity of bound tPA in the presence of 6AHA. Lower rates of plasmin generation were observed at all tPA concentrations, consistent with the observations in Fig 4. Binding was again found to be saturable, but now the data could not be fitted to a two-binding site model, being fully consistent with binding to a single site with a kd of 660 nmol/L (Fig 5). Thus, under these conditions, the activity of tPA bound to the high-affinity site is apparently not detected. These observations suggest that it is the activity of tPA bound to the high-affinity site that is greatly reduced in the presence of 6AHA, ie, that tPA bound to this site activates cell-bound plasminogen very efficiently, whereas the activity of tPA bound to the lower affinity site(s) is largely unaffected (Table 2).
Binding Constants for the Interaction of tPA and K2P With VSMCs
| . | +/− 6AHA . | High Affinity . | Low Affinity . | ||
|---|---|---|---|---|---|
| . | . | App. kd . | Max. Pln. Gen.† . | App. kd . | Max. Pln. Gen.† . |
| . | . | (nmol/L)* . | . | (nmol/L)* . | . |
| tPA | − | 25 ± 5 | 190 ± 30 | 300 ± 80 | 210 ± 25 |
| tPA | + | ND | ND | 660 ± 90 | 135 ± 10 |
| K2P | − | ND | ND | 235 ± 45 | 140 ± 10 |
| K2P | + | ND | ND | 810 ± 55 | 45 ± 5 |
| . | +/− 6AHA . | High Affinity . | Low Affinity . | ||
|---|---|---|---|---|---|
| . | . | App. kd . | Max. Pln. Gen.† . | App. kd . | Max. Pln. Gen.† . |
| . | . | (nmol/L)* . | . | (nmol/L)* . | . |
| tPA | − | 25 ± 5 | 190 ± 30 | 300 ± 80 | 210 ± 25 |
| tPA | + | ND | ND | 660 ± 90 | 135 ± 10 |
| K2P | − | ND | ND | 235 ± 45 | 140 ± 10 |
| K2P | + | ND | ND | 810 ± 55 | 45 ± 5 |
Binding constants derived from the data shown in Figs 5 and 6 are shown. Because of the functional nature of the binding assay, Bmax is equivalent to the maximum rate of plasmin generation by tPA (or K2P) bound to that site. By comparison, uPA binding to uPAR on these cells gives a maximum plasmin generation rate of 24 (±4) pmol/L⋅min−1 in the absence of 6AHA.
Abbreviation: ND, not detected.
Because the binding has not been formally shown to reach equilibrium under these experimental conditions, the parameter determined is not a true equilibrium constant and is thus referred to as an apparent kd.
Expressed as pmol/L⋅min−1.
An alternative interpretation of these data is that 6AHA causes the dissociation of tPA from the high-affinity site via an interaction with the lysine-binding kringle 2 module of tPA. However, this does not appear to be the case, because, as shown in Fig 4, the rate of plasmin generation did not decrease during the course of the assay (which was initiated by the addition of plasminogen together with 6AHA) and, furthermore, any dissociated tPA would still be present and its activity detected in the assay system. The question of whether such dissociation contributed to the lack of detection of the high-affinity site was also addressed directly by including 6AHA during the preincubation of the cells with tPA and subsequently assaying bound tPA in the absence of 6AHA. The binding of tPA was essentially unchanged under these conditions, with both classes of binding site being detected (data not shown).
Therefore, analysis of the functional binding of tPA to VSMCs shows the presence of two classes of tPA binding sites associated with VSMCs. tPA binding to the high-affinity site leads to a large increase in tPA activity, whereas tPA binding to the low-affinity site does not significantly affect its activity. Direct comparison of plasmin generation by the two plasminogen activators shows that, under saturating conditions, the binding of tPA to the high-affinity binding site is responsible for eightfold more plasmin generation than is uPA bound to uPAR on the same cells (Table 2).
Efforts to detect these binding sites in cell lysates by chemical cross-linking to 125I-labeled tPA using a variety of homo-bifunctional cross-linkers were unsuccessful (data not shown). However, such negative data do not argue against a specific binding site because, for instance, although the uPA/uPAR complex can be efficiently cross-linked using human proteins, the complex between the murine proteins cannot.37
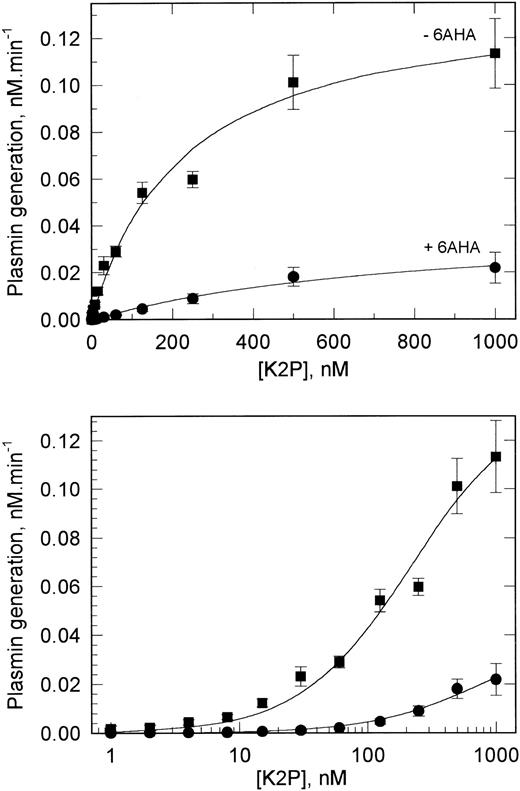
K2P binding to VSMCs.In an attempt to further discriminate between the two classes of tPA binding sites, experiments similar to those described above were also performed with the domain deletion mutant K2P, which is lacking the fibronectin type-I, EGF-like, and kringle 1 modules of tPA. Figure 6 shows that K2P also bound saturably to VSMCs, although the maximum plasmin generation was approximately three times lower than at saturating concentrations of tPA. When these data were analyzed, the binding of K2P to only a single class of binding site was detected. The kd of this interaction was 230 nmol/L, suggesting that K2P binds to the same low-affinity sites as intact tPA but does not interact with the high-affinity tPA binding site.
Binding of K2P to VSMCs determined by functional assay. The experiments were performed on confluent monolayers of VSMCs as described in the legend to Fig 5. The data obtained assaying for plasmin generation both in the absence (▪) or presence (•) of 6AHA best fit a single binding-site model (solid lines shown). The binding constants derived from these data are shown in Table 2.
Binding of K2P to VSMCs determined by functional assay. The experiments were performed on confluent monolayers of VSMCs as described in the legend to Fig 5. The data obtained assaying for plasmin generation both in the absence (▪) or presence (•) of 6AHA best fit a single binding-site model (solid lines shown). The binding constants derived from these data are shown in Table 2.
When assayed in the presence of 6AHA, small decreases in both the affinity and activity of the low-affinity site were observed with K2P, with these differences being of a similar magnitude to those observed with tPA (Table 2). These decreases may be partly accounted for by K2P dissociation from the cellular binding sites brought about by 6AHA, because inclusion of 6AHA during preincubation of the cells with K2P led to an up to fourfold lower binding and this binding showed no evidence of saturability up to 1 μmol/L (data not shown). Therefore, in contrast to the binding of tPA, the binding of K2P to VSMCs appears to be mediated primarily by the kringle 2 lysine-binding site.
Role of ECM in mediating VSMC tPA-catalyzed plasminogen activation.To determine if the observed functional binding of tPA to VSMCs involved interactions with components of the ECM, similar experiments were performed on the matrix exposed after removal of the cells. At low concentrations of tPA, less than 20% of the total plasmin generation was found to be associated with the ECM, and this increased to approximately 40% at the highest tPA concentrations (Fig 7). Analysis of these data showed the binding of tPA to a single class of binding site on the ECM with a kd of 590 nmol/L. In the experiment shown, the maximum capacity of the ECM for plasmin generation accounted for approximately 60% of the total low-affinity capacity of the cells to support tPA-catalyzed plasminogen activation. Therefore, the majority of the low-affinity, but not the high-affinity, binding of tPA to VSMCs is mediated by components of the ECM. However, it should be noted that this does not exclude an involvement of components derived from the plasma membrane because these may be present together with true ECM components.
Involvement of VSMC ECM in the functional binding of tPA. The ECM remaining after removal of VSMCs by lysis was preincubated with tPA and assayed for plasmin generation as in Fig 5. In the experiment shown, the tPA binding constants in the presence of cells were determined to be kd1 = 16 nmol/L (175 [pmol/L]⋅min−1) and kd2 = 1,000 nmol/L (500 [pmol/L]⋅min−1) and in the absence of cells a single class of site kd = 590 nmol/L (305 [pmol/L]⋅min−1).
Involvement of VSMC ECM in the functional binding of tPA. The ECM remaining after removal of VSMCs by lysis was preincubated with tPA and assayed for plasmin generation as in Fig 5. In the experiment shown, the tPA binding constants in the presence of cells were determined to be kd1 = 16 nmol/L (175 [pmol/L]⋅min−1) and kd2 = 1,000 nmol/L (500 [pmol/L]⋅min−1) and in the absence of cells a single class of site kd = 590 nmol/L (305 [pmol/L]⋅min−1).
Competition of tPA Binding to VSMCs
| Competitor . | Concentration . | Plasmin Generation (% of control) . | |
|---|---|---|---|
| . | . | High-Affinity Site . | Low-Affinity Site . |
| None | — | 100 | 100 |
| Ser478→Ala tPA | 1 μmol/L | 13 (±1) | ND |
| aPMSF-uPA | 1 μmol/L | 107 (±9) | 130 (±15) |
| PMSF-plasmin | 1 μmol/L | 98 (±6) | ND |
| RAP | 50 nmol/L | 130 (±15) | 125 (±17) |
| Anti-annexin II | 100 μg/mL | 97 (±7) | ND |
| Mannose | 25 mmol/L | 96 (±5) | 97 (±9) |
| Heparin | 50 μg/mL | 225 (±20)3-150 | 180 (±25) |
| Heparinase | 1 U/mL3-151 | 113 (±10) | 95 (±15) |
| Competitor . | Concentration . | Plasmin Generation (% of control) . | |
|---|---|---|---|
| . | . | High-Affinity Site . | Low-Affinity Site . |
| None | — | 100 | 100 |
| Ser478→Ala tPA | 1 μmol/L | 13 (±1) | ND |
| aPMSF-uPA | 1 μmol/L | 107 (±9) | 130 (±15) |
| PMSF-plasmin | 1 μmol/L | 98 (±6) | ND |
| RAP | 50 nmol/L | 130 (±15) | 125 (±17) |
| Anti-annexin II | 100 μg/mL | 97 (±7) | ND |
| Mannose | 25 mmol/L | 96 (±5) | 97 (±9) |
| Heparin | 50 μg/mL | 225 (±20)3-150 | 180 (±25) |
| Heparinase | 1 U/mL3-151 | 113 (±10) | 95 (±15) |
The effect of various potential competitors on tPA binding to the high- and low-affinity sites on VSMCs was determined at tPA concentrations of 10 nmol/L and 1 μmol/L and assay of plasmin generation in the absence and presence of 6-AHA, respectively. The binding of tPA was competed by preincubation of cells with the competitors for 10 minutes before further incubation with tPA as described in the legend to Fig 5. Data shown (mean ± SD of triplicate determinations) are expressed relative to the activity of tPA bound in the absence of competitor under the two experimental conditions.
Abbreviation: ND, not determined.
The apparent increase in tPA activity in the presence of heparin appears to be due to a direct potentiation of tPA activity as reported by others39 and was also observed if heparin was included in the plasminogen activation assay rather than in the preincubation with tPA.
Preincubation with heparinase for 60 minutes before washing and further incubation with tPA.
Role of LRP in mediating VSMC cell surface plasminogen activation.In addition to binding and internalizing the complexes uPA and tPA with PAI-1, LRP has also been reported to bind uncomplexed proteases.38 Because VSMCs have been shown to express LRP, we speculated that it may account for part of the observed functional binding of tPA. To address this issue, we preincubated the cells with RAP, the broad spectrum antagonist of ligand binding to LRP, before incubation with tPA. This had no inhibitory effect on tPA binding to either the high- or low-affinity binding sites (Table 3).
A variety of other known competitors of tPA binding to various cell types, including MoAbs and polyclonal antibodies against annexin II, also failed to inhibit the functional binding of tPA to VSMCs, whereas the tPA active site mutant (Ser478 → Ala) inhibited the high-affinity binding by greater than 85% (Table 3). Heparinase treatment of the cells also had no effect on subsequent tPA binding. Heparin increased the activity of tPA bound to both the high- and low-affinity sites; however, this was shown to occur through its known effect as a modulator of tPA activity.39
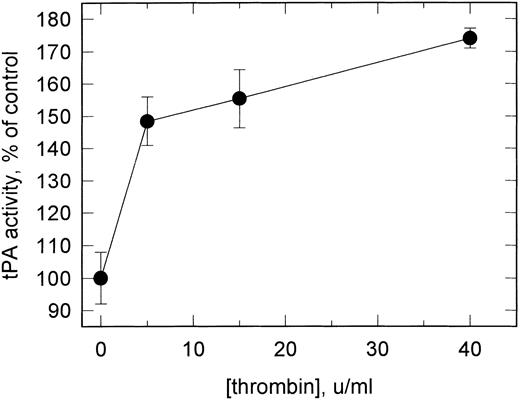
Effect of thrombin stimulation of VSMCs on cell surface plasminogen activation.The effect of thrombin on the expression of uPAR and the high-affinity tPA binding sites on VSMCs and consequently on plasmin generation was studied on quiescent cells stimulated with varying concentrations of thrombin for 18 hours. No effect on pro-uPA–mediated plasminogen activation was observed subsequent to this treatment, but plasminogen activation catalyzed by tPA bound to the high-affinity site was increased to 180% of controls at 40 U/mL thrombin. Thrombin dose-response curves showed this to be the maximum effect, with half-maximal stimulation at approximately 2.5 U/mL (Fig 8). Treatment of the cells with PMA led to a similar increase in tPA binding, with again no effect on uPA binding activity.
Effect of thrombin stimulation on the functional binding of tPA to VSMCs. Serum-deprived cells were stimulated with thrombin for 18 hours at the concentrations shown under serum-free conditions. The plasminogen activator activity of bound tPA was determined as in Fig 5 under conditions preferential for the detection of the high-affinity site (preincubation with 10 nmol/L tPA). Cells were also assayed in suspension (as in Fig 4) with similar results. Using conditions preferential for detection of the low-affinity site (see legend to Table 3), plasmin generation was essentially unchanged (<5% increase). Data shown represent the mean ± SD (n = 4).
Effect of thrombin stimulation on the functional binding of tPA to VSMCs. Serum-deprived cells were stimulated with thrombin for 18 hours at the concentrations shown under serum-free conditions. The plasminogen activator activity of bound tPA was determined as in Fig 5 under conditions preferential for the detection of the high-affinity site (preincubation with 10 nmol/L tPA). Cells were also assayed in suspension (as in Fig 4) with similar results. Using conditions preferential for detection of the low-affinity site (see legend to Table 3), plasmin generation was essentially unchanged (<5% increase). Data shown represent the mean ± SD (n = 4).
DISCUSSION
Proteolytic enzymes are increasingly being recognized as playing an important role in the migration and proliferation of VSMCs associated with the development and progression of vascular lesions. In many other pathophysiologic conditions involving invasive cell migration, the generation of plasmin activity is thought to be a determining step due to its manifold effects. The results of our study support the contention that plasmin generation may also be a determining step in mobilizing VSMC proteolytic activity by showing that these cells possess two independent receptor-mediated mechanisms for promoting cell surface plasmin generation. This is facilitated by specific cellular binding sites for each of the plasminogen activators, uPA and tPA; the former being uPAR, the well-characterized glycolipid-anchored uPA receptor, and the latter being a novel high-affinity, specific binding site for tPA.
tPA was found to bind to three distinct classes of binding site on VSMCs. The highest affinity interaction, detected by 125I-labeled tPA binding, was the binding of tPA to PAI-1, as has previously been observed on both this cell type21 and endothelial cells.40-43 The other sites were not detected by this methodology, this probably being precluded by the high affinity of the PAI-1 sites and the high level of apparently nonspecific binding observed (as discussed below). To overcome this and to detect the binding of functionally active tPA, we endeavored to determine bound tPA by plasminogen activation. This functional assay enabled us to detect the two additional classes of tPA binding. The lower affinity site bound both tPA and the deletion mutant K2P. The activity of tPA bound to this site was not significantly affected by the cellular binding of plasminogen and a large proportion of the binding capacity appeared to reside in the ECM. In contrast, the higher affinity site bound only tPA, binding could be inhibited greater than 85% by inactive tPA, binding was intimately associated with the cells, and the activity of bound tPA was increased at least 90- to 100-fold on plasminogen binding. This latter observation, by analogy with the uPAR-mediated system, is a consequence of the preferential activation of cell-bound plasminogen by the bound tPA. The lack of binding of K2P to the high-affinity site implicates regions N-terminal to kringle 2 in the high-affinity binding of tPA. Of these, the fibronectin type-I and EGF-like modules appear to have the characteristics necessary to mediate this interaction, because the fibronectin type-I module is involved in tPA binding to fibrin and the EGF-like module is thought to be involved in the clearance of tPA by hepatic parenchymal cells.44 However, an auxiliary involvement of the lysine-binding kringle 2 cannot be excluded by the present experiments.
The binding parameter Bmax determined by functional assay shows the maximum rate of plasmin generation by bound tPA, rather than the maximum number of binding sites. In principle, discrimination between multiple classes of binding site is only possible when their Bmaxs are of the same order of magnitude and their affinities are sufficiently different.45 This is the case for the two classes of binding sites determined here by functional assay, because both mediate approximately equivalent levels of plasminogen activation. However, due to the potentiation of plasmin generation by tPA bound to the high-affinity site, it must of necessity involve a proportionally lower number of binding sites. This accounts for the lack of discrimination of the high-affinity site by radioligand binding when the criteria described above are not fulfilled. Although a direct comparison between the activities of free and bound tPA was not possible, estimates of bound tPA can be made by comparison with the plasminogen activator activity of tPA in the solution phase. On this basis, the low-affinity site is equivalent to 1.4 × 106 binding sites/cell, and because tPA activity did not appear to be affected by binding to this site, this may be a reliable approximation. From the 90- to 100-fold increase in plasmin generation observed on tPA binding to the high-affinity site, it can be estimated that this binding represents approximately 15,000 sites/cell.
Comparison of maximal activities of tPA and uPA on these cells shows that, at saturating concentrations of the activators, the high-affinity tPA binding site accounts for approximately eightfold more plasmin generation than does uPAR. This advantage of the tPA site will be lessened at equal (nonsaturating) concentrations of the two activators, due to the higher affinity of the uPA:uPAR interaction. However, tPA has been reported to be up to 50-fold more abundant than uPA in the vessel wall after injury.7,46 Additionally, in the present study, thrombin, an important mediator of VSMC migration and proliferation, was found to increase tPA, but not uPA, binding (although it has previously been reported to increase uPAR levels in bovine VSMCs22 ). These observations suggest that tPA binding to the specific high-affinity site may be the primary mechanism for cell surface plasminogen activation, and therefore also for the overall generation of proteolytic activity, on VSMCs.
The high-affinity tPA binding site we detect on VSMCs is distinct from those previously reported by others on endothelial cells. Some of these investigators observed the binding of tPA only to PAI-1 on endothelial cells,40,43 whereas others have reported the active-site independent binding of tPA to a non–PAI-1 site.41,42,47,48 Varying affinities have been reported for this binding, with kds from 18 nmol/L41 to 170 nmol/L,42 but in all instances tPA binding could be efficiently competed by an excess of either uPA41,42,47 or plasminogen,48 effects that we do not observe on VSMCs. Also, in comparison to the 90- to 100-fold potentiation of the activity of bound tPA that we observe on VSMCs, the effects found on endothelial cells were small, ranging from none42 to fourfold,41 and therefore comparable to the effect observed here for the low-affinity binding site. However, the latter investigators have reported a 60-fold increase in the catalytic efficiency of tPA in the presence of a purified endothelial cell tPA-binding protein, which was found to be identical to annexin II.49 Within the vasculature, annexin II has been reported to be expressed only by endothelial cells50 and not to be expressed in vascular50 or indeed other types51 of smooth muscle. Furthermore, we have found no evidence for a specific site on endothelial cells that facilitates cell-surface plasminogen activation with the characteristics described here for the VSMC site (unpublished observations) and antibodies to annexin II did not compete the binding of tPA. In contrast to the PAI-1–mediated binding of tPA to VSMCs,21 the functional binding that we observe does not involve LRP, because binding was not inhibited by RAP and there was no evidence for internalization of tPA during the course of the activity assays. Therefore, the high-affinity binding of tPA to VSMCs appears to be mediated by a novel tPA receptor that may have a major role in tPA function.
tPA has been reported to be a potent mitogen for VSMCs.52 This effect is dependent on the catalytic activity of tPA, but not plasmin generation, and was found to saturate at a tPA concentration of approximately 30 nmol/L, similar to the high-affinity binding observed here. The necessity for tPA catalytic activity suggests that the mitogenic effect may involve a protease-activated receptor analogous to the thrombin receptor,53 although this remains to be determined. It is possible that the two tPA binding phenomena are related and that the binding of tPA to its high-affinity binding site on VSMCs not only increases the functional activity of the protease, and thus contributes to ECM degradation and cell migration, but also induces proliferation of these cells.
The characteristics of uPA binding to VSMCs are in accordance with those expected for an interaction mediated by uPAR. The effects of this binding on overall plasmin generation are also both qualitatively and quantitatively similar to those that we have previously observed on monocytes and monocytoid cell lines.18,20 The recent observation that uPAR is localized in caveolae in two cell types that are abundant in this membrane specialization, melanoma cells34 and VSMCs,33 has led to the proposal that the high efficiency of uPAR-mediated cell surface plasmin generation is due to the clustering of uPAR in this membrane compartment.34 However, we find no evidence to support this, because disruption of these structures did not interfere with the functional activity of bound uPA (or tPA; data not shown). This is consistent with our previous studies that have characterized the potentiation of the uPA-catalyzed plasminogen activation system on monocytes and monocytoid cell lines,18,20 cells that neither form caveolae nor have a uPAR immunostaining pattern consistent with such a specialized distribution.54 We have also shown in model systems that the effects of cell surface uPAR on plasmin generation do not require clustering24 or the presence of a GPI moiety.55 Thus, the GPI-mediated incorporation of uPAR into caveolae does not appear to affect its function in facilitating cell surface plasminogen activation on VSMCs.
The plasminogen activators have generally been understood to have quite distinct biologic roles; tPA in fibrin dissolution and the maintenance of vascular patency and uPA in the generation of pericellular proteolytic activity in tissues,19 based both on their respective distribution in vivo and the mechanisms by which their otherwise poor activities are enhanced. tPA is expressed in very few tissues or cell types, the primary source probably being the endothelium, and its activity is stimulated by binding to fibrin. uPA is expressed by many cell types and in various tissues and its activity is potentiated by binding to cell surface uPAR. However, recent observations in transgenic mice with targeted disruption of the genes for tPA and uPA have shown that there is potential overlap in the function of the two activators as uPA can compensate for a lack of tPA, at least in the removal of fibrin deposits.15 The demonstration that VSMCs have two independent mechanisms for the generation of cell surface plasmin activity shows a previously unsuspected role for tPA-catalyzed cell surface plasminogen activation in ECM degradation and cell migration. Although the distribution of tPA is very limited compared with that of uPA, VSMCs are one of the few cell types that express both tPA and uPA in vivo, and the expression of both is upregulated in response to vascular injury and in atherosclerotic vessels.5-7 Together, these observations provide strong evidence to support the hypothesis that VSMCs use tPA in an autocrine manner to facilitate plasminogen activation by a receptor-dependent mechanism, analogous to the uPA/uPAR system that is also operative in these cells. Characterization of the protein responsible for the specific high-affinity binding of tPA to VSMCs will help to further elucidate the role of tPA binding in VSMC function.
ACKNOWLEDGMENT
The authors thank Dr Jan Verheijen for the gift of K2P, Prof Roger Lijnen for Ser478 → Ala tPA, Prof Katherine Hajjar for anti-annexin II, Dr Dudley Strickland for RAP, Dr Jack Henkin for ATF, and Dr Michael Ploug for recombinant uPAR. We also thank Ulla Dennehy and Sally Mill for expert assistance with VSMC culture and Dr Michael Scully and Prof Fedor Bachmann for helpful comments and discussion.
Supported by the British Heart Foundation (Grants No. PG95029 and PG96041 to V.E.).
Address reprint requests to Vincent Ellis, PhD, Thrombosis Research Institute, Emmanuel Kaye Building, Manresa Road, London SW3 6LR, UK.

![Fig. 7. Involvement of VSMC ECM in the functional binding of tPA. The ECM remaining after removal of VSMCs by lysis was preincubated with tPA and assayed for plasmin generation as in Fig 5. In the experiment shown, the tPA binding constants in the presence of cells were determined to be kd1 = 16 nmol/L (175 [pmol/L]⋅min−1) and kd2 = 1,000 nmol/L (500 [pmol/L]⋅min−1) and in the absence of cells a single class of site kd = 590 nmol/L (305 [pmol/L]⋅min−1).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/6/10.1182_blood.v90.6.2312/3/m_bl_0026f7.jpeg?Expires=1766869912&Signature=bhNpblZCYuYIi8MgbLCBO6E87Sq3wf9Z0wcr6sH7T6NFU2eDnwVixjzNSYI0tU937bWPX-zS~3fP47VsJrwHUo3NB0jHh8usfsZNHVPStTAd6-P9fNVQDP7GhXcHps750Zwv34mGl990aMBdvO9EYHfDVmMHhz60mMlkEBhuEqWEQ7v93l34pzicLRKQyDivcHmTmszPSgofYjkqf21P1ssIQ7uV73mcIcxkLNms6CTdy6oBnswTgPJPLNCIVq9wsPaj0viQ9xV~wetDJS2a~FnPjly1y2meycULVYoXnzSndW8OqODbbMwLNR0Fw5WL38rC1PV8Y9qKYg3ouMpl9A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal