Abstract
The production of interleukin-1β (IL-1β), a powerful mediator of inflammation, is tightly regulated at several levels. However, in some pathologic conditions, a pharmacologic treatment is required to control the toxicity of excessive extracellular IL-1β. Because of the heavy side effects of most therapies used in IL-1β–mediated pathologies, a goal of pharmacologic research is the development of selective anti–IL-1β drugs. We show here that the sulfonylurea glyburide, currently used in the oral therapy of noninsulin dependent diabetes, is an inhibitor of IL-1β secretion from human monocytes and mouse macrophages. Glyburide reduces dramatically the recovery of extracellular 17-kD IL-1β in the absence of toxic effects on the cells and without affecting the synthesis or processing of the IL-1β precursor. IL-1β belongs to the family of leaderless secretory proteins released from the cell by a nonclassical secretory route. In bacteria and yeast Atp binding cassette (ABC) transporters are involved in the secretion of leaderless secretory proteins. Interestingly, glyburide blocks the anion exchanger function of ABC1, a mammalian member of the family of ABC transporters. We thus investigated the involvement of ABC1 in IL-1β secretion, through the analysis of the effects of drugs known to inhibit IL-1β secretion, on the activity of ABC1 and in turn the ability of known inhibitors of ABC1 of blocking IL-1β secretion. Our data show that IL-1β secretion and the function of ABC1 as an anion exchanger are sensitive to the same drugs, therefore suggesting an involvement of the ABC1 transporter in the secretion of leaderless proteins in mammals.
INTERLEUKIN-1β (IL-1β) is one of the most effective mediators of inflammatory reactions primarily produced by activated monocytes or macrophages. Agents able to impair its production or secretion are therefore of high therapeutic impact. However, unfortunately, the molecular mechanism underlying IL-1β secretion is still obscure.1-3
IL-1β is unique among cytokines, because it is not secreted via the classical exocytic pathway. The lack of a secretory signal peptide hampers its targeting to the endoplasmic reticulum. Large amounts of IL-1β, synthesized as a precursor polypeptide of 33 kD, are found in the cytosol, from which the cytokine gains access to the extracellular medium. An additional step is required, because the propolypeptide has to be proteolitycally cleaved by the IL-1β converting enzyme (ICE or caspase I)4,5 to the mature, fully active 17-kD form. ICE-mediated processing and the secretory step appear to occur cotemporally and lead to the exclusive recovery of the mature form in the extracellular medium. The subcellular site where maturation takes place is still an open question. Indeed, whereas ICE and IL-1β colocalize in the cytosol of IL-1β–secreting macrophages, the enzymatic activity of ICE is undetectable in the cells.6 Recently, Singer et al7 have proposed on the basis of IEM data that the maturation takes place during the translocation of the precursor (proIL-1β) along a transmembrane channel in which active ICE had previously or concomitantly docked.
Because similar pathways in prokaryotes and lower eukaryotes use dedicated Atp binding cassette (ABC) translocators, it is reasonable to hypothesize that mammalian ABC transporters act as exporters for IL-1β and other leaderless secretory proteins (see for review Kucher et al8 ). Indeed, ABC transporters can handle a wide variety of substrates and are able to mediate energy-dependent membrane translocation of proteins of fairly large molecular weight such as toxins secreted by gram-negative bacteria.9 We have recently characterized a novel member of this family of protein, ABC1, conserved in mice and humans.10 ABC1 is expressed by macrophages, where it is required during the engulfment of apoptotic corpses.11 When expressed in the heterologous system of Xenopus oocytes, ABC1 behaves as an electroneutral anion exchanger with a characteristic pharmacologic profile.12
We report here on the dramatic inhibitory effect of the sulfonylurea glyburide on IL-1β secretion both in the human and mouse systems. Glyburide happens to be one of the most potent inhibitors of ABC1 activity as an anion transporter in X oocytes and in mouse peritoneal macrophages. Hence, we extended the investigation to other ABC1 blockers and, in turn, to inhibitors of IL-1β secretion13-15 to show that these two effects share a similar pharmacology.
MATERIALS AND METHODS
Preparation of mouse peritoneal macrophages. Thioglycollate-elicited peritoneal macrophages (2 mL intraperitoneal injection of thioglycollate medium 4 days previously) were harvested by peritoneal washes from 8- to 12-week-old B6/CBA mice, extensively washed in complete medium (RPMI 1640 supplemented with 10% fetal calf serum [FCS]), and plated at 2 × 105 cells/well in 96-well microtiter plates for secretion studies or at 4 × 105/well in 48-well plates for efflux studies. After incubation at 37°C for 2 hours, nonadherent cells were removed and monolayers were incubated overnight at 37°C. When required, lipopolysaccharide (LPS) stimulation was performed for 3 hours at 1 μg/mL, followed by 30 minutes of incubation in the presence of 5 mmol/L ATP.
Cytokine detection. The levels of murine cytokines (IL-1β, IL-1α, tumor necrosis factor α [TNFα], and IL-6) were measured by specific enzyme-linked immunosorbent assay (ELISA) kits (Genzyme, Cambridge, MA), following the manufacturer's instructions, on the supernatants of the 30-minute ATP stimulation. When necessary, the same peritoneal macrophage supernatants were used for the simultaneous detection of the four cytokines. The drugs were added at the indicated concentrations during the 30 minutes of ATP incubation. Each experimental point was assessed on four individual wells.
Preparation of human monocytes and assessment of cytokine release by Western blotting. Peripheral blood mononuclear cells (PBMC) were isolated from buffy coats from healthy donors by Ficoll-Hypaque gradients, resuspended at 107/mL in RPMI medium containing 10% FCS, and plated as described.2 After 1 hour at 37°C, nonadherent cells were removed and adherent cells were activated for 1 hour with 5 μg/mL LPS (Sigma, St Louis, MO) in RPMI/FCS. Supernatants were then replaced with RPMI 1% Nutridoma (Boehringer Mannheim, Mannheim, Germany) and incubation was performed for additional 30 minutes in the presence of 1 mmol/L ATP or 4 hours in the absence of ATP.16 At the end of incubation, supernatants were concentrated by precipitation with 10% trichloroacetic acid (TCA) and cells were lysed in 0.25% NP40. Aliquots of cell lysates and supernatants were boiled in Laemmli sample buffer containing 2β mercaptoethanol (2ME), resolved by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and blotted to Hybond-ECL (Amersham International, Little Chalfont, UK), as described.17 Blots were probed with anti–IL-1β monoclonal antibody 3ZD or with the polyclonal goat anti–IL-6 (generous gift of F. Cozzolino, Rome, Italy), followed by peroxidase-conjugated secondary antibody (Dakopatts, Glostrup, Denmark) and developed with the ECL system (Amersham) following the manufacturer's instructions.
Oocytes preparation. Stage V Xenopus laevis oocytes were prepared and injected with ABC1 encoding cRNA or water as previously described in detail.12 Each batch of oocytes was tested for faithful expression of ABC1 transporter by immunoprecipitation with anti-ABC1 specific antibody.
Iodide efflux measurements. The procedure for I125 efflux measurements from X oocytes is described in detail in Becq et al.12 For peritoneal macrophages, the procedure is essentially as described in Becq et al.18 Briefly, macrophages cultured overnight in complete medium in 48-well plates were washed twice in modified Earle's salt solution (ESS) and loaded with 1 mL of 1 μmol/L KI (0.1 μCi/mL of NaI125 ) in ESS for 30 minutes at 37°C. After a few washes to achieve equilibration, the amount of radioactive iodide in the efflux medium was measured at 1-minute intervals for 11 minutes. The first two points were used to establish a stable efflux baseline. At the end of incubation (t8), the medium was recovered and the amount of cell associated radioactivity was counted after lysis of the monolayers in 1 N NaOH. The drugs to be tested were dissolved in dimethyl sulfoxide (DMSO; final concentration, 0.1%) and added during the ATP incubation and the efflux washes. For both oocytes and macrophages experiments, efflux curves were generated by plotting the percentage of total radioactivity released for each time point versus time. Data are presented as the mean ± SD for n observations. Statistical significance is assessed at the 95% confidence level with the Student's t-test.
Chemicals. Glyburide, 4,4′-diisothiocyanostilbene-2,2′-disulfonic acid (DIDS), sulphobromophtaleine (BSP), and ATP were purchased from Sigma; Tenidap and UK5099 were provided by Pfizer (Grotow, CT); and the peptide inhibitor of ICE, AcYVAD-chloromethylketone, was purchased from Bachem Biochemica (Bubendorf, CH). When necessary, the compounds were dissolved in DMSO and used at a final DMSO concentration never exceeding 0.1%. LPS (Escherichia coli strain 0111:B4) was purchased from Calbiochem (La Jolla, CA).
RESULTS
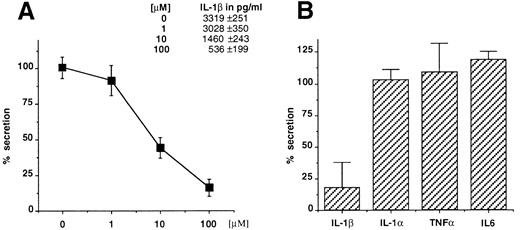
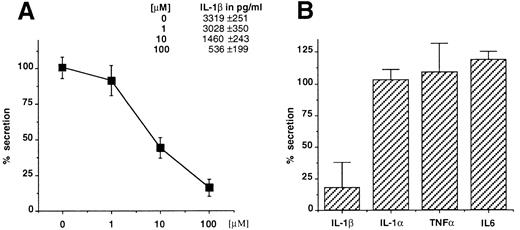
Glyburide blocks IL-1β secretion from mouse macrophages and human monocytes. Thioglycollate-elicited mouse peritoneal macrophages were stimulated with LPS (1 μg/mL for 3 hours) and ATP (5 mmol/L for 30 minutes) in the presence of increasing concentrations of glyburide (1, 10, or 100 μmol/L), and the amount of IL-1β recovered in the supernatant of the ATP incubation was measured by ELISA (Fig 1A). The results showed that glyburide induces a dose-dependent reduction of IL-1β secretion. To control the specificity of this effect, the supernatants at the most effective doses were used to measure the secretion of IL-1α, which also leaves the cells via unusual mechanisms, and of TNFα and IL-6, which are exocytosed by the classical ER-Golgi pathway.19 In each experiment, four individual samples were measured and averaged for each experimental point. As shown in Fig 1B, glyburide treatment leaves almost unaffected the amounts of secreted IL-1α (n = 4), TNFα (n = 6), or IL-6 (n = 4).
Glyburide inhibits the secretion of IL-1β from mouse peritoneal macrophages without affecting the release of IL-1α, TNFα, or IL-6. (A) Dose-dependent inhibition of IL-1β release from macrophage monolayers as assessed by ELISA. Results are averaged from four individual assays and expressed as a percentage of release in the absence of the drug. Absolute values in picograms per milliliter are indicated. (B) Comparative effect of 100 μmol/L glyburide on the secretion of IL-1β IL-1α, TNFα, or IL-6. Results (averaged from 6 experiments for IL-1β and TNFα and from 4 for IL-1α and IL-6) are expressed as a percentage of the amount released in the absence of drug.
Glyburide inhibits the secretion of IL-1β from mouse peritoneal macrophages without affecting the release of IL-1α, TNFα, or IL-6. (A) Dose-dependent inhibition of IL-1β release from macrophage monolayers as assessed by ELISA. Results are averaged from four individual assays and expressed as a percentage of release in the absence of the drug. Absolute values in picograms per milliliter are indicated. (B) Comparative effect of 100 μmol/L glyburide on the secretion of IL-1β IL-1α, TNFα, or IL-6. Results (averaged from 6 experiments for IL-1β and TNFα and from 4 for IL-1α and IL-6) are expressed as a percentage of the amount released in the absence of drug.
Similar experiments were performed on metabolically labeled cells and the analysis of immunoprecipitated IL-1β showed no effect of glyburide on the intracellular amounts of the cytokine, whereas the recovery of extracellular 17-kD IL-1β mirrored the results of the ELISA (not shown).
Glyburide exerts a similar inhibition on IL-1β secretion from activated human monocytes, as assessed by Western blot analysis, both after long-term LPS stimulation or after ATP enhancement (Fig 2A and B). As already reported,1 extracellular IL-1α was undetectable in both conditions (not shown). No modifications on IL-6 secretion were detected after treatment with glyburide (Fig 2C).
Glyburide inhibits IL-1β release from human monocytes without affecting the processing. Cell lysates (cyt) and supernatants (sup) from untreated human activated monocytes (U) or cells exposed to Glyburide (G; 100 μmol/L) were analyzed using Western blotting and probed with an anti–IL-1β specific antibody ([A] after LPS and ATP stimulation and [B] after long-term LPS stimulation). Reduced amounts of the 17-kD mature form of IL-1β are recovered in the supernatants of treated cells in the absence of a detectable difference in the intracellular profiles. In (C), the absence of effect of glyburide on the secretion of IL-6 is shown. In (D), supernatants from human activated monocytes either untreated (U) or treated with 100 μmol/L glyburide (G) or 100 μmol/L AcYVAD-CHO or both were analyzed using Western blotting. Whereas AcYVAD treatment leads to extracellular accumulation of proIL-1β and blocks its processing to the mature form, the treatment with glyburide clearly impairs the recovery of the precursor both in the absence and in the presence of ICE inhibitors.
Glyburide inhibits IL-1β release from human monocytes without affecting the processing. Cell lysates (cyt) and supernatants (sup) from untreated human activated monocytes (U) or cells exposed to Glyburide (G; 100 μmol/L) were analyzed using Western blotting and probed with an anti–IL-1β specific antibody ([A] after LPS and ATP stimulation and [B] after long-term LPS stimulation). Reduced amounts of the 17-kD mature form of IL-1β are recovered in the supernatants of treated cells in the absence of a detectable difference in the intracellular profiles. In (C), the absence of effect of glyburide on the secretion of IL-6 is shown. In (D), supernatants from human activated monocytes either untreated (U) or treated with 100 μmol/L glyburide (G) or 100 μmol/L AcYVAD-CHO or both were analyzed using Western blotting. Whereas AcYVAD treatment leads to extracellular accumulation of proIL-1β and blocks its processing to the mature form, the treatment with glyburide clearly impairs the recovery of the precursor both in the absence and in the presence of ICE inhibitors.
Glyburide had no effect on the activity of recombinant human ICE in vitro (not shown), thus ruling out the possibility that the reduced recovery of the 17-kD IL-1β resulted from pharmacologic interference with ICE-mediated processing.
In addition, we compared the molecular forms of IL-1β secreted by monocytes treated with acYVAD-CHO, as a specific inhibitor of ICE,20 in the presence or absence of glyburide (100 μmol/L). As shown in Fig 2D, the treatment with acYVAD-CHO leads to the accumulation in the extracellular medium of the unprocessed 33-kD proIL-1β form21; this accumulation is effectively prevented by glyburide treatment.
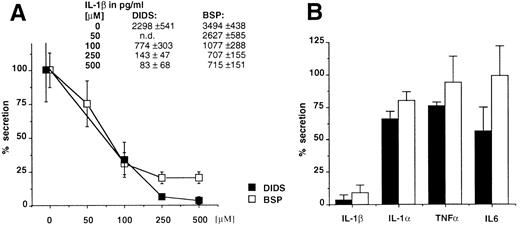
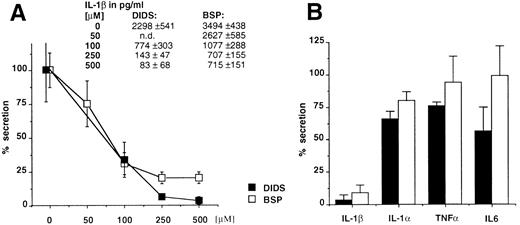
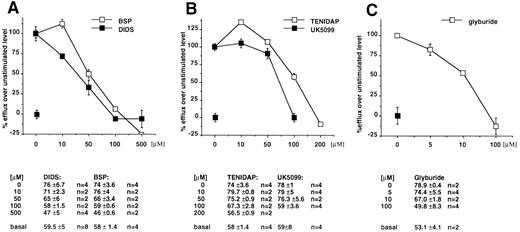
The anion transporter activity of ABC1 and IL-1β secretion are affected by the same drugs. As already reported, the expression of ABC1 transporter in X oocytes generates a specific anion efflux that can be inhibited in a dose-dependent fashion by glyburide and other compounds belonging to different pharmacologic classes, such as DIDS and BSP (inhibition of 88%, 84%, and 79%, respectively).12 We therefore analyzed the effect of the latter compounds on the secretion of IL-1β, IL-1α, TNFα, or IL-6. Figure 3A and B shows that these ABC1 blockers also inhibit IL-1β secretion while only marginally affecting the secretion of the other cytokines. On the contrary, the classical MDR reversal agent verapamil had no effect on the secretion of IL-1β (data not shown). These data indicate a clear pharmacologic correlation between ABC1 activity and IL-1β secretion.
(A) The ABC1 blockers, DIDS and BSP, inhibit the secretion of IL-1β in a dose-dependent fashion. Results are expressed as a percentage of the secretion from untreated macrophage monolayers. Absolute amounts (average from 4 individual points) of IL-1β in picograms per milliliter are reported. (B) Comparative effect of DIDS and BSP on the secretion of IL-1β, IL-1α, TNFα, or IL-6. Results (averaged from 6 experiments for IL-1β and TNFα and from 4 for IL-1α and IL-6) are expressed as a percentage of the amount released in the absence of drug.
(A) The ABC1 blockers, DIDS and BSP, inhibit the secretion of IL-1β in a dose-dependent fashion. Results are expressed as a percentage of the secretion from untreated macrophage monolayers. Absolute amounts (average from 4 individual points) of IL-1β in picograms per milliliter are reported. (B) Comparative effect of DIDS and BSP on the secretion of IL-1β, IL-1α, TNFα, or IL-6. Results (averaged from 6 experiments for IL-1β and TNFα and from 4 for IL-1α and IL-6) are expressed as a percentage of the amount released in the absence of drug.
We then tested the effect on ABC1 activity of two anti-inflammatory agent, Tenidap and UK 5099, known to reduce IL-1β secretion possibly via the perturbation of anionic fluxes that they provoke.15 Both drugs show a dose-dependent inhibition of the ABC1-mediated anion efflux from X oocytes (maximal inhibition at 100 μmol/L; data not shown).
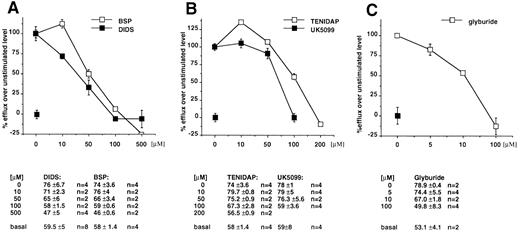
To investigate the activity of ABC1 in cells physiologically expressing the transporter, we investigated the anion efflux from ex vivo peritoneal macrophages in basal conditions and after LPS/ATP stimulation.16,22 23 An anion efflux of 51.7% ± 6.1% (n = 16) can be detected at t8 in basal conditions, and this is increased to 67.7% ± 6.6% (n = 20) after LPS/ATP stimulation. We then tested the effect of the various drugs on the basal and induced anion flux. Our data showed that a fraction of this efflux, which increases after stimulation, is sensitive to the panel of compounds. In fact, in basal conditions, efflux values at t8 were 33% ± 1% (n = 2) in the presence of DIDS (500 μmol/L); 42% ± 2% (n = 2) in the presence of BSP (500 μmol/L); and 49% ± 1% (n = 2) in the presence of glyburide (100 μmol/L).
After stimulation with LPS and ATP, DIDS reduces the efflux to 32.5% ± 3% (n = 6), BSP to 46.8% ± 2% (n = 3), and glyburide to 35.7% ± 12% (n = 3). In these conditions, the incubation with Tenidap (200 μmol/L) and UK5099 (100 μmol/L) reduces anion effluxes to 44.6% ± 5% (n = 5) and 46% ± 6% (n = 2). All the compounds are inhibitory in a dose-dependent fashion, as shown in Fig 4A through C. We concluded from these results that, in mouse peritoneal macrophages, a fraction of the anion effluxes is dependent from the activity of ABC1 transporter.
Sensitivity profile of anion fluxes from mouse peritoneal macrophages to DIDS and BSP (A), Tenidap and UK5099 (B), and Glyburide (C). I125 efflux was measured in the presence of the indicated concentrations of drugs from cells stimulated by LPS/ATP and in basal conditions. The difference between the flux in the absence of the drug from stimulated and unstimulated cells was considered the reference value. The effect of the drug is reported as a percentage of the reference flux. Absolute values are reported below each panel.
Sensitivity profile of anion fluxes from mouse peritoneal macrophages to DIDS and BSP (A), Tenidap and UK5099 (B), and Glyburide (C). I125 efflux was measured in the presence of the indicated concentrations of drugs from cells stimulated by LPS/ATP and in basal conditions. The difference between the flux in the absence of the drug from stimulated and unstimulated cells was considered the reference value. The effect of the drug is reported as a percentage of the reference flux. Absolute values are reported below each panel.
DISCUSSION
In this report, we show that glyburide is a potent inhibitor of the secretion of the leaderless cytokine IL-1β. The inhibition is selective, because secretion via the classical exocytic pathway is not impaired and is devoid of toxic effect, because macrophage viability and the rate of protein synthesis are unaffected. We have previously reported that glyburide is one of the inhibitors of the anion exchanger activity of the ABC transporter, ABC1, in the heterologous expression system of X oocytes.16 The data presented here show a precise correlation between the pharmacologic inhibition of the ABC1 activity and of the secretion of IL-1β. Indeed, in addition to glyburide, BSP and DIDS also inhibit both ABC1 and IL-1β secretion. In turn, two other compounds known to affect IL-1β secretion also inhibit ABC1.
The finding that ABC1 exerts a detectable anion exchanger function in the physiologic system of mouse peritoneal macrophages and that, in these cells, both IL-1β secretion and ABC1 activity are impaired by the same drugs suggests the involvement of ABC1 in IL-1β secretion.
The hypothesis that a member of the ABC transporter family may play a role in leaderless secretion in mammals arises from the evidence of their involvement in analogous pathways both in yeast and procaryotes. However, experiments trying to unambiguously demonstrate a direct involvement have failed so far. Moreover, drugs able to inhibit the activity of known ABC transporters such as MDR do not affect IL-1β secretion (A.R. and M.F.L., unpublished result). This is in line with our observation that ABC1 sensitivity profile is original among ABC transporters, because verapamil, a typical MDR reversing agent, does not affect ABC1 function; conversely, cystic fibrosis transmembrane regulator (CFTR) is insensitive to the ABC1 inhibitor DIDS. Although glyburide sensitivity is not exclusive to ABC1 but it is shared by CFTR and sulfonylurea receptor (SUR), the involvement of CFTR can be ruled out due to its DIDS insensitivity, whereas SUR involvement is unlikely because of its different sensitivity to glyburide and its barely detectable expression in cells of the macrophage lineage.24
Whereas glyburide profoundly affects IL-1β secretion, it only sligthly decreases, in the mouse system, the release of IL-1α, another leaderless secretory protein. Whether this means that IL-1α follows a distinct route remains to be elucidated. However, it is of note that, at least in the human system, IL-1β is the major secreted form, with IL-1α being almost undetectable extracellularly.
The molecular mechanism underlying the inhibitory effect of ABC1 blockers on IL-1β secretion is still unclear. However, the reduced secretion of IL-1β did not result from altered synthesis or increased instability of intracellular pools, because the treatment with these drugs did not modify the amounts of the intracellular cytokine. Moreover, we could exclude any effect of glyburide on the processing of the precursor by ICE. Indeed, glyburide shows no inhibitory effect on recombinant ICE in vitro and prevents the extracellular accumulation of proIL-1β induced by ICE inhibitors. Our data support the hypothesis that proIL-1β is a secretory competent form and imply that the molecular step affected by ABC1 inhibitory drugs is dissociated from the proteolytic maturation of IL-1β. Thus, our results allow to surmize an implication of ABC1 or a closely related transporter in the alternative secretory pathway for the export of the cytokine. Whether this transporter is indeed the channel through which IL-1β translocates the membrane or fulfills a permissive role for the process to take place remains to be investigated.
ACKNOWLEDGMENT
The authors thank A. Diu-Hercend and O. Krebs (Roussel-Uclaf ) for testing the effects of glyburide, DIDS, and BSP on recombinant human ICE; C. Gabel (Pfizer Inc) for discussion and for having provided Tenidap and UK5099; F. Cozzolino for the anti–IL-6 antiserum; C. Reynolds for the 3ZD antibody; and B. Verrier and M. Gola for discussion. The Centro Trasfusionale of Gaslini Hospital (Genova, Italy) is also thanked for the generous gift of buffy coats.
Supported by institutional grants from INSERM and CNRS and specific grants from ARC, AIRC, and LLNC. F.B. was supported by a postdoctoral fellowship from AFLM.
Address reprint requests to Giovanna Chimini, MD, Centre d'Immunologie de Marseille-Luminy, Parc Scientifique de Luminy, Case 906, 13288 Marseille Cedex 09, France.

![Fig. 2. Glyburide inhibits IL-1β release from human monocytes without affecting the processing. Cell lysates (cyt) and supernatants (sup) from untreated human activated monocytes (U) or cells exposed to Glyburide (G; 100 μmol/L) were analyzed using Western blotting and probed with an anti–IL-1β specific antibody ([A] after LPS and ATP stimulation and [B] after long-term LPS stimulation). Reduced amounts of the 17-kD mature form of IL-1β are recovered in the supernatants of treated cells in the absence of a detectable difference in the intracellular profiles. In (C), the absence of effect of glyburide on the secretion of IL-6 is shown. In (D), supernatants from human activated monocytes either untreated (U) or treated with 100 μmol/L glyburide (G) or 100 μmol/L AcYVAD-CHO or both were analyzed using Western blotting. Whereas AcYVAD treatment leads to extracellular accumulation of proIL-1β and blocks its processing to the mature form, the treatment with glyburide clearly impairs the recovery of the precursor both in the absence and in the presence of ICE inhibitors.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/8/10.1182_blood.v90.8.2911/4/m_bl_0049f2.jpeg?Expires=1764017350&Signature=sdN5vhFjDr41aIGkyPlWhbccGqIK47cBkOiCTxwaW0fgFdRjgPI9lXUsBuMBa4b0CVDi5LxF4o5484nvq-zzg8GVOGl3gFcTdN5J-BRSNNDa-XEGm7lgCXagvTe4A8otZ4Zvz7T4tgNYknwxKgU2xUGDsBcYOky32QNk14Ynip4Vp9-UoiAAWo28HjPv4mlWuzQMvHGnsHZUu0buAIjsEgcT10uWGTN0detmCX0fpNdH0LAN01FklFcbFW9KMX4SD50S8yWlvS1gretN5oHmhaU98i75J-Jzmv7anFcZiJ9-2DplXNYoQJZxNDsI4QAPZDT~ugbKeaGqjCiHIiosKQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 2. Glyburide inhibits IL-1β release from human monocytes without affecting the processing. Cell lysates (cyt) and supernatants (sup) from untreated human activated monocytes (U) or cells exposed to Glyburide (G; 100 μmol/L) were analyzed using Western blotting and probed with an anti–IL-1β specific antibody ([A] after LPS and ATP stimulation and [B] after long-term LPS stimulation). Reduced amounts of the 17-kD mature form of IL-1β are recovered in the supernatants of treated cells in the absence of a detectable difference in the intracellular profiles. In (C), the absence of effect of glyburide on the secretion of IL-6 is shown. In (D), supernatants from human activated monocytes either untreated (U) or treated with 100 μmol/L glyburide (G) or 100 μmol/L AcYVAD-CHO or both were analyzed using Western blotting. Whereas AcYVAD treatment leads to extracellular accumulation of proIL-1β and blocks its processing to the mature form, the treatment with glyburide clearly impairs the recovery of the precursor both in the absence and in the presence of ICE inhibitors.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/8/10.1182_blood.v90.8.2911/4/m_bl_0049f2.jpeg?Expires=1764017351&Signature=n5zIH9cAlmgi6QcEn9PoTkTSSVocbgpURWUaWFqBseoibj3-njkKyqYK8Txbpw9HCK3RwBKFHRUuhPrWY0A4ELjRP8E43GC25VL1cEBVB0ej-CCG8ABKmw9srzh5WeWJ~hSHOsEGxt21DhXdtwadRH6TvpSXQZmPgbH0XOpc80NWlhgvvbLdATugVsv~bdjyaBuBSe7IBwmq7NZ6rIS7AjizZxuWWKCQcz3tZVKsAtf1wieesUDMLBZWuB-eju7CCYAbaQfqgy24vIwLF8tPIA-pFjikp4~JZqZ76J97QxqlSz9cqUSfsr1EUKTT4Zq23JDhZwpZjYlld05Vo2f5cg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)