Abstract
Studies of hematopoietic progenitor cell development in vivo, ex vivo, and in factor-dependent cell lines have shown that c-kit promotes proliferation through synergistic effects with at least certain type 1 cytokine receptors, including the erythropoietin (Epo) receptor. Presently, c-kit is shown to efficiently support both mitogenesis and survival in the FDCP1 cell subline, FDC2. In this system, mitogenic synergy with c-kit was observed for ectopically expressed wild-type Epo receptors (wt-ER), an epidermal growth factor (EGF) receptor/Epo receptor chimera, and a highly truncated Epo receptor construct ER-Bx1. Thus, the Epo receptor cytoplasmic box 1 subdomain appears, at least in part, to mediate mitogenic synergy with c-kit. In studies of potential effectors of this response, Jak2 tyrosine phosphorylation was shown to be induced by Epo, but not by stem cell factor (SCF). In addition and in contrast to signaling in Mo7e and BM6 cell lines, in FDC2-ER cells SCF and Epo each were shown to rapidly activate Pim 1 gene expression. Recently, roles also have been suggested for the nuclear trans-factor GATA-1 in regulating progenitor cell proliferation. In FDC2-ER cells, the ectopic expression of GATA-1 had no detectable effect on Epo inhibition of apoptosis. However, GATA-1 expression did result in a selective and marked inhibition in mitogenic responsiveness to SCF and to a decrease in c-kit transcript expression. These studies of SCF and Epo signaling in FDC2–wt-ER cells serve to functionally map the ERB1 region as a c-kit–interactive domain, suggest that Pim1 might contribute to SCF and Epo mitogenic synergy and support the notion that SCF and Epo may act in opposing ways during red cell differentiation.
THE GROWTH and survival of erythroid progenitor cells is mediated in part by the lineage-restricted, stage-specific expression of select cytokine receptors. In studies of progenitor cells ex vivo the development of burst-forming unit–erythroid (BFU-E) has been shown to depend on signaling through the receptors for stem cell factor (SCF),1 interleukin-3 (IL-3),2 granulocyte-macrophage colony-stimulating factor (GM-CSF),3 and erythropoietin (Epo),4 whereas the subsequent production and maturation of colony-forming unit–erythroid (CFU-E) is supported by SCF, Epo, and insulin-like growth factor-I (IGF-I).5,6 In these progenitor cells, the proliferative actions of SCF, IL-3, GM-CSF, IGF-I, and Epo involve effects on both mitogenesis and survival.7,8 For SCF and Epo in particular, important roles in promoting red cell production have been established through studies in mice with mutations at W (white-spotting, c-kit) and Sl (steel, SCF) loci9,10 and through recent studies of Epo receptor disruption in chimeric mice.11,12 In steel and white-spotting mice, erythropoiesis is compromised significantly9,10 and macrocytic anemia is observed in mice homozygous for the well-studied Sld allele.10 Also compromised is the production of early pluripotent progenitor cells, tissue mast cells, and at least certain myeloid progenitor cells. By comparison, in mice in which the expression of the Epo receptor gene has been disrupted BFU-E and CFU-E are produced, but in erythropoiesis beyond the CFU-E stage is blocked.11,12 Taken together, these findings suggest that SCF and Epo cotarget erythroid progenitor cells at the late BFU-E or early CFU-E boundary. Consistent with this notion is the observed ability of SCF and Epo to costimulate mitogenesis in progenitor cells ex vivo13 and in at least certain factor-dependent hematopoietic cell lines.14-16
Among hematopoietic cell lines used to study SCF and Epo cosignaling to date, HCD57 cells perhaps have been best studied.15,16 In this murine erythroleukemic line, c-kit and the Epo receptor each are expressed and each have been shown to support mitogenesis. In addition, in recent studies in this model15,16 SCF interestingly has been shown to activate the tyrosine phosphorylation of the Epo receptor. However, molecular mechanisms that mediate this apparent trans-phosphorylation event and its functional significance are largely undefined. In addition, in recent studies by Jacobs et al16 SCF has been observed to fail to effectively support HCD57 cell survival. Finally, mitogenic synergy between SCF and Epo also has been shown in MBO2 cells,14 and in P815 mastocytomic cells SCF and Epo cosignaling events have been studied.15 In this latter model, however, the interpretations of possible mechanisms are complicated by the consideration that in P815 cells c-kit is mutated at Tyr814, and this mutation reportedly results in constitutive activation.17 18
In an effort to provide an advantageous model for mechanisms of c-kit/Epo receptor interactions, we presently have assessed the extent to which mitogenesis and survival might be supported by SCF and Epo in murine myeloid FDCP1 cell subclone FDC2.19 In this system, Epo-dependent mitogenesis20,21 and inhibition of apoptosis22 previously have been shown to be supported by transfected Epo receptor forms. We now show that endogenous receptor for SCF likewise efficiently mediates mitogenic and survival responses in this system and demonstrate that mitogenic synergy in derived FDC2-ER cells is supported by c-kit and the wild-type Epo receptor (wt-ER). In addition, studies of the activity of minimal Epo receptor forms suggest a primary role for the Epo receptor cytoplasmic box 1 domain in mediating this synergy, and possible roles for effectors that are activated via the box 1 domain (Jak2, pim 1, and c-myc) are considered experimentally.
Recently, significant effects on progenitor cell growth and survival also have been suggested to be exerted by the erythroid trans-factor, GATA-1.23,24 In studies by Weiss et al23 evidence for a role for GATA-1 in survival has been suggested based on the observation that programmed cell death is activated in a factor-dependent cell line derived from GATA-1–deficient ES cells, and is repressed on the forced expression of wt GATA-1. By comparison, mitogenesis in 3T3 fibroblasts recently has been shown to be inhibited on the retroviral-mediated expression of GATA-1, with an associated delay in cell cycle progression observed at the G1 to S boundary.24 Based on these reports, possible effects of the ectopic expression of GATA-1 on SCF and Epo signaling in FDC2-ER cells also were investigated. No detectable effects of GATA-1 expression on the Epo-dependent inhibition of apoptosis were observed. However, GATA-1 enforced a selective inhibition of mitogenic signaling by SCF, and this was associated with a decrease in levels of c-kit transcripts. Overall, the present studies establish FDC2-ER cells as an advantageous model for investigations of SCF and Epo mitogenic synergy and begin to address the nature of molecular mechanisms that mediate this important example of integrated signaling between hematopoietic type-1 cytokine receptors and c-kit.
MATERIALS AND METHODS
Epo receptor and GATA-1 constructs and expression vectors. Epo receptor constructs used in this study include the murine wt-ER,25 a form truncated at residues to delete the box 2 subdomain and all distal cytoplasmic residues (ER-Bx1),19 and a chimeric construct (EE483) in which the extracellular domain of the epidermal growth factor (EGF) receptor (residues Leu1-Cys620 ) was fused directly to the transmembrane and full-length cytoplasmic domains of the murine Epo receptor.26 For expression, modified pXM vectors were used for wt-ER and ER-Bx1 constructs.19,20 For EE483, a previously constructed EGF receptor-Epo receptor chimeric cDNA was cloned into pGEM5Zf+ at Not I and Sal I sites to acquire a 5′ Spe I site. This cDNA was then cloned at 5′-Nhe I and 3′-Sal I sites into a modified pCINeo vector (pCIneo BII).26 For GATA-1, a full-length murine GATA-1 cDNA27 was cloned from pBlueScript-II into 5′Kpn I and 3′Not I sites within a pXM expression vector.
Electrotransfection and culture of FDC2 cells and derived cell lines. The IL-3–dependent murine myeloid cell line used in this study, FDC2, is a subclone (FDCWEHI-2) of FDCP1 cells28 and originally was isolated based on resistance in transformation to factor-independent growth. FDC2 cells and derived cell lines routinely were maintained in OptiMEM medium, 8% fetal bovine serum (FBS), 10 μmol/L 2-mercaptoethanol supplemented with conditioned medium from WEHI-3B cells (W3CM) as a source of IL-3. FDC2 lines ectopically expressing Epo receptor forms were prepared by stable coelectrotransfection with the above pXM expression vectors (50 μg) and pCINeo (2.5 μg) as described previously.20 FDC2 cells ectopically expressing the above EGF receptor-Epo receptor chimera were prepared by stable transfection with 60 μg of pCINeo-EE483. Lines ectopically expressing Epo receptor forms were established by selection in G418 (1 mg/mL, 14 days) and EPO (50 U/mL, 5 passages), whereas lines expressing pCINeo-EE483 were established by selection in G418 (1 mg/mL, 14 days) and EGF (25 ng/mL, 5 passages). FDC2 cell lines ectopically coexpressing wt-ER and GATA-1 were prepared by cotransfection with pXMwtER (5 μg) and pXMG1 vectors (55 μg) and selection in Epo. Clonal lines were isolated by limiting dilution.
Assays of cytokine-induced mitogenesis. Cytokine-induced mitogenesis of FDC2 cells and derived cell lines was assayed based on stimulated rates of the reduction of MTS [3-(4,5-dimethylthiazol-2-yl) -5- (3-carboxymethoxyphenyl) -2- (4-sulfophenyl-2H-tetrazolium] to formazan (Promega, Madison, WI),19 or the incorporation of [3H]thymidine.20 Briefly, cells (3 × 105 cells per mL, 50 μL per well, 96-well plate) were exposed to cytokines (50 μL) for 48 hours (37°C, 5% CO2 ). MTS and phenazone methosulfate were added, and at 2 hours of incubation absorbance (A490 ) was measured (microplate reader, Model 550, Bio-Rad, Rockville Centre, NY). Alternatively, incubations were with [3H]thymidine (1 μCi per well for 2 hours), and scintillation counting of harvested cells was performed using a 1205 Betaplate counter (KBL Pharmacia, Piscataway, NJ).
Assays of apoptosis-associated DNA fragmentation. In assays of cytokine-inhibited apoptosis of FDC2 cells and derived cell lines, exponentially growing cells (6 to 9 × 105 cells/mL) were washed twice in OptiMEM medium and were adjusted to 8 × 105 in preequilibrated OptiMEM medium, 1% FBS, 10 μmol/L 2-mercaptoethanol. Cells then were incubated for 7 hours in the presence of cytokines at the concentrations indicated, and DNA was isolated using a Trevigen extraction system (Gaithersburg, MD). Internucleosomally fragmented DNA was assayed by end-labeling with [32P]-α-adenosine triphosphate (ATP; 800 Ci/mmol) and DNA polymerase I (Klenow fragment; 5 minute reaction, 23°C). Reactions were terminated by the addition of Na2EDTA, pH 8.0 (50 mmol/L final concentration) and heating (15 minutes at 70°C). Products were electrophoresed (1.4% Trevigel 500 agarose gel in 4 mmol/L Tris, 2 mmol/L NaC2H3O2 , 1 mmol/L Na2EDTA, pH 7.8) (Trevigel, Gaithersburg, MD), gels were stained with ethidium bromide to confirm equivalency in loading, DNA was transferred to Nytran membranes, and was analyzed quantitatively by phosphor-imaging.
Jak2 activation assays. FDC2–wt-ER cells (at 8 × 105 cells/mL) were washed in OptiMEM and incubated for 12 hours at 37°C, 5% CO2 in medium containing 1% FBS. Na3VO4 (500 μmol/L) then was added for a 20-minute period, followed by Epo or SCF as indicated. Cells were chilled to 0°C, washed once with OptiMEM, and lysed in 0.2 mL lysis buffer (1.1% NP-40, 150 mmol/L NaCl, 50 mmol/L Tris, 1 mmol/L Na2EDTA, 0.02% NaDOC, 1 mmol/L Na3VO4 , 2 mmol/L NaF, pH 7.5, 0.5 μg/mL leupeptin, 0.7 μg/mL pepstatin, 50 μg/mL phenylmethylsulfonyl fluoride). Cleared lysates were incubated with Jak2 antiserum (3 hours, 4°C; UBI, Lake Placid, NY) and with protein A-Sepharose CL4B (1.5 hours, 4°C). Immune complexes were washed twice with wash buffer (lysis buffer containing NP-40 at 0.55%) eluted in sample buffer (3.4% sodium dodecyl sulfate (SDS), 0.2 mol/L dithiothreitol, 0.05 mmol/L bromophenyl blue, 10% glycerol, and 0.12 mol/L Tris-HCl, pH 6.8 [100°C, 5 minutes]), electrophoresed (7.5% acrylamide/0.2% bisacrylamide SDS gels), and transferred to Nitro-Plus membranes (MSI, Westboro, MA). Membranes were blocked (1% powered milk, 3% bovine serum albumin [BSA], 0.1% Tween-20, in 10 mmol/L Tris Base, 150 mmol/L NaCl, 0.05% Tween-20) and probed with phosphotyrosine antibody 4G10 (UBI). Complexes were detected by ECL (Amersham, Arlington Heights, IL).19 For reprobing, membranes were stripped in 100 mmol/L 2-mercaptoethanol, 2% SDS, 62.5 mmol/L Tris-HCl, pH 6.7 (50°C, 20 minutes).
RNA isolation and Northern blot analyses. For use in Northern blotting, total RNA was isolated from FDC2 cells and derived cell lines by the method of Chomczynski and Sacchi.29 Cells from exponentially growing cultures were processed in TRIzol reagent (1 × 107 cells per mL of reagent; Life Technologies, Gaithersburg, MD). RNA (from 5 × 106 cells) was electrophoresed in 1.25% agarose gels containing 6% formaldehyde (with 3% electrophoresis buffer) and was blotted to Nytran membranes (Schleicher and Schuell, Keene, NH) by downward capillary transfer. Membranes were fixed by UV-irradiation (312 nm for 3 minutes) and heating (1 hour at 68°C under vacuum). For use in hybridization, 32P-labeled probes were prepared by random priming (Prime-a-Gene System, Promega) using DNA polymerase I (Klenow fragment), 50 μCi of [α-32P]dATP (3,000 Ci/mmol), and 25 ng of the following cDNA fragments: murine Epo receptor, 1.5 kb Xho I fragment of pXM–wt-ER25; murine GATA-1, 1.8 kb Kpn I-Not I fragment of pXM–GATA-127; murine EKLF, 1.2 kb Xba I fragment of pBOSEKLF30; murine c-kit, 1.6 kb Sph I-Sca I fragment of pCDM8–c-kit.31 Murine Pim-1, 1.3 kb fragment of pCMP2332; murine myc, 1.5 kb fragment of pSVLC-myc33; and murine glyceraldehyde phosphate dehydrogenase, 0.8 kb fragment of pSPgapdh. For detection of βmaj globin transcripts, a 1,100-bp murine βmaj-globin 5′ Bgl II 3′ Xba I fragment was prepared by polymerase chain reaction (PCR) using a genomic clone,34 and the following primers: 5′-CTGACAGATGCTCTCTTGGG-3′, 3′-CACAACCCCAGAAACAGACA-5′. For detection of 7S transcripts, a 7S rRNA cDNA fragment35 was prepared by PCR using the following primers: 5′-TGTAGTTCCAGCTACTCGGGAGGCT-3′, and 5′-TCCGCCTGGTCGTTCACCCCT-3′. 32P-labeled probes were isolated using Sephadex G-50 micro-columns (Pharmacia Biotech, Piscataway, NJ). Hybridizations were performed for 2 hours at 68°C in QuickHyb solution (Stratagene, La Jolla, CA) using 3 × 106 cpm of probe. Membranes were washed in 0.2× SSC, 0.1% SDS, at 50°C, and were exposed to X-OMAT AR film (Kodak, Rochester, NY). For reprobing, membranes were stripped in 0.1× SSC, 0.5% SDS at 80°C.
RESULTS
c-kit– and Epo receptor–signaling of FDC2 cell mitogenesis and survival. In primary experiments, the relative abilities of c-kit and the Epo receptor to promote mitogenesis in FDC2 cells were investigated. In nontransfected parental cells, mitogenesis was activated through endogenous c-kit at rates that were essentially equivalent to those activated by IL-3, the cytokine used to establish this factor-dependent line28 (Fig 1A). Upon stable transfection, the wt-ER likewise efficiently supported mitogenesis, and in derived FDC2–wt-ER cells the signaling capacity of c-kit was retained. In normal progenitor cells, SCF and Epo also have been shown to exert important effects on progenitor cell survival,19 and the ability of each to inhibit apoptosis in FDC2–wt-ER cells therefore also was investigated. As assayed based on inhibition of internucleosomal DNA fragmentation, SCF and Epo each were observed to effectively inhibit apoptosis in a concentration-dependent fashion (Fig 1B). In these initial experiments Epo was used at 20 U/mL (4 nmol/L; maximal mitogenic dose), whereas SCF was used at 220 ng/mL (6.6 nmol/L). In addition, the long-term growth of FDC2–wt-ER cells was supported by either Epo or SCF at essentially equivalent rates over a test period of several months (A.L.W., February 1997, data not shown). Thus, these primary studies establish FDC2–wt-ER cells as an appropriate model system for studies of proliferative signaling by c-kit and the cloned Epo receptor.
SCF- and Epo-dependent mitogenesis and inhibition of apoptosis in FDC2 and FDC2–wt-ER cells. Parental FDCP1-WEHI2 cells (ie, FDC2 cells) were transfected stably with an Epo receptor expression vector (pXM-ER) and were selected in Epo to yield FDC2–wt-ER cells. In FDC2–wt-ER cells and parental FDC2 cells, the ability of IL-3, Epo, and SCF to promote mitogenesis and/or survival was then tested. (A) Mitogenic signaling was assayed based on cytokine-stimulated rates of [3H] thymidine incorporation. To account for any minor differences in plating, values were normalized versus maximal responsiveness to IL-3 in each cell line. (B) FDC2 and FDC2–wt-ER cells were cultured for 7 hours in Optimem medium, 1% FBS in the presence (or absence) of SCF (220 ng/mL or 6.6 nmol/L), Epo (20 U/mL or 4 nmol/L), or IL-3 (20% W3CM, ≥ maximal mitogenic dose). Apoptosis then was assayed based on observed levels of intranucleosomal DNA fragmentation. Levels of fragmentation were analyzed quantitatively by phosphor-imaging.
SCF- and Epo-dependent mitogenesis and inhibition of apoptosis in FDC2 and FDC2–wt-ER cells. Parental FDCP1-WEHI2 cells (ie, FDC2 cells) were transfected stably with an Epo receptor expression vector (pXM-ER) and were selected in Epo to yield FDC2–wt-ER cells. In FDC2–wt-ER cells and parental FDC2 cells, the ability of IL-3, Epo, and SCF to promote mitogenesis and/or survival was then tested. (A) Mitogenic signaling was assayed based on cytokine-stimulated rates of [3H] thymidine incorporation. To account for any minor differences in plating, values were normalized versus maximal responsiveness to IL-3 in each cell line. (B) FDC2 and FDC2–wt-ER cells were cultured for 7 hours in Optimem medium, 1% FBS in the presence (or absence) of SCF (220 ng/mL or 6.6 nmol/L), Epo (20 U/mL or 4 nmol/L), or IL-3 (20% W3CM, ≥ maximal mitogenic dose). Apoptosis then was assayed based on observed levels of intranucleosomal DNA fragmentation. Levels of fragmentation were analyzed quantitatively by phosphor-imaging.
Beyond their independent effects on mitogenesis and survival, Epo and SCF are known to exert synergistic effects on hematopoietic progenitor cell proliferation.13 Therefore, experiments were performed to test for this synergy in FDC2–wt-ER cells and to define Epo receptor domains that might support any such effects. The approach taken here was to express select Epo receptor constructs in FDC2 cells and to assay their abilities to cofunction with c-kit in promoting mitogenesis at rates above calculated additive levels. In these experiments, three Epo receptor constructs were studied: the wt-ER, a highly truncated ER-Bx1 construct, and a chimeric construct (EE483) in which the Epo receptor extracellular and transmembrane domains were replaced with those of the human EGF receptor (Fig 2A). Primary analyses served to establish the mitogenic activities of the ERBx1 and EE483 Epo receptor forms. In the Epo receptor form ER-Bx1, the conserved box 1 cytoplasmic subdomain is retained while the adjacent box 2 domain and all distal carboxyl-terminal residues are deleted. This includes the deletion of eight (phospho)tyrosine sites for the binding of SH2 domain-encoding effectors; therefore, ER-Bx1 comprises a minimal test construct. In derived FDC2-ERBx1 cells, this minimal receptor form proved to possess significant mitogenic activity and supported Epo-induced [3H] thymidine incorporation at rates approximating 25% of maximal levels supported by the wt-ER (Fig 2B). By comparison, the chimeric construct EE483, which was developed to test possible roles for Epo receptor extracellular domains in cosignaling with c-kit, likewise was active in stably transfected FDC2-EE483 cells and mediated ligand-induced mitogenesis with a dose-response profile that paralleled that of the wt-ER (Fig 2B).
Construction, expression, and mitogenic activity of Epo receptor forms used to study Epo and SCF coinduced FDC2 cell proliferation. (A) In experiments aimed at testing possible mechanisms of Epo and SCF cosignaling, two minimal Epo receptor forms were constructed, expressed in FDC2 cells, and assayed initially for their activities in mediating ligand-induced mitogenesis in derived cell lines. In the construct ER-Bx1, 178 of a total of 236 cytoplasmic residues of the wt-ER have been deleted, including the conserved box 2 domain and eight (phospho)tyrosine sites for effector recruitment. The construct EE483 is a chimeric receptor form in which the extracellular domain of the Epo receptor was replaced by that of the human EGF receptor. (B) The mitogenic activity of the above receptor forms in FDC2-derived cell lines (FDC2, FDC2–wt-ER, FDC2–ER-Bx1, FDC2-EE483) was assayed based on cytokine-stimulated rates of [3H] thymidine incorporation. To account for any minor differences in plating, values were normalized versus maximal responsiveness to IL-3 in each cell line. Northern blotting of Epo and EE483 transcripts served to show apparent equivalence in receptor expression (B.J., August 1996, data not shown) and for the wt-ER and ER-Bx1 this has been confirmed through [125I] Epo equilibrium binding assays (400 to 600 receptors per cell).19 25
Construction, expression, and mitogenic activity of Epo receptor forms used to study Epo and SCF coinduced FDC2 cell proliferation. (A) In experiments aimed at testing possible mechanisms of Epo and SCF cosignaling, two minimal Epo receptor forms were constructed, expressed in FDC2 cells, and assayed initially for their activities in mediating ligand-induced mitogenesis in derived cell lines. In the construct ER-Bx1, 178 of a total of 236 cytoplasmic residues of the wt-ER have been deleted, including the conserved box 2 domain and eight (phospho)tyrosine sites for effector recruitment. The construct EE483 is a chimeric receptor form in which the extracellular domain of the Epo receptor was replaced by that of the human EGF receptor. (B) The mitogenic activity of the above receptor forms in FDC2-derived cell lines (FDC2, FDC2–wt-ER, FDC2–ER-Bx1, FDC2-EE483) was assayed based on cytokine-stimulated rates of [3H] thymidine incorporation. To account for any minor differences in plating, values were normalized versus maximal responsiveness to IL-3 in each cell line. Northern blotting of Epo and EE483 transcripts served to show apparent equivalence in receptor expression (B.J., August 1996, data not shown) and for the wt-ER and ER-Bx1 this has been confirmed through [125I] Epo equilibrium binding assays (400 to 600 receptors per cell).19 25
The ability of the above-defined Epo receptor forms to function synergistically was next investigated. In these experiments, FDC2–wt-ER, FDC2-ERBx1 and FDC2-EE483 cells were exposed to combined doses of SCF and Epo, and mitogenic effects first were assayed (Fig 3). In FDC2–wt-ER cells interactive signaling was observed, and levels of SCF- and Epo-stimulated mitogenesis were supported at rates as high as fivefold above calculated additive levels (Fig 3A). In FDC2-ERBx1 cells, synergy in SCF and Epo mitogenic signaling also was observed (Fig 3B). However, levels of synergy were diminished to approximately threefold above additive effects. In part this diminution might be accounted for by a modest yet reproducible increase in responsiveness to SCF that was observed selectively in FDC2-ERBx1 cells. Finally, in FDC2-EE483 cells, synergy between SCF and EGF likewise was observed at levels as high as threefold to fourfold above calculated additive effects (Fig 3C). This latter finding suggests that the synergistic cosignaling of mitogenesis by SCF and Epo apparently does not involve extracellular domains of the Epo receptor (or the contributions of putative subunits that may interact through these domains).
Mitogenic synergy between c-kit and stably transfected Epo receptor forms in FDC2–wt-ER, FDC2–ER-Bx1 and FDC2-EE483 cells. In experiments aimed at testing the ability of the wt-ER and the receptor forms ER-Bx1 and EE483 to act synergistically with SCF, FDC2-derived cell lines were exposed to SCF, Epo, or both cytokines. Levels of induced mitogenesis then were assayed as above (see Fig 2 and Materials and Methods). In each line, synergy in mitogenic signaling was observed upon Epo and SCF costimulation at all cytokine concentrations tested (SCF, 0, 6.8, 13.5, 27 ng/mL or 0, 0.2, 0.4, 0.8 nmol/L; Epo, 0, 0.11, 0.22 U/mL or 0, 0.022, 0.044 nmol/L; EGF 0, 0.5, 1.0 ng/mL or 0, 0.1, 0.2 nmol/L). Levels of synergy are indexed above histograms, and represent the fold increase in rates of [3H]thymidine incorporation over calculated additive values for Epo and SCF, or EGF and SCF.
Mitogenic synergy between c-kit and stably transfected Epo receptor forms in FDC2–wt-ER, FDC2–ER-Bx1 and FDC2-EE483 cells. In experiments aimed at testing the ability of the wt-ER and the receptor forms ER-Bx1 and EE483 to act synergistically with SCF, FDC2-derived cell lines were exposed to SCF, Epo, or both cytokines. Levels of induced mitogenesis then were assayed as above (see Fig 2 and Materials and Methods). In each line, synergy in mitogenic signaling was observed upon Epo and SCF costimulation at all cytokine concentrations tested (SCF, 0, 6.8, 13.5, 27 ng/mL or 0, 0.2, 0.4, 0.8 nmol/L; Epo, 0, 0.11, 0.22 U/mL or 0, 0.022, 0.044 nmol/L; EGF 0, 0.5, 1.0 ng/mL or 0, 0.1, 0.2 nmol/L). Levels of synergy are indexed above histograms, and represent the fold increase in rates of [3H]thymidine incorporation over calculated additive values for Epo and SCF, or EGF and SCF.
In addition to their effects on mitogenesis, Epo and SCF each exert important survival effects in both FDC2–wt-ER cells (see Fig 1 above) and in normal progenitor cells.7 8 Therefore, FDC2–wt-ER cells were used to test whether SCF and Epo might comodulate survival. In the above experiments (Fig 1) cytokines were used at concentrations that supported proliferation at or above maximal rates. In experiments designed to test whether SCF and Epo might act synergistically in supporting survival, limiting concentrations of factors were used. Specifically, conditions first were established wherein apoptosis in FDC2–wt-ER cells was partially inhibited independently by Epo or by SCF (Fig 4). Cells then were exposed under these conditions to varying concentrations of Epo (25 U/mL or 5 nmol/L, Fig 4B; 10 U/mL or 2 nmol/L, Fig 4C) in the presence or absence of SCF at 33 ng/mL (1 nmol/L; ie, approximately one-half maximal mitogenic dose). In these experiments, however, apoptosis was not detectably inhibited by coexposure to SCF and Epo beyond additive effects.
Inhibition of apoptosis in FDC2–wt-ER cells by SCF and Epo is not subject to synergy. Based on the observed synergy of SCF and Epo costimulated mitogenesis in FDC2–wt-ER cells (Fig 3, above) studies were performed to test whether this might involve effects on the inhibition of apoptosis. FDC2–wt-ER cells in Optimem medium, 1% FBS were cultured for 7 hours in the presence of SCF (33 ng/mL or 1 nmol/L), and in the presence or absence of Epo (Epo2 25 U/mL or 5 nmol/L, Epo1 10 U/mL or 2 nmol/L). Apoptosis then was assayed based on levels of intranucleosomal DNA fragmentation and products were analyzed quantitatively by phosphor-imaging. Values are means of three independent scans (±SE).
Inhibition of apoptosis in FDC2–wt-ER cells by SCF and Epo is not subject to synergy. Based on the observed synergy of SCF and Epo costimulated mitogenesis in FDC2–wt-ER cells (Fig 3, above) studies were performed to test whether this might involve effects on the inhibition of apoptosis. FDC2–wt-ER cells in Optimem medium, 1% FBS were cultured for 7 hours in the presence of SCF (33 ng/mL or 1 nmol/L), and in the presence or absence of Epo (Epo2 25 U/mL or 5 nmol/L, Epo1 10 U/mL or 2 nmol/L). Apoptosis then was assayed based on levels of intranucleosomal DNA fragmentation and products were analyzed quantitatively by phosphor-imaging. Values are means of three independent scans (±SE).
Possible molecular mechanisms of SCF and Epo proliferative cosignaling of FDC2–wt-ER cells. In the above experiments, mitogenic synergy between SCF and Epo in FDC2–wt-ER cells was shown to depend at least in part on the ERBx1 subdomain. In related experiments, signaling factors that might support this response were investigated. Recently, our laboratory has shown that the ERBx1 domain not only specifies Jak2 kinase activation,19,36 but also is necessary and sufficient for the Epo-induced expression of c-myc and the serine-threonine kinase, Pim 1.19 By comparison, whether SCF signaling depends on Jak2 is a matter of controversy,16,37-39 and in the mastocytoma cell line BM640 and in Mo7E cells41 Pim 1 expression reportedly is not activated through c-kit. Based on these considerations, studies in FDC2–wt-ER cells of SCF and Epo signaling mechanisms focused on the activation of Jak2, and pim1 and c-myc gene expression.
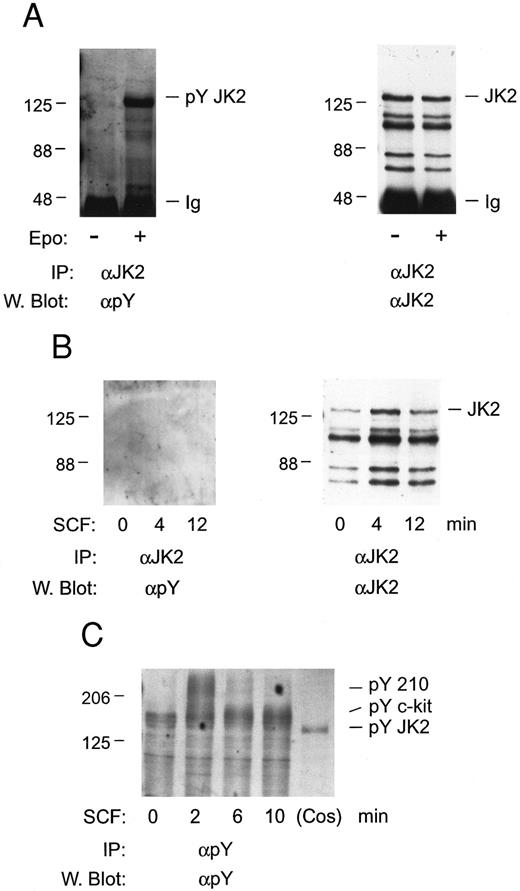
In studies of Jak2 activation, whether c-kit mediates the tyrosine phosphorylation of this Janus kinase in FDC2–wt-ER cells first was investigated. FDC2–wt-ER cells were expanded in SCF, cultured in the absence of cytokines for 7 hours, and stimulated with either SCF or Epo. Jak2 then was immunoprecipitated and tyrosine phosphorylation was assayed by Western blotting (Fig 5). Despite the expression of Epo receptors at low levels in FDC2–wt-ER cells (400 to 600 per cell)19 Epo efficiently induced the tyrosine phosphorylation of Jak2 (Fig 5A). In contrast, Jak2 tyrosine phosphorylation was not induced at detectable levels in cells exposed to SCF at high concentrations (200 ng/mL; 6 nmol/L) for varied intervals (Fig 5B). In addition, immunoprecipitations with phosphotyrosine antibodies indicated that c-kit was activated in SCF-exposed FDC2–wt-ER cells at 2 to 6 minutes of exposure, and that this was preceded by the rapid transient tyrosine phosphorylation of a Mr 210,000 protein (Fig 5C). Finally, no SCF-induced tyrosine phosphorylation of Jak2 was observed when FDC2–wt-ER cells were expanded in IL-3 or Epo (negative results, unpublished observation). Thus, these experiments suggest that mitogenic synergy between c-kit and the Epo receptor in FDC–wt-ER cells does not depend upon SCF activation of Jak2.
Tyrosine phosphorylation of Jak2 is induced efficiently by Epo, but not SCF, in FDC2–wt-ER cells. To test for the possible involvement of Jak2 in SCF signaling FDC2–wt-ER cells in exponential growth were cultured in the absence of cytokines to induce G0/G1 synergy and were then exposed to Epo (as a positive control; 50 U/mL or 10 nmol/L, 8 minutes) or SCF (200 ng/mL or 6 nmol/L, 0, 4, 12 minutes; Panel B). Jak2 was then immunoprecipitated from Triton-X-100 lysates (IP), and was assayed for tyrosine phosphorylation by Western blotting (α Py). In addition, blots were stripped and reprobed with antibodies to Jak2 to confirm equivalence in recoveries and loading (A and B, right panels). Based on the absence of detectable tyrosine phosphorylation of Jak2 in response to SCF (Panel B), lysates also were incubated in parallel with antibodies to phosphotyrosine, and were Western blotted with αpY antibodies (Panel C). Here, the rapid SCF-induced tyrosine phosphorylation of proteins corresponding in molecular weight to c-kit and to a previously described Mr 210,000 protein47 was observed.
Tyrosine phosphorylation of Jak2 is induced efficiently by Epo, but not SCF, in FDC2–wt-ER cells. To test for the possible involvement of Jak2 in SCF signaling FDC2–wt-ER cells in exponential growth were cultured in the absence of cytokines to induce G0/G1 synergy and were then exposed to Epo (as a positive control; 50 U/mL or 10 nmol/L, 8 minutes) or SCF (200 ng/mL or 6 nmol/L, 0, 4, 12 minutes; Panel B). Jak2 was then immunoprecipitated from Triton-X-100 lysates (IP), and was assayed for tyrosine phosphorylation by Western blotting (α Py). In addition, blots were stripped and reprobed with antibodies to Jak2 to confirm equivalence in recoveries and loading (A and B, right panels). Based on the absence of detectable tyrosine phosphorylation of Jak2 in response to SCF (Panel B), lysates also were incubated in parallel with antibodies to phosphotyrosine, and were Western blotted with αpY antibodies (Panel C). Here, the rapid SCF-induced tyrosine phosphorylation of proteins corresponding in molecular weight to c-kit and to a previously described Mr 210,000 protein47 was observed.
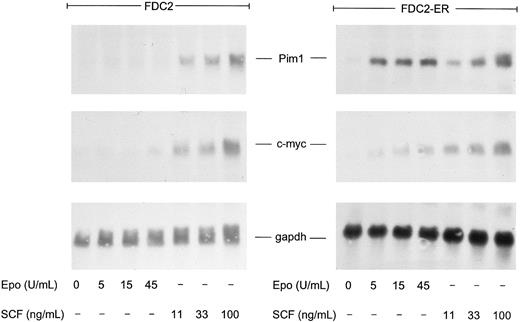
In studies of Epo- and SCF-induced expression of pim1 and/or c-myc transcripts, FDC2–wt-ER cells (and FDC2 cells as a control) were synchronized by withdrawal of cytokines and were exposed for 60 minutes to SCF or Epo at increasing concentrations. Levels of pim1 and c-myc transcripts then were assayed by Northern blotting (Fig 6). As expected, in FDC2–wt-ER cells Epo induced the rapid accumulation of pim1 and c-myc transcripts. Somewhat unexpectedly, however, pim1 transcript accumulation also was stimulated by SCF (both in FDC2 and FDC2–wt-ER cells). Thus, this result differs from findings in BM640 and MO7E cells,41 correlates with the activity of SCF in promoting survival in FDC2–wt-ER cells (see above, Figs 1 and 6), and is at least consistent with the prospect that Pim1 may play a role in mediating cosignaling of mitogenesis by SCF and Epo. Interestingly, pim1 transcript expression in FDC2–wt-ER cells also was observed to be activated efficiently by Epo at concentrations that were relatively inefficient in activating c-myc transcript expression (and mitogenesis). Thus, it might be speculated that the Epo-induced pathways to Pim1 expression may be independent from Epo-induced pathways to c-myc transcription.
SCF-induced expression of pim1 transcripts in FDC2–wt-ER cells. To test whether the expression of pim1 transcripts might be modulated by SCF in FDC2 and FDC2–wt-ER cells, cytokines were withdrawn to promote accumulation in G0/G1 and cells were exposed to SCF at 11, 33, and 100 ng/mL (0.3, 1, and 3 nmol/L) for 60 minutes or to Epo at 0, 5, 15, and 45 U/mL (0, 1, 3, and 9 nmol/L) as a positive control. Total RNA then was isolated and levels of pim1 transcripts were assayed by Northern blotting. For comparison, c-myc transcript levels also were analyzed and equivalence in loading was confirmed using a probe for glyceraldehyde phosphate dehydrogenase (gapdh).
SCF-induced expression of pim1 transcripts in FDC2–wt-ER cells. To test whether the expression of pim1 transcripts might be modulated by SCF in FDC2 and FDC2–wt-ER cells, cytokines were withdrawn to promote accumulation in G0/G1 and cells were exposed to SCF at 11, 33, and 100 ng/mL (0.3, 1, and 3 nmol/L) for 60 minutes or to Epo at 0, 5, 15, and 45 U/mL (0, 1, 3, and 9 nmol/L) as a positive control. Total RNA then was isolated and levels of pim1 transcripts were assayed by Northern blotting. For comparison, c-myc transcript levels also were analyzed and equivalence in loading was confirmed using a probe for glyceraldehyde phosphate dehydrogenase (gapdh).
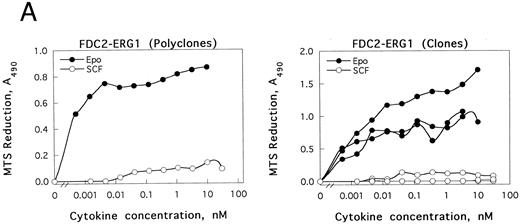
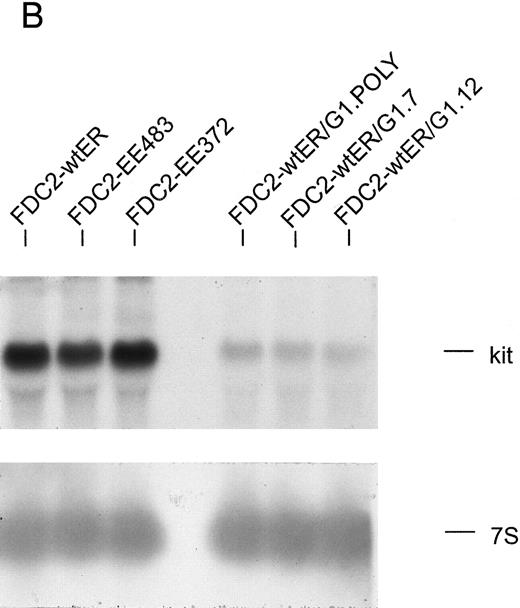
GATA-1 modulation of SCF and Epo signaling in FDC2–wt-ER cells.As introduced above, the erythroid transcription factor GATA-1 has been suggested to modulate both the survival23 and growth24 of hematopoietic progenitor cells. To test whether GATA-1 might affect SCF- and/or Epo-dependent proliferation in FDC2–wt-ER cells, murine wt–GATA-1 was expressed stably in this myeloid system, and possible effects on Epo-dependent inhibition of apoptosis first were studied. In DNA fragmentation assays, however, no significant effects of GATA-1 expression on survival were observed (negative results not shown, B.J.). Subsequently, possible effects of GATA-1 expression on SCF- and Epo-induced mitogenesis were assayed. In these experiments, a selective inhibition (>20-fold) of SCF-induced proliferation (v Epo-induced proliferation as a control) was observed both in polyclonal FDCER-G1 cells and derived clonal lines (FDC2-ER/G1-C7 and FDC2-ER/G1-C12; Fig 7A). To initially address mechanisms that regulate this effect, c-kit transcript levels were assayed by Northern blotting (Fig 7B). In polyclonal and clonal FDC2-ER/G1 lines, a twofold to threefold decrease in c-kit transcript level was observed as compared with FDC2–wt-ER cells. Thus, GATA-1–dependent repression of SCF signaling in this system may be explained at least in part by a GATA-1–dependent selective inhibition of c-kit gene expression. Based on this observed inhibition of proliferative signaling, the possibility that a program of erythroid gene expression might be activated in FDCER-G1 cells also was assessed. Northern blot analyses revealed the expression in FDC2–wt-ER/G1 cells (but not FDC2 or FDC2–wt-ER cells) of endogenous GATA-1, EKLF, βmaj-globin genes (Fig 7C). One explanation for this observed activated expression of red cell genes could be that the forced expression of GATA-1 (together with the Epo receptor) in myeloid FDC2 cells induced their de novo expression. Alternatively, the possibility exists that the culture of FDC2-ER/G1 cells in Epo resulted in the outgrowth of a previously undetected erythroid subpopulation. This latter possibility cannot be discounted by data presented here, and experiments aimed at resolving these possibilities are ongoing. Nonetheless, in the context of SCF and Epo cosignaling the defined correlation between commitment to erythroid differentiation and the observed selective loss of responsiveness to SCF is of interest to consider.
The expression of GATA-1 in FDC2–wt-ER cells inhibits SCF mitogenic signaling and reduces c-kit transcript levels. (A) In FDC2–wt-ER and derived FDC2-ER/G1 cells, mitogenic responsiveness to Epo and SCF was assayed based on cytokine-stimulated reduction of MTS. For FDC2-ER/G1 cells, polyclonal (left panel) as well as derived clonal lines (right panel) were analyzed. (B) In FDC2–wt-ER and FDC2-ER/G1 cells, levels of c-kit transcripts were analyzed by Northern blotting. Total RNA (20 mg per lane) was used, and equivalence in loading was confirmed using a cDNA probe for 7S RNA.33 Cell lines analyzed include parental control FDC2–wt-ER, FDC2-EE483, FDC2-EE372 cells,26 and FDC2-ER/G1 cells (polyclonal, “poly” clonal lines, −7 and −12).
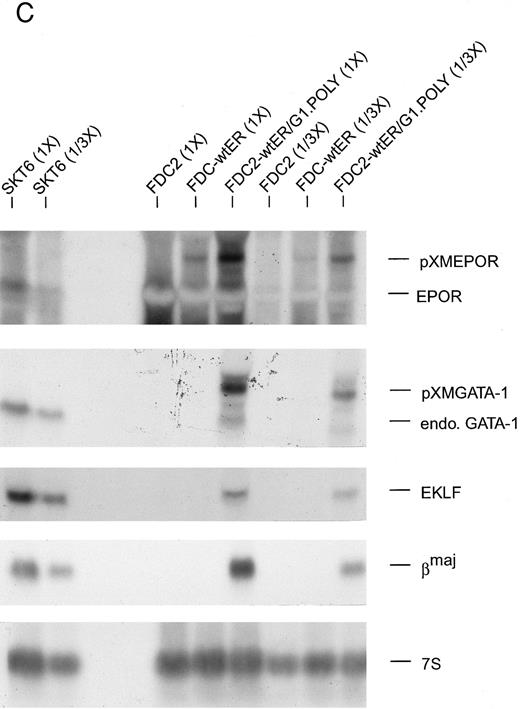
(C) Ectopic expression of GATA-1 in FDC2–wt-ER cells is associated with the transcription of endogenous GATA-1, EKLF and βmaj genes. Based on the above-observed inhibition of SCF signaling in FDC2–wt-ER cells expressing GATA-1 (ie, FDC2-ER/G1 cells) possible effects on lineage commitment were investigated. Total RNA was isolated from FDC2, FDC2–wt-ER, and FDC2-ER/G1 cells and levels of ectopically expressed Epo receptor mRNA, ectopically expressed GATA-1 mRNA, and endogenous GATA-1, EKLF, and βmaj-globin transcripts were assayed by Northern blotting. GATA-1 mRNA expressed from a pXM vector is larger than, and resolves from, endogenous GATA-1 transcripts. In polyclonal FDC2-ER/G1 cells (FDC2-ER/G1.Poly) expression of endogenous genes for GATA-1, EKLF, and βmaj-globin was activated. In contrast, no detectable levels of transcripts for GATA-1, EKLF, or βmaj-globin were observed in control FDC2 or FDC2–wt-ER cells. As a positive control, RNA from murine erythroleukemic SKT6 cells also was analyzed at two levels of loading (1X, 1/3X; left most lanes).
The expression of GATA-1 in FDC2–wt-ER cells inhibits SCF mitogenic signaling and reduces c-kit transcript levels. (A) In FDC2–wt-ER and derived FDC2-ER/G1 cells, mitogenic responsiveness to Epo and SCF was assayed based on cytokine-stimulated reduction of MTS. For FDC2-ER/G1 cells, polyclonal (left panel) as well as derived clonal lines (right panel) were analyzed. (B) In FDC2–wt-ER and FDC2-ER/G1 cells, levels of c-kit transcripts were analyzed by Northern blotting. Total RNA (20 mg per lane) was used, and equivalence in loading was confirmed using a cDNA probe for 7S RNA.33 Cell lines analyzed include parental control FDC2–wt-ER, FDC2-EE483, FDC2-EE372 cells,26 and FDC2-ER/G1 cells (polyclonal, “poly” clonal lines, −7 and −12).
(C) Ectopic expression of GATA-1 in FDC2–wt-ER cells is associated with the transcription of endogenous GATA-1, EKLF and βmaj genes. Based on the above-observed inhibition of SCF signaling in FDC2–wt-ER cells expressing GATA-1 (ie, FDC2-ER/G1 cells) possible effects on lineage commitment were investigated. Total RNA was isolated from FDC2, FDC2–wt-ER, and FDC2-ER/G1 cells and levels of ectopically expressed Epo receptor mRNA, ectopically expressed GATA-1 mRNA, and endogenous GATA-1, EKLF, and βmaj-globin transcripts were assayed by Northern blotting. GATA-1 mRNA expressed from a pXM vector is larger than, and resolves from, endogenous GATA-1 transcripts. In polyclonal FDC2-ER/G1 cells (FDC2-ER/G1.Poly) expression of endogenous genes for GATA-1, EKLF, and βmaj-globin was activated. In contrast, no detectable levels of transcripts for GATA-1, EKLF, or βmaj-globin were observed in control FDC2 or FDC2–wt-ER cells. As a positive control, RNA from murine erythroleukemic SKT6 cells also was analyzed at two levels of loading (1X, 1/3X; left most lanes).
DISCUSSION
The present studies of mechanisms of SCF and Epo cosignaling in FDC2 cells were prompted by three considerations. First, and as introduced above, SCF has been shown in hematopoietic progenitor cells ex vivo9,10,11 and in isolated erythroid progenitor cells in vitro5 to act synergistically with Epo in promoting the expansion of late BFU-E. However, little is understood regarding underlying mechanisms. Second, and as emphasized recently by Wu et al15 and Jacobs et al,16 the availability of cell lines that are useful for mechanistic studies of SCF and Epo coaction is limited, and in at least certain of these models SCF signaling of proliferation may be compromised.17,18 By comparison, SCF-dependent mitogenesis and survival pathways presently are shown to be supported efficiently in FDC2 cells and derived cell lines. Third, novel roles recently have been suggested for GATA-1 in regulating progenitor cell growth24 and survival,23 and FDC2 cells also comprise a useful null yet relevant background to test whether GATA-1 might exert effects selectively on SCF and/or Epo proliferative signaling.
In primary experiments, endogenous SCF receptors in FDC2 cells were shown to cofunction with stably transfected Epo receptor forms, and mitogenic synergy was shown to be supported by the wt-ER, a chimeric receptor form lacking the Epo receptor extracellular domain (EECA), and a highly truncated Epo receptor form, ER-Bx1. Synergy exerted between c-kit and these Epo receptor forms ranged from threefold to fivefold above additive effects, and for each receptor construct synergy was observed at all concentrations of cytokines tested. In this context, the activity of the chimera EECA suggests first that extracellular domains of the Epo receptor apparently are dispensable for cooperative signaling with c-kit. The nontrivial nature of this result is underlined by studies in which roles for Epo receptor extracellular domains have been implicated in the signaling of globin gene expression in BaF/3 cells,42 and in the downmodulation of Epo signaling by the IL-3 receptor.43 Second, the ability of the ER-Bx1 receptor construct to support mitogenic synergy with c-kit supports the notion that this effect is mediated at least in part by the ER-Bx1 domain. In recent studies our laboratory has shown that the box 1 domain mediates the specific recruitment of Jak2,36 and that Epo receptor forms containing only the box 1 domain (and directly flanking residues) are highly efficient in mediating the expression of c-myc and the serine-threonine kinase Pim1.19 Therefore, in the present context of SCF and Epo cosignaling Jak2 activation and pim1 and c-myc transcript expression in response to SCF and Epo were investigated. In FDC2–wt-ER cells, SCF-induced tyrosine phosphorylation of Jak2 was not detectable, and this was despite exposure to SCF at concentrations (200 ng/mL, 6 nmol/L) above those required to fully support mitogenesis, survival, and long-term growth. These results suggest that at least in FDC2–wt-ER cells, Jak2 activation by SCF may not occur at appreciable levels and that any such activation does not correlate with proliferative responses. In previous studies of potential roles for Jak2 in SCF signaling, its induced tyrosine phosphorylation has been detected in Mo7e cells, FDCP-1 cells, and human fetal hepatocytes with the most convincing results obtained in Mo7e cells. However, using Mo7e cells Tang et al39 were unable to show this response, and in studies by Jacobs-Helber et al16 in HCD57 cells SCF-induced tyrosine phosphorylation of Jak2 likewise was not detected. Also, in studies by Weiler et al37 and by Brizzi et al38 in Mo7e and T18 cells, possible correlations between SCF-induced Jak2 phosphorylation and SCF-induced proliferation were not established. Thus, although SCF activation of Jak2 phosphorylation can be detected in at least certain cell lines, our present studies in FDC2–wt-ER cells (and studies by Jacobs-Helber et al in HCD57 cells)16 argue that the possible engagement of Jak2 by c-kit is nonessential to SCF-induced mitogenesis and survival.
With regard to possible roles for Pim1 in SCF and Epo costimulated proliferation, the observation that SCF induces pim1 transcript expression merits discussion. The potential importance of this observation is underlined first by our recent finding that pim1 (and c-myc) transcript expression is mediated by the ER-Bx1 domain19 (ie, that domain that presently is shown to mediate mitogenic synergy with c-kit). In addition, the activation of pim1 transcript expression by SCF previously has not been detected in studies in Mo7e41 or BM6,40 or (to our knowledge) through the stimulation of any receptor tyrosine kinase to date. However, in Eμ-Pim1 transgenic and Pim1-deficient mice, mean erythrocyte volumes correlate with Pim1 expression44 and IL-3 responsiveness is impaired.45 In Eμ-Pim1 mice, significant effects of Pim1 on apoptosis in lpr lymphoid cells also have been shown.46 Based on these reports and the present findings, studies are in progress to define possible roles for Pim1 in Epo and SCF cosignaling through its altered expression in FDC2-ER cells.
Finally, effects of GATA-1 expression on Epo and SCF signaling in FDC2–wt-ER cells have been investigated. Little if any effects of GATA-1 on Epo-dependent inhibition of apoptosis were detected. However, it should be noted that this (as well as the lack of detectable synergy in Epo and SCF inhibition of apoptosis) are negative results that may be attributable to assay sensitivity. Specifically, the DNA fragment end-labeling assay used, although sensitive, provides only an overall index of apoptosis. To overcome this limitation, TUNEL assays48 presently are being applied in this system. In contrast, GATA-1 expression did lead to a marked and selective inhibition of SCF-induced mitogenesis. Interestingly, in purified human erythroid progenitor cells a corresponding antagonism between c-kit and Epo receptor signaling recently has been described whereby Epo-stimulated differentiation events are retarded by SCF.6 In FDC2-ER/G1 cells, specific mechanisms involved in the inhibition of c-kit expression and SCF mitogenic signaling presently are unclear, and this is complicated somewhat by the observation that expression of endogenous genes for GATA-1, EKLF, and βmaj-globin apparently is activated in this myeloid cell line on the coexpression of GATA-1 and the Epo receptor. Nonetheless, if this program of erythroid gene expression proves to be activated de novo, FDC2-ER/G1 cells should also prove valuable as a novel system for studies of erythroid lineage commitment.
ACKNOWLEDGMENT
The authors thank Amgen (and Drs Steve Elliot and Robert Pacifici) for the provision of recombinant human EPO. We also thank Rick Gregory and Tammy Reese for the construction of the receptor form EE483, and Drs Peter Besmer, Ed Prochownik, and Jos Domen for the generous provision of murine c-kit, c-myc, and pim1 cDNAs, respectively.
This work was supported by National Institutes of Health Grant Nos. DK 40242, RCDA HL 03042, and ACS IM774 (D.M.W.); and by a Sigma Xi Grant-in-Aid (B.J.).
Address reprint requests to Don M. Wojchowski, PhD, 115 William L. Henning Bldg, The Pennsylvania State University, University Park, PA 16802.

![Fig. 1. SCF- and Epo-dependent mitogenesis and inhibition of apoptosis in FDC2 and FDC2–wt-ER cells. Parental FDCP1-WEHI2 cells (ie, FDC2 cells) were transfected stably with an Epo receptor expression vector (pXM-ER) and were selected in Epo to yield FDC2–wt-ER cells. In FDC2–wt-ER cells and parental FDC2 cells, the ability of IL-3, Epo, and SCF to promote mitogenesis and/or survival was then tested. (A) Mitogenic signaling was assayed based on cytokine-stimulated rates of [3H] thymidine incorporation. To account for any minor differences in plating, values were normalized versus maximal responsiveness to IL-3 in each cell line. (B) FDC2 and FDC2–wt-ER cells were cultured for 7 hours in Optimem medium, 1% FBS in the presence (or absence) of SCF (220 ng/mL or 6.6 nmol/L), Epo (20 U/mL or 4 nmol/L), or IL-3 (20% W3CM, ≥ maximal mitogenic dose). Apoptosis then was assayed based on observed levels of intranucleosomal DNA fragmentation. Levels of fragmentation were analyzed quantitatively by phosphor-imaging.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/9/10.1182_blood.v90.9.3533/4/m_bl_0047f1a.jpeg?Expires=1768189191&Signature=wR4A-NaEdgO1u8IsHO7ge8mqrZDubgdnXf~IR1oowjVyMcpj9f-aoXRcWkOkPq8onZOfVDUbItIBXJiVKX7XbhiRrrjLZLMziq8NkaK9VrRbcWyils9-z88QfV8OFKtn9SBmb8E4tcntNJjAIRXvU~U4ZO5KpUo98zALMq~KXLaxdHZkQjeUr62mGbbepCfaOgEEPV5UsBNA2ZpHsqR72t7bPifHdVyrVVN7oE6nflirfwKYA71dkqATtCph7-EaxYPYwmtPnRwldPhyMF2U1YE~gIBXr83m~wDCnF~~QFDsMVLkCZ-lX1Fl4dR4R0KUDyHtu7H~10l5KPIGQOhBaA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. SCF- and Epo-dependent mitogenesis and inhibition of apoptosis in FDC2 and FDC2–wt-ER cells. Parental FDCP1-WEHI2 cells (ie, FDC2 cells) were transfected stably with an Epo receptor expression vector (pXM-ER) and were selected in Epo to yield FDC2–wt-ER cells. In FDC2–wt-ER cells and parental FDC2 cells, the ability of IL-3, Epo, and SCF to promote mitogenesis and/or survival was then tested. (A) Mitogenic signaling was assayed based on cytokine-stimulated rates of [3H] thymidine incorporation. To account for any minor differences in plating, values were normalized versus maximal responsiveness to IL-3 in each cell line. (B) FDC2 and FDC2–wt-ER cells were cultured for 7 hours in Optimem medium, 1% FBS in the presence (or absence) of SCF (220 ng/mL or 6.6 nmol/L), Epo (20 U/mL or 4 nmol/L), or IL-3 (20% W3CM, ≥ maximal mitogenic dose). Apoptosis then was assayed based on observed levels of intranucleosomal DNA fragmentation. Levels of fragmentation were analyzed quantitatively by phosphor-imaging.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/9/10.1182_blood.v90.9.3533/4/m_bl_0047f1b.jpeg?Expires=1768189191&Signature=Qxa9XCTsCnJRuyUq~vYQUz6gRQa9z~rhHKdJyuAN7jqUmRWHPFI9LBg1UaNkGu6z3b05Vl2MfzY3zXDDMKoLSqk-jPzcs9-tgBzlZdh~L8BZFWMFVBXXtfzbQF3XX6tCaBxYhwbda2qzcxd-wJiJNwZJV~KjuclXrhoqTuXAOQeFxb~AxjzyVGb-WkavGCRq4cFebLHFxTycIfpjBtTCZRn-JKYAzdC4ruNIapw4-ThQv7xJ9o14nrjsNmSSEWz1-4IBLfgJKLQle4nCKZORrZub4-I-qDCOeWRrkis2tDQZte9dOerfaTmAmlTRDyB~V-NgA4dQbnhXNWlOgI2kzw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Construction, expression, and mitogenic activity of Epo receptor forms used to study Epo and SCF coinduced FDC2 cell proliferation. (A) In experiments aimed at testing possible mechanisms of Epo and SCF cosignaling, two minimal Epo receptor forms were constructed, expressed in FDC2 cells, and assayed initially for their activities in mediating ligand-induced mitogenesis in derived cell lines. In the construct ER-Bx1, 178 of a total of 236 cytoplasmic residues of the wt-ER have been deleted, including the conserved box 2 domain and eight (phospho)tyrosine sites for effector recruitment. The construct EE483 is a chimeric receptor form in which the extracellular domain of the Epo receptor was replaced by that of the human EGF receptor. (B) The mitogenic activity of the above receptor forms in FDC2-derived cell lines (FDC2, FDC2–wt-ER, FDC2–ER-Bx1, FDC2-EE483) was assayed based on cytokine-stimulated rates of [3H] thymidine incorporation. To account for any minor differences in plating, values were normalized versus maximal responsiveness to IL-3 in each cell line. Northern blotting of Epo and EE483 transcripts served to show apparent equivalence in receptor expression (B.J., August 1996, data not shown) and for the wt-ER and ER-Bx1 this has been confirmed through [125I] Epo equilibrium binding assays (400 to 600 receptors per cell).1925](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/9/10.1182_blood.v90.9.3533/4/m_bl_0047f2a.jpeg?Expires=1768189191&Signature=FXtfCBDotJQE9wxVTEh6fdFGJ~wWgj4Egx9znLaXZy5TszF3uGjOnSbloz0CKj953zgQEK5n~S~hYoy8jgwzc-a4mfWPmUHNLk4TF~z4KF8CRjaOt3VioyF-c7ilfjG8P0L1qVR1BV36OHPRveQG9KNcoz-8mUlLpTZn5ansunBdKYeDP4R4uMvsXd8h-OL1Z3bcSjVTY6Pb5rWLa4RTcoiKhNsGfiEizsnGxCuOsMlmjHLa4~ekFm5OFVT4vnHOlPytqOv8-ZqG1CqS~yoIAviF5IjWB8BDeUrbeTnAXj1KDzhejpdTjizEGcc-zBF3-IN7w7HFqsGnto7zYxg2MQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Construction, expression, and mitogenic activity of Epo receptor forms used to study Epo and SCF coinduced FDC2 cell proliferation. (A) In experiments aimed at testing possible mechanisms of Epo and SCF cosignaling, two minimal Epo receptor forms were constructed, expressed in FDC2 cells, and assayed initially for their activities in mediating ligand-induced mitogenesis in derived cell lines. In the construct ER-Bx1, 178 of a total of 236 cytoplasmic residues of the wt-ER have been deleted, including the conserved box 2 domain and eight (phospho)tyrosine sites for effector recruitment. The construct EE483 is a chimeric receptor form in which the extracellular domain of the Epo receptor was replaced by that of the human EGF receptor. (B) The mitogenic activity of the above receptor forms in FDC2-derived cell lines (FDC2, FDC2–wt-ER, FDC2–ER-Bx1, FDC2-EE483) was assayed based on cytokine-stimulated rates of [3H] thymidine incorporation. To account for any minor differences in plating, values were normalized versus maximal responsiveness to IL-3 in each cell line. Northern blotting of Epo and EE483 transcripts served to show apparent equivalence in receptor expression (B.J., August 1996, data not shown) and for the wt-ER and ER-Bx1 this has been confirmed through [125I] Epo equilibrium binding assays (400 to 600 receptors per cell).1925](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/9/10.1182_blood.v90.9.3533/4/m_bl_0047f2b.jpeg?Expires=1768189191&Signature=w00I2l~VQF4h5Km0fjcjNZjkLTOOYpByAinNlRHPoLm8YgJDUxGettXweRrLqBAmVuTVCzmCROTg9-THdW~HcFO4DQRgEp7MqABVRXT3eWjjpOvwlshhabFHO49wgviR7b4T6s4VNLUmn22p0v8qjztBKUm-Dqb2thxfLHy9MI~vxZ9c75QenGFjNTQuzSvSWVrp3bxlSDTHMKfGmUe2J1zO2Zaytvs-jwUZ7cSRTuzF~srdxRcsuF1MqHjIpPRZLq-m3w0Nc4V3It2fsWthFtAkY4gZoCHiJGNoXo2qQygXm4dv4u6g9YfcVZGdIM-MfGRe1E-nkXWmDtQrwNGcUg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Mitogenic synergy between c-kit and stably transfected Epo receptor forms in FDC2–wt-ER, FDC2–ER-Bx1 and FDC2-EE483 cells. In experiments aimed at testing the ability of the wt-ER and the receptor forms ER-Bx1 and EE483 to act synergistically with SCF, FDC2-derived cell lines were exposed to SCF, Epo, or both cytokines. Levels of induced mitogenesis then were assayed as above (see Fig 2 and Materials and Methods). In each line, synergy in mitogenic signaling was observed upon Epo and SCF costimulation at all cytokine concentrations tested (SCF, 0, 6.8, 13.5, 27 ng/mL or 0, 0.2, 0.4, 0.8 nmol/L; Epo, 0, 0.11, 0.22 U/mL or 0, 0.022, 0.044 nmol/L; EGF 0, 0.5, 1.0 ng/mL or 0, 0.1, 0.2 nmol/L). Levels of synergy are indexed above histograms, and represent the fold increase in rates of [3H]thymidine incorporation over calculated additive values for Epo and SCF, or EGF and SCF.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/9/10.1182_blood.v90.9.3533/4/m_bl_0047f3.jpeg?Expires=1768189191&Signature=ttA8PHiQcNppHXwSDv1LlSxuZlz18pZj7iMkCQydp6Xsc3e~iczAUpBbT0IJDfPbW32uq2IILyeY7-9hRI5mAxT~ni38P7aP-LHHjvSJ93h3O~qB8B65nX~~UkS9W6BWz~H1ihNcC5ZvtKhQYtuhnEnbUW-n6ehfgtEdx1RNDqJ~zLNr~TPHj4~P1~ZIsIYaoqGs3oKLPotPgQk8Z~N6T24vXMim5ISW2XRlDUvPhhNgiqyY37DAyp6nomsRKzWmd-dC3gwDdATQtVJ6MuItj9DZH0rOddDwK7ZlPgqyQ-xMo99PbjCbBA1x3PfgL2-zH7IU8g-EqOvqomfiPfLhmw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)