Abstract
Antifolates such as methotrexate (MTX) and trimetrexate (TMTX) are widely used in the treatment of cancer and nonmalignant disorders. Transient, yet sometimes severe myelosuppression is an important limitation to the use of these drugs. It has previously been shown that clonogenic myeloid progenitors and colony-forming units-spleen are resistant to antifolates, suggesting that myelotoxicity occurs late in hematopoietic development. The goal of this study was to define the mechanisms by which primitive hematopoietic cells resist the toxic effects of antifolate drugs. To test the hypothesis that myeloid progenitors may salvage extracellular nucleotide precursors to resist TMTX toxicity, a defined liquid culture system was developed to measure TMTX toxicity in expanding progenitor populations. These in vitro experiments showed that both human and murine progenitors can resist TMTX toxicity by importing thymidine and hypoxanthine from the serum. As predicted from these findings, several drugs that block thymidine transport sensitized progenitors to TMTX in vitro, although to differing degrees. These nucleoside transport inhibitors were used to test whether progenitors and hematopoietic stem cells (HSCs) could be sensitized to TMTX in vivo. Treatment of mice with TMTX and nitrobenzylmercaptopurineriboside phosphate (NBMPR-P), a potent transport inhibitor, caused significant depletions of clonogenic progenitors within the bone marrow (20-fold) and spleen (6-fold). Furthermore, NBMPR-P administration dramatically sensitized HSCs to TMTX, with dual-treated mice showing a greater than 90% reduction in bone marrow repopulating activity. These studies demonstrate that both myeloid progenitor cells and HSCs resist TMTX by using nucleotide salvage mechanisms and that these pathways can be pharmacologically blocked in vivo using nucleoside transport inhibitors. These results have important implications regarding the use of transport inhibitors for cancer therapy and for using variants of dihydrofolate reductase for in vivo selection of genetically modified HSCs.
ANTIFOLATES such as methotrexate (MTX) are among the first compounds to be effectively used in cancer treatment and are also used to treat a variety of nonmalignant conditions. These drugs act by inhibiting various enzymes involved in nucleotide biosynthesis, ultimately causing interruption of DNA replication. In the absence of rescue with leucovorin, the dose-limiting toxicity of antifolates is myelosuppression. This toxicity appears to exclusively affect cells at relatively late stages of hematopoietic development. In mice treated with MTX or the nonclassical antifolate trimetrexate (TMTX), at doses that caused severe bone marrow (BM) hypocellularity, the content of myeloid colony-forming cells (CFU-Cs) was reduced less than twofold in most cases.1-3 Although some studies have shown greater depletions in the CFU-spleen (CFU-S) pool in antifolate-treated mice,3,4 high proliferative potential murine colony-forming cells were antifolate resistant.5 Because antifolates kill only actively dividing cells, one potential explanation for these findings is that most myeloid progenitors may be quiescent during the period of drug exposure.6 However, recent evidence has shown that cell-cycle dormancy is not the sole cause of resistance in primitive myeloid cells. In mice treated with pegylated stem cell factor to increase the proportion of cycling progenitors,7 both CFU-Cs and CFU-Ss remained resistant to MTX and trimetrexate (TMTX).8 The mechanisms by which early myeloid progenitor cells escape antifolate toxicity have remained unclear and are the focus of this study.
We hypothesized that the relative resistance of primitive myeloid cells may be caused by utilization of preformed nucleotide precursors that are present in serum. Antifolates act by inhibiting the de novo biosynthesis of thymidylate and purine nucleotides. It is well known that many cells can compensate for this effect by importing thymidine and purine bases such as hypoxanthine from the extracellular environment. There are several lines of evidence to suggest that these salvage mechanisms play an important role in modulating antifolate-induced BM toxicity. Addition of thymidine and hypoxanthine to semisolid cultures of BM cells increases the plating efficiency of human CFU-granulocyte-macrophages (CFU-GMs) in the presence of MTX.9 The relatively high local concentration of hypoxanthine in the BM suggests that thymidine uptake may be a rate-limiting determinant of myelotoxicity.10 Consistent with this idea, thymidine infusions have been used to rescue patients from the myelosuppressive effects of high-dose MTX.11 Under the normal physiologic conditions present in vivo, it is not known to what degree nucleotide salvage mechanisms contribute to the relative antifolate resistance seen in primitive myeloid cells.
A powerful tool to study this issue is the use of drugs that inhibit thymidine transport. Nucleosides such as thymidine can enter cells through facilitative carriers such as the recently cloned es transporter,12 or through sodium-dependent concentrative transporters.13 Drugs such as dipyridamole, draflazine, and nitrobenzylmercaptopurineriboside (NBMPR) are inhibitors of the facilitative nucleoside transporters.14 Several of these inhibitors can decrease the plating efficiency of murine CFU-GM in the presence of MTX,15 suggesting that interruption of thymidine transport can sensitize myeloid cells to antifolates. In vivo data from clinical trials also indicate that transport inhibitors can potentiate MTX-induced myelosuppression,16 although the stage of myelopoiesis at which toxicity was occurring was not defined.
The main goal of this study was to determine the role of nucleotide salvage pathways in modulating the antifolate sensitivity of myeloid progenitors and repopulating HSCs. These studies have relevance to the use of nucleoside transport inhibitors in cancer therapy. In vitro experiments have shown that NBMPR and dipyridamole can enhance the tumor-cell killing associated with antimetabolite drugs.17-19 If progenitors and hematopoietic stem cells (HSCs) use thymidine salvage pathways to a significant extent, hematopoietic toxicity could limit the clinical application of this approach. Our studies are also pertinent to the development of methods for the in vivo selection of genetically modified HSCs.20 Efficient enrichment of stem cells that express dihydrofolate reductase (DHFR) vectors will require that the drugs used for selection eliminate unmodified HSCs.8 Therefore, determining the susceptibility of HSCs to antifolates and potentially enhancing drug sensitivity with transport inhibitors could lead to useful strategies for amplifying genetically corrected hematopoietic cells.
MATERIALS AND METHODS
Drug treatment of mice. Female mice were purchased from the Jackson Laboratories (Bar Harbor, ME) and were used for experiments between 8 and 14 weeks of age. TMTX base was received from the Drug Synthesis & Chemistry Branch, Developmental Therapeutics Program, Division of Cancer Treatment, NCI (Bethesda, MD) and converted to the glucuronate as previously described.21 The TMTX salt was then dissolved in sterile water and administered by intraperitoneal (ip) injection. 5-Fluorouracil (5FU) was purchased from Sigma (St Louis, MO), dissolved in phosphate-buffered saline (PBS; BioWhittaker, Walkersville, MD), and administered as an intravenous bolus by tail-vein injection. An aqueous solution of racemic draflazine (R75231, 50% active draflazine) was kindly provided by Dr H. Van Belle from the Janssen Research Foundation (Turnhout Seweg, Belgium) and administered as an ip injection. NBMPR was purchased from Sigma and NBMPR-P was prepared as previously described.22 Lyophilized NBMPR-P was then dissolved in sterile water immediately before use and administered as an ip injection.
Assays for progenitor and stem cell killing. BM cells were obtained from femurs of untreated and treated mice 24 hours after the final drug dose by flushing with PBS containing 2% fetal bovine serum (FBS) (Hyclone, Logan, UT). Fixed volumes from the femurs of untreated and treated mice were assayed to measure the progenitor or HSC content in the marrow space. For all treated mice, cells were dispersed from both femurs into single-cell suspensions using a 21-gauge needle and resuspended in a final volume of 1.0 mL. In one set of experiments, a volume equivalent to 8 × 105 cells from untreated mice was transplanted via lateral tail vein into WB/B6F1 W/Wv recipients (see Fig 1). In the second set of experiments shown in Fig 7, BM cells from treated C57Bl/6J mice were mixed with an equal volume of BM from untreated B6.C-H-1b/ByJ mice (termed HW80). W/Wv recipient mice then received a total volume of BM cells equivalent to 2 × 107 cells from untreated donors (1 × 107 cell equivalents from each donor type). For experiments measuring progenitor content (see Fig 6), a volume of marrow equivalent to 1 × 105 BM cells from untreated mice was plated in 3 mL of TMTX-free methylcellulose culture media (Methocult 3430; Stem Cell Technologies, Vancouver, Canada), supplemented with 3 U/mL recombinant human erythropoietin (Amgen, Thousand Oaks, CA). The number of progenitors was determined by scoring colonies after 7 to 12 days of culture at 37°C in 5% CO2 .
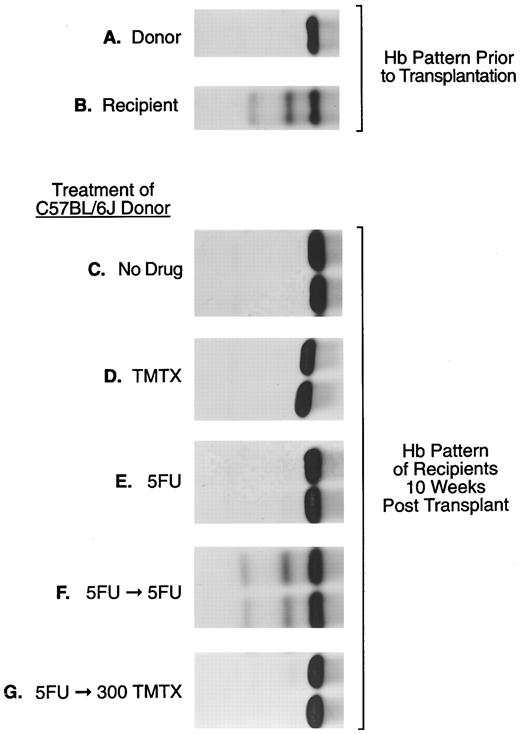
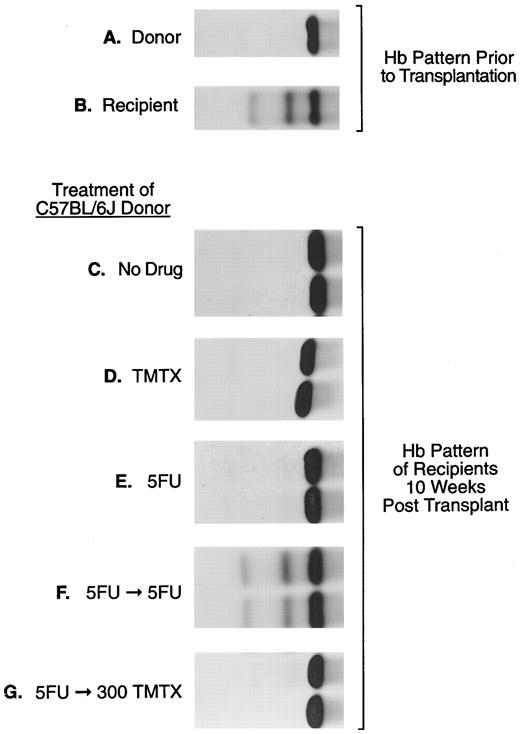
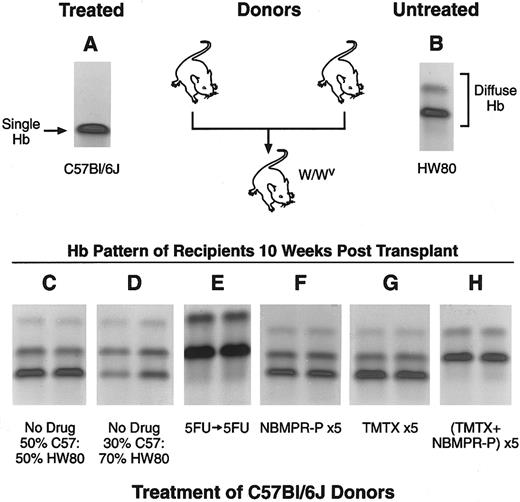
The sensitivity of murine repopulating HSCs to TMTX in vivo. Donor C57Bl/6J mice, homozygous for the single Hbb s allele (panel A), were treated as indicated: (panel C), no drug; (panel D), 300 mg/kg TMTX ip; (panel E), 150 mg/kg 5FU intravenously; (panel F ), 150 mg/kg 5FU followed 5 days later by 150 mg/kg 5FU; (panel G), 150 mg/kg 5FU followed 5 days later by 300 mg/kg TMTX. Twenty-four hours after the final treatment BM cells were obtained for transplant. Panels (C) through (G) show the hemoglobin phenotype in recipient mice 10 weeks after transplantation. In this figure samples from two representative recipient mice are shown in each panel.
The sensitivity of murine repopulating HSCs to TMTX in vivo. Donor C57Bl/6J mice, homozygous for the single Hbb s allele (panel A), were treated as indicated: (panel C), no drug; (panel D), 300 mg/kg TMTX ip; (panel E), 150 mg/kg 5FU intravenously; (panel F ), 150 mg/kg 5FU followed 5 days later by 150 mg/kg 5FU; (panel G), 150 mg/kg 5FU followed 5 days later by 300 mg/kg TMTX. Twenty-four hours after the final treatment BM cells were obtained for transplant. Panels (C) through (G) show the hemoglobin phenotype in recipient mice 10 weeks after transplantation. In this figure samples from two representative recipient mice are shown in each panel.
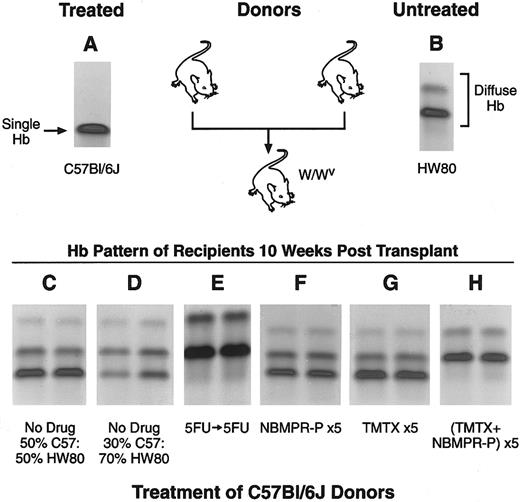
Stem cell toxicity in mice treated with TMTX plus NBMPR-P. Hemoglobin electrophoresis patterns of donor mice (panels A and B) and recipient mice after transplant (panels C through H) are shown. Genetically anemic W/Wv mice were transplanted with mixtures of BM cells from C57Bl/6J mice, homozygous for the single hemoglobin allele (panel A), and from HW80 mice, homozygous for the diffuse hemoglobin allele (panel B). The different hemoglobin patterns seen in recipients 10 weeks after transplant reflect the relative stem cell content from each of the donor grafts (panels C through H). Marrows from untreated C57Bl/6J and HW80 donors were mixed in a 50%:50% or a 30%:70% volume:volume ratio and a total of 2 × 107 nucleated cells were injected in recipient mice (panels C and D). C57Bl/6J donors were also treated with the various drug regimens indicated in panels E through H and described in Results. These mixtures were injected into recipients and the reconstitution patterns seen 10 weeks after transplantation are shown in panels E through H.
Stem cell toxicity in mice treated with TMTX plus NBMPR-P. Hemoglobin electrophoresis patterns of donor mice (panels A and B) and recipient mice after transplant (panels C through H) are shown. Genetically anemic W/Wv mice were transplanted with mixtures of BM cells from C57Bl/6J mice, homozygous for the single hemoglobin allele (panel A), and from HW80 mice, homozygous for the diffuse hemoglobin allele (panel B). The different hemoglobin patterns seen in recipients 10 weeks after transplant reflect the relative stem cell content from each of the donor grafts (panels C through H). Marrows from untreated C57Bl/6J and HW80 donors were mixed in a 50%:50% or a 30%:70% volume:volume ratio and a total of 2 × 107 nucleated cells were injected in recipient mice (panels C and D). C57Bl/6J donors were also treated with the various drug regimens indicated in panels E through H and described in Results. These mixtures were injected into recipients and the reconstitution patterns seen 10 weeks after transplantation are shown in panels E through H.
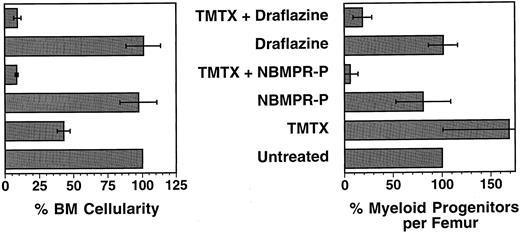
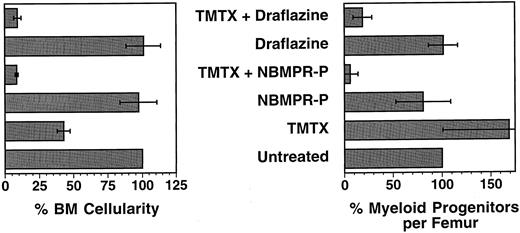
Bone marrow cellularity and progenitor content in mice treated with TMTX and either NBMPR-P or draflazine. C57Bl/6J mice were treated for 5 consecutive days with either TMTX 130 mg/kg/d, NBMPR-P 20 mg/kg/d, draflazine 20 mg/kg/d, or with combinations of either TMTX (130 mg/kg/d) plus NBMPR-P (20 mg/kg/d) or TMTX (130 mg/kg/d) plus draflazine (20 mg/kg/d of racemic mixture). Twenty-four hours after the final treatment, the femurs were flushed to determine both total cellularity and progenitor content per femur. All values are expressed as percentages relative to untreated mice. Each bar represents the mean ±1 SD of three independent experiments. Each experiment contained two to three mice per group.
Bone marrow cellularity and progenitor content in mice treated with TMTX and either NBMPR-P or draflazine. C57Bl/6J mice were treated for 5 consecutive days with either TMTX 130 mg/kg/d, NBMPR-P 20 mg/kg/d, draflazine 20 mg/kg/d, or with combinations of either TMTX (130 mg/kg/d) plus NBMPR-P (20 mg/kg/d) or TMTX (130 mg/kg/d) plus draflazine (20 mg/kg/d of racemic mixture). Twenty-four hours after the final treatment, the femurs were flushed to determine both total cellularity and progenitor content per femur. All values are expressed as percentages relative to untreated mice. Each bar represents the mean ±1 SD of three independent experiments. Each experiment contained two to three mice per group.
Hemoglobin electrophoresis. Analysis of hemoglobin phenotype of recipient erythroid cells was performed according to a previously described protocol.23 Briefly, packed red blood cells that were collected in heparinized microcapillary tubes from the retro-orbital sinus were lysed in cystamine, and hemoglobins were separated on cellulose acetate plates that were processed according to the manufacturer's instructions (Helena Laboratories, Beaumont, TX). The proportion of donor and recipient hemoglobins were quantitated using an IS 1000 Digital Imaging System (Alpha Innotech Corp, San Leandro, CA).
In vitro myeloid progenitor suspension cultures. Unseparated BM cells from C57Bl/6J, HW80 or WB/B6F1 +/+ mice (wild type at the W locus), were cultured in 6-well plates (Corning, Corning, NY) at 1 × 106 cells/mL in Dulbecco's modified Eagle's medium (DMEM; BioWhittaker), 20 ng/mL murine interleukin-3 (IL-3), 50 ng/mL human IL-6, 50 ng/mL rat stem cell factor (SCF ) (all kindly provided as a gift from Amgen), 4 mmol/L glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin (GIBCO-BRL, Gaithersburg, MD) and 15% FBS. Dialyzed FBS (Hyclone) was purchased as a single lot that contained undetectable levels of thymidine and hypoxanthine according to the manufacturer's specification sheet. Undialyzed FBS was also used in some experiments (Hyclone). Stock solutions of thymidine and hypoxanthine (both from Sigma) were prepared in sterile water and PBS, respectively, and then added to the culture medium at the indicated concentrations. Dipyridamole and NBMPR (both from Sigma) were dissolved in dimethyl sulfoxide (DMSO) before addition to the culture medium. A pre-prepared stock solution of draflazine was also added to some cultures. Thymidine-free cultures contained dialyzed FBS that was treated for 16 hours with 1 U/mL thymidine phosphorylase (TP) (Sigma) to convert any residual thymidine to thymine. All suspension cultures were incubated at 37°C, 5% CO2 for 4 days. Nonadherent and adherent cells were procured, washed extensively, and resuspended in equal volumes of PBS. The volume equivalent of 1 × 104 BM cells from TMTX-free cultures was added to 3 mL of Methocult media. Note that TMTX was added only to various suspension cultures but never to the Methocult media itself. The number of progenitors was determined by scoring colonies of greater than 50 cells after 7 to 12 days.
Surplus human hematopoietic progenitors from the apheresis products of two breast cancer patients following chemotherapy and granulocyte colony-stimulating factor (G-CSF ) mobilization were obtained after informed consent. CD34+ cells were immunoselected using a commercially available kit according to the manufacturer's instructions (Ceprate; Cell Pro, Bothel, WA). Cells were cultured in the same conditions as described for murine cells except that human IL-3 and human SCF were used (R&D systems, Minneapolis, MN). After 4 days of culture, cell volumes equivalent to 1 × 103 human cells from TMTX-free cultures were plated in 3 mL of Methocult H4534 (Stem Cell Technologies) supplemented with 3 U/mL human erythropoietin. Colonies were scored after incubation for 14 days at 37°C, 5% CO2 .
Retroviral-mediated BM transduction. BM transduction experiments were performed as previously described.21 Briefly, unseparated BM from C57Bl/6J or WB/B6F1-+/+ mice was prestimulated for 48 hours in DMEM containing 15% FBS plus 20 ng/mL murine IL-3, 50 ng/mL human IL-6, and 50 ng/mL rat SCF at 37°C, 5% CO2 . BM cells were obtained and placed in coculture with ecotropic retroviral producing cells21 24 with the above cytokines and 6 μg/mL polybrene. Forty-eight hours later, nonadherent cells were procured and placed into the in vitro suspension cultures described above.
Statistical methods. The probability of statistical differences between experimental groups was determined by a two-tailed Student's t-test using InStat2.03 software for Macintosh (Cupertino, CA).
RESULTS
Resting murine stem cells resist killing with TMTX in vivo. To examine the susceptibility of murine HSCs to antifolates, TMTX was administered to donor mice and HSC survival was monitored in a repopulation assay. BM cells were procured from drug-treated C57Bl/6J mice 24 hours after treatment and injected into WB/B6F1 W/Wv recipients. Donor engraftment was monitored based on the hemoglobin pattern appearing in recipient mice 10 weeks after transplant. Posttransplant conversion of the recipient pattern (Fig 1 panel B) to that of the donor (Fig 1 panel A) indicated that viable HSCs survived drug treatment in the donor mice. For each experimental group shown in panels C through G, between two and six mice were transplanted and all gave the same result. As a positive control, 8 × 105 cells from untreated donors fully reconstituted W/Wv recipients, as evidenced by conversion to 100% donor hemoglobin in transplanted recipients (Fig 1 panel C). The same marrow volume was transplanted from donor mice treated with a variety of drug regimens (Fig 1 panels D through G). When donors were treated with a single bolus dose of either TMTX or 5FU, full donor reconstitution was seen in all recipients, indicating that neither treatment reduced the number of HSCs below that required for engraftment (Fig 1 panels D and E).
Stem cells induced to cycle with 5FU also resist killing with TMTX in vivo. One possible explanation for the above finding is that most HSCs are quiescent and therefore not susceptible to killing with S-phase–specific drugs.6 It is known that 5FU treatment draws HSCs into cycle,25,26 thereby rendering them susceptible to a subsequent dose of 5FU.27 To test whether induction of HSC cycling would similarly increase susceptibility to TMTX, donor mice were treated with TMTX administered 5 days after a single bolus of 5FU. Even after induction of cycling with 5FU, TMTX was not toxic to donor HSCs (Fig 1 panel G). In contrast, treatment with sequential 5FU doses spaced 5 days apart resulted in complete killing of donor HSCs, as indicated by maintenance of the recipient hemoglobin pattern (Fig 1 panel F ).
These results were confirmed by Southern blot analysis using the EcoRI polymorphism that distinguishes between donor and recipient-globin alleles. In recipients transplanted with marrow from 5FU/TMTX-treated donors, DNA from the BM, spleen, and thymus showed the expected 10-kb band representing the single hemoglobin allele,28 showing that all hematopoietic organs had been reconstituted with stem cells from the treated donor (data not shown). A second series of experiments was performed where volume equivalents of 2 × 106 untreated BM cells were transplanted instead of 8 × 105 cell equivalents. Six recipients received marrow from 5FU/TMTX-treated donors and two recipients from donors treated with TMTX only. Both groups showed full reconstitution with donor marrow, again showing that HSCs are resistant to TMTX in vivo (data not shown).
Thus, murine repopulating HSCs are resistant to high doses of TMTX in vivo via a mechanism that is independent of cell cycle status.
Mechanism of TMTX resistance in myeloid progenitors. Murine myeloid progenitors, as scored by myeloid colony formation in semisolid media, were also resistant to TMTX in vivo (data not shown), confirming the results of Blau et al.8 To determine the means by which immature hematopoietic cells resist killing with TMTX, we focused on myeloid progenitors, hypothesizing that the underlying mechanism in HSCs may be the same. Prior data have shown that differentiating myeloid cells15,29 and certain tumor cells18 30 can resist MTX by uptake of the exogenous nucleotide precursors thymidine and hypoxanthine. To test whether proliferating myeloid progenitors can resist TMTX by a similar mechanism, we designed an in vitro assay to measure TMTX sensitivity using defined concentrations of thymidine and hypoxanthine. Unseparated murine BM cells were incubated in TMTX-containing suspension cultures with either dialyzed FBS, which contained undetectable levels of thymidine and hypoxanthine, or with medium supplemented with known concentrations of these nucleotide precursors. The cells were incubated in TMTX for 4 days in the presence of IL-3, IL-6, and SCF to induce proliferation of myeloid progenitors. After 4 days, the cells were washed and plated in drug-free semisolid media to score CFUs per volume of suspension culture.
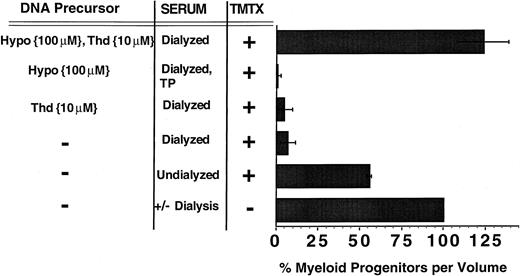
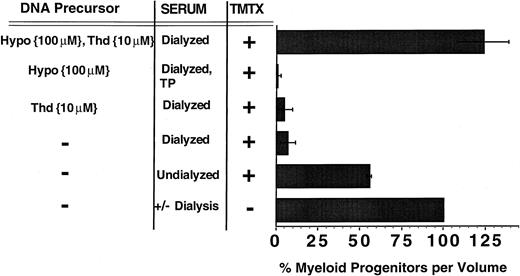
In the absence of TMTX, the number of progenitors increased by an average of 10-fold over the 4-day culture period and this expansion was not significantly different using either dialyzed or undialyzed serum. When 150 nmol/L TMTX was added to cultures containing undialyzed serum, less than 50% of clonogenic progenitors were killed (Fig 2). In contrast, TMTX killed 93% of the progenitors in cultures with dialyzed serum, indicating that dialyzable serum components contribute to TMTX resistance. Addition of either 10 μmol/L thymidine or 100 μmol/L hypoxanthine alone to dialyzed serum did not confer any protection from TMTX. In contrast, addition of both thymidine and hypoxanthine completely restored progenitor survival in the presence of TMTX (P = .0002 v dialyzed serum only). Control experiments showed that addition of 10 μmol/L thymidine and/or 100 μmol/L hypoxanthine had no effect on the growth of myeloid progenitors in the absence of TMTX (data not shown). Progenitor colonies were of uniform size between various experimental conditions, presumably because TMTX was not present in the methylcellulose cultures. These data indicate that myeloid progenitors can transport exogenous hypoxanthine and thymidine to overcome TMTX-induced toxicity and that both pyrimidine and purine precursors are required.
The role of thymidine and hypoxanthine uptake in TMTX-resistance of murine myeloid progenitors in vitro. Unseparated murine BM cells were cultured in suspension dishes in the presence of cytokines and either undialyzed or dialyzed FBS. Some cultures also contained 10 μmol/L thymidine (Thd), 100 μmol/L hypoxanthine (Hypo), and/or 150 nmol/L TMTX. After 4 days of culture, equal volumes of cells were washed and plated in TMTX-free semisolid cultures to determine the myeloid progenitor content. Progenitor survival was calculated by dividing the number of CFU-Cs per volume from cultures containing TMTX by the number per volume from drug-free cultures, then multiplying by 100. The designation TP indicates that the serum was treated with thymidine phosphorylase. Each bar represents the mean of at least three independent experiments and the line shows ±1 SD from the mean.
The role of thymidine and hypoxanthine uptake in TMTX-resistance of murine myeloid progenitors in vitro. Unseparated murine BM cells were cultured in suspension dishes in the presence of cytokines and either undialyzed or dialyzed FBS. Some cultures also contained 10 μmol/L thymidine (Thd), 100 μmol/L hypoxanthine (Hypo), and/or 150 nmol/L TMTX. After 4 days of culture, equal volumes of cells were washed and plated in TMTX-free semisolid cultures to determine the myeloid progenitor content. Progenitor survival was calculated by dividing the number of CFU-Cs per volume from cultures containing TMTX by the number per volume from drug-free cultures, then multiplying by 100. The designation TP indicates that the serum was treated with thymidine phosphorylase. Each bar represents the mean of at least three independent experiments and the line shows ±1 SD from the mean.
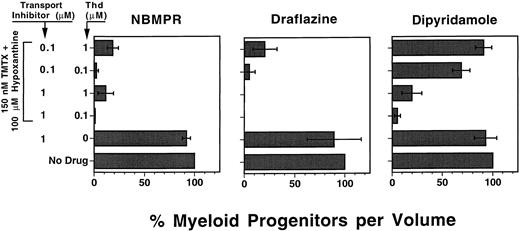
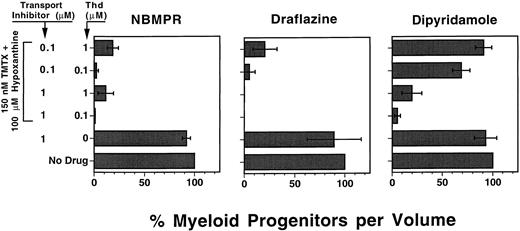
Nucleoside transport inhibitors sensitize myeloid progenitors to TMTX in vitro. Draflazine, NBMPR, and dipyridamole are drugs that inhibit certain facilitative nucleoside transport mechanisms involved in thymidine rescue.13 We used our defined culture system to determine whether these drugs would sensitize murine myeloid progenitors to TMTX at physiologically relevant concentrations of thymidine. Each transport inhibitor was tested in cultures containing either 0.1 or 1.0 μmol/L thymidine, approximating the serum thymidine levels measured in humans and mice, respectively.9 31 Figure 3 shows that in the absence of TMTX, the transport inhibitors alone had no effect on myeloid progenitor survival. In the presence of 150 nmol/L TMTX and exogenous thymidine, all three drugs enhanced progenitor killing when used at a concentration of 1.0 μmol/L. When the inhibitors were used at a lower concentration of 0.1 μmol/L, NBMPR and draflazine maintained their ability to sensitize progenitors at both thymidine concentrations. In contrast, 0.1 μmol/L dipyridamole was much less potent in the presence of 0.1 μmol/L thymidine and had no effect at 1.0 μmol/L thymidine (P < .0001 v NBMPR and draflazine under equivalent conditions). These data indicate that both NBMPR and draflazine potently sensitize murine myeloid progenitors to TMTX, reversing the sparing effects seen with physiologic levels of thymidine.
The effects of nucleoside transport inhibitors on TMTX killing of murine progenitors. Unseparated murine BM cells were added to in vitro suspension cultures containing cytokines, dialyzed FBS, hypoxanthine, TMTX, and thymidine (Thd) in various amounts as indicated. Each panel shows data from cultures which also contained the indicated concentrations of one of the following nucleoside transport inhibitors: NBMPR, draflazine (amount of active stereoisomer), or dipyridamole. Equal volumes of cells were plated in methylcellulose after 4 days of suspension culture to determine the myeloid progenitor content. Progenitor survival is expressed as a percentage of CFU-C relative to TMTX-free conditions as described in Fig 2. Each bar represents the mean ±1 SD for three independent experiments.
The effects of nucleoside transport inhibitors on TMTX killing of murine progenitors. Unseparated murine BM cells were added to in vitro suspension cultures containing cytokines, dialyzed FBS, hypoxanthine, TMTX, and thymidine (Thd) in various amounts as indicated. Each panel shows data from cultures which also contained the indicated concentrations of one of the following nucleoside transport inhibitors: NBMPR, draflazine (amount of active stereoisomer), or dipyridamole. Equal volumes of cells were plated in methylcellulose after 4 days of suspension culture to determine the myeloid progenitor content. Progenitor survival is expressed as a percentage of CFU-C relative to TMTX-free conditions as described in Fig 2. Each bar represents the mean ±1 SD for three independent experiments.
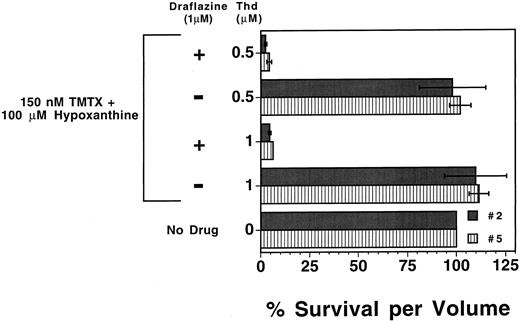
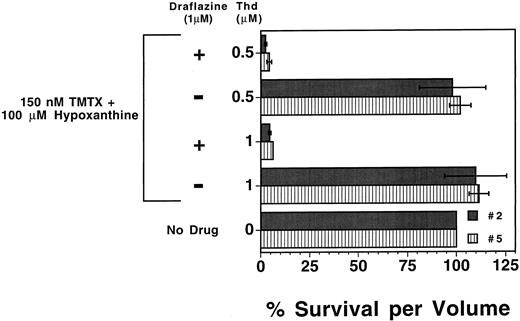
We next tested whether human myeloid progenitors could also be sensitized to TMTX with nucleoside transport inhibitors. Draflazine was chosen based on prior clinical data showing it can effectively inhibit nucleoside transport in vivo in humans.32 Human CD34+ enriched cells, immunoselected from peripheral blood apheresis products of two different patients, were added to the defined in vitro suspension culture system with or without 1.0 μmol/L draflazine. As was observed with murine progenitors, TMTX alone was toxic to human myeloid progenitors cultured in dialyzed FBS (data not shown), but this toxicity can be circumvented by the addition of thymidine (1.0 μmol/L or 0.5 μmol/L) and 100 μmol/L hypoxanthine (Fig 4). Although draflazine alone had no effect on proliferating progenitors (data not shown), draflazine markedly sensitized human myeloid progenitors to TMTX at both thymidine concentrations (P ≤ .01 v no draflazine). These data indicate that thymidine rescue from TMTX toxicity occurs in both murine and human myeloid progenitors and that this function can be blocked by transport inhibitors in both cases.
Effects of draflazine on TMTX toxicity in proliferating human myeloid progenitors. Mobilized peripheral blood CD34+ cells from two separate patients (nos. 2 and 5) were cultured in the presence of dialyzed FBS and cytokines. Cultures also contained 150 nmol/L TMTX, 100 μmol/L hypoxanthine, 1 μmol/L draflazine (amount of active stereoisomer), and differing amounts of thymidine (Thd) as indicated. After 4 days of culture, cells were washed and equal volumes of cells were plated in methylcellulose to determine the myeloid progenitor content. Progenitor survival is expressed as a percentage of CFU-C relative to TMTX-free conditions as in Fig 2. Dark and light bars represent the data obtained with samples from the two different patients. Each bar represents the mean of two experiments ±1 SD.
Effects of draflazine on TMTX toxicity in proliferating human myeloid progenitors. Mobilized peripheral blood CD34+ cells from two separate patients (nos. 2 and 5) were cultured in the presence of dialyzed FBS and cytokines. Cultures also contained 150 nmol/L TMTX, 100 μmol/L hypoxanthine, 1 μmol/L draflazine (amount of active stereoisomer), and differing amounts of thymidine (Thd) as indicated. After 4 days of culture, cells were washed and equal volumes of cells were plated in methylcellulose to determine the myeloid progenitor content. Progenitor survival is expressed as a percentage of CFU-C relative to TMTX-free conditions as in Fig 2. Dark and light bars represent the data obtained with samples from the two different patients. Each bar represents the mean of two experiments ±1 SD.
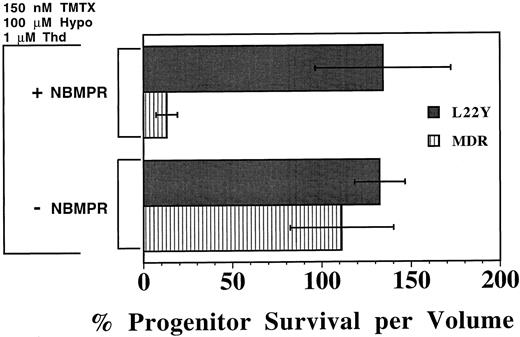
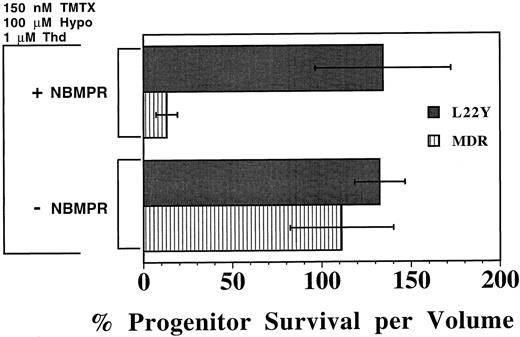
A drug resistant DHFR gene protects murine progenitors from TMTX and NBMPR. To validate that the toxicity of the combination of TMTX and NBMPR was caused by inhibition of nucleotide biosynthesis, we next tested whether retroviral-mediated transfer of a drug-resistant DHFR gene would protect myeloid progenitors. Murine BM cells were transduced with a retroviral vector expressing the antifolate-resistant DHFR variant L22Y21 or with a vector containing the human MDR1 cDNA as a control.24 After coculture with vector-producing cells, nonadherent transduced BM cells were procured, washed, and incubated in liquid culture with TMTX in the presence or absence of NBMPR. As shown in Fig 5, both control and L22Y-transduced progenitors were resistant to TMTX in cultures which contained thymidine and hypoxanthine but lacked NBMPR. However, when 1.0 μmol/L NBMPR was added, L22Y-modified progenitors remained completely TMTX resistant whereas 87% of the MDR1-modified progenitors were killed. This sensitization was statistically significant for MDR1 control cells (P < .005) but not for DHFR-transduced cells. Thus, the toxicity of TMTX plus NBMPR can be completely reversed in vitro by transfer of a resistance-conferring DHFR gene, indicating that restoration of de novo nucleotide synthesis can circumvent the toxicity of this drug combination.
Protection of murine progenitors from the combined toxicity of TMTX plus NBMPR using a DHFR retroviral vector. Murine BM cells were first transduced with either a retroviral vector expressing the L22Y-DHFR gene (L22Y) or, as a control, the human multidrug resistance 1 gene (MDR). Cells were subsequently cultured in medium containing dialyzed serum, 150 nmol/L TMTX, 100 μmol/L hypoxanthine (hypo), and 1 μmol/L thymidine (Thd), in the presence or absence of 1 μmol/L NBMPR. Equal volumes of cells were washed and plated in methylcellulose after 4 days of suspension culture to determine the myeloid progenitor content. Progenitor survival is expressed as a percentage of progenitors per volume relative to TMTX-free conditions. Each bar represents the mean ±1 SD from three independent experiments.
Protection of murine progenitors from the combined toxicity of TMTX plus NBMPR using a DHFR retroviral vector. Murine BM cells were first transduced with either a retroviral vector expressing the L22Y-DHFR gene (L22Y) or, as a control, the human multidrug resistance 1 gene (MDR). Cells were subsequently cultured in medium containing dialyzed serum, 150 nmol/L TMTX, 100 μmol/L hypoxanthine (hypo), and 1 μmol/L thymidine (Thd), in the presence or absence of 1 μmol/L NBMPR. Equal volumes of cells were washed and plated in methylcellulose after 4 days of suspension culture to determine the myeloid progenitor content. Progenitor survival is expressed as a percentage of progenitors per volume relative to TMTX-free conditions. Each bar represents the mean ±1 SD from three independent experiments.
In vivo sensitization of myeloid progenitors to TMTX using nucleoside transport inhibitors. To determine whether nucleoside transport inhibitors could be used to sensitize myeloid progenitors to TMTX in vivo, untransplanted C57Bl/6J mice were given TMTX daily for 5 days together with either draflazine or the 5′ monophosphate of NBMPR (NBMPR-P). Twenty-four hours after the final treatment, the BM cellularity and progenitor content were determined. Progenitors were quantified using a standard CFU-C assay and expressed as clonogenic cells per volume of femur, relative to untreated controls. Figure 6 shows the data obtained from the 27 of 32 mice that survived drug treatment (three experiments with two to three mice per group). TMTX alone at a dose of 130 mg/kg/d for 5 consecutive days reduced BM cellularity by 57%, but actually caused an increase in the number of myeloid progenitors per femur. In contrast, when either NBMPR-P or draflazine was given together with the same dose of TMTX, severe reductions were seen both in the BM cellularity and progenitor content. The combination of TMTX plus NBMPR-P reduced the marrow progenitor content to 6.0% of that seen in untreated controls. These reductions were statistically significant when compared to progenitor survival with either transport inhibitor alone (P < .05 in both cases). The effects of the drug combinations were clearly synergistic given that neither inhibitor alone significantly reduced BM cellularity or progenitor content. Treatment with TMTX plus NBMPR-P also reduced the numbers of progenitors in the spleen to 17% of that seen in untreated mice (data not shown). Thus, both NBMPR-P or draflazine can sensitize myeloid progenitors to TMTX in vivo, thereby allowing significant progenitor depletion.
NBMPR-P also sensitizes HSCs to TMTX in vivo. We next tested if transport inhibitors could be used to sensitize repopulating HSCs to TMTX. In an experiment analogous to that shown in Fig 1, donor C57BL/6J mice were treated with 130 mg/kg/d of TMTX plus 20 mg/kg/d of NBMPR-P for 5 consecutive days. BM cells were obtained 24 hours after the final treatment and transplanted into WB/B6F1 W/Wv recipients. In contrast to the results obtained using TMTX alone, the combined use of TMTX and NBMPR-P led to a complete lack of donor reconstitution, indicating that both drugs together caused significant killing of HSCs (data not shown).
To confirm this observation, we used a more sensitive competitive repopulating assay to determine HSC toxicity.33 Donor C57Bl/6J mice were treated on 5 consecutive days with either TMTX alone at a dose of 130 mg/kg/d, NBMPR-P alone at a dose of 20 mg/kg/d, or both drugs together. BM cells were obtained 24 hours after the final treatment and mixed with an equal volume of BM cells from untreated congenic B6.C-H-1b/ByJ (HW80) mice. These mixtures were injected into recipient W/Wv mice and reconstitution was monitored by hemoglobin electrophoresis 10 weeks after transplant. The ratio of the single (Fig 7 panel A) to diffuse (Fig 7 panel B) hemoglobins in reconstituted recipients was used to measure the relative HSC content within each donor marrow. As a control, mice were transplanted with 50%:50% and 30%:70% volume:volume mixtures from untreated C57Bl/6J and HW80 marrows, respectively. The hemoglobin phenotypes in recipients accurately reflected the proportions of each donor marrow used in these mixtures (Fig 7 panels C and D). For each of the experimental arms shown in Fig 7 panels C through H, between three and eight mice were transplanted. All mice within a group showed identical reconstitution patterns and each panel shows representative data from two recipients.
Sequential 5FU treatment completely eliminated HSCs from the C57Bl/6J graft, as shown in the analysis of transplant recipients in Fig 7 panel E. In this group, all recipients showed a 100% diffuse hemoglobin pattern, indicating that the reconstituted hematopoietic system was derived entirely from the untreated HW80 graft. As expected, no HSC toxicity was seen in C57Bl/6J mice treated with either NBMPR-P or TMTX alone for 5 consecutive days. Recipients receiving these treated marrows showed equal contributions to reconstitution from treated and untreated donors (Fig 7 panels F and G). In contrast, the use of TMTX plus NBMPR-P together completely eliminated reconstituting activity from the C57Bl/6J graft (Fig 7 panel H), to the same degree as that seen with sequential 5FU treatment. These results were obtained in two independent experiments, one of which is shown in Fig 7. These data show that treatment with NBMPR-P causes dramatic in vivo sensitization of repopulating HSCs to TMTX toxicity.
DISCUSSION
Prior studies have shown that myeloid progenitors can resist TMTX through a cell-cycle–independent mechanism.8 We have confirmed these observations and extended them to show that repopulating stem cells are also resistant to TMTX, even when induced to cycle with 5FU. By using both in vitro and in vivo assays, we have further shown that nucleotide salvage mechanisms are responsible for the resistance seen in primitive myeloid cells. Taken together, these results suggest that the degree of TMTX sensitivity at different stages of myeloid development is determined, at least in part, by differential utilization of nucleotide salvage pathways. Our data indicate that TMTX normally exerts toxicity at a stage distal to CFU-C formation, probably at the level of differentiating myeloid precursors. Toxicity at this stage of development, when the myeloid compartment size is dramatically expanding, is consistent with the severe BM hypocellularity caused by TMTX. In addition, our data are consistent with those obtained by Blau et al using MTX,8 suggesting that this toxicity profile may be characteristic of antifolates in general. The protection from myelosuppression afforded by transfer of DHFR vectors21,34 35 is likely to be exerted at this relatively late stage of myelopoiesis. This model also predicts that the increase in drug sensitivity occurring with myeloid differentiation may be due to relatively reduced utilization of nucleotide salvage mechanisms in more mature myeloid cells. It is not known which pathways may be downregulated or which biochemical steps are rate limiting. During myeloid differentiation, decreased expression could occur for any number of gene products necessary for the salvage mechanism, such as thymidine transporters, purine base transporters, thymidine kinase, or hypoxanthine guanine phosphoribosyl-transferase.
Our results illustrate several interesting points regarding thymidine salvage in myeloid cells. The observation that NBMPR-P can sensitize both progenitors and HSCs to TMTX shows that primitive myeloid cells express mainly the NBMPR-sensitive es facilitative transporter. If primitive cells were significantly using either concentrative thymidine transport mechanisms or the ei NBMPR-insensitive facilitative transporter, NBMPR would not block these pathways and therefore could not cause sensitization to antifolates. Secondly, in contrast to what is observed with antifolates, thymidine salvage does not appear to protect HSCs and myeloid progenitor cells against 5FU toxicity. If 5FU killed progenitor cells mainly by inhibition of de novo thymidylate synthesis, the same thymidine salvage mechanisms that confer resistance to TMTX should also provide protection from 5FU. The lack of such protection suggests that the hematopoietic toxicity of 5FU is not mediated by inhibition of thymidylate synthase, but rather through other mechanisms such as direct inhibition of RNA synthesis.36 Thymidylate synthase-independent toxicity would be predicted by the dosing schedule of 5FU used.37 Whatever the mechanism of cytotoxicity, a single dose of 5FU induces stem cells to cycle,25 27 presumably by killing progenitors and drawing HSCs into division. Our results suggest that an equivalent effect can be achieved with the combination of TMTX and NBMPR-P. The elimination of repopulating HSCs with five consecutive daily doses of TMTX and NBMPR-P could only occur if stem cells were drawn into cycle, presumably by the initial treatment doses, and thereby rendered susceptible to S-phase–dependent toxicity occurring with later doses.
Our study shows that severe myelotoxicity could limit the use of nucleoside transport inhibitors for improving the antitumor responses obtained using antifolates. Transport inhibitors can sensitize certain human tumor cells to MTX, including lines derived from lymphoid leukemias, sarcomas, and colon carcinomas.17,18,30,38 Based on these findings, dipyridamole has been used clinically to increase the antitumor effects of MTX. These phase 1 clinical trials have yielded disappointing results, showing increased hematopoietic toxicity with no evidence for improved tumor responses.16,39 The lack of tumor sensitization could be due to the high degree of serum protein binding of dipyridamole,40 as well as the limited potency of dipyridamole for transport inhibition. A more potent inhibitor like NBMPR-P should induce better tumor sensitization, but our results predict that accompanying sensitization of primitive myeloid cells would lead to severe myelosuppression. Clearly, methods to protect hematopoiesis from the combined effects of NBMPR-P and antifolates would greatly facilitate this approach. It should be possible to use DHFR vectors to protect myeloid cells from the combined toxicity of NBMPR-P and TMTX, in a fashion analogous to using DNA alkyltransferase gene transfer and O6-benzylguanine to widen the therapeutic ratio of nitrosoureas.41,42 The availability of DHFR variants that confer high levels of resistance to antifolates21 35 will allow testing of this approach in animal models.
Another potential application for the combined use of TMTX, NBMPR-P, and DHFR gene transfer is for the in vivo selection of genetically modified HSCs. In vivo selection is a strategy whereby cytotoxic drugs are administered after autologous stem cell transplant to increase the number of transduced myeloid cells.20,24 Evidence for in vivo selection of DHFR-transduced myeloid cells in MTX-treated mice has been equivocal.43,44 It has been suggested that lack of selection is predicted by the intrinsic antifolate resistance seen in untransduced myeloid progenitors and CFU-S.8 Our findings support this contention by showing that untransduced HSCs resist TMTX, even when induced to cycle. Without elimination of unmodified HSCs, antifolates alone cannot provide stable selection for myeloid cells that express DHFR resistance vectors. This obstacle may be surmountable by sensitizing progenitors and HSCs to TMTX with coadministration of transport inhibitors such as NBMPR or draflazine. Using retroviral vectors that are known to express transferred genes in primitive stem cells,45 46 we predict that TMTX plus NBMPR could be used to enrich for stem cell clones that express resistance-conferring DHFR cDNAs.
ACKNOWLEDGMENT
We thank Drs Jacques Galipeau and Kevin Bunting for helpful discussions, Dr Lee Schwartzberg for providing the human peripheral blood cells used in these studies, and Dr Arthur Nienhuis for his critical review of the manuscript.
Supported in part by National Heart, Lung, and Blood Institute Program Project Grant No. P01 53749-02 (B.P.S.), The James S. McDonnell Foundation Grant No. 94-50 (B.P.S.), The John H. Sununno Postdoctoral Fellowship (J.A.A.), US Public Health Service Grants No. P01 CA 31922 (R.L.B.) and R01CA 55056 (J.A.B.), Cancer Center Support Grant No. P30 CA 21765 (R.L.B., J.A.B., and B.P.S.), and by the American Lebanese Syrian Associated Charities (ALSAC).
Address reprint requests to Brian P. Sorrentino, MD, Division of Experimental Hematology, St Jude Children's Research Hospital, 332 N Lauderdale, Memphis, TN 38101.