Abstract
In addition to their major function in antigen presentation and natural killer cell activity regulation, HLA class I molecules may modulate T-cell activation and proliferation. Monoclonal antibodies (MoAbs) that recognize distinct epitopes of HLA class I molecules were reported to interfere with T-cell proliferation. We show here that two MoAbs (mouse MoAb90 and rat YTH862) that bind to an epitope of the α1 domain of HLA class I heavy chain induce apoptotic cell death of activated, but not resting, peripheral T lymphocytes. Other reference anti-HLA class I antibodies specific for distinct epitopes of the α1 (B9.12.1), α2 (W6/32), or α3 (TP25.99) domains of the heavy chain decreased T-cell proliferation but had little or no apoptotic effect. Apoptosis shown by DNA fragmentation, phosphatidylserine externalization, and decrease of mitochondrial transmembrane potential was observed whatever the type of T-cell activator. Apoptosis did not result from Fas/Fas-L interaction and distinct though partly overlapping populations of activated T cells were susceptible to Fas– and HLA class I–mediated apoptosis, respectively. Induction of apoptosis did not require HLA class I cross-linking inasmuch as it could be observed with monovalent Fab′ fragments. The data indicate that MoAb90 and YTH862 directed against the α1 domain of HLA class I trigger apoptosis of activated T lymphocytes by a pathway which does not involve Fas-ligand.
HLA CLASS I molecules are heterodimers made of a 45-kD α-chain with three extracellular domains noncovalently associated with the invariant 12-kD β2-microglobulin (β2m).1-3 Their main biological function is the presentation of antigenic peptides to CD8+ T cells. Nine-mer peptides generated by the proteasomes of the antigen-presenting cells are bound to the peptide groove formed by the highly polymorphic regions of the α1 and α2 domains.4 Interaction between HLA class I and TCRαβ is further stabilized by the binding of CD8 to the less polymorphic α3 domain.5-7 In addition to their role in antigen presentation, HLA class I molecules expressed on T cells may also be involved in signal transduction and cellular activation. The intracellular signaling events after HLA class I ligation include protein tyrosine phosphorylation and increase in intracellular free calcium.8-12 Engagement of HLA class I molecules on human T cells by either soluble or cross-linked antibodies but also by cell surface-bound CD8 molecules was reported to either inhibit or induce cellular proliferation, depending on the conditions.13-24 In addition, several studies have shown that HLA class I ligation on T cells may result in growth arrest, anergy, and eventually apoptosis induction.25-28
In the immune system, apoptosis induced by surface receptors, such as Fas (APO-1, CD95) and tumor necrosis factor receptor (TNF-R) is a central mechanism for the homeostasis of T and B lymphocytes, and for lysis of target cells by cytotoxic T lymphocytes (CTL) and natural killer (NK) cells.29,30 Regarding human T cells, apoptosis can be triggered by several membrane receptors including three members of the TNF-R family,31 Fas,32-34 TNF-RI,35 and CD30,36 but also CD2,37,38 CD45,39 CTLA4,40 and HLA class I molecules.27 28
In murine models of allogeneic bone marrow (BM) transplantation, it has been shown that antigen-presenting cells, isolated from BM or spleen, that express CD8 could induce anergy or apoptosis of alloreactive CTL precursors, an effect designated as the “veto” phenomenon.41 Sambhara and Miller42 reported that two anti-class I monoclonal antibodies (MoAbs) that recognize the H-2D α3 domain could inhibit the generation of alloreactive CTL and induce apoptosis of both CD8+ CTL and CD4+ helper T-cell precursors. Apoptosis was similarly achieved by incubation of activated CD4+ or CD8+ T cells with cells that expressed CD8.42 Interestingly apoptosis was restricted to activated T cells but fully differentiated effector CTL and Th cells were resistant.
In the present study we looked for a possible human counterpart of the veto phenomenon by testing the capacity of various anti-HLA class I MoAbs to induce apoptosis of activated T cells. Recent reports indicate that cross-linking MoAbs that recognize β2m28 or the α3 domain of the heavy chain27 can induce T-cell apoptosis, but no selectivity toward preactivated T cells was documented in these studies. In this report we extend to peripheral T cells our previous observations made on B cells43 and report that two antibodies (mouse MoAb90 and rat YTH862) directed against an epitope of the α1 domain of HLA class I heavy chain can induce apoptosis of activated but not resting T cells. This effect does not involve Fas (Apo-1, CD95)/Fas-ligand interaction and does not require cross-linking.
MATERIALS AND METHODS
MoAbs and Reagents
MoAb90 was produced by immunization with consecutive intraperitoneal injections of human tonsil cells (106 cells) followed by the standard procedure of Köhler and Milstein.44 MoAb90 (IgG1) was purified from ascites fluids by protein A chromatography. YTH862 MoAb (IgG2b) was produced by immunization of rats (DA strain) with consecutive intraperitoneal injections of phytohemagglutinin (PHA)-activated human peripheral blood lymphocyte (PBL) (20 × 106 cells) and fusion with the Y3 hybridoma. MoAb90 bound at very high levels (>100-fold above background) to 97% to 100% of PBL from all human donors tested (n > 70). Double-immunofluorescent staining further showed that MoAb90 binds to 100% of human peripheral blood T cells, B cells, monocytes, and NK cells. MoAb90 binds to 30 different hematopoietic cell lines but not to Daudi (β2m deficient) and K562 that do not express HLA class I molecules. The HLA class I specificity of MoAb90 was further demonstrated by binding to HLA class I transfected cells as well as immunoprecipitation of a 45-kD HLA class I heavy chain in HLA-A, -B, -C, and -G-transfected murine fibroblasts (data not shown). The YTH862 MoAb reacts with all human cell lines tested except Daudi and precipitates class I and β2m from 125I-labeled PHA blast membranes. The anti-HLA class I MoAbs, B9.12.1 (anti-HLA-A, -B, and -C, IgG2a), B1.23.2 (anti-HLA-B and -C, IgG2a), and the anti-β2m, B1G6 (IgG2a),45 were kindly provided by Dr P. Le Bouteiller. HC10 (anti-HLA-B, IgG2a)46 was a gift from Prof H. Ploegh (MIT, Cambridge, MA). TP25.99 (anti-HLA-A, -B, and -C, IgG1)45 was provided by Dr R. Buelow (SangStat Medical Corp, Menlo Park, CA) and W6/32 (anti-HLA-A, -B, and -C, IgG2a) was obtained from American Type Culture Collection (ATCC; Rockville, MD). The CD3 MoAb OKT3 was from Cilag Laboratories (Levallois-Perret, France). The agonist antihuman Fas MoAb CH11 (IgM) was from Immunotech (Marseille, France). The antagonist antihuman Fas MoAb ZB4 (IgG1) was from Kamiya Biomedical Co (Thousand Oaks, CA). The anti-β2m MoAb BE104 (IgG2a) was prepared by C. Vincent and the anti-CD8 MoAb BL15 and the anti-CD4 MoAb BL4 by J. Brochier in our laboratory. Two irrelevant IgG1 and IgG2a MoAbs were used as isotype controls. The CD45RA (MEM-56) MoAb was from Monosan (Uden, The Netherlands) and the CD45RO (UCHL-1) MoAb was from Valbiotech (Paris, France). Fluorescein isothiocyanate (FITC)-conjugated anti-CD45RO, phycoerythrin (PE)-conjugated anti-CD45RA, and FITC-conjugated anti-Fas (UB2) were from Immunotech. FITC-conjugated anti-CD25, anti-CD69, anti-CD71, anti-CD4, and PE-conjugated anti-CD8 were from Becton Dickinson (Mountain View, CA). Staphylococcus aureus enterotoxin B (SEB), pockweed mitogen (PWM), PHA, phorbol myristate acetate (PMA), ionomycin, and concanavalin A (Con A) were obtained from Sigma (St Louis, MO).
Cells and Transfectants
PBL. PB was collected from healthy donors in the presence of sodium citrate. Blood was defibrinated, then mononuclear cells were isolated by centrifugation on a layer of histopaque (Sigma). Those cell suspensions referred to as PBL contained 1.8% ± 0.4% monocytes as defined by expression of CD14. In some experiments PB mononuclear cells (PBMC) were isolated from heparinized blood by centrifugation on histopaque.
For preparation of CD4+ and CD8+ cells, PBL were depleted from CD8+ and CD4+ cells, respectively, by adherence onto plastic surfaces coated with anti-CD4 (BL4) and anti-CD8 (BL15) MoAbs. The percentages of CD8+ and CD4+ cells in those suspensions were 91.4% ± 2.4% and 95.2% ± 2.5%, respectively.
For preparation of CD45RA+ and CD45RO+ cells, PBL were depleted from CD45RO+ and CD45RA+ cells, respectively, by adherence onto plastic surfaces coated with anti-CD45RO (UCHL-1) and anti-CD45RA (MEM-56) MoAbs. The percentages of CD45RA+ cells from CD45RA suspension and CD45RO+ cells from CD45RO suspension were 93% ± 3.2% and 94.3% ± 5.1%, respectively.
Cell lines. The origin, phenotypic characteristics, and susceptibility to Fas-mediated apoptosis of Burkitt's lymphoma cell lines (Ramos and Raji), B-lymphoblastoid cell lines (IARC970, Dakiki, RPMI8866), and T-cell lines (SupT-1, CEM, Molt-4, Sud HL-1, Jurkat) are listed in a previous report.47 Six human T-cell lines (three CD4+ and three CD8+) derived from human tonsils were provided by J. Banchereau.
C1R transfectant cells. Transfectants of the HLA-A, -B negative human B-lymphoblastoid cell line C1R that express HLA-A2.1 (C1R-A2) or hybrid mouse/human class I molecules, DAA, ADA, AAD, and ADD were a generous gift from V.H. Engelhard and have been previously described.48 Briefly, interspecies hybrid class I molecules were created by reciprocal exchange of single exons encoding the three extracellular coding regions (α1, α2, and α3) between the genomic clones of HLA-A2.1 and H-2Dd. The hybrid class I genes have three-letter designations, such that the first letter indicates the origin of the promoter, leader, and α1 domains; the second letter indicates the origin of the α2 domain; and the third letter indicates the origin of the α3, transmembrane, and cytoplasmic domains. An “A” represents a domain from HLA-A2.1 and “D” represents contributions from H2-Dd.
Culture medium and cell proliferation. PBL were resuspended in RPMI 1640 (Sigma) supplemented with 10% fetal calf serum (FCS), 2 mmol/L L-glutamine, and antibiotics (penicillin 100 U/mL, streptomycin 100 μg/mL). For proliferation assay, cells (106/mL) were incubated in 96-well microplates (Costar, Cambridge, MA) coated with the CD3 MoAb OKT3 (5 μg/mL) or in the presence of PHA (5 μg/mL), Con A (10 μg/mL), PWM (1/300), SEB (50 ng/mL), PMA (10 ng/mL) with or without MoAbs. Mixed lymphocyte reaction (MLR) were performed using mitomycin treated Raji cells as previously described.49 Cultures were maintained in a humid atmosphere at 37°C containing 5% CO2 for 3 or 5 days of mitogenic or MLR culture, respectively. During the last 12 hours of incubation they were pulsed with (methyl-3H)thymidine ([3H]TdR; Amersham) at 0.5 μCi/well. 3H-TdR uptake was measured using a Packard direct beta counter (Packard, Meriden, CT) after harvesting.
Immunofluorescence staining. Cells were washed with isotonic NaCl/Pi buffer containing 1% bovine serum albumin (BSA) and 0.2% NaN3 (phosphate-buffered saline [PBS]/BSA/Azide). Cells (5 × 105) were incubated with 20 μL of nonlabeled MoAbs for 30 minutes at 4°C. After two washes in PBS/BSA/Azide buffer, cells were incubated with FITC-goat antimouse Ig (Dako, 1/50) for 30 minutes at 4°C. After washes, cells were fixed with 1% formaldehyde in PBS/BSA/Azide buffer and analyzed by flow cytometry with a FACScan (Becton Dickinson).
Competition epitope analysis. For competitive cross-blocking studies, PBL cells were incubated with blocking MoAb or control Ig isotypes for 30 minutes at 4°C. Biotinylated MoAb90 was added to the cells for an additional 30 minutes at 4°C. The cells were washed and incubated with fluorescein-conjugated streptavidin (Zymed Laboratories, San Francisco, CA) for 30 minutes at 4°C. After subsequent washes, cells were analyzed by flow cytometry.
Preparation of F(ab)′2 and Fab′ fragments. MoAb90 was digested by Ficin to yield F(ab)′2 and Fab′ fragments following the instructions of the manufacturer (Pierce, Rockford, IL). F(ab)′2 and Fab′ fragments were purified by chomatography using a protein A column and the purity [98% for F(ab′)2 and 96% for Fab′ preparation] was controlled by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under nonreducing conditions.
Measurement of apoptosis. After 3 days of culture, PHA-activated PBL were obtained. Dead cells were removed by centrifugation on a layer of histopaque (Sigma) and washed in Hanks' balanced salt solution. Viable cells (106/mL) were incubated in 96-well microplates with various MoAbs. After 24 hours of incubation, cell death was evaluated by fluorescence microscopy after staining with Hoechst 33342 (Sigma) at 10 μg/mL50 and by flow cytometry after addition of biotinylated annexin V following previously described methods.51 Based on these measurements, results were expressed either as percentage of apoptotic cells or percentage of specific apoptosis according to the following formula:
In addition, counts of viable cells (by trypan blue exclusion) and measurement of [3H]-TdR incorporation over the last 8 hours were performed on the same suspensions at indicated time. The morphological features of the cells after various treatments were also observed under transmission electronic microscopy. After two washings, cells were fixed in 2% osmium tetroxide in 0.1 mol/L cacodylate buffer pH 7.4, dehydrated, and Epon embedded. Thin sections were cut and after lead citrate and uranyl acetate contrasting, they were observed in a JEOL 100CS electronic microscope (Tokyo, Japan).
DNA fragmentation assays. Cells were incubated in RPMI medium for 24 hours with the agents indicated. DNA preparations were obtained following a procedure previously described.52 Electrophoresis was performed in 2% agarose gel containing 0.1 μg/mL ethidium bromide (Sigma). After electrophoresis, gels were examined under UV light.
Evaluation of mitochondrial transmembrane potential. To evaluate the mitochondrial transmembrane potential (Dψm ), cells (5 × 105/mL) were incubated with 3,3′-dihexyloxacarbocyanine [DiOC6(3); 40 nmol/L in PBS; Molecular Probes, Inc, Eugene, OR] for 15 minutes at 37°C, followed by analysis on a cytofluorometer (Becton Dickinson; l. Ex. Max., 488 nmol/L; l. EM. Max., 525 nm). The decrease in Dψm is a characteristic of apoptotic cells.53
RESULTS
MoAb90 and YTH862 Bind to the α1 Domain of HLA Class I
Transfected C1R cells that express HLA-A2.1 (C1R-A2) or hybrid mouse/human exon-shuffled HLA-A2.1/H-2Dd genes were stained with different anti–class I MoAbs and analyzed by flow cytometry. As shown in Table 1, MoAb90, YTH862, and B9.12.1 bound to HLA-A2.1–transfected cells and to C1R-ADA, -AAD, and -ADD, that express hybrid class I major histocompatibility complex (MHC) molecules with the human α1 domain from HLA-A2.1, but not to transfected C1R-DAA cells (α1 domain from H-2Dd and α2 and α3 domain from HLA-A2.1), suggesting that they recognize a determinant that maps to the α1 domain of the HLA class I heavy chain. These experiments confirmed the binding of TP25.99 and W6/32 to the α3 and the α2 domains, respectively.45 Competitive cross-blocking studies confirmed these results. The binding of biotinylated MoAb90 was equally inhibited by unlabeled MoAb90 and YTH862, suggesting that both antibodies may recognize identical or overlapping epitopes. Some inhibition was observed with the two anti-β2m MoAbs BE104 and B1G6. The B9.12.1 MoAb (α1 domain) induced borderline inhibition whereas the HC10 (α1/α2 domains), W6/32 (α2), TP25.99 (α3), and B1.23.2 MoAbs did not compete with MoAb90 binding (Table 2). It was concluded that MoAb90 and YTH862 recognize a nonpolymorphic epitope of the HLA class I α1 domain that maps close to β2m and distinct from the epitopes recognized by B9.12.1 and HC10.
Several Anti-HLA Class I Antibodies Inhibit Mitogen-Induced PBL Proliferation
The functional effects of anti-HLA class I MoAbs (at 10 μg/mL) on the DNA synthesis of activated PBL were then tested. Whatever the mitogen used, MoAb90 and to a greater extent YTH862 added at the onset of culture inhibited T-cell proliferation by 46% to 99%, whereas W6/32 and TP25.99 were less efficient (Table 3). However, unlike W6/32 and TP25.99, only MoAb90 and YTH862 also inhibited the T-cell proliferation induced by PMA. The four MoAbs strongly inhibited the allogeneic MLR. Most anti–class I MoAbs known to inhibit T-cell proliferative responses were reported to interfere with early activation events.16 18-22 The expression of the early activation markers CD69, CD25, CD71, and CD95 (Fas/Apo-1) was therefore studied in T-cell suspensions incubated during 24 hours with PHA and various anti–class I MoAbs. W6/32 slightly but significantly inhibited the expression of G1 phase markers whereas MoAb90, YTH862, and TP25.99 did not (data not shown). Therefore, the latter antibodies are unlikely to interfere with early T-cell activation events.
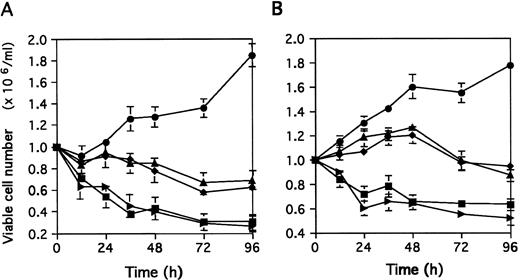
The simultaneous addition of PHA and MoAb90 or YTH862 slightly decreased the number of viable cells at 12 hours and no more than 30% of cells seeded in culture were still viable after 96 hours (Fig 1A). In the same conditions, the addition of W6/32 or TP25.99 inhibited the proliferation induced by PHA, but did not significantly decrease viable cell recovery during the first 48 hours of culture. At 72 to 96 hours, viable cell recovery was 65% to 70% of cells seeded in culture (Fig 1A), comparable with that of nonactivated PBL in absence of MoAb.
Effect of MoAb90 and YTH862 added at day 0 (A) or day 3 (B) on viable cell recovery. Viable cell numbers (determined by trypan blue exclusion) are expressed as mean ± SD of three independent experiments. (A) PBL were stimulated with PHA (5 μg/mL) in the presence of various MoAbs (10 μg/mL) for indicated time. (B) PBL were activated with PHA (5 μg/mL) for 3 days, then 106 viable cells/mL were cultured with various MoAbs (10 μg/mL) for indicated time. (•), Control; (▪), MoAb90; (▸), YTH862; (▴), W6/32; (♦), TP25.99.
Effect of MoAb90 and YTH862 added at day 0 (A) or day 3 (B) on viable cell recovery. Viable cell numbers (determined by trypan blue exclusion) are expressed as mean ± SD of three independent experiments. (A) PBL were stimulated with PHA (5 μg/mL) in the presence of various MoAbs (10 μg/mL) for indicated time. (B) PBL were activated with PHA (5 μg/mL) for 3 days, then 106 viable cells/mL were cultured with various MoAbs (10 μg/mL) for indicated time. (•), Control; (▪), MoAb90; (▸), YTH862; (▴), W6/32; (♦), TP25.99.
Addition of MoAb90 or YTH862 to day 3 PHA-activated cells resulted in a rapid decrease of the viable cell recovery during the first 24 hours (Fig 1B) whereas W6/32 or TP25.99 decreased the proliferation induced by PHA but induced only a minimal cell loss (5% to 10%) that could not be detected before 96 hours of culture (Fig 1B). In the same experimental conditions, DNA synthesis was markedly inhibited by MoAb90 and YTH862, and to a lesser extent by B9.12.1 and B1.G6 (Table 2).
MoAb90 and YTH862 Induce Apoptosis of Activated T Cells
To study the mechanisms by which MoAb90 and YTH862 decrease viable cell recovery, several parameters of apoptotic cell death were assessed. As shown in Fig 2A, MoAb90 induces double-strand DNA breaks yielding a typical ladder only in PHA-activated but not in resting cells. No DNA fragmentation was observed in cells treated with control IgG1 and W6/32 MoAbs (Fig 2A).
MoAb90 induces apoptosis of activated T lymphocytes. (A) DNA fragmentation: PBL were incubated 3 days with medium alone (lanes 1 through 3) or PHA (5 μg/mL) (lanes 4 through 6). Dead cells were removed and viable cells were treated for 24 hours with control IgG1 (lanes 1 and 4), W6/32 (lanes 2 and 5), or MoAb90 (lanes 3 and 6) at 10 μg/mL. (B through D) Morphology of 3-day PHA-activated PBL after Hoescht 33342 staining. After 3 days of culture, PHA-activated PBL were obtained. Dead cells were removed and viable cells (106/mL) were incubated in 96-well microplates in the presence of the MoAbs. Morphology was analyzed by fluorescence microscopy. (E through G) Electronic microscopy of 3-day PHA-activated PBL. PBL were cultured during 3 days with PHA (5 μg/mL). Viable cells were then treated for 24 hours with control IgG1 (10 μg/mL) (B and E), W6/32 (10 μg/mL) (C and F ), or MoAb90 (10 μg/mL) (D and G).
MoAb90 induces apoptosis of activated T lymphocytes. (A) DNA fragmentation: PBL were incubated 3 days with medium alone (lanes 1 through 3) or PHA (5 μg/mL) (lanes 4 through 6). Dead cells were removed and viable cells were treated for 24 hours with control IgG1 (lanes 1 and 4), W6/32 (lanes 2 and 5), or MoAb90 (lanes 3 and 6) at 10 μg/mL. (B through D) Morphology of 3-day PHA-activated PBL after Hoescht 33342 staining. After 3 days of culture, PHA-activated PBL were obtained. Dead cells were removed and viable cells (106/mL) were incubated in 96-well microplates in the presence of the MoAbs. Morphology was analyzed by fluorescence microscopy. (E through G) Electronic microscopy of 3-day PHA-activated PBL. PBL were cultured during 3 days with PHA (5 μg/mL). Viable cells were then treated for 24 hours with control IgG1 (10 μg/mL) (B and E), W6/32 (10 μg/mL) (C and F ), or MoAb90 (10 μg/mL) (D and G).
PHA-activated cells treated with MoAb90 for 24 hours (Fig 2D) but not cells treated with control MoAbs (Fig 2B and C) show nuclear fragmentation as typical features of apoptotic cells. No features of apoptotic cells were observed in resting T cells treated with MoAb90 (data not shown). Transmission electronic microscopy further confirmed that MoAb90-treated cells present typical features of apoptosis with undamaged cytoplasmic membrane, an irregular chromatin condensation, or as a rim inside the nuclear membrane and a disruption of the nucleus into discrete fragments (Fig 2G). Cells treated with control IgG1 or W6/32 did not show nuclear injury (Fig 2E and F ). It should be stressed that no necrotic cells were found on examination of a large number of MoAb90-treated cells. YTH862 MoAb that cross-reacts with MoAb90 induced a strong apoptotic effect on PHA-activated PBL (Table 2). Among the other anti-β2m and anti–α-chain MoAbs tested, only B9.12.1 and TP25.99 induced a slight apoptotic effect, which remained minimal as compared with that of MoAb90 or YTH862.
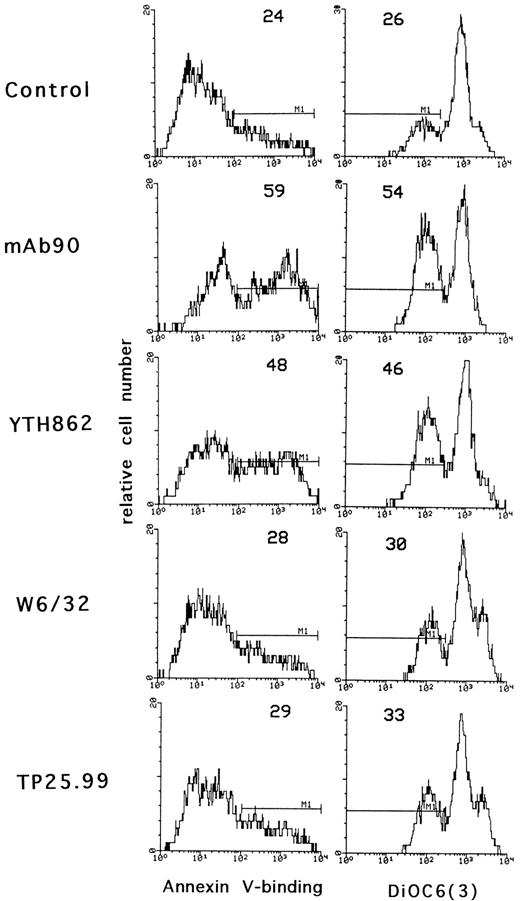
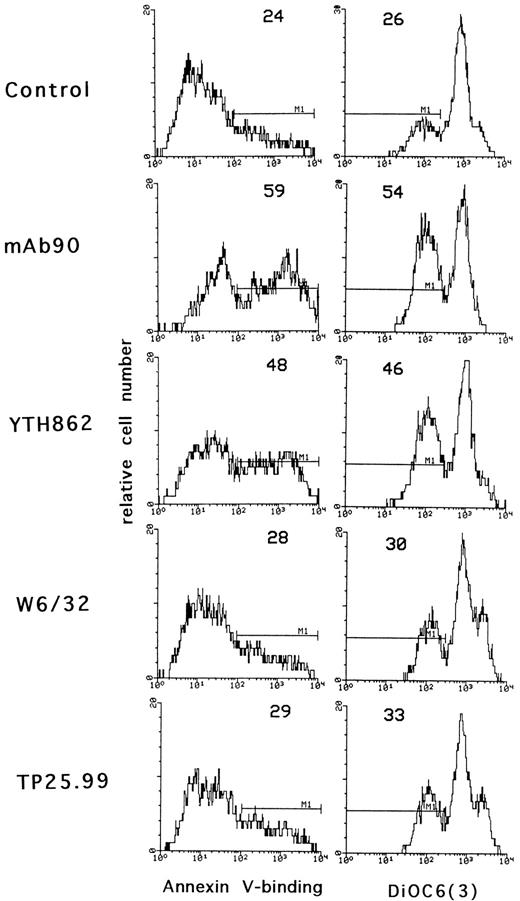
To confirm that only MoAb90 and YTH862 induced apoptosis of activated T lymphocytes, we used two other methods based on different parameters characteristic of apoptosis: externalization of phosphatidylserine and alterations in the mitochondrial transmembrane potential, measured by binding of biotinylated-annexin V and DiOC6(3) fluorescence, respectively. As shown in Fig 3, MoAb90 and YTH862 induced significant binding of annexin V and decrease of mitochondrial transmembrane potential of PHA-activated cells whereas W6/32 and TP25.99 had only minimal effect.
Effect of anti–HLA class I MoAbs on phosphatidylserine externalization and mitochondrial transmembrane potential. Three-day PHA-activated PBL were treated with control IgG1, MoAb90, YTH862, W6/32, or TP25.99 at 10 μg/mL for 24 hours. Externalization of phosphatidylserine was shown by annexin V-binding and Δψm was evaluated after staining with DiOC6(3) as described in Materials and Methods. The percentage of cells that bind annexin V and that of cells with decreased mitochondrial transmembrane potential is indicated for each hictogram. In these conditions, the percentage of apoptotic cells was not modified in resting PBL.
Effect of anti–HLA class I MoAbs on phosphatidylserine externalization and mitochondrial transmembrane potential. Three-day PHA-activated PBL were treated with control IgG1, MoAb90, YTH862, W6/32, or TP25.99 at 10 μg/mL for 24 hours. Externalization of phosphatidylserine was shown by annexin V-binding and Δψm was evaluated after staining with DiOC6(3) as described in Materials and Methods. The percentage of cells that bind annexin V and that of cells with decreased mitochondrial transmembrane potential is indicated for each hictogram. In these conditions, the percentage of apoptotic cells was not modified in resting PBL.
Knowing that MoAb90 and YTH862 induced apoptosis of activated but not resting T cells, we determined their activity on Burkitt's lymphoma cell lines, lymphoblastoid cell lines, leukemic T-cell lines, and T-cell lines derived from tonsils stimulated with PHA and interleukin-2 (IL-2). No consistent apoptosis could be detected on any of these cell lines.
Kinetics and Dose-Response of MoAb90-Induced Apoptosis
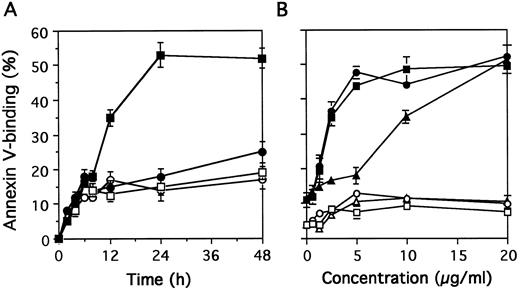
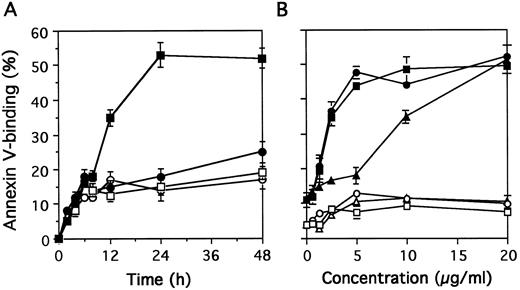
The percentage of apoptotic cells measured by binding of biotinylated annexin-V increased from 8 hours to 24 hours of exposure to MoAb 90 (Fig 4A). Maximum apoptosis of activated T cells induced by MoAb90 was observed with 5 μg/mL (Fig 4B). Resting T cells were resistant to MoAb90 even at concentrations as high as 50 μg/mL. Interestingly, F(ab′)2 as well as monovalent Fab′ fragments also induced apoptosis of activated PBL. The AC50 (concentration required to induce 50% of maximal apoptosis) of intact IgG and F(ab′)2 was 2.1 μg/mL and that of Fab′ fragments 8.5 μg/mL (Fig 4B). As a possible contamination of Fab′ fragments by F(ab′)2 represents less than 4% (as determined by SDS-PAGE gel electrophoresis, data not shown), we conclude that HLA class I–dependent apoptosis does not require receptor cross-linking. Kinetics and dose-response studies with YTH862 MoAb showed similar results (data not shown).
Kinetics and dose-response of MoAb90-induced apoptosis. (A) Resting T cells (open symbols) or 3-day PHA-activated cells (closed symbols) were treated with control IgG1 (circles) or MoAb90 (squares) for indicated time. (B) Resting T cells (open symbols) or 3 day-PHA-activated cells (closed symbols) were treated with dose range of intact MoAb90 (squares), F(ab′)2 fragments (circles), and Fab′ (triangles) for 24 hours. The percentage of apoptotic cells was determined by flow cytometry after staining with annexin V. Results are expressed as mean ± SD of three independent experiments.
Kinetics and dose-response of MoAb90-induced apoptosis. (A) Resting T cells (open symbols) or 3-day PHA-activated cells (closed symbols) were treated with control IgG1 (circles) or MoAb90 (squares) for indicated time. (B) Resting T cells (open symbols) or 3 day-PHA-activated cells (closed symbols) were treated with dose range of intact MoAb90 (squares), F(ab′)2 fragments (circles), and Fab′ (triangles) for 24 hours. The percentage of apoptotic cells was determined by flow cytometry after staining with annexin V. Results are expressed as mean ± SD of three independent experiments.
HLA Class I–Induced Apoptosis Is Not Dependent on Fas/Fas-Ligand Interaction
Because the apoptotic activity of MoAb90 and YTH862 was effective only on activated T cells that are known to express Fas, we studied whether HLA class I–induced apoptosis was dependent on interaction of Fas with Fas-ligand (Fas-L). To this end, PHA-activated T cells were preincubated for 1 hour with the antagonist anti-Fas MoAb ZB4 that blocks the interaction between Fas and Fas-L, before addition of either the MoAb90, YTH862, or the agonist anti-Fas MoAb CH11. Although similar levels of apoptosis and proliferation inhibition were induced by anti–HLA class I MoAbs and the agonist CH11 anti-Fas MoAb, only anti–Fas-induced apoptosis was blocked by ZB4 (Fig 5). Analysis by Northern blot further showed that MoAb90 as well as W6/32 did not increase levels of Fas-L mRNA (data not shown).
Effect of the antagonist anti-Fas MoAb ZB4 on HLA class I–induced apoptosis. PBL were activated with PHA (5 μg/mL) for 3 days. Viable cells were pre-incubated with the antagonist anti-Fas MoAb ZB4 for 1 hour and then treated with medium, YTH862 (10 μg/mL), MoAb90 (10 μg/mL), or the agonist anti-Fas MoAb CH11 (1 μg/mL) for 20 hours. The percentage of apoptotic cells was determined by fluorescent microscopy after staining with Hoescht 33342. In parallel, inhibition of [3H]TdR uptake during the last 8 hours of culture was measured. Results are expressed as mean ± SD of four independent experiments.
Effect of the antagonist anti-Fas MoAb ZB4 on HLA class I–induced apoptosis. PBL were activated with PHA (5 μg/mL) for 3 days. Viable cells were pre-incubated with the antagonist anti-Fas MoAb ZB4 for 1 hour and then treated with medium, YTH862 (10 μg/mL), MoAb90 (10 μg/mL), or the agonist anti-Fas MoAb CH11 (1 μg/mL) for 20 hours. The percentage of apoptotic cells was determined by fluorescent microscopy after staining with Hoescht 33342. In parallel, inhibition of [3H]TdR uptake during the last 8 hours of culture was measured. Results are expressed as mean ± SD of four independent experiments.
Differential Susceptibility of CD45RA+ and CD45RO+ T-Cell Subsets to Fas- and HLA Class I–Mediated Apoptosis
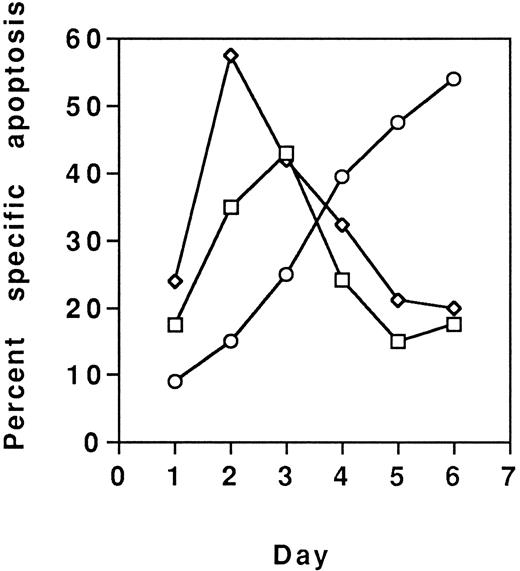
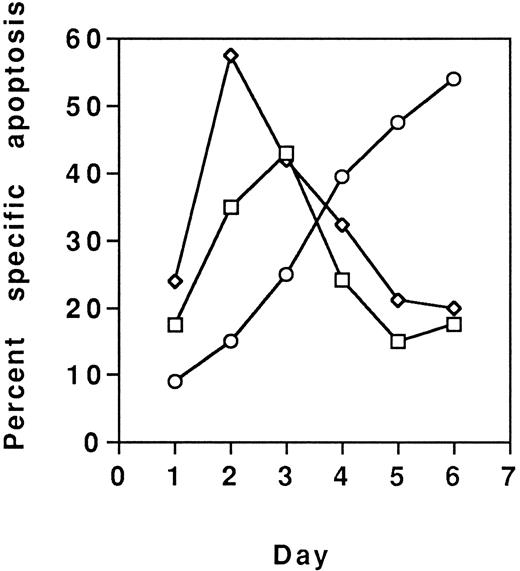
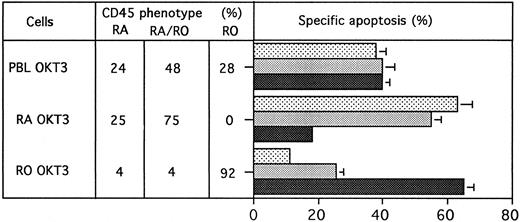
Because MoAb90 and YTH862 killed approximately 40% to 50% of cells, their effects on various T-cell subsets were analyzed. PHA-activated CD4+- and CD8+-enriched T-cell populations were found equally sensitive (data not shown). However, results showed that the CD45RO+ enriched T-cell subset activated with anti-CD3 was less sensitive to HLA class I–induced apoptosis (eg, ≈10% of specific apoptosis) than the CD45RA+ subset (eg, ≈70% of specific apoptosis) (Fig 6). Conversely, the CD45RO+ subset was most sensitive to Fas-mediated apoptosis (Fig 6). Further evidence that distinct subsets of activated T cells were targeted by anti-Fas and anti–HLA class I antibodies, respectively, was brought by the additive effects of the two types of MoAbs (Fig 7), and by the observation that susceptibility of T cells to HLA class I–mediated apoptosis is maximal after 2 or 3 days of activation and return to the basal level on day 6, whereas susceptibility to Fas-mediated apoptosis is maximal on day 6 (Fig 8).
Effect of MoAb90 on T-cell subpopulations. CD45RA+ and CD45RO+ T-cell subpopulations were prepared as described in Materials and Methods. After 3 days of culture in the presence of immobilized-anti-CD3 (OKT3), viable cells from PBL or CD45RA and CD45RO T-cell subsets were treated with MoAb90 (▧, 10 μg/mL), YTH862 (, 10 μg/mL), or anti-Fas MoAb CH11 (, 1 μg/mL). The percentage of CD45RA, CD45RO, and double-positive CD45RA/RO-positive cells was assessed by fluorescence-activated cell sorting (FACS) analysis, and after 24 hours of incubation with antibodies, apoptosis was evaluated by fluorescence microscopy after Hoescht 33342 staining. Results are expressed as mean ± SD of three independent experiments.
Effect of MoAb90 on T-cell subpopulations. CD45RA+ and CD45RO+ T-cell subpopulations were prepared as described in Materials and Methods. After 3 days of culture in the presence of immobilized-anti-CD3 (OKT3), viable cells from PBL or CD45RA and CD45RO T-cell subsets were treated with MoAb90 (▧, 10 μg/mL), YTH862 (, 10 μg/mL), or anti-Fas MoAb CH11 (, 1 μg/mL). The percentage of CD45RA, CD45RO, and double-positive CD45RA/RO-positive cells was assessed by fluorescence-activated cell sorting (FACS) analysis, and after 24 hours of incubation with antibodies, apoptosis was evaluated by fluorescence microscopy after Hoescht 33342 staining. Results are expressed as mean ± SD of three independent experiments.
PBL were activated with PHA (5 μg/mL) for 3 days. Viable cells were then treated by antibodies MoAb90 (10 μg/mL), YTH862 (10 μg/mL), or anti-Fas MoAb CH11 (1 μg/mL) alone or in association and measurements of apoptosis were performed as in Fig 6. In parallel, CD45RA and CD45RO phenotype after 3 days of culture was evaluated by FACS analysis (CD45RA+ 22 [2.4%], CD4RO+ 32 [0.5%], CD45RA+RO+ 32 [0.5%]) (SEM in brackets).
PBL were activated with PHA (5 μg/mL) for 3 days. Viable cells were then treated by antibodies MoAb90 (10 μg/mL), YTH862 (10 μg/mL), or anti-Fas MoAb CH11 (1 μg/mL) alone or in association and measurements of apoptosis were performed as in Fig 6. In parallel, CD45RA and CD45RO phenotype after 3 days of culture was evaluated by FACS analysis (CD45RA+ 22 [2.4%], CD4RO+ 32 [0.5%], CD45RA+RO+ 32 [0.5%]) (SEM in brackets).
PBL were activated with PHA (5 mg/mL) and each day, from day 1 to day 6, aliquots of viable cells were treated with MoAb90 (10 μg/mL, □), YTH862 (10 μg/mL, ⋄), or anti-Fas MoAb CH11 (1 μg/mL, ○), and measurements of apoptosis were performed as in Fig 6.
PBL were activated with PHA (5 mg/mL) and each day, from day 1 to day 6, aliquots of viable cells were treated with MoAb90 (10 μg/mL, □), YTH862 (10 μg/mL, ⋄), or anti-Fas MoAb CH11 (1 μg/mL, ○), and measurements of apoptosis were performed as in Fig 6.
DISCUSSION
In agreement with previous reports,15-22,24 we show in the present study that anti–HLA class I antibodies, including W6/32 and TP25.99, which recognize epitopes in the α2 and α3 domains, respectively, inhibited T-cell proliferation induced by various mitogens in the presence of accessory cells, but only MoAb90 and YTH862 inhibited proliferation induced by phorbol esters and triggered apoptosis of activated T cells, irrespective of the type of activation. Both MoAb90 and YTH862 bind to the α1 domain of the HLA class I and recognize closely associated or identical epitopes(s) according to competitive cross-blocking experiments. Because the epitope(s) recognized by these antibodies is(are) expressed on HLA-A, -B, -C, and -G molecules (P. Le Bouteiiler, data not shown) it is(there are) quite unlikely to be associated with allele-specific determinants. Futhermore, both antibodies were found to bind to PBL and to induce apoptosis of activated T cells in 70 individuals tested at random. Owing to the polymorphism of HLA class I molecules,3 such data do not formally exclude some type of allelic preference but strongly suggest that these antibodies react with (a) nonpolymorphic epitope(s). This hypothesis is further strengthened by the finding that anti-β2m antibodies interfere with the binding of these two anti-α1 domain MoAbs. Further characterization of the epitope(s) recognized by these MoAbs is in progress, but their lack of reactivity in Western blot suggests that they recognize (a) conformational epitope(s), and that inhibition by peptide is unlikely to be informative.
MoAb90 and YTH862 antibodies, as intact Ig but also F(ab)′2 and Fab′ fragments, did not interfere with early activation signals but induced apoptosis of activated T cells, as shown by the initial decrease in viable cell counts, typical nuclear alterations, DNA fragmentation, externalization of phosphatidylserine, and decrease of transmembrane mitochondrial potential. Among nine anti–HLA class I or anti-β2m MoAbs tested, only these two MoAbs induced apoptosis. However, induction of apoptosis of human T cells by anti–HLA class I molecules was very recently reported by two other groups using antibodies directed against either the β2m28 or the α3 domain of HLA class I molecules.27 An important finding of the present study is that α1 domain-induced apoptosis does not require cross-linking, as shown by the fully functional effect of monovalent Fab′ fragments. This excludes a possible Fc-receptor mediated antibody-dependent cell-cytotoxicity mechanism and, interestingly, suggests that the putative α1 ligand acts in a monovalent fashion. Inhibition of OKT3- or lectin-induced proliferation by F(ab)′2 and Fab′ fragments of anti–HLA class I MoAbs was already reported,15,19,20 but with regard to apoptosis, the effect of MoAb90 and YTH862 reported here differ from the effect of 5H7 MoAb and the rabbit anti-β2m antibody which induced efficient apoptosis only when cross-linked. Similarly, the RE2 MoAb, an antimouse MHC class I MoAb, but not its Fab′ fragments, induces a novel type of cell death without DNA fragmentation or swelling of mitochondria of transformed T- and B-cell lines, as well as activated T and B lymphocytes.54
Unlike 5H7 MoAb and anti-β2m antibodies, which induced apoptosis of Epstein-Barr virus (EBV)-transformed lymphoblastoid cell lines, normal PBL, or Jurkat cell line,27,28 MoAb90 and YTH862 induced apoptosis of activated peripheral T lymphocytes while resting lymphocytes, tumor, or T-cell lines are resistant. In parallel, it was shown that naive, memory, and germinal center B cells underwent apoptosis in the presence of MoAb90 or YTH862 only if activated through surface CD40.43 Because the epitope(s) recognized by MoAb90 and YTH862 is (are) expressed on both resting and activated T cells, it was important to assess at which stage of activation T cells became susceptible to HLA class I–mediated apoptosis. Activated T cells require IL-2 or IL-4 but not transition from G1 to S phase of the cell cycle to undergo apoptosis in the presence of these MoAbs.55
The present results are at variance from data reported in the human system where the cross-linking of class I MHC molecule with 5H7 MoAb induces apoptosis in absence of T- or B-cell receptor signaling.27 However, the identification of early activated T cells (CD45RA+) as the main target of anti–HLA class I bears striking similarities with the functional effects of anti–MHC class I MoAb reported in murine systems. Indeed, class I MHC–reactive anti-H2 MoAbs were shown to inhibit murine T-cell proliferation in primary but not secondary allogeneic MLR, suggesting a possible resistance of preactivated memory T cells.56 In addition, anti-α3 domain MoAbs were shown to induce apoptosis of activated CD4+ and CD8+ precursors while mature effector T cells that had undergone extensive proliferation in vitro were resistant.42
MoAb90- and YTH862-induced apoptosis should be clearly distinguished from activation-induced cell death (AICD) because MoAb90 and YTH862 do not trigger T-cell activation and neither Fas-L mRNA and protein expression nor TNF-α, which have been involved in AICD,57-59 could be detected in MoAb90- or YTH862-treated cells (data not shown). Moreover, blocking Fas/Fas-L interaction by the antagonist anti-Fas MoAb ZB4 did not interfere with HLA class I–mediated apoptosis. A mere cross-reactivity of MoAb90 and YTH862 with Fas is excluded by the fact that these MoAbs do not bind to the Daudi cell line which expresses high levels of Fas antigen. Furthermore, apoptosis is triggered by monovalent Fab′ fragments whereas Fas-mediated apoptosis requires oligomerization of surface Fas molecules60 and none of the anti–class I MoAbs induced apoptosis of Fas-sensitive cell lines (data not shown). Finally, the differential susceptibility among activated T cells according to the kinetics and to the expression of CD45RA or RO isoforms provides further evidence that Fas and HLA class I mediate apoptosis in partially overlapping but distinct subsets of target cells. The additive effect of anti–HLA class I antibodies fits with the hypothesis of distinct target cells according to their stage of activation, as does the observation that IL-4 is sufficient to confer susceptibility to apoptosis by anti–HLA class I antibodies but not by anti-Fas antibodies.55
Because of its restriction toward activated lymphocytes, the α1 domain induced apoptosis of T cells described herein may represent the basis for novel therapeutic interventions where humanized MoAb90 or YTH862, or derived fragments, may be used to induce selective deletion of antigen-specific activated T cells that appear after organ or hematopoietic cell allografts. An advantage of MoAb90 and YTH862 over MoAbs that trigger activated T-cell apoptosis, as do anti-CD3 MoAbs59 or association of CD2,37 38 is the lack of intrinsic T-cell–activating properties that account for severe systemic inflammatory reactions, and their capacity to delete early activated T lymphocytes that are not yet susceptible to Fas-mediated apoptosis.
ACKNOWLEDGMENT
We thank G. Panaye for expert assistance in flow cytometry and M. Mutin for electronic microscopy studies.
G.M. was supported by Fondation Merieux. This work was supported in part by a contract from the Région Rhône-Alpes (H0987 30000) and from the European Biotech Program “In Vitro Immunotoxicology” (Bio 2. CT 92-0316).
Address reprint requests to Jean Pierre Revillard, MD, INSERM U80, Hôpital E. Herriot, Pav. P, 5 place d'Arsonval 69437 Lyon cedex 03, France.



![Fig. 5. Effect of the antagonist anti-Fas MoAb ZB4 on HLA class I–induced apoptosis. PBL were activated with PHA (5 μg/mL) for 3 days. Viable cells were pre-incubated with the antagonist anti-Fas MoAb ZB4 for 1 hour and then treated with medium, YTH862 (10 μg/mL), MoAb90 (10 μg/mL), or the agonist anti-Fas MoAb CH11 (1 μg/mL) for 20 hours. The percentage of apoptotic cells was determined by fluorescent microscopy after staining with Hoescht 33342. In parallel, inhibition of [3H]TdR uptake during the last 8 hours of culture was measured. Results are expressed as mean ± SD of four independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/9/10.1182_blood.v90.9.3629/4/m_bl_0035f5.jpeg?Expires=1768020862&Signature=pqyJXBeYheaVBk~f46g5~MFMrSjCd~8bw5y~dae4EfvJplUjgldCL5morokL5XNirPw~Jo1izLGWNgbWqzIrMBI3nB~ZWbSyRp~is9SEQtis8AQyx88iVqaevfSlmTh6Aop7UaIsf3TWBpJYvefvsP~AxiUS~s5Xzl4TIg1uQpxFWbEHQt9uaEXY2DiZs93VAj7L8KxSmnRpEuRwBDxXlCd5rgbhXlgsfvB1lvkZMUj7vUMWWaPYx9b4hDU2hCuS39tuoXbpCCShEsRc-ACfyTxbJewCa6sDY9Gbvlitjx~Sc~qqpv6vh~-wZCvwMv5XrXo5My3uyeohdC9bhmz2-A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 7. PBL were activated with PHA (5 μg/mL) for 3 days. Viable cells were then treated by antibodies MoAb90 (10 μg/mL), YTH862 (10 μg/mL), or anti-Fas MoAb CH11 (1 μg/mL) alone or in association and measurements of apoptosis were performed as in Fig 6. In parallel, CD45RA and CD45RO phenotype after 3 days of culture was evaluated by FACS analysis (CD45RA+ 22 [2.4%], CD4RO+ 32 [0.5%], CD45RA+RO+ 32 [0.5%]) (SEM in brackets).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/9/10.1182_blood.v90.9.3629/4/m_bl_0035f7.jpeg?Expires=1768020862&Signature=0Ww~08kE60gBf~eKhCZ1H5R4~5d-i9hnd9MtRSS3mduiVBmdCr2iwBSzpWw9CQBs8UyWjBIFB1a63OFzCCJYM~m~Nm5v9uQo1xdQztXYXqxx1BvA4zk~VcGUB8TpXcdCK8fYqmXOZYkeyPKTtVOe-0D6t2Tb1N8SSZUhQ00n0zwX5F~cWVr1er8-PCSxtMM76lRM9r3yO8Y2dA74-riz-XZE9bVdW~J-~bZBxZBDeICnMwNkPVwJzWYtbl7aXfQWInomtHxVBr5RbB83yNBEdp~IAA6h4IX9ipq3S1539Ls2yyp1~UW~z8r-b9NJOBMXe1rhIGS5Pu7F1rwsVUs8Yg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Fig. 5. Effect of the antagonist anti-Fas MoAb ZB4 on HLA class I–induced apoptosis. PBL were activated with PHA (5 μg/mL) for 3 days. Viable cells were pre-incubated with the antagonist anti-Fas MoAb ZB4 for 1 hour and then treated with medium, YTH862 (10 μg/mL), MoAb90 (10 μg/mL), or the agonist anti-Fas MoAb CH11 (1 μg/mL) for 20 hours. The percentage of apoptotic cells was determined by fluorescent microscopy after staining with Hoescht 33342. In parallel, inhibition of [3H]TdR uptake during the last 8 hours of culture was measured. Results are expressed as mean ± SD of four independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/9/10.1182_blood.v90.9.3629/4/m_bl_0035f5.jpeg?Expires=1768644484&Signature=FLidzyqYD9T1DF40NSDAAp4ivLPkpY2fviXjBcZvgbAWReevxosdd8nIw8hwfZGiOEO7rKGeo2LCnI99xJqhySKIlkKqnll7zDWFZKLeRxj2thzMck4btxs-cqF26EqzSCkfwKBrMpZEXWr4n8kQbu-LKk5ax7YQTkqIBzr-buFDeNXDwJkxmXbD8SIgRD3W5XpyIVJftRqjA21ypVDIjh9lgkDnzpzZMp1CL0s3StjLiABEw3iBNmal7XhBD-tbYR98MvpfPJFT3p~nmu-FT7n3gKlIq3S~bxVjDaPCcp66xtyGiwiZzvb4s1jsDAhl4tBaKr0v7Po7Sxyl5JPPAA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. PBL were activated with PHA (5 μg/mL) for 3 days. Viable cells were then treated by antibodies MoAb90 (10 μg/mL), YTH862 (10 μg/mL), or anti-Fas MoAb CH11 (1 μg/mL) alone or in association and measurements of apoptosis were performed as in Fig 6. In parallel, CD45RA and CD45RO phenotype after 3 days of culture was evaluated by FACS analysis (CD45RA+ 22 [2.4%], CD4RO+ 32 [0.5%], CD45RA+RO+ 32 [0.5%]) (SEM in brackets).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/9/10.1182_blood.v90.9.3629/4/m_bl_0035f7.jpeg?Expires=1768644484&Signature=0Xw3NzVTqer5kOaXzERMD4UqyiY0PgnkQX1Irj9pzC9m7WJWjAeIbQJBDCXI1BYERjnfzBKE1TLT4ehi61-hzECXkJIt1U36uK9bxUi-W5W6MywgmzMw9ELbkxlrJ-2Mru-1VslcSCo71OIcsiF816k5D7XkWYfyK7A1Lx6fJ4wMqTlir1a7qH0Zbu8wvJsUdp-midCMmJWghr~I6VMttagi665MMqrppnBOR9hyRTDsGS0VUMinbbuMItIy7RK86L3wINtnCI01UT-9~n8ls4dQugYL7dFT4tuSXonHaxLQKlcRufAZaIYFQFH48o9xxWC2hqWq6pR3E2q5SjnW1g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)