Abstract
CD95 (APO-1/Fas)-mediated apoptosis is pivotal in normal lymphocyte homeostasis and mutations of CD95 cause a benign autoimmune lymphoproliferation syndrome (ALPS) in humans and mice. However, tumors only rarely develop in these patients, and no CD95 mutations have yet been directly implicated in tumorigenesis. We therefore examined 81 de novo childhood T-lineage acute lymphoblastic leukemias (T-ALL) including 54 steroid-poor responders, 10 relapsed T-ALL, and 10 leukemic T-cell lines, for the presence of CD95 mutations using single-strand confirmation polymorphism and sequence analysis. In leukemic blasts and normal T cells of one patient, a heterozygous mutation in exon 3 of CD95 causing a 68Pro → 68Leu change associated with decreased CD95-mediated apoptosis was found. In leukemic blasts and normal T cells of a second patient, a homozygous mutation in the promoter of CD95 causing disruption of a consensus sequence for AP-2 binding without decreasing constitutive CD95 expression was detected. No large intragenic alterations of CD95 were found, no homozygous loss was detected in the cell lines, and no CD95 mutations were detected in the relapses. The data presented here show that CD95 mutations occur in some T-ALL and may be of biological importance.
APO-1 (CD95/FAS)-MEDIATED apoptosis is a major mechanism of growth control, at least in lymphoid cells and perhaps in other cells. CD95 is expressed in many normal and malignant human cells,1 including T-cell leukemias.2-5 CD95 expression has been associated with a better prognosis in B-cell lymphomas6 and with responsiveness to chemotherapy in acute myeloid leukemia,7whereas most primary T-cell leukemias are constitutively resistant against CD95-induced apoptosis.4,5 8
The role of the CD95 system in tumorigenesis in animal models is complex. Production of CD95 ligand in tissue compartments inducing apoptosis in T cells9 may explain the existence of immunologically privileged sites like the central nervous system (CNS), long known as a sanctuary site for acute lymphoblastic leukemia (ALL). A similar effect is exerted by CD95 ligand producing tumor cells like melanoma,10 hepatocellular carcinoma,11 colon carcinoma,12,13 and lung carcinoma,14 which may explain the phenomenon of tumors as immunologically privileged tissues. Interestingly, CD95-deficient lpr/lpr mice do not develop malignancy unless they exhibit a concomitant T-cell defect, which favors the development of B-cell lymphomas, suggesting a tumor suppressor function of CD95.15 In a similar way,lpr/lpr mice show accelerated development of T-cell lymphomas only in the presence of additional oncogenic events such as overexpression of L-MYC.16
Progression of tumors may be facilitated by production of soluble (s) CD95 in the tumor via alternative splicing. sCD95 secreted by this mechanism neutralizes Fas ligand, thus causing resistance against CD95-mediated apoptosis.17 sCD95 was detected in a human T-cell leukemia cell line18 and found to be increased in the serum of patients with lymphoid but not myeloid malignancies.19 20
Mutations in CD95 with defective CD95-mediated T-cell apoptosis have been described in patients with autoimmune lymphoproliferative syndrome (ALPS).21-25 Their phenotype with massive benign lymphadenopathy, autoimmunity, and increased number of CD3+CD4-CD8- lymphocytes is similar to the lpr syndrome described in mice.26 Most of the mutations, of which the majority were point mutations in the death domain, were heterozygous, showed a dominant negative phenotype, or required additional genetic or other factors to result in ALPS. Of note, only 3 patients developed malignancies: hepatitis-associated hepatocellular carcinoma, multiple neoplasia, and osteosarcoma.23,24 A CD95 germline mutation, causing an apoptosis defect and associated with ALPS and Hodgkin's disease, has been described in 1 family.25
In addition to its role in negative physiological growth control, considerable evidence from different laboratories suggests that apoptosis induced by anticancer drugs involves activation of the CD95 system,27-32 whereas others found chemotherapy-induced apoptosis to be CD95-independent.33
Because the incidence of CD95 mutations in malignancies has not been investigated yet with sensitive methods, the relevance of CD95 mutations for tumorigenesis and tumor progression is unknown. We therefore investigated childhood T-lineage ALLs (T-ALL) for CD95 mutations. We show in this report that CD95 is mutated in some T-ALL. These mutations, which differ from those causing ALPS, may be of biological significance.
MATERIALS AND METHODS
Patients and case reports.
Samples from 81 randomly chosen children with de novo T-ALL were investigated. In addition 10 children with relapsed T-ALL were examined, in 5 of which the initial disease was investigated as well. From 60 children entered on the German ALL-BFM 90 trial34complete clinical data were available (Table 1). Immunologic marker analysis was performed at the central reference laboratory of the ALL-BFM trials (Robert-Rössle-Klinik, Humboldt Universität, Berlin, Germany). Cell surface and intracytoplasmic (cy)/intranuclear antigens were detected by standard direct or indirect immunofluorescence assays as previously described.35,36 Immunophenotypic subgroups of T-ALL were defined as follows: pro–T-ALL: cyCD3+, CD7+; pre–T-ALL: cyCD3+, CD7+, CD2+ and/or CD5+ and/or CD8+; cortical T-ALL: cy or membrane CD3+, CD7+, CD1a+; and mature T-ALL: membrane CD3+, CD1a-.37 Because more samples were available from children with initially high leukocyte counts the selection was biased towards high-risk patients, which included a high number of steroid-poor responders.38
Patient 7 was a boy found to be microcephalic and micrognathic in infancy who at the age of 4 years developed a pharyngeal epithelial carcinoma for which he was treated with radiation therapy, methotrexate, and a single dose of cyclophosphamide. At the age of 14 years, he presented with lymphadenopathy, marked hepatosplenomegaly, a white blood cell count (WBC) of 98200/μL with 84% lymphoid blasts, hemoglobin 4.5 g/dL, platelet count of 127000/uL, and CNS disease. Immunophenotyping showed an early T-ALL. No cytogenetic and molecular tests were performed. He responded poorly to steroid induction and died of pneumonia after 11 months of palliative therapy. The maternal grandfather had died of Hodgkin's disease.
Patient 14, a girl, has previously been partially described.39 She presented at the age of 18 months with massive hepatosplenomegaly, a WBC of 743000/μL with 94% lymphoid blasts, hemoglobin 7.6 g/dL, platelet count of 52000/μL, and CNS disease. The immunophenotype showed a T-cell ALL with an intermediate thymocyte phenotype. Karyotype was 45, XX, −11, −14, and a marker chromosome, most likely der11. The T-cell receptor (TCR) showed a germline configuration of the β-gene by Southern blot analysis. Molecular analysis showed a heterozygous deletion of the ALL-1gene, eliminating exon 8.39 She responded poorly to steroids, blasts persisted in her bone marrow at day 15 but the deletion was undetectable in her bone marrow at day 33. Fourteen months after initial diagnosis she developed T-ALL, whose immunophenotypic and molecular features differed from her initial disease. Immunological markers had changed to a mature T-cell phenotype. The blasts now expressed TCR α and β proteins as determined by flow cytometry. NoALL-1 deletion was found. Despite intensive chemotherapy, she did not achieve remission and died of sepsis. There was no familiy history of malignant disease. The parents were not consanguinous.
Patient cells and cell lines.
Leukemic blasts were obtained by Ficoll-Hypopaque separation of blood or bone marrow at the time of diagnosis. Mycoplasma-free T-cell lines Jurkat 16, Molt 4, CEM, CEMDOXOR (a doxorubicine-resistant CEM derivative cross-resistant to APO-1) and CEMCD95R (an APO-1–resistant CEM derivative cross-resistant to doxorubicine),27 HPB, HUT 78, Walser, SKW 3, and H9 are routinely maintained in our laboratory and were kept in continuous suspension culture as previously described.40
DNA and RNA isolation.
Genomic DNA and total RNA were extracted using Quiagen Genomic-tips and RNEasy kit, respectively, according to the manufacturer's directions (Quiagen, Hilden, Germany). DNA from 50 unrelated donors without hematologic or immunologic disease was provided by Dr Bartram (Heidelberg, Germany).
Long-distance polymerase chain reaction (PCR) and restriction enzyme digestion.
CD95 was amplified by long-distance PCR using genomic DNA from patient cells. Two overlapping primer pairs were constructed according to the published sequence of CD9541-43: CD95LoFlank, 5′-ATTAGATGCTCAGAGTGTGTGCACAAGGCTGG-3′ with CD95LoInt2A, 5′-ACATACCTGGAGGACAGGAGTTGATGTCAGTC-3′, spanning the 5′ flanking region to intron 2 and CD95LoInt2B; 5′-CTGAGATCCAAACTGCTATACAAGTGACCTGC-3′ with CD95LoEx9, 5′-GGCTGTGCTCATTGACATGGGAGAAAGTCATG-3′, spanning intron 2 with the untranslated region of exon 9. Primers CD95LoFlank and CD95LoInt2A were used with the Expand Long Template PCR System (Boehringer Mannheim, Mannheim, Germany). Two hundred fifty ng of DNA were amplified according to the manufacturer's directions using buffer #3 and 0.75 μL of enzyme mix. Primers CD95LoInt2B and CD95LoEx9 were used with the Advantage-GC Genomic PCR kit (Clontech, Heidelberg, Germany) according to the manufacturer's directions, with a final GC-Melt concentration of 1 mol/L. PCR conditions for both primer pairs were as follows: denaturing for 2 minutes at 94°C; 10 cycles of 94°C for 10 seconds, 65°C for 30 seconds, and 68°C for 12 minutes; followed by 26 cycles of 94°C for 10 seconds, 65°C for 30 seconds, and 68°C for 12 minutes, with an increment of 20 seconds per cycle; and finished by a final extension at 68°C for 7 minutes. PCR was performed with PTC 200 thermocyclers (MJ Research, Watertown, MA). PCR products were digested with BamHI andHindIII (Promega, Madison, WI), separated on 0.7% agarose gels, stained with ethidium bromide, and visualized under ultraviolet light.
PCR and single-strand conformation polymorphism analysis (SSCP).
The CD95 gene consists of nine exons.41 Genomic DNA from patients and cell lines was amplified with primer pairs covering and flanking the coding region (Table 2). For positive SSCP controls, mutant PCR products were made by substituting a single base within one primer of each primer pair, creating either an inversion or a transversion. Amplification was done with 100 ng human DNA; 300 nmol/L of each primer; 50 μmol/L each of dCTP, dGTP, and dTTP; 40μmol/L dATP; 1μCi [α-33P] dATP; 25 or 75 mmol/L KCl; 1.5 or 3.5 mmol/L MgCl2; 10 mmol/L Tris, pH 8.3, 8.8, or 9.2; and 1.2 U Taq DNA polymerase (MBI Fermentas, St Leon-Rot, Germany). The samples were denatured at 95°C for 3 minutes; amplified for 15 cycles, with each cycle consisting of 45 seconds at 95°C and 1 minute at 70°C, with a decrease of 0.7°C per cycle; followed by 25 cycles, with each cycle consisting of 45 seconds at 95°C, 30 seconds at 50°C or 55°C, and 1 minute at 72°; followed by 5 minutes at 72°C. Two μL of amplified DNA was diluted with 8 μL of 95% formamide, 10 mmol/L NaOH, 0.25% bromophenol blue, and 0.25% xylene cyanole; denatured for 5 minutes at 95°C; and flash cooled on ice. Seven μL of the denatured sample were loaded on a gel either with or without 5% glycerol. The gels consisted of 0.5 × MDE gel solution (AT Biochem, Malvern, PA) and 0.6 × TBE (1×= 0.09 mol/L Tris, 0.09 boric acid, 0.002 mol/L EDTA, pH 8.0). Electrophoresis conditions were 6 to 8 W for 15 to 17 hours at 25°C. Gels were dried and autoradiography was performed at −70°C. Mutant bands were cut from the dried gel, the DNA eluated into water overnight, and reamplified and sequenced using the same primers applied for initial amplification.
Sequence analysis.
Cycle sequencing was performed with the fmol DNA cycle sequencing system (Promega, Madison, WI) according to the manufacturer's specifications.
Dinucleotide repeat analysis.
Two polymorphic dinucleotide repeats flanking the CD95 gene, AFM 205xe3 and AFMb362yg5, were chosen from the Genome Data Base and primers synthesized according to the sequences provided. PCR was performed as described for SSCP-PCR except that one primer was endlabeled with γ-33P using polynucleotide kinase. Amplimers were separated using a 8% denaturing polyacrylamide gel run with 60 W. Gels were dried and autoradiography performed.
Reverse transcriptase (RT)-PCR.
CD95 cDNA synthesis was performed as previously described.40 A 311 bp fragment of CD95 cDNA was amplified using primers 5′-TCAAGGAATGCACACTCACCAGC-3′ and 5′-GGCTTCATTGACACCATTCTTTCG-3′. To control for RNA integrity and to quantitate, a 600-bp fragment of GAPDH cDNA was amplified. Radioactive touchdown PCR was performed as described above, with 50 mmol/L KCl, 1.5 mmol/L MgCl2, 10 mmol/L Tris pH 8.3, an annealing temperature of 55°C, and 26 final cycles. Amplification was within the exponential range (data not shown). PCR products were separated on a 4% polyacrylamide gel. Films were exposed to the dried gels overnight.
CD95 expression on ALL-blasts.
Cryopreserved ALL-blasts were resuspended in RPMI 1640 supplemented with 10% fetal calf serum (FCS), penicillin/streptomycin, L-glutamine, and HEPES buffer at a concentration of 2 × 106cells/mL. Immunophenotyping was performed with biotinylated anti–APO-1 (IgG3) monoclonal antibody (MoAb) and streptavidin-phycoerythrin, in conjunction with CD7 fluorescein isothiocyanate (FITC) and CD3 PerCP mouse MoAb (Becton Dickinson, Heidelberg, Germany).44
Separation of T-ALL blasts from T lymphocytes.
CD7+CD3- T-ALL blasts were separated from CD3+ T cells by negative selection using an anti-CD3 mouse MoAb (OKT3) and goat-antimouse antibody-coated magnetic beads (Dynabeads M-450; Dynal, Hamburg, Germany) as described.45
Apoptosis assay.
Apoptosis of cryopreserved leukemic blasts from six patients and the Jurkat 16 cell line was measured by assessment of plasma membrane integrity as determined by uptake of the DNA intercalating dye propidium iodide (PI; 2.5 μg/μL final concentration) analyzed by flow cytometry. Percentage of specific cell death following incubation with anti-CD95 MoAb (anti–APO-1 IgG3, 10 μg/mL) and Protein A (5 ng/ml; Pharmacia, Uppsala, Sweden) was calculated as follows: 100 × (experimental PI uptake [% of cells]) −spontanous PI uptake of cells in medium (% of cells) divided through (100% − spontaneous PI uptake [% of cells]). A minimum of 10,000 events were analyzed in each experiment.
RESULTS
Lack of large insertions or deletions of the CD95 gene in pediatric T-cell leukemia.
To look for larger intragenic alterations, the complete CD95 gene, successfully amplified in 65 patient leukemias by overlapping long-distance PCR, was subjected to restriction endonuclease analysis. Double-digestion with BamHI and HindIII did not show alterations on ethidium bromide–stained agarose gels (data not shown).
Abnormal SSCP patterns are found in some pediatric T-cell leukemias.
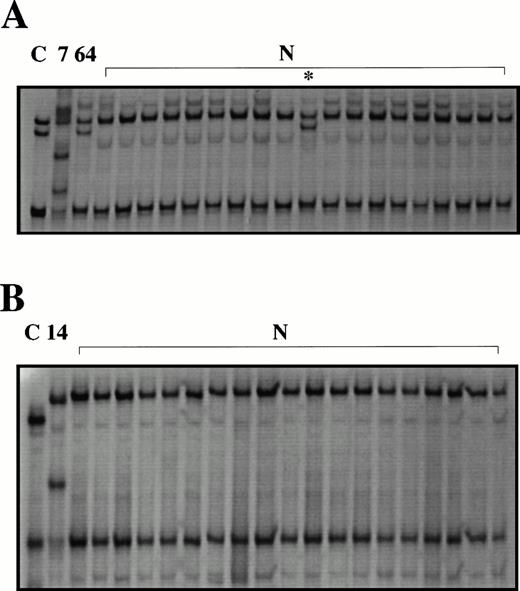
To estimate the sensitivity of the SSCP assay, the positive controls made for each primer pair by using a single-base mismatched primer were subjected to SSCP. The SSCP assay detected 15 of 18 (83%) of the mutant PCR products (data not shown). To search for point mutations and small deletions or insertions, PCR-SSCP analysis was performed on the complete coding region and the 3′ promoter of the CD95 gene in the patient samples and leukemic cell lines. Abnormal SSCP patterns were found in exon 3, (Fig 1A). The pattern seen in patient 7 was unique for this patient, because it was not detected in 100 chromosomes of normal probands. A second pattern (patient 64) was found in 6 of 81 patients and in several healthy controls, suggesting a polymorphism. SSCP analysis of the 3′ promoter showed abnormal bands in 1 patient (patient 14) not seen in the 50 normal controls (Fig 1B). All other exons were normal in all patient samples and cell lines studied.
Abnormal SSCP patterns are found in some pediatric T-cell leukemias. PCR-SSCP analysis of exon 3 (A) and the proximal promoter (B) of CD95. C, normal human genomic DNA amplified using a mutant primer with a single base mismatch (positive control);7,14,64, patient leukemias; N, peripheral blood from healthy controls. Abnormal migrating bands are seen in the positive controls, the leukemias and in one healthy control (*).
Abnormal SSCP patterns are found in some pediatric T-cell leukemias. PCR-SSCP analysis of exon 3 (A) and the proximal promoter (B) of CD95. C, normal human genomic DNA amplified using a mutant primer with a single base mismatch (positive control);7,14,64, patient leukemias; N, peripheral blood from healthy controls. Abnormal migrating bands are seen in the positive controls, the leukemias and in one healthy control (*).
A heterozygous germline mutation in exon 3 of CD95 causing a 68Pro → 68Leu change is associated with decreased CD95-mediated apoptosis.
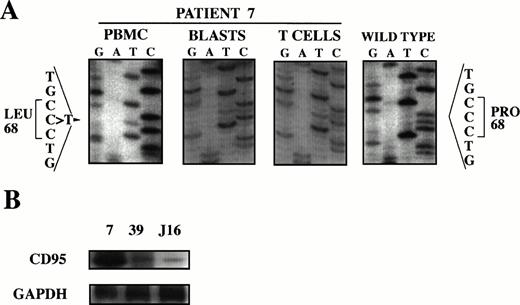
In patient 7, a C → T transition was found at position 251 of the cDNA causing a Pro68 → Leu68 substitution (the locations of cDNA and protein are given in respect to the ATG start codon and the start of the mature protein, respectively, according to Itoh et al46) (Fig 2A). To determine zygosity and germline involvement of the mutation, the patient's mature T lymphocytes were immunomagnetically purified from the T-ALL blasts based on CD3 expression. With this separation method we routinely obtain greater than 95% purity of both cell populations of interest. Sequencing of exon 3 amplificates of both cell populations showed the mutation to be heterozygous and to involve the patient's germline (Fig 2A). To assess whether CD95 expression was altered in this patient in addition to the presence of the missense mutation, CD95 mRNA of the blasts was determined by RT-PCR. The blasts strongly expressed CD95 compared with a different cryopreserved patient leukemia and Jurkat cells not harboring CD95 mutations (Fig 2B). To determine whether this mutation affected CD95-mediated apoptosis, specific apoptosis was measured by flow cytometry after incubation of blasts with anti–APO-1 and found to be markedly decreased (Table 3).
Mutation analysis of patient 7. (A) Heterozygous germline mutation in exon 3 of CD95 causing a 68Pro → 68Leu change. Genomic DNA of PBMC was subjected to PCR using primers flanking exon 3 of CD95, amplificates were subjected to SSCP analysis, abnormal migrating bands were cut-out from the gel, reamplified, and sequenced. T-ALL blasts and mature T lymphocytes were immunomagnetically purified, exon 3 of CD95 amplified by PCR and sequenced. Nucleotide sequence is compared to wild type. Protein is numbered in respect to the start of the mature protein (according to Itoh et al46). (B) CD95 mRNA is strongly expressed compared with cryopreserved blasts from a different patient and thawed Jurkat cells. mRNA was determined by RT-PCR and compared with GAPDH.
Mutation analysis of patient 7. (A) Heterozygous germline mutation in exon 3 of CD95 causing a 68Pro → 68Leu change. Genomic DNA of PBMC was subjected to PCR using primers flanking exon 3 of CD95, amplificates were subjected to SSCP analysis, abnormal migrating bands were cut-out from the gel, reamplified, and sequenced. T-ALL blasts and mature T lymphocytes were immunomagnetically purified, exon 3 of CD95 amplified by PCR and sequenced. Nucleotide sequence is compared to wild type. Protein is numbered in respect to the start of the mature protein (according to Itoh et al46). (B) CD95 mRNA is strongly expressed compared with cryopreserved blasts from a different patient and thawed Jurkat cells. mRNA was determined by RT-PCR and compared with GAPDH.
A homozygous germline mutation in the promoter of CD95 causes disruption of an AP-2 binding consensus sequence, but does not decrease constitutive CD95 expression.
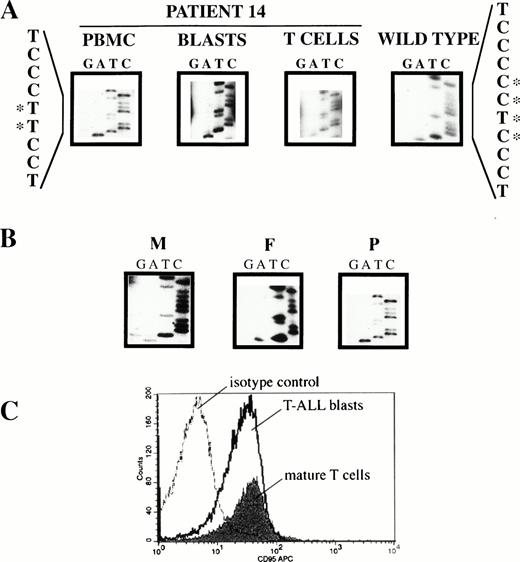
A deletion/insertion was found in patient 14, involving nt −350 to nt −347 of the CD95 promoter and destroying an AP-2–binding consensus sequence (location of promoter nucleotides relative to the ATG start codon according to Behrmann et al41) (Fig 3A). To determine zygosity and germline involvement of the mutation, the patient's mature T lymphocytes were immunomagnetically purified from the leukemic blasts. Sequencing of appropriate promoter amplificates of both cell populations showed the mutation to be homozygous and to involve the patient's germline (Fig 3A). To define the pattern of inheritance of the mutation, the appropriate promoter region was sequenced in the parents. As shown in Fig 3B, the father was homozygous for the mutation and the mother homozygous for the wild type. To rule out paternal disomy, two polymorphic markers flanking the CD95 gene, AFM205xe3 and AFMb362yg5, were analyzed. The patient showed both paternal and maternal alleles, thus ruling out paternal disomy (data not shown). To assesss whether this promoter mutation affected expression, CD95 was assayed on immunomagnetically separated leukemic blasts and mature T cells of the patient from the time of initial presentation. CD95 was expressed (Fig 3C) and was not decreased compared with patients' leukemias not harboring this or other CD95 mutations (Table 4).
Mutation analysis of patient 14. (A) Homozygous germline mutation in the promoter of CD95 causing disruption of a consensus sequence for AP-2 binding. Genomic DNA of PBMC was amplified by PCR using primers 1-1F/1-1R (covering nt −408 to nt −202 of the CD95 promoter; nucleotides numbered relative to the ATG start codon according to Behrmann et al41). The amplificate was subjected to SSCP analysis, abnormal migrating bands were cut-out from the gel, reamplified, and sequenced. T-ALL blasts and mature T lymphocytes were immunomagnetically purified, PCR-amplified as above, and sequenced. Nucleotide sequence is compared to wild type. Bases involved in the mutation are highlighted by asterisks. (B) The mutation is inherited from the father. Genomic DNA of PBMC from the mother (M), the father (F), and the patient (P) were amplified as under (A).The mutation is inherited from the homozygous father, whereas the mother has wild-type alleles only. (C) The mutation in the CD95 promoter does not decrease constitutive CD95 expression. CD95 expression on cryopreserved PBMC was determined by three-color immunofluorescence analysis as described in Materials and Methods. For electronic gating T-ALL blasts were identified by forward/side scatter characteristics of viable lymphocytes and surface expression of CD7 but not CD3. Mature T cells were CD3+ and CD7+.
Mutation analysis of patient 14. (A) Homozygous germline mutation in the promoter of CD95 causing disruption of a consensus sequence for AP-2 binding. Genomic DNA of PBMC was amplified by PCR using primers 1-1F/1-1R (covering nt −408 to nt −202 of the CD95 promoter; nucleotides numbered relative to the ATG start codon according to Behrmann et al41). The amplificate was subjected to SSCP analysis, abnormal migrating bands were cut-out from the gel, reamplified, and sequenced. T-ALL blasts and mature T lymphocytes were immunomagnetically purified, PCR-amplified as above, and sequenced. Nucleotide sequence is compared to wild type. Bases involved in the mutation are highlighted by asterisks. (B) The mutation is inherited from the father. Genomic DNA of PBMC from the mother (M), the father (F), and the patient (P) were amplified as under (A).The mutation is inherited from the homozygous father, whereas the mother has wild-type alleles only. (C) The mutation in the CD95 promoter does not decrease constitutive CD95 expression. CD95 expression on cryopreserved PBMC was determined by three-color immunofluorescence analysis as described in Materials and Methods. For electronic gating T-ALL blasts were identified by forward/side scatter characteristics of viable lymphocytes and surface expression of CD7 but not CD3. Mature T cells were CD3+ and CD7+.
No homozygous loss of CD95 in T-ALL cell lines.
No homozygous loss of CD95 was present in the cell lines because the gene could be amplified in all cell lines examined (data not shown).
No CD95 mutations in relapsed T-ALL.
To determine if CD95 mutations are acquired during disease evolution, relapsed T-ALL were investigated. No mutations were found by SSCP in the 10 relapses studied (data not shown).
A known polymorphism is detected with the expected frequency.
Sequence analysis of the abnormal SSCP bands detected as the same pattern in exon 3 in 6 patients and 1 healthy control showed the presence of a polymorphism already described47: 222A → G (data not shown; numbering of cDNA in respect to the ATG start codon, according to Itoh et al46).
DISCUSSION
We hypothesized that CD95 (APO-1/Fas) mutations may occur in childhood T-lineage leukemia based on several lines of evidence. First, CD95 is of major importance for controlling T-cell homeostasis. Second, the existence of benign lymphoproliferative syndromes shows that dysfunction of CD95 causes accumulation of T lymphocytes. Third, most primary T-lineage leukemias are resistant to CD95-mediated apoptosis. Fourth, evidence is emerging that lymphoid malignancies may be associated with CD95 mutations.48 Finally, the CD95 system mediates cytotoxicity of important drugs used in the therapy of ALL and drug resistance is common in T-ALL.27-32
We found mutations in 2 of 81 patients. It is unlikely that many mutations have been missed because our PCR-SSCP assay has a sensitivity of at least 83% for detecting point mutations, as estimated by the detection of positive controls created by single-base mismatched primers. This sensitivity is comparable with most published SSCP assays. Nevertheless, detection of primer mutations are not necessarily predictive of the detectability of mutations elsewhere within the amplicon. Therefore, as in all SSCP assays, some mutations might have been missed. The long PCR/restriction enzyme assay will detect most gross intragenic alterations of CD95 such as large deletions (unless they involve a primer binding site) or insertions (as long as their size does not prevent amplification), although rearrangements such as translocations would not be amplified. Homozygous loss of CD95 could have been masked by contamination of the leukemic samples with normal cells. This, however, is unlikely, because no homozygous loss was detected in the homogenous cell lines. Because we have examined only the 3′ promoter and did not examine the majority of cases for CD95 expression, we cannot rule out a higher incidence of CD95 alterations outside the coding and 3′ promoter region. However, most T-ALL have been shown to express CD95.5
In T-ALL with CD95 mutations, these mutations might contribute to leukemogenesis and chemoresistance. In patient 7, the heterozygous alteration of CD95 was not detected in 100 chromosomes of normal probands, making a polymorphism unlikely. This mutation without loss of expression was associated with apoptosis resistance. Interestingly, this mutation is located adjacent to a stretch of DNA that encodes for amino acid residues important for ligand binding to CD95.49It remains to be proven whether this mutation decreases ligand binding, interferes with receptor signaling or is silent. Whether the development of two unrelated malignancies at young age, one of which very rare at this age, is related to the germline mutation of this dysmorphic patient with a family history of Hodgkin's disease, has to be investigated.
In patient 14, a homozygous alteration was found in the promoter. This alteration was not found in 50 normal individuals suggesting a bona fide mutation rather than a polymorphism. The mutation's unusual feature of involving the germline in a homozygous fashion prompted us to investigate the parents. Surprisingly, the mutation was inherited from the homozygous father. We ruled out uniparental (paternal) disomy although a small partial paternal disomy might escape detection if the distance of the flanking polymorphic markers is large enough. Alternatively, the maternal allele might have been lost by microdeletion during early zygote development. This mutation destroys a consensus sequence for binding the transcription factor AP-2 without affecting constitutive CD95 expression. In T cells, AP-2 has been shown to influence the expression of the tumor necrosis factor α (TNF-α) gene50,51 and might play a role in the constitutive expression of the IL3 gene.52 Drug treatment of leukemic cells increases CD95 expression and susceptibility to CD95-mediated apoptosis.27,28 Leukemia cells cross-resistant to APO-1 and cytotoxic drugs show diminished upregulation of CD95 that contributes to drug resistance in these cells.29 30 It has to be shown whether the chemoresistant blasts of this patient show diminished CD95 upregulation and, if so, whether the destruction of the AP-2 binding site contributes to this regulation defect. The similarity between this patient with two T-ALL in rapid succession, a CD95 germline mutation with possible attenuation of CD95 upregulation, an ALL-1 gene deletion at initial presentation, and the lymphoma-bearing lpr/lpr mouse overexpressing L-MYC16 is intriguing and warrants further study to show a permissive effect of this CD95 mutation on leukemogenesis.
It is surprising that both mutations identifed in this study were present in the germline, as germline mutations in other oncogenes and tumor-suppressor genes are rare in childhood ALL. Interestingly, the death domain, which is mutated in most cases of ALPS with CD95 mutations,21-25 is not altered in T-ALL.
The two ALL patients with CD95 mutations were both drug resistant. This might indicate that CD95 mutations are more important for chemoresistance than for leukemogenesis. As mentioned, recent evidence suggests that the CD95 system mediates cytotoxic drug action; and CEM cells cross-resistant to APO-1 and cytotoxic drugs show a defect in activation of the CD95 system.27 However, CD95 in these cells was not mutated in the present study, indicating that chemoresistance due to alterations in the CD95 system may be determined by mechanisms other than CD95 mutations. Our study population included a high percentage of steroid-poor responders. Steroid sensitivity is mediated by mechanisms other than the CD95 system. It is therefore conceivable that, despite the predictive value of the steroid response for therapy failure, alterations in cytotoxic mechanisms different from the steroid pathway may contribute to therapy failure. Thus, examining more chemoresistant patients might show a higher incidence of CD95 mutations.
The low incidence of CD95 mutations in untreated T-ALL in this cohort, heavily biased towards high-risk patients, suggests that mutations in CD95 do not contribute to leukemogenesis in most T-ALL. This is in line with the absence of reported cytogenetic or molecular genetic aberrations in T ALL involving 10q23 where CD95 is located.53 Also, coinheritance of a lymphoid malignancy with a germline CD95 mutation causing impaired apoptosis has been reported in only one familiy with ALPS and Hodgkin's disease.22 Furthermore, in mouse models, loss of CD95 does not give rise to malignancy unless a second insult is provided.15,16 We found an APO-1–resistant phenotype without mutations in CD95 and with normal CD95 expression in one leukemia (patient 36). This finding is supported by the fact that most T-ALL are resistant to CD95-mediated apoptosis,4 5 whereas only very few have mutated CD95. Thus, apoptosis defects in T-ALL may be caused by molecules other than CD95. In a limited number of relapses, half of them paired with their initial disease, no CD95 mutations were found. This argues against CD95 mutations being accumulated in late stages of disease evolution.
In conclusion, CD95 is mutated in some de novo childhood T-ALL and these alterations, which differ markedly from those described in ALPS, might be of functional relevance. In most de novo T-ALL, CD95 alterations do not contribute to leukemogenesis and, in a limited number of relapsed T-ALL, no involvement of CD95 mutations in the evolution of the disease was seen. It remains to be determined whether CD95 mutations are involved in chemoresistance of T-ALL.
ACKNOWLEDGMENT
We thank Dr B. Jannsen, Institut für Humangenetik und Anthropologie, Universität Heidelberg, Germany for DNA of healthy controls and helpful discussions, and Dr Bender-Götze, Kinderpoliklinik der Universität München, Germany and Dr I. Richter, Kinderklinik und Poliklinik der Universität Rostock, Germany for providing patient specimens and information.
Supported by grants from the Deutsche Forschungsgemeinschaft (DFG) and the Tumorzentrum Heidelberg/Mannheim, Germany.
Address correspondence to Klaus-Michael Debatin, MD, Universitäts-Kinderklinik Ulm, Prittwitzstrasse 43, 89075 Ulm, Germany.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.