Abstract
Remarkable structural and functional similarities exist between theDrosophila Toll/Cactus/Dorsal signaling pathway and the mammalian cytokine-mediated interleukin-1 receptor (IL-1R)/I-κB/NF-κB activation cascade. In addition to a role regulating dorsal-ventral polarity in the developing Drosophilaembryo, signaling through Drosophila Toll (dToll) activates the nonclonal, or innate, immune response in the adult fly. Recent evidence indicates that a human homologue of the dToll protein participates in the regulation of both innate and adaptive human immunity through the activation of NF-κB and the expression of the NF-κB–controlled genes IL-1, IL-6, and IL-8, thus affirming the evolutionary conservation of this host defense pathway. We report here the cloning of two novel human genes, TIL3 and TIL4 (Toll/IL-1R–like-3, -4) that exhibit homology to both the leucine-rich repeat extracellular domains and the IL-1R–like intracellular domains of human andDrosophila Toll. Northern analysis showed distinctly different tissue distribution patterns with TIL3 expressed predominantly in ovary, peripheral blood leukocytes, and prostate, and TIL4 expressed primarily in peripheral blood leukocytes and spleen. Chromosomal mapping by fluorescence in situ hybridization localized the TIL3 gene to chromosome 1q41-42 and TIL4 to chromosome 4q31.3-32. Functional studies showed that both TIL3 and TIL4 are able to activate NF-κB, though in a cell type–dependent fashion. Together with human Toll, TIL3 and TIL4 encode a family of genes with conserved structural and functional features involved in immune modulation.
THE DROSOPHILA TOLL gene (dToll) encodes a transmembrane protein with features of a signal-transducing receptor.1,2 The intracellular and extracellular domains of dToll exhibit striking structural and functional similarities with proteins of two distinct families: the interleukin-1 receptor (IL-1R)-like family3,4 and the superfamily of leucine-rich repeat (LRR) proteins.5 dToll is involved in establishing the dorsal/ventral axis of the developing Drosophila embryo through a conserved signaling pathway involving the downstream effectors dorsal, an Rel family member with homology to the transcription factor NF-κB,6 and cactus, a protein with structural homology to I-κB.7
The dToll signaling pathway is also involved in the innate nonspecificDrosophila immune response through the induction of genes encoding antibacterial8 and antifungal9peptides. The immune response modulated by dToll reflects an ancestral conserved system that has homologous components in plants10and vertebrates, as exemplified by the IL-1/NF-κB–mediated induction of the mammalian inflammatory response.11 Mutagenesis and deletion analyses have shown that amino acid residues conserved between the IL-1R and dToll cytoplasmic domains are essential for signal transduction by these receptors.12,13 Experiments involving the deletion of the extracellular LRR regions of dToll showed a dominant gain-of-function activity, suggesting that dToll functions as a receptor whose intrinsic intracellular signaling activity is regulated by its extracellular domain.13 14
Two human genes with homology to the Drosophila Toll sequence have recently been described. Human Toll (hToll) is a type I transmembrane protein with an extracellular domain consisting of LRRs and a cytoplasmic domain homologous to the cytoplasmic domain of the human IL-1R protein.15 A constitutively active hToll mutant induced the activation of NF-κB and the expression of NF-κB–controlled genes for inflammatory cytokines. These results support the conservation of a host-defense pathway betweenDrosophila and humans.15 A second gene, TIL (Toll/IL-1R–like), also encodes a protein with regions homologous to the intracellular and extracellular domains of dToll.16However, TIL was unable to activate NF-κB through a chimeric IL-1R/TIL receptor complex.17
Three additional Drosophila genes, 18-wheeler, tlr (Toll-like receptor) and MstProx, exhibit homology to dToll involving both the intracellular IL-1R–like domain and the extracellular LRRs. Eighteen-wheeler is required for Drosophila morphogenesis and is thought to function as a cell adhesion or receptor molecule that facilitates cell movements.18Drosophila tlr is also involved in embryogenesis with a potential role in cell-to-cell interactions at critical boundaries during development.19MstProx is a recently cloned Drosophila gene that has not been extensively characterized.17
Based on the pivotal role of Drosophila Toll in both development and immune regulation, we undertook a database search to identify other human genes with sequence homology to dToll. We reasoned that evidence of a family of Toll-like genes in Drosophilacould indicate a similar family of genes in humans. Two novel sequences were identified that we have named TIL3 and TIL4 (Toll/IL-1R–like-3 and -4). In this study we report the isolation and characterization of the full-length cDNAs for TIL3 and TIL4, including their chromosomal assignments and tissue expression patterns. We also show that TIL3 and TIL4 activate NF-κB in a cell type–dependent fashion. Together with hToll, TIL3 and TIL4 encode a family of genes with structural and functional similarities that may serve to modulate the human immune response.
MATERIALS AND METHODS
Cell culture and general methods.
DNA manipulations including transformation, plasmid preparation, and gel electrophoresis were performed according to standard procedures.20 Restriction and modification enzymes (Boehringer Mannheim, Inc, Life Technologies, Rockville, MD) were used in accordance with the manufacturers' recommendations. MCF-7 breast carcinoma cells and 293T cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Life Technologies) supplemented with 10% fetal calf serum. BHK (baby hamster kidney) cells were grown in DMEM supplemented with 5% fetal calf serum.
DNA sequencing and analysis.
Sequencing was performed by the dideoxy chain-termination method using Taq dye primer and dye terminator kits (Applied Biosystems, Foster City, CA). The nucleotide sequences were analyzed with an ABI 377 automated sequencer (Applied Biosystems). The final sequences were confirmed by sequencing both strands. Sequence homology searches and comparisons were performed using BLASTN and BLASTX algorithms on the National Center for Biotechnology Information (NCBI) web-server. Sequence assembly was performed using the Phred, Phrap, and Consed programs (http://chimera.biotech.washington.edu/UWGC/).
Cloning of TIL3 and TIL4.
Several human sequences in the NCBI expressed-sequence-tag (EST) database were identified with statistically significant homology to theDrosophila Toll gene. One EST (IMAGE Consortium Clone 684668) corresponded to the published human gene KIAA0012/TIL (Genbank IDD13637). One EST (IMAGE Consortium Clone ID 202057) corresponded to a published human gene, hToll (Genbank accession U93091). Two others (IMAGE Consortium Clone Ids 80633 and 277229) showing homology to dToll were obtained (Genome Systems, St Louis, MO) and sequenced in their entirety. Each comprised a partial open reading frame with homology to dToll and hToll. Rapid amplification of cDNA ends (RACE) was used to obtain full-length cDNAs using human peripheral blood leukocyte and prostate cDNA as template (Clontech, Palo Alto, CA) with gene specific primers TX360L 5′-CTCTGATGGATTGATGTTTCATC-3′ for TIL3 and TW236L 5′GAGAGTCACACAGGTAATTTGCTGG 3′ for TIL4. RACE products were cloned using the T/A cloning method according to the manufacturer's instructions (Invitrogen, La Jolla, CA), sequenced, and assembled as described above.
Northern blot analysis.
TIL3 (nt 1211-1620) and TIL4 (nt 667-3288) cDNA probes were labeled with 32P-dCTP (DuPont NEN, Boston, MA) using a random priming labeling kit following the manufacturers instructions (Life Technologies). Adult poly(A)+ multiple-tissue Northern blots (Clontech) were hybridized in Quick-hyb solution (Clontech) at 68°C according to the manufacturer's instructions. The filters were washed in three changes of 2× SSC with 0.05% sodium dodecyl sulfate (SDS) at room temperature for 30 minutes each followed by two changes of 0.1× SSC with 0.1% SDS at 50°C for 20 minutes each. Equal mRNA loading was assessed by stripping the filter and reprobing with a human 32P-labeled β-actin probe. Hybridization and washing conditions were as described above.
Chromosomal localization by fluorescence in situ hybridization (FISH).
TIL3 and TIL4 cDNA probes were labeled as described above and were hybridized to filters of an arrayed human BAC genomic library (Research Genetics, Huntsville, AL). Positive clones were identified and subsequently verified by polymerase chain reaction using TIL3 and TIL4 gene-specific amplification primers. BAC and plasmid DNA clones were biotinylated by nick translation, prehybridized in the presence of human Cot1 DNA, and hybridized (at 10 and 50 ng/uL, respectively) to metaphase spreads of a normal male following procedures described in detail elsewhere.21 After hybridization and washing, the hybridization sites were labeled with fluorescein-conjugated avidin. The chromosomes, which had previously been released from an early-S methotrexate block in the presence of BrdU, were counterstained with DAPI to produce a QFH-like banding pattern. Digital image processing was performed as described elsewhere.22 The locations of hybridization signals were analyzed in 10 well-spread, well-banded metaphases for each sample.
Chimeric constructions.
Expression vectors encoding the Fas-hToll, Fas-TIL3, and Fas-TIL4 fusion proteins were made by joining the nucleotides encoding the extracellular domain of human Fas receptor (aa 1 to 169) to the nucleotides encoding the transmembrane and cytoplasmic tail of hToll (aa 629 to 841), TIL3 (aa 630 to 858), and TIL4 (aa 584 to 784), respectively. The chimeric constructs were cloned into the pCDNA3 expression vector (Invitrogen).
NF-κB assay.
For the NF-κB reporter assay, 8 × 104 MCF7 human breast cancer cells were transfected in triplicate with 500 ng of the test constructs or empty vector along with 250 ng of a NF-κB reporter construct23 and a LacZ reporter construct (Invitrogen) in a 24-well plate using Superfect (Qiagen, Valencia, CA) following the manufacturer's instructions. Forty-eight hours after transfection, cells were lysed and luciferase activity was measured using the luciferase assay reagent (Promega, Madison, WI) as described previously.23 Baby hamster kidney fibroblast cells (BHK) and transformed human epithelial kidney 293T cells were plated as described above and transfected using the calcium phosphate precipitation method20 with luciferase analysis performed after 48 or 24 hours, respectively.
RESULTS
TIL3 and TIL4 are homologs of the Drosophila Toll gene with homology to IL-1R cytoplasmic domain (IL-1Rcd) and LRR extracellular domain.
Two human cDNA sequences in the NCBI database of expressed sequence tags were identified that exhibited significant sequence homology to the Drosophila Toll protein. These clones were found to contain partial open reading frames (ORFs) corresponding to TIL3 and TIL4. The RACE methodology was used to obtain full-length cDNA clones of these genes.
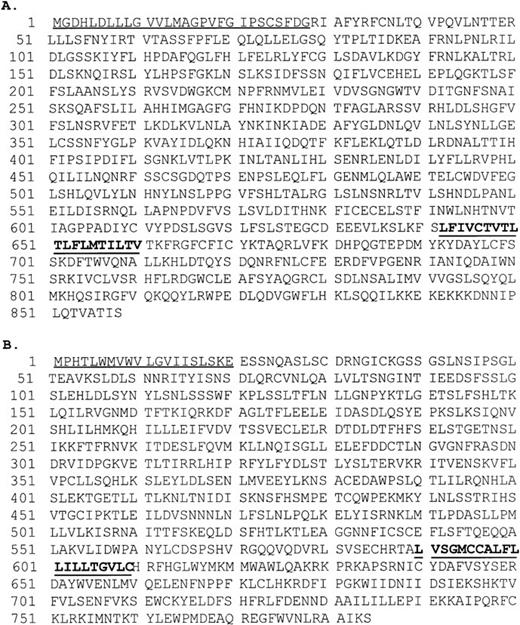
The nucleotide sequence of the TIL3 cDNA contains an open reading frame of 2,574 bp that starts at the first methionine codon preceded by an appropriate Kozak consensus sequence for the initiation of translation.24 The predicted protein sequence derived from the open reading frame produces an 858-amino acid polypeptide (Fig1A). The nucleotide sequence of the TIL4 cDNA contains an open reading frame of 2,352 bp that starts at the first methionine codon preceded by an appropriate Kozak consensus sequence for the initiation of translation. The predicted protein sequence derived from the open reading frame produces a 784-amino acid polypeptide (Fig 1B).
(A) Amino acid sequence of TIL3. The predicted signal peptide (residues 1 to 30) is underlined. The predicted transmembrane segment (residues 642 to 660) is in bold and underlined. (B) Amino acid sequence of TIL4. The predicted signal peptide (residues 1 to 20) is underlined. The predicted transmembrane segment (residues 590 to 609) is in bold and underlined.
(A) Amino acid sequence of TIL3. The predicted signal peptide (residues 1 to 30) is underlined. The predicted transmembrane segment (residues 642 to 660) is in bold and underlined. (B) Amino acid sequence of TIL4. The predicted signal peptide (residues 1 to 20) is underlined. The predicted transmembrane segment (residues 590 to 609) is in bold and underlined.
The TIL3 and TIL4 amino acid sequences exhibit features of type I integral membrane proteins. Each contains two regions of hydrophobicity corresponding to a signal peptide located at the amino terminus, and a transmembrane region near the midportion (Fig 1). TIL3 and TIL4 share 40% overall amino acid similarity and 24% amino acid identity (Fig2). They exhibit homology with both the intracellular and extracellular regions of the DrosophilaToll,1 human Toll,15 and TIL25proteins (Fig 2).
(A) Alignment of the amino acid sequence of the cytoplasmic domains of the IL-1R and the Toll/IL-1R–like family members: Drosophila wheeler and Toll, human Toll, TIL, TIL3, and TIL4. Alignments were performed using the Clustal algorithm49 and Boxshade (http://ulrec3.unil.ch/software/BOX_faq.html). Sequence identity (black) or similarity (gray) between at least 40% of the sequence members are shaded. Inactivating dToll mutations are marked with an asterisk (*).13 Critical amino acid residues for IL-1R activation of IL-2 are denoted by a solid circle (•),12,42 for IL-1R activation of IL-8 by a cross (+),45 and IL-1R activation of NF-κB by a box (□).43 (B) Alignment of the extracellular LRR terminal-flanking sequences of the LRR proteins TIL3, TIL4, TIL, hToll, dToll, human platelet glycoprotein 1b-α (Gp1b-α) and 1b-β (gp1b-β),27,47 platelet glycoprotein IX (gpIX),28 leucine-rich glycoprotein (LRG),29and the oncofetal antigen 5T4 (ofg-5T4).30 The extracellular region of dToll contains two cysteine-rich LRR domains (dToll #1 and dToll#2). Sequence identity (black) or similarity (gray) between at least 40% of the sequence members are shaded. The mutations responsible for the dominant, constitutively active dToll proteins are denoted by asterisk (*) and are located in the second dToll terminal repeat.13
(A) Alignment of the amino acid sequence of the cytoplasmic domains of the IL-1R and the Toll/IL-1R–like family members: Drosophila wheeler and Toll, human Toll, TIL, TIL3, and TIL4. Alignments were performed using the Clustal algorithm49 and Boxshade (http://ulrec3.unil.ch/software/BOX_faq.html). Sequence identity (black) or similarity (gray) between at least 40% of the sequence members are shaded. Inactivating dToll mutations are marked with an asterisk (*).13 Critical amino acid residues for IL-1R activation of IL-2 are denoted by a solid circle (•),12,42 for IL-1R activation of IL-8 by a cross (+),45 and IL-1R activation of NF-κB by a box (□).43 (B) Alignment of the extracellular LRR terminal-flanking sequences of the LRR proteins TIL3, TIL4, TIL, hToll, dToll, human platelet glycoprotein 1b-α (Gp1b-α) and 1b-β (gp1b-β),27,47 platelet glycoprotein IX (gpIX),28 leucine-rich glycoprotein (LRG),29and the oncofetal antigen 5T4 (ofg-5T4).30 The extracellular region of dToll contains two cysteine-rich LRR domains (dToll #1 and dToll#2). Sequence identity (black) or similarity (gray) between at least 40% of the sequence members are shaded. The mutations responsible for the dominant, constitutively active dToll proteins are denoted by asterisk (*) and are located in the second dToll terminal repeat.13
The TIL3 and TIL4 polypeptides contain the two distinct structural/functional motifs characteristic of dToll: the IL-1R–like region in the cytoplasmic portion of the protein and the LRR domain in the extracellular portion. An amino acid multiple sequence alignment of the cytoplasmic IL-1R–like regions of TIL3, TIL4, and other IL-1R family members is shown in Fig 2A. Several of the specific amino acid residues shown to be of functional importance in IL-1R–mediated activities such as activation of IL-2, IL-8, and NF-κB are conserved in the predicted TIL3 and TIL4 protein sequences (Fig 2A). However, several of these critical amino acids diverge significantly between the IL-1R and the IL-1R–related proteins. For example, two of six residues involved in IL-1R–mediated NF-κB activation, amino acids 518 and 519, are divergent in the hToll sequence. Nonetheless, hToll has been shown to activate NF-κB.15 Further, of three inactivating mutations found in the cytoplasmic portion of theDrosophila Toll protein,13 all three wild-type functional residues are conserved in the TIL3 and TIL sequences, but only two retain conservation in TIL4, hToll, and IL-1R (Fig 2A).
The similarities among the deduced amino acid sequences of the LRR-flanking regions of the extracellular TIL3 and TIL4 proteins and other LRR family members are shown in Fig 2B. Previous reports have emphasized the similarity between the dToll protein and the human membrane receptor platelet glycoprotein 1b-α (Gp1b-α) by virtue of their common LRRs1 and a sequence of 60 amino acids containing two to four conserved cysteine residues located immediately C-terminal to the block of LRRs.13,26 This sequence is conserved in several other human proteins, including platelet glycoprotein 1b-β,27 platelet glycoprotein IX,28 serum leucine-rich glycoprotein (LRG),29and the oncofetal antigen 5T4 (ofg-5T4).30 The high level of sequence conservation indicates that this region is likely to be of structural and/or functional significance. A role for the conserved cysteine residues in the formation of disulfide-linked extracellular domains that may be involved in cell adhesion and ligand binding has been suggested.26 31
Expression distribution of TIL3 and TIL4.
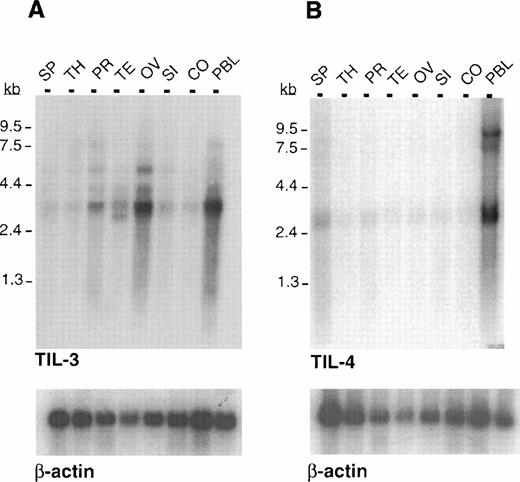
To examine the expression pattern of TIL3 and TIL4, cDNA fragments for each gene were used to probe Northern blots containing poly(A)+ RNA from several human tissues. The TIL3 cDNA hybridized to an mRNA species of approximately 3.3 kb, which was present predominantly in peripheral blood leukocytes (PBL), ovary, and prostate, and showed lower expression in other tissues (Fig3A). Several less prominent bands of higher molecular weight were also detected. The TIL4 cDNA hybridized to an mRNA species of approximately 2.8 kb present predominantly in PBL and spleen with weak expression in the remaining tissues (Fig 3B). The predominant message sizes correspond to the full-length TIL3 and TIL4 cDNA clones we have isolated by RACE.
Multiple tissue Northern analysis of poly (A)+ RNA with TIL3 and TIL4. (A) TIL3 is predominantly expressed in PBL and ovary with a lower expression in prostate and testis. (B) TIL4 is predominantly expressed in PBL. Tissues examined: SP, spleen; TH, thymus; PR, prostate; TE, testis; OV, ovary; SI, small intestine; CO, colon; PBL, peripheral blood leukocytes. A human β-actin probe was used as a control for equivalent RNA loading.
Multiple tissue Northern analysis of poly (A)+ RNA with TIL3 and TIL4. (A) TIL3 is predominantly expressed in PBL and ovary with a lower expression in prostate and testis. (B) TIL4 is predominantly expressed in PBL. Tissues examined: SP, spleen; TH, thymus; PR, prostate; TE, testis; OV, ovary; SI, small intestine; CO, colon; PBL, peripheral blood leukocytes. A human β-actin probe was used as a control for equivalent RNA loading.
Chromosomal localization of TIL3 and TIL4.
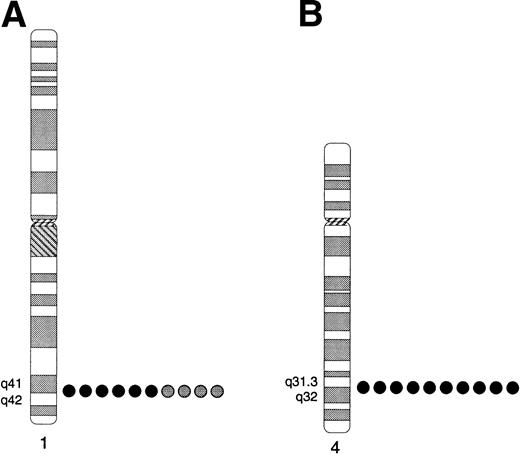
TIL3 and TIL4 were mapped to their chromosome location by FISH. BACs 22D07 and 21K16, containing TIL3 and TIL4, respectively, were biotinylated and hybridized to metaphase spreads of a normal male donor. The TIL3 BAC hybridized to three locations, 1q41-q42, 9p12, and 9q12-13 (not shown), suggesting that the BAC either is chimeric or contains sequences that are present at multiple locations. To determine which of these locations harbors the TIL3 gene, a plasmid, TX667U3288L, containing 2.6 kb of the TIL3 sequence was used for FISH (Fig4A). Signals were observed with the plasmid only at 1q41-q42, thereby localizing the TIL3 gene to this location. FISH analyses of BAC 21K16 place the TIL4 gene at 4q31.3-32 (Fig 4B).
Localization of TIL3 and TIL4 to human chromosome regions 1q41-q42 and 4q31.3-q32, respectively, by FISH. (A) TIL3. Summary of the locations of hybridization signals observed among 10 mitotic cells hybridized with plasmid TX667U3288L. Signals were observed in the 1q41-q42 region on both homologs (black circles) of 6 cells and only a single homolog (gray circles) in 4 cells, for an overall efficiency of 80%. Signals were not observed consistently at any other location. (B) TIL4. Summary of the locations of FISH signals observed among 10 mitotic cells hybridized with BAC 21K16, which contains TIL4. Hybridization signals were observed at 4q31.3-q32 on both homologs in each cell.
Localization of TIL3 and TIL4 to human chromosome regions 1q41-q42 and 4q31.3-q32, respectively, by FISH. (A) TIL3. Summary of the locations of hybridization signals observed among 10 mitotic cells hybridized with plasmid TX667U3288L. Signals were observed in the 1q41-q42 region on both homologs (black circles) of 6 cells and only a single homolog (gray circles) in 4 cells, for an overall efficiency of 80%. Signals were not observed consistently at any other location. (B) TIL4. Summary of the locations of FISH signals observed among 10 mitotic cells hybridized with BAC 21K16, which contains TIL4. Hybridization signals were observed at 4q31.3-q32 on both homologs in each cell.
TIL3 and TIL4 activate NF-κB in a cell type–specific manner.
The sequence similarity of the cytoplasmic domains of TIL3 and TIL4 with the type IL-1R suggests that the TIL proteins are signal transducing molecules that may participate in similar intracellular signaling pathways. Stimulation of the IL-1R is known to result in activation of the transcription factor NF-κB, and this activity has been localized to a critical region spanning amino acids 508 to 529 of the IL-1R cytoplasmic domain exhibiting considerable homology to both dToll and hToll.32 As hToll was recently shown to activate NF-κB and upregulate inflammatory cytokines,15 we investigated whether the sequence conservation observed between TIL3, TIL4, and the IL-1R also reflects a functional conservation with regard to downstream signaling events. Because the natural ligands for hToll, TIL3, and TIL4 have not been identified, we constructed chimeric receptors comprising the transmembrane and intracellular regions of TIL3, TIL4, and hToll, each joined to the extracellular domain of the Fas (CD95) receptor. Fas, a member of the tumor necrosis factor (TNF) family of receptors, has been shown to spontaneously aggregate and activate in a ligand-independent fashion when overexpressed in mammalian cells.33
The chimeric receptors were tested for their ability to activate NF-κB in vivo in an overexpression assay. The constructs were transiently transfected into MCF7 human breast carcinoma cells, along with a NF-κB/luciferase reporter plasmid. Forty-eight hours later, cells were lysed and assayed for luciferase activity. As shown in Fig5A, all three human Toll-like proteins were able to activate NF-κB when overexpressed in MCF7 cells. The strongest activation was seen with the Fas-hToll construct. TIL3 and TIL4 were also able to activate NF-κB in BHK cells (hToll was not assayed) (Fig 5B). A tissue-specific difference in the signaling pathways used by the TIL proteins is indicated by the NF-κB activation in 293T cells. hToll activated NF-κB effectively in these cells, but TIL4 failed to activate NF-κB, and TIL3 showed only a weak response (Fig 5C). We also compared the ability of TIL3 and TIL4 to activate NF-κB relative to DR3/WSL/TRAMP/APO3 (hereafter DR3), an NF-κB–inducing member of the tumor necrosis factor receptor family.34 Although all three receptors could activate NF-κB in MCF7 cells, NF-κB activation by DR3 was an order of magnitude greater than that seen with TIL3 and TIL4 (Fig 5D).
NF-κB activation by hToll, TIL3, and TIL4. Expression plasmids encoding Fas-hToll, Fas-TIL3, Fas-TIL4, or empty vector were cotransfected with a β-galactosidase (LacZ) expression plasmid into different cell lines in triplicate. Forty-eight hours later cells were lysed from two of the wells and luciferase activity measured as described previously.23 Cells from the third well were fixed with glutaraldehyde (0.5% in phosphate-buffered saline) and stained with X-gal (5-bromo-4-chloro-3-indoxyl-b-D-galactosidase) to obtain relative transfection efficiency.
NF-κB activation by hToll, TIL3, and TIL4. Expression plasmids encoding Fas-hToll, Fas-TIL3, Fas-TIL4, or empty vector were cotransfected with a β-galactosidase (LacZ) expression plasmid into different cell lines in triplicate. Forty-eight hours later cells were lysed from two of the wells and luciferase activity measured as described previously.23 Cells from the third well were fixed with glutaraldehyde (0.5% in phosphate-buffered saline) and stained with X-gal (5-bromo-4-chloro-3-indoxyl-b-D-galactosidase) to obtain relative transfection efficiency.
DISCUSSION
We have isolated two human cDNAs, TIL3 and TIL4, that exhibit extensive sequence homology with the Drosophila Toll protein in both the IL-1Rcd and the LRR extracellular domain. TIL3 and TIL4 are also very similar to the previously described human Toll-like genes, hToll and TIL.15,16 After submission of this manuscript, the cloning and expression profiles of four complete and one partial human Toll-like receptors (TLRs) were described.35 These genes were designated TLRs 1 through 5. The sequence of TIL3 is similar to the partial clone termed TLR5, whereas TIL4 and hToll correspond to TLR2 and TLR4, respectively. In addition to dToll, three additional Drosophila proteins, designated 18-wheeler, tlr, and MstProx, exhibit both IL1-Rcd and LRR homology. Together, these Toll/IL-1R–like genes encode a novel family of signal transducing transmembrane proteins with putative functions involving the immune response, morphogenesis, and cell adhesion. The structure of the Toll family of proteins is biologically unique in combining both the IL-1R and LRR domains in a single molecule. This configuration has significant functional implications in view of the many proteins from diverse biological systems that exhibit homology to these motifs.
The sequence similarity observed between dToll and the IL-1Rcd is highly significant in view of the striking evolutionary conservation of their respective signal transduction pathways involving theDrosophila genes dorsal, cactus, pelle, and their human counterparts NF-κB, I-κB, IRAK, and IRAK2.34,36 In addition to a functional role in establishing dorsal-ventral polarity in the developing Drosophila embryo, the dToll signaling pathway participates in the adult Drosophila innate immune response via induction of antifungal and antibacterial peptides.9 The recent demonstration of the ability of hToll to activate NF-κB and induce effector cytokines such as IL-1, IL-6, and IL-8 provided the first direct evidence for the conservation of this pathway of innate immunity in vertebrates.15 We have shown here that TIL3 and TIL4 resemble hToll in their ability to activate NF-κB, thereby providing further evidence of the conservation of Toll signaling in mammals. This result is consistent with the high degree of sequence homology in the cytoplasmic domains of these three proteins and the cytoplasmic domains of other IL-1R–like proteins, which are known activators of NF-κB in mammals.
The cytoplasmic domains of TIL3, TIL4, and hToll are also homologous to the cytoplasmic domain of the human gene TIL (Fig 2A). Previous studies with TIL in the form of a chimeric IL-1R extracellular domain and TIL intracellular domain failed to activate NF-κB in COS7 cells.17 There are several explanations for this result. The NF-κB/luciferase reporter assay used in the present study is much more sensitive than the gel-shift assay used in the TIL study, and a weak activation of NF-κB might not have been detected. Alternatively, although TIL may not activate NF-κB in COS7 cells, it may show activity in other cell types. This possibility is exemplified by the ability of TIL4 to activate NF-κB in MCF7 and BHK cells, but not 293T cells.
We also observed a difference in the relative level of activation affected by TIL3 and TIL4 in the different cell lines tested. These cell-dependent behaviors may reflect a difference in the accessory/adapter molecules that interact with these proteins and play critical roles in their signal transduction pathways. The distinct tissue expression patterns exhibited by these receptors lend support to cell type–specific activity. Studies with hToll have shown expression in a wide spectrum of human tissues with the highest level in spleen and peripheral blood leukocytes including monocytes, macrophages, dendritic cells, B cells, and T cells.15 TIL3 is expressed in PBLs, ovary, and prostate, and TIL4 is predominantly expressed in PBLs and spleen. The observed amino acid sequence divergences between these proteins may also influence receptor function by providing alternative protein conformations leading to distinct binding affinities for interacting proteins.
If the signaling pathway involving these Toll/IL-1R–like proteins mirrors the IL-1R pathway, then upstream and downstream events will likely involve molecules homologous to those in the IL-1R pathway. The IL-1R is a multisubunit complex,37 as shown by the identification of the IL-1R accessory protein.38 The downstream signaling events involve two IL-1R–associated kinases, IRAK39 and IRAK2,40 and the recently described putative IL-1R adapter/regulator, MyD88.40 Interestingly, the dToll and IL-1 signal pathways retain their striking conservation with the finding that IRAK and IRAK2 are homologous to Pelle, a protein kinase in the dToll pathway.39
The IL-1 and TNF receptor families represent the two known families of cell-surface receptor proteins that can lead to activation of the NF-κB pathway in mammalian cells.41 Our study suggests that although both families can activate NF-κB, they differ in their relative activity. The TNF receptor family member DR3 exhibits much stronger stronger NF-κB activation than do TIL3 or TIL4. This result may be supported by recent reports suggesting the presence of distinct downstream adapter proteins, such as TRAF2 and TRAF6, that participate in the signal transduction of these two receptor families.41 However, this conclusion is based on ligand-independent receptor activation in a transient transfection-over-expression system. It is possible that the observed difference reflects a variation in the abilities of these two receptor types to self-activate when overexpressed, and not in their inherent abilities to activate NF-κB in the presence of their cognate ligands. The identification of ligands for TIL3, TIL4, and hToll will help to define signal specificity and regulation through the native receptor complex.
The essential functional regions of the dToll and IL-1R proteins have been delineated through detailed deletion and mutational analysis experiments. Seven conserved residues of the IL-1Rcd are critical for full signal transduction as assayed by IL-2 activation,12,42 and six of seven of these residues are also conserved in the dToll protein. The human Toll/IL-1–like genes retain 3 or 4 of these residues, but only hToll Phe-807 is conserved in all of the Toll/IL-1R–like proteins and IL-1R (Fig 2A). Several of these conserved residues have also been shown to be essential for IL-1R activation of NF-κB.43 The region corresponding to aa 435 to 484 of IL-1R is similar in sequence to the box 1- and box2-like elements present in gp130, the signal-transducing subunit of the IL-6R family.44 Amino acid mutagenesis of the hydrophobic elements within the gp130 homologous region in the IL-1R identified several residues critical for the capacity to induce IL-8 gene expression.45 These residues are highly conserved in the Toll/IL-1R–like protein family (Fig 2A).
Deletion of the extracellular LRR portion of dToll leads to a constitutively active protein.14 Examination ofDrosophila embryo mutants revealed nine gain-of-function mutations, all located in the extracellular domain. Of these mutations, three involve cysteine-to-tyrosine residue changes in the region located immediately outside the transmembrane domain.13These cysteines, adjacent to the LRRs, are thought to be involved in the formation of intramolecular disulfide bonds,13 and are conserved in all of the Toll/IL-1R–like proteins that we have examined.
LRRs are short protein modules of 20 to 29 amino acids characterized by a periodic arrangement of hydrophobic residues that are found in a diverse group of extracellular, membrane, and cytoplasmic proteins.46 A subset of the LRR protein superfamily comprises proteins with LRR flanking cysteine clusters, an arrangement that appears to be a property of adhesive proteins and receptors. This group includes the TIL/IL-1R–like proteins, leucine-rich glycoprotein,29 the metastasis-associated oncofetal antigen 5T4,30 and components of the platelet glycoprotein 1b-V-IX complex.27,28 Platelet glycoprotein 1b is a membrane receptor involved in the adhesion of platelets to subendothelial connective tissue at sites of endothelial damage.47 dToll has also been shown to promote cell adhesion in either a direct manner involving the LRR domains, or indirectly by transducing an extracellular signal that causes activation of other adhesion molecules on the cell surface.26
In view of their sequence homology with the LRR extracellular domains of dToll and the platelet glycoproteins, it is likely that the human Toll/IL-1R proteins may also have adhesive properties. It is interesting to speculate how the adhesive and immunoregulatory functions of Toll/IL-1R proteins could be linked. In view of dToll's diverse functions in development, immune regulation, and adhesion, the Toll/IL-1 signaling pathway may not be dedicated to specific target genes, but instead may control completely divergent genes involved in distinct processes.48 The intimate involvement of Toll in developmental events coupled with the striking conservation of the Toll signaling pathway from plants to vertebrates should make the Toll/IL-1R–related genes hToll, TIL, TIL3, and TIL4 prime candidates for the investigation of developmentally associated diseases linked to their respective chromosomal locations.
ACKNOWLEDGMENT
We thank Steve Lasky and Carol Loretz for sequencing assistance.
Supported by the CaPCURE Foundation and the Stowers Institute for Medical Research, a postdoctoral fellowship from the Damon Runyon-Walter Winchell Foundation (P.M.C.), and a grant (DE-GF03-96ER62173) from the United States Department of Energy (O.N., H.F.M., B.J.T.).
Address reprint requests to Peter S. Nelson, MD, Department of Molecular Biotechnology, Box 357730 HSB K-360, University of Washington, Seattle, Washington 98195.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.