Abstract
β2-Glycoprotein I (β2GPI) is a highly glycosylated plasma protein with the ability to bind negatively charged substances such as DNA, heparin, dextran sulfate, and negatively charged phospholipids. The most relevant physiological role of β2GPI is supposed to be the regulation of the function of anionic phospholipids like cardiolipin (CL). β2GPI consists of a single polypeptide chain (326 amino acid residues) with a molecular mass of about 50 kD and with five tandem repeated domains (I, II, III, IV, and V). In the previous study, we found that factor Xa can produce the nicked form by cleaving Lys 317-Thr 318, using recombinant human domain V (r-Domain V). However, the reaction was extremely slow. In the present paper, we found that plasmin can produce the nicked form of domain V, using recombinant domain V (r-Domain V) and β2GPI from human plasma. On sodium dodecyl sulfate-polyacrylamide gel electrophoresis, r-Domain V was rapidly cleaved into a nicked form by plasmin, very slowly by factor Xa, but not by thrombin, tissue-type plasminogen activator, urokinase, and tissue factor/factor VIIa. The cleavage site of r-Domain V and β2GPI by plasmin was proved to be Lys 317-Thr 318 by amino acid sequence analysis of the digest and of the C-terminal peptide isolated by high-performance liquid chromatography. The cleavage was completely inhibited by plasmin inhibitor (α2PI). The nicked form was demonstrated to show reduced affinity for CL with a dissociation constant of one order of magnitude larger than that of the intact β2GPI. To determine whether the specific cleavage of β2GPI by plasmin can occur also in plasma, human plasma was first acid-treated to inactivate α2PI and then incubated with urokinase. About 12% of β2GPI in plasma was nicked when α2PI activity decreased to 80%. The nicked form was not generated in plasminogen-depleted plasma. These results suggest that plasmin can produce the nicked form of β2GPI with the reduced ability to bind phospholipids in vivo.
β-GLYCOPROTEIN I (β2GPI) is a highly glycosylated2plasma protein with the ability to bind negatively charged substances such as DNA, heparin, dextran sulfate, and negatively charged phospholipids.1-4 The most relevant physiological role of β2GPI is thought to be the binding with the anionic phospholipids, including cardiolipin (CL). Anticardiolipin antibodies or lupus anticoagulants are strongly associated with thrombosis. In these autoimmune diseases with anti-phospholipid antibody syndrome, β2GPI is a cofactor in the recognition of the phospholipid antigen, CL, by anti-CL antibodies.5-7 β2GPI consists of a single polypeptide chain (326 amino acid residues) with a molecular mass of about 50 kD and with five tandem repeated domains (I, II, III, IV, and V), which have a common motif named as the complement control protein or Sushi domain superfamily.8-10 We reported that both domain I and domain V of β2GPI are important for the interaction with CL, using bovine β2GPI.11Particularly, the importance of domain V has been proved by several lines of evidence. Steinkasserer et al12 found that recombinant human domain V (r-domain V) inhibited the interaction of β2GPI with CL. Hunt et al13 reported that the commercial preparation of human β2GPI contained a nicked form in domain V, which displayed a lower interaction with CL.
The generation of a nicked form of β2GPI in vivo may regulate the function of β2GPI. However, it is unknown what proteases generate the nicked form by cleaving domain V of β2GPI. Hunt et al13 found two cleavage sites in domain V of commercial human β2GPI, one major site as Lys 317-Thr 318 and another minor site as Ala 314-Phe 315. We expressed human r-domain V with a signal peptide containing factor Xa recognition site and prepared the intact domain V by removing the signal peptide, using factor Xa.14 During the course of that study, we found that a nicked form of domain V was produced by a long incubation with factor Xa by the cleavage of Lys 317-Thr 318 bond and that the nicked form lost the ability to interact with CL by the specific cleavage. Although the study presented clearly the evidence that factor Xa can produce a nicked form of domain V, the reaction was extremely slow. In the present study, to examine the protease responsible for producing the nicked form in vivo, we incubated r-Domain V with various proteases from plasma and found that plasmin produced the nicked form by cleaving Lys 317-Thr 318. Here, we report the evidence that plasmin can produce the nicked form of β2GPI by the incubation of purified β2GPI with plasmin and also by the activation of plasminogen in plasma by urokinase.
MATERIALS AND METHODS
Materials.
Human proteases were purchased from the following manufacturers; thrombin from Protogen AG (Laüfelfingen, Switzerland), plasmin from American Diagnostica (Greenwhich, CT), UK from BioPur AG (Bubendorf, Switzerland), t-PA from Chromogenix (Mölndal, Sweden), α2PI from Biopool AB (Umea, Sweden), and factor Xa from Enzyme Research Laboratories, Inc (South Bend, IN). Platelin (rabbit brain phospholipids) was purchased from Organon Teknika Co (Durham, NC). HiTrap Heparin was purchased from Pharmacia Biotechnology (Uppsala, Sweden). Soluble tissue factor (sTF) was kindly supplied by Dr Toshiyuki Miyata (National Cardiovascular Center Research Institute). Factor VIIa was kindly supplied by Dr Tomohiro Nakagaki (Chemo-Sero-Therapeutic Research Institute, Kumamoto, Japan). CL and PC were purchased from Avanti Polar Lipids (Alabaster, AL).
Preparation of human β2 GPI and r-Domain V.
r-Domain V with signal peptide, which consists of extra amino acid sequence of Tyr-Val-Glu-Phe-Met-Ile-Glu-Gly-Arg-Thr with the amino terminal Lys 242 of human β2GPI, was expressed in methylotrophic yeast, Pichia pastoris, and then purified using anion-exchange and reversed-phase chromatographies, as described in the previous paper.14 r-Domain V was obtained by treatment with factor Xa, giving it an extra Thr at the N-terminus. The numbers of amino acid residues of intact β2-GPI were employed for the numbering of the amino acid residues of r-Domain V, neglecting the extra Thr at the N-terminus. Human β2GPI was prepared as described by Polz et al.2 Briefly, fresh human plasma was treated with 1.3% perchloric acid at 4°C for 15 minutes and centrifuged at 5,000g for 10 minutes. The supernatant was neutralized by adding 1/3 vol of 1.0 mol/L Tris-HCl (pH 8.0) and concentrated by Centricon-30 (Amicon Co, Beverly, MA). The concentrate was applied to a column of HiTrap Heparin. After washing the column with 10 mmol/L Tris-HCl (pH 8.0), β2GPI was eluted with a linear gradient of 10 mmol/L Tris-HCl and the same buffer containing 1 mol/L NaCl.
Cleavage of human β2GPI and r-Domain V by various proteases.
All enzymatic reactions were performed in 0.1 mol/L Tris-HCl (pH 7.5) containing 20 mmol/L NaCl and 0.3 mmol/L CaCl2 at 37°C. About 1 μg of r-Domain V was reacted with factor Xa, human thrombin, human plasmin, UK, t-PA, and sTF/ VIIa. In the case of sTF/VIIa, the reaction was performed in the presence of 60 μg/mL of platelin. The molar ratios of enzymes to substrates were 1:50. At the specified times, aliquots were removed from the reaction mixture. These samples were reduced by 2-mercaptoethanol and subjected to 10% to 20% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).15 Separation of the peptide chains of plasmin-treated r-Domain V was carried out using reversed-phase high-performance liquid chromatograpyy (HPLC). r-Domain V (50 μg) was incubated with 7.2 μg of plasmin, with molar ratio of plasmin to substrate of 1 to 50. The reaction was stopped by adding twice volume of 8 mol/L guanidium hydrochloride at each reaction time. Next, the samples were reduced by the final concentration of 3 mmol/L of dithiothreitol for 3 hours. The reduced samples were pyridylethylated by the final concentration of 10 mmol/L of 4-vinylpyridine for 2 hours. Each reduced-pyridylethylated sample was subjected to 5C18-AR reversed-phase HPLC column (4.6 × 20 mm; Nacalai Tesque, Kyoto, Japan). Peptides were eluted with a linear gradient from 7% to 55% acetonitrile.
Separation of the nicked form of β2GPI from the intact protein was performed using HiTrap Heparin column. β2GPI (660 μg) was incubated with 19 μg of plasmin with molar ratio of plasmin to substrate of 1 to 56 in the presence or absence of 5.4 μg/mL of α2PI. The reaction was stopped by adding twice volume of 8 mol/L guanidium hydrochloride at each reaction time. Then, the samples were diluted 10 times with 10 mmol/L Tris-HCl buffer (pH 8.0) and subjected to HiTrap Heparin column (1 mL). Proteins were eluted with a linear concentration gradient formed by the solutions containing 0.1 mol/L and 1 mol/L NaCl with a flow rate of 1.0 mL/min. The peak areas were calculated using a Waters 911J Photodiode array system controller (Millipore, Milford, MA).
The N-terminal amino acid sequences of the samples were analyzed by a protein sequencer, model 473A (Applied Biosystem, Foster City, CA).
Liposome preparation and binding assay.
Large unilamellar vesicles consisting of CL and PC at molar ratio of 1:1 were prepared, and analysis of the binding affinity for liposome membranes was performed as described previously.11 The dissociation constant (kd) and the number of phospholipids in the binding site (n) were estimated by curve-fitting of the plot of percentage bound versus phospholipid concentration according to the method described previously.11
UK activation of human plasma.
Plasminogen-depleted plasma was prepared by applying 20 mL of normal plasma to a column (1.2 × 5 cm) of Lysine-Sepharose 4B (Pharmacia Biotechnology), which had been equilibrated with 50 mmol/L sodium phosphate buffer, pH 7.5, and by collecting the nonadsorbed fraction. Normal plasma or plasminogen-depleted plasma (3.8 mL) was dialyzed against 2 L of 10 mmol/L sodium acetate buffer, pH 4.0, at 4°C for 12 hours, and then against 5 L of 3 mmol/L Tris-HCl buffer, pH 8.0, containing 50 mmol/L NaCl for 12 hours. The dialysates were mixed with 30 IU of UK (Midori Jyuji, Osaka, Japan) and incubated at 37°C for 12 hours. To examine the effect of α2PI in plasma on the cleavage of β2GPI by plasmin, the acid-treated plasma was mixed with nontreated plasma in a various ratio and the mixtures were incubated with UK. α2PI activity in the mixtures was estimated according to the method described previously.16 17
The intact and nicked forms of β2GPI were separated by the treatment with perchloric acid and by heparin affinity column chromatography as described above.
RESULTS
Degradation of r-Domain V and β2GPI by plasmin.
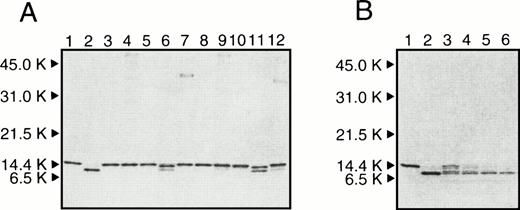
Figure 1A shows a profile of SDS-PAGE of r-Domain V incubated with factor Xa, thrombin, UK, t-PA, and sTF/VIIa with enzyme to substrate mole ratio of 1 to 50, for 8 hours or 24 hours. Lanes 1 and 2 are the intact r-Domain V and nicked r-Domain V prepared as described in the previous report,14respectively. As reported in the previous paper,14 very slow cleavage of r-Domain V with factor Xa was observed (lanes 6 and 11). Thrombin, t-PA, UK, and sTF/VIIa did not cleave r-Domain V under this condition. Figure 1B shows SDS-PAGE of r-Domain V incubated with plasmin at an enzyme to substrate mole ratio of 1 to 50. After a 30-minute incubation, more than 50% of r-Domain V band was decreased and a new band that corresponded to the nicked r-Domain V was appeared. After 1-hour incubation, the intact r-Domain V was almost disappeared. These results indicate that plasmin can convert r-Domain V into a nicked Domain V very quickly. The cleavage of r-Domain V by plasmin was not significantly affected in the presence of phospholipid and calcium (data not shown).
(A) SDS-PAGE of r-Domain V incubated with various proteases at 37°C for 8 hours (lanes 3 to 7) and 24 hours (lanes 8 to 12). r-Domain V was incubated with UK (lanes 3 and 8), t-PA (lanes 4 and 9), thrombin (lanes 5 and 10), factor Xa (lanes 6 and 11), and sTF/VIIa (lanes 7 and 12) in 0.1 mol/L Tris-HCl (pH 7.5) containing 20 mmol/L NaCl and 0.3 mmol/L CaCl2. Molar ratios of r-Domain V to proteases were 50:1. (B) SDS-PAGE of r-Domain V incubated with plasmin. r-Domain V was incubated with plasmin with a molar ratio of r-Domain V to plasmin was 50:1 at 37 °C. Lanes 3 to 6 are samples taken at 0.25, 0.5, 1, and 2 hours, respectively. For comparison, intact r-Domain V and nicked r-Domain V as prepared in the previous paper14 are shown in lanes 1 and 2 in (A) and (B), respectively. The positions of molecular-weight markers are shown at the left of the gel.
(A) SDS-PAGE of r-Domain V incubated with various proteases at 37°C for 8 hours (lanes 3 to 7) and 24 hours (lanes 8 to 12). r-Domain V was incubated with UK (lanes 3 and 8), t-PA (lanes 4 and 9), thrombin (lanes 5 and 10), factor Xa (lanes 6 and 11), and sTF/VIIa (lanes 7 and 12) in 0.1 mol/L Tris-HCl (pH 7.5) containing 20 mmol/L NaCl and 0.3 mmol/L CaCl2. Molar ratios of r-Domain V to proteases were 50:1. (B) SDS-PAGE of r-Domain V incubated with plasmin. r-Domain V was incubated with plasmin with a molar ratio of r-Domain V to plasmin was 50:1 at 37 °C. Lanes 3 to 6 are samples taken at 0.25, 0.5, 1, and 2 hours, respectively. For comparison, intact r-Domain V and nicked r-Domain V as prepared in the previous paper14 are shown in lanes 1 and 2 in (A) and (B), respectively. The positions of molecular-weight markers are shown at the left of the gel.
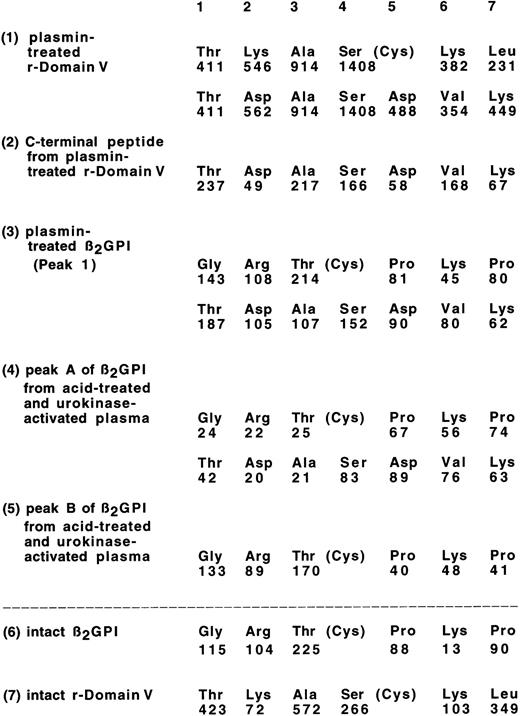
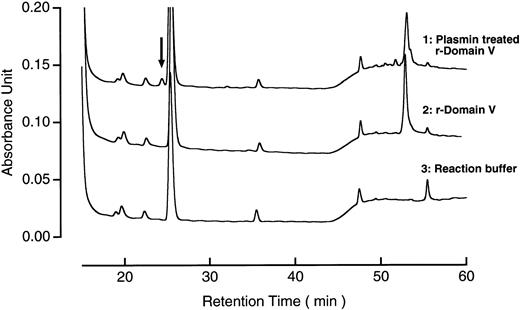
The r-Domain V was incubated with plasmin for 2 hours with enzyme/substrate molar ratio of 1:50; the N-terminal amino acid sequence of the digest was examined (Fig2[1]), comparing with that of intact r-Domain V (Fig 2[7]). From plasmin-digest of r-Domain V, a new amino acid sequence was found which started from Thr 318, in addition to the N-terminal amino acid sequence of r-Domain V. Figure 3 shows reversed-phase HPLC patterns of r-Domain V and plasmin-digest of Domain V after reduction and pyridylethylation. The peak shown by the arrow indicates the position of a new peptide generated by plasmin digestion. The N-terminal amino acid sequence of the peptide from the plasmin digest was found to be Thr-Asp-Ala-Ser-Asp-Val-Lys-Pro, as shown in Fig2(2), confirming that this peptide was derived from the C-terminal part of the nicked r-Domain V. These results indicate that plasmin cleaved a peptide bond of Lys 317-Thr 318 in r-Domain V, which was the same cleavage site by factor Xa as reported previously.14
Amino acid sequences of plasmin digests of β2GPI and r-Domain V. Each value under the amino acid sequence identified shows the amount of PTH-amino acid (in picomoles) recovered using a gas-phase sequencer. Any other sequence was not observed: (1), plasmin digest of r-Domain V; (2), C-terminal peptide of plasmin-treated r-Domain V (see Fig 3); (3), Peak 1 from plasmin-treated β2GPI (see Fig 4A); (4), peak A of β2GPI from acid-treated and UK-activated plasma (see Fig7); (5), peak B of β2GPI from acid-treated and UK-activated plasma; (6), intact β2GPI; and (7), intact r-Domain V.
Amino acid sequences of plasmin digests of β2GPI and r-Domain V. Each value under the amino acid sequence identified shows the amount of PTH-amino acid (in picomoles) recovered using a gas-phase sequencer. Any other sequence was not observed: (1), plasmin digest of r-Domain V; (2), C-terminal peptide of plasmin-treated r-Domain V (see Fig 3); (3), Peak 1 from plasmin-treated β2GPI (see Fig 4A); (4), peak A of β2GPI from acid-treated and UK-activated plasma (see Fig7); (5), peak B of β2GPI from acid-treated and UK-activated plasma; (6), intact β2GPI; and (7), intact r-Domain V.
Reversed-phase HPLC patterns of plasmin-treated r-Domain V. The digest of r-Domain V (1.4 μg) was subjected to reversed-phase HPLC, as described in Materials and Methods. Arrow indicates the peak corresponding to the C-terminal peptide of the digest.
Reversed-phase HPLC patterns of plasmin-treated r-Domain V. The digest of r-Domain V (1.4 μg) was subjected to reversed-phase HPLC, as described in Materials and Methods. Arrow indicates the peak corresponding to the C-terminal peptide of the digest.
β2GPI was then incubated with plasmin at enzyme/substrate mole ratio of 1:56 for each time. When the digests of β2GPI by plasmin were subjected to HiTrap Heparin column chromatography, two distinct peaks were separated (Fig 4A). Peak 1 was found to have a new N-terminal amino acid sequence that started from Thr 318, in addition to the N-terminal amino acid sequence of β2GPI as shown in Fig 2(3), confirming that peak 1 corresponded to the nicked β2GPI. Peak 2 was shown to be the intact β2GPI by the amino acid sequence analysis.
(A) HiTrap Heparin column chromatography patterns of plasmin-treated β2GPI. A total of 660 μg of β2GPI was incubated with 19 μg of plasmin at 37°C, where molar ratio of plasmin to substrate was 1:56. A total of 60 μg of the digest of β2GPI was subjected to HiTrap Heparin column chromatography, as described in Materials and Methods. (B) Time course of the increase of nicked β2GPI (peak 1 in [A]). Horizontal axis and vertical axis correspond to the reaction time (hours) and area of peaks of nicked β2GPI. The experiment was performed in the presence (•) or absence of α2PI (▪).
(A) HiTrap Heparin column chromatography patterns of plasmin-treated β2GPI. A total of 660 μg of β2GPI was incubated with 19 μg of plasmin at 37°C, where molar ratio of plasmin to substrate was 1:56. A total of 60 μg of the digest of β2GPI was subjected to HiTrap Heparin column chromatography, as described in Materials and Methods. (B) Time course of the increase of nicked β2GPI (peak 1 in [A]). Horizontal axis and vertical axis correspond to the reaction time (hours) and area of peaks of nicked β2GPI. The experiment was performed in the presence (•) or absence of α2PI (▪).
Figure 4B shows the increases of the nicked β2GPI after the incubation with plasmin, which was calculated by the area of peak 1 shown in Fig 4A. The half-time of the cleavage reaction of β2GPI was about 30 minutes. The maximum cleavage was obtained about at 2-hour incubation. The slow decrease of the nicked β2GPI after 2-hour incubation indicates that the other peptide bonds in β2GPI were cleaved by plasmin after the longer incubation. In the presence of α2PI, β2GPI was not cleaved at all (closed circles in Fig 4B).
Interaction of intact and nicked forms of β2GPI with CL.
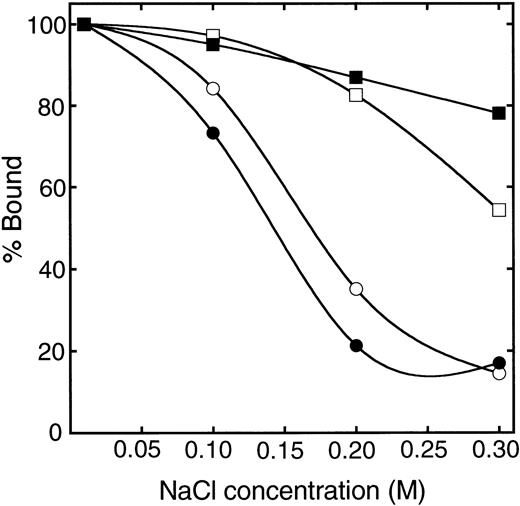
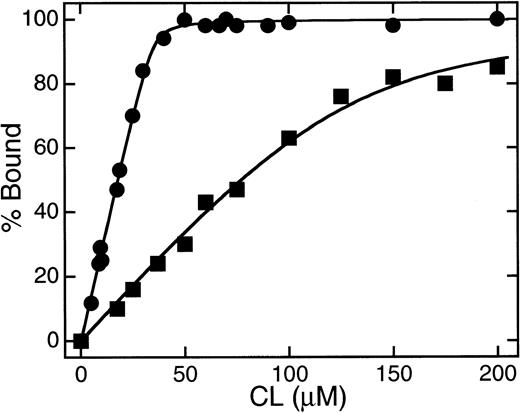
Figure 5 shows the effect of NaCl concentration on the bindings of β2GPI and r-Domain V and of their nicked forms to liposomes containing CL. The affinity of nicked forms decreased markedly with the increase of NaCl concentrations, whereas intact proteins retained their affinity as reported previously.11 These results indicate that the affinity of β2GPI for CL was reduced after the cleavage of Lys 317-Thr 318 in domain V by plasmin. Figure 6 shows the bindings of intact and nicked forms of β2GPI with liposomes containing CL as a function of the phospholipid concentration. The dissociation constant (kd) and the number of phospholipids in the binding sites (n) in the interaction of β2GPI with CL were estimated by the curve-fitting procedure as described previously.11 As shown in Table 1, the approximate kd value of the nicked form was one order of magnitude larger than that of the intact β2GPI and n value of the nicked form was three times larger than that of the intact protein. These values of human intact and nicked β2GPI were similar to those of bovine intact and nicked β2GPI, as reported respectively.11
Effect of NaCl concentration on the bindings of β2GPI and r-Domain V and of their nicked forms to liposomes containing CL. Solutions containing intact β2GPI (▪), nicked β2GPI (•), intact r-Domain V (□), or nicked (○) and various concentrations of NaCl in 10 mmol/L Tris-HCl, pH 7.5, were mixed with liposomes containing CL and PC. After the incubation at 25°C for 30 minutes, the mixtures were centrifuged at 120,000g for 1 hour. The amount of proteins in the supernatant was measured as described by Hagihara et al.11 The amount of proteins in the absence of liposomes at the indicated NaCl concentration was taken to be 100%.
Effect of NaCl concentration on the bindings of β2GPI and r-Domain V and of their nicked forms to liposomes containing CL. Solutions containing intact β2GPI (▪), nicked β2GPI (•), intact r-Domain V (□), or nicked (○) and various concentrations of NaCl in 10 mmol/L Tris-HCl, pH 7.5, were mixed with liposomes containing CL and PC. After the incubation at 25°C for 30 minutes, the mixtures were centrifuged at 120,000g for 1 hour. The amount of proteins in the supernatant was measured as described by Hagihara et al.11 The amount of proteins in the absence of liposomes at the indicated NaCl concentration was taken to be 100%.
Plots of the binding of β2GPI to liposome membranes containing CL as a function of the concentration of CL. One micromolar of intact (▪) or nicked (•) β2GPI was incubated with CL/PC (1:1)-LUV at 25°C in 10 mmol/L Tris-HCl buffer containing 10 mmol/L NaCl. The amounts of proteins bound to liposomes were measured as described by Hagihara et al.11
Plots of the binding of β2GPI to liposome membranes containing CL as a function of the concentration of CL. One micromolar of intact (▪) or nicked (•) β2GPI was incubated with CL/PC (1:1)-LUV at 25°C in 10 mmol/L Tris-HCl buffer containing 10 mmol/L NaCl. The amounts of proteins bound to liposomes were measured as described by Hagihara et al.11
Cleavage of β2GPI by plasmin in plasma.
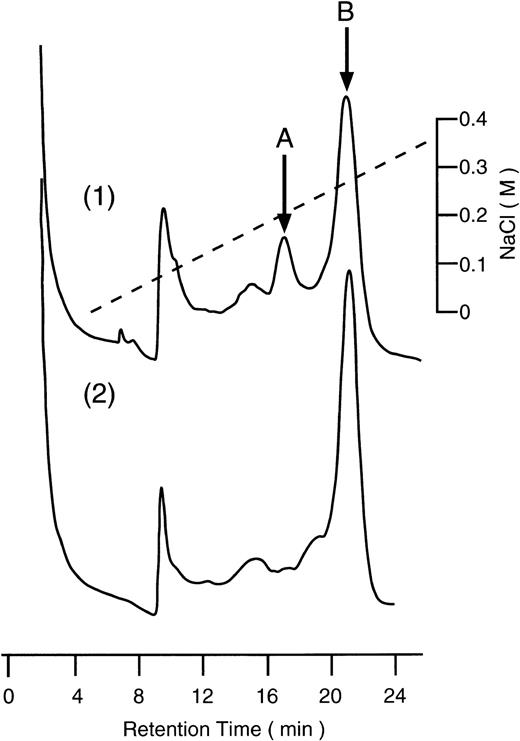
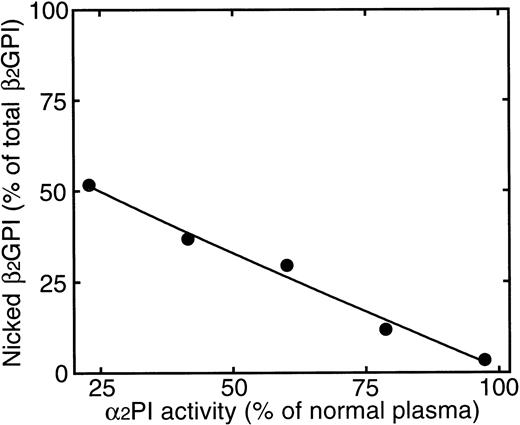
To prove that the same specific cleavage of β2GPI by plasmin can occur also in plasma, acid-treated plasma was incubated with UK, and intact and nicked form of β2GPI were separated using heparin affinity column chromatography, as described in Materials and Methods. As shown in Fig7(1), a small peak (peak A) and a large peak (peak B) were separated. The amino acid sequence of peak A was found to be the same as the plasmin-digest of β2GPI, as shown in Fig 2. The amino acid sequence of peak B was found to be the same as intact β2GPI (Fig 2). Without the addition of UK to the acid-treated plasma, peak A was not detected (data not shown). When plasminogen-depleted plasma was used, instead of normal plasma, the peak A was also not detected (Fig 7[2]). To examine the effect of α2PI in plasma on the cleavage of β2GPI, the acid treated plasma was mixed with normal plasma in a various ratio and treated with UK. The acid-treated plasma retained 22.9% of α2PI activity. As shown in Fig 8, a nicked form of β2GPI was generated with the decrease of α2PI activity in plasma. On the plasma with 80% of α2PI activity of normal plasma, about 12% of β2GPI was detected as nicked β2GPI after 16 hours incubation with UK. These results indicate that a nicked domain V of β2GPI could be generated in plasma by the action of plasmin after activation of plasminogen, particularly when α2PI activity in plasma decreased.
Heparin affinity chromatography of β2GPI from acid-treated and UK-activated plasma. Both normal and plasminogen-depleted human plasmas were treated with acid and activated with UK as described in Materials and Methods. After the treatment with perchloric acid, sample was applied to a column of HiTrap Heparin and eluted with a linear gradient of NaCl. (1), normal plasma; (2), plasminogen-depleted plasma.
Heparin affinity chromatography of β2GPI from acid-treated and UK-activated plasma. Both normal and plasminogen-depleted human plasmas were treated with acid and activated with UK as described in Materials and Methods. After the treatment with perchloric acid, sample was applied to a column of HiTrap Heparin and eluted with a linear gradient of NaCl. (1), normal plasma; (2), plasminogen-depleted plasma.
Relationship between the formation of nicked β2GPI and α2PI activity in plasma. After 16-hour incubation of samples with 120 IU of UK at 37°C, the intact and nicked form of β2GPI were separated as described in Materials and Methods. Horizontal axis and vertical axis correspond to α2PI activity and amount of nicked β2GPI (percentage of total β2GPI) in the plasma. α2PI activity was expressed as the percentage of normal plasma.
Relationship between the formation of nicked β2GPI and α2PI activity in plasma. After 16-hour incubation of samples with 120 IU of UK at 37°C, the intact and nicked form of β2GPI were separated as described in Materials and Methods. Horizontal axis and vertical axis correspond to α2PI activity and amount of nicked β2GPI (percentage of total β2GPI) in the plasma. α2PI activity was expressed as the percentage of normal plasma.
DISCUSSION
In the present work we showed that plasmin generated quickly a nicked form of β2GPI by the limited cleavage of a peptide bond of domain V, Lys 317-Thr 318, by using recombinant domain V and β2GPI from human plasma. Cleavage of the peptide bond by factor Xa was extremely slow. Cleavage by thrombin, UK, t-PA, and sTF/VIIa was not detected. The nicked form of β2GPI was shown to have a reduced affinity for CL by the quantitative analysis of the binding with liposomes containing CL. The importance of domain V in β2GPI has been demonstrated for the interaction with CL and other phospholipids.18 Sheng et al19demonstrated that Lys 284, Lys 286, and Lys 287 in domain V are critical for the binding with phospholipid by using mutant proteins. We elucidate that cleavage of Lys 317-Thr 318 by plasmin causes the conformational change of the domain V, leading to the reduced interaction with phospholipid, although the conformational change was not significantly detected by circular dichroism and fluorescent measurements.14
Cleavage of the peptide bond in domain V by plasmin was also demonstrated in plasma. The nicked form of β2GPI was isolated from UK-treated plasma. We could not detect the cleavage of Ala 314-Phe 315 in β2GPI isolated from plasma after the activation of plasminogen, as reported in commercial preparation of human β2GPI by Hunt et al.13 Other proteases in plasma or from cells may participate in the cleavage of the peptide bond. The activation of plasminogen occurs after the formation of fibrin, because both plasminogen and plasminogen activator associate with fibrin.20,21 Then, plasmin can degrade fibrin and other plasma proteins. In the fluid phase, most of plasmin generated is inactivated by α2 plasmin inhibitor. This study demonstrated the significant cleavage of β2GPI in acid-treated plasma, which exhibited 23% of α2PI activity of normal plasma. Our study also indicated that about 12% of β2GPI was nicked when α2PI activity in plasma decreased to 80% (Fig 8). Therefore, the in vivo limited proteolysis of β2GPI by plasmin could occur significantly when plasmin inhibitor level decreases. Brighton et al22 demonstrated that β2GPI levels in plasma were significantly reduced in patients with thrombosis or DIC. It is highly probable that plasmin inhibitor decreased in these patients and then plasmin could generate the nicked β2GPI. The nicked form of β2GPI may be rapidly cleared from circulation than native β2GPI.
It has been reported that plasminogen binds to extracellular matrix (ECM) synthesized by endothelial cell monolayers and activated by t-PA or UK on the matrix.23,24 Plasmin generated on the matrix is protected from inhibition by α2 plasmin inhibitor. It has been also reported that β2GPI binds to heparin.2 The extracellular matrix produced by endothelial cell is a complex array of glycoproteins and glycosaminoglycans. Therefore, it is conceivable that β2GPI binds to heparin-like glycosaminoglycans and cleaved by plasmin in the extracellular matrix. The possibility that β2GPI binds to fibrin and then proteolyzed by plasmin also intrigues us. We have shown that radiolabeled β2GPI was incorporated into fibrin (Ohkura N, Kato H, unpublished observations), although further investigation should be performed. Recently, Kochl et al25 showed the interaction of β2GPI with apolipoprotein (a) by using the yeast two-hybrid interaction trap system. These investigators also demonstrated the interaction in plasma by using co-immunoprecipitation experiments. These results suggest that lipoprotein (a) potentiates the association of β2GPI with fibrin, as apolipoprotein (a) has been shown to associate with fibrin.
Although further investigation is needed to demonstrate the in vivo significance of the limited cleavage of β2GPI by plasmin, the present data indicate the possibility that plasmin can produce the nicked form of β2GPI and reduce the function of β2GPI in vivo. β2GPI has been shown to interact with phospholipid and many other negatively charged substances and to modulate the activation of the intrinsic pathway, prothrombinase activity and phospholipid-dependent protein Ca activity.26-29 The phopholipid-bound β2GPI can be cleaved by plasmin, since the cleavage of β2GPI by plasmin was not affected in the presence of phospholipid. The cleavage of β2GPI by plasmin demonstrated in the current study may give a new insight into the function of β2GPI.
N.O. and Y.H. contributed equally to this work.
Supported by Grants-in-Aid for Scientific Research from the Ministry of Education Science, Sports and Culture of Japan, and by Fellowships of the Japan Society for the Promotion of Science for Japanese Junior Scientists.
Address reprint requests to Hisao Kato, PhD, National Cardiovascular Center Research Institute, Fujishirodai-5, Suita, Osaka 565, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 4. (A) HiTrap Heparin column chromatography patterns of plasmin-treated β2GPI. A total of 660 μg of β2GPI was incubated with 19 μg of plasmin at 37°C, where molar ratio of plasmin to substrate was 1:56. A total of 60 μg of the digest of β2GPI was subjected to HiTrap Heparin column chromatography, as described in Materials and Methods. (B) Time course of the increase of nicked β2GPI (peak 1 in [A]). Horizontal axis and vertical axis correspond to the reaction time (hours) and area of peaks of nicked β2GPI. The experiment was performed in the presence (•) or absence of α2PI (▪).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/11/10.1182_blood.v91.11.4173/4/m_blod41135004ax.jpeg?Expires=1769423003&Signature=DEVQ5tHVNR5zFmJ6PBOrif6n3vqOh5CyLRNEBbGXTmygrPQAJwbiM2B62I3suSzyAduaFaYnr4~mguJlONYL1jcolMbij4KPs2SUCC1e31hb7iZYoiDELpHSVaa4KOWQ7m6I3b9JtmCpR6kuEGjaDI5Gygemb61AOJ-9Lg~MOAl2FBT~~IxdBTztBnACl3g1-389EXPgawUMQmX1EI9LLOXUw0YUIo47qywLHC0kwXqlG7jDfU2bMiSaDBGANJc8xRB5XGUyMp~26oHsQi5Al0IZ2Gcta~2j~aA3WE-kj-65QfSERNfUw54WD-Sneex2cTZDiZCipf5wpc~dDNp95A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. (A) HiTrap Heparin column chromatography patterns of plasmin-treated β2GPI. A total of 660 μg of β2GPI was incubated with 19 μg of plasmin at 37°C, where molar ratio of plasmin to substrate was 1:56. A total of 60 μg of the digest of β2GPI was subjected to HiTrap Heparin column chromatography, as described in Materials and Methods. (B) Time course of the increase of nicked β2GPI (peak 1 in [A]). Horizontal axis and vertical axis correspond to the reaction time (hours) and area of peaks of nicked β2GPI. The experiment was performed in the presence (•) or absence of α2PI (▪).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/11/10.1182_blood.v91.11.4173/4/m_blod41135004bx.jpeg?Expires=1769423003&Signature=xJpq5uuE46TUZiowCjcEXvD62Phh4fwVfX0a5v~VGQZkrwDuawMplBbIECBHzpnw4UclxPYKPMNcY6CNZRBb~rpA~6feza4X42XrIrm9SInmKOkzhDVzIDUyMvykIZC590Qg0~XRMBK-7aOT48~injdt00QQyxcYTSgo5W3CjkB5YW0~-T2gmIcEJ5Coodu8sZDJ06RCsNhphZvw-Geb1jWtQpIoU~a4W25czGqdJLFpz2kv99ReV2QYbM3aZjeoanwlvU6nUhx2d-gdZAp3AP6~lT8J1ld3bu~jRBjUYqClk87e6L1EDJlbu~AUHhvrC1CJdhiPspHpi2vtIQUXrg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)