Abstract
Hemodynamic forces modulate various endothelial cell functions under gene regulation. Previously, we have shown that fibrinolytic activity of endothelial cells is enhanced by the synergistic effects of shear stress and cytokines. In this study, we investigated the effect of shear stress on tumor necrosis factor (TNF)-α–induced tissue factor (TF) expression in cultured human umbilical vein endothelial cells (HUVECs), using a modified cone-plate viscometer. Shear stresses at physiological levels reduced TNF-α (100 U/mL)–induced TF expression at both mRNA and antigen levels, in a shear-intensity and exposure-time dependent manner, whereas shear stress itself did not induce TF expression in HUVECs. TF expressed on the cell surfaces measured by flow cytometry using an anti-TF monoclonal antibody (HTF-K180) was also decreased to one third by shear force applied at 18 dynes/cm2 for 15 hours before and 6 hours after TNF-α stimulation. Furthermore, functional activity of TF, as assessed by the activation of factor X in the presence of FVIIa and Ca2+, was also decreased by shear application. However, the stability of TF mRNA was not decreased in the presence of shear stress. These results suggest that shear force acts as an important regulator of TF expression in endothelium at the transcriptional level.
BY VIRTUE OF THEIR contact with blood flow, endothelial cells are continuously exposed to fluid shear stress, a tangential force generated by the velocity gradient in viscous fluid flow. Recently, several lines of evidence have identified shear stress as an important regulator of the structure and function of endothelial cells.1-3 In addition, some of these flow-induced changes in endothelial function were shown to be regulated at the transcriptional level.1-6 Common promoter elements interacting with shear stress-induced transcriptional factors, known as shear stress responsive element (SSRE) have been reported, including SSRE found in the platelet-derived growth factor (PDGF)-B gene4 and phorbol ester TPA (phorbol 12-tetradecanoate 13-acetate)-responsive elements (TRE) found in the bovine monocyte chemotactic protein (MCP)-1 gene.6 Anti- and prothrombotic properties are also modulated in response to shear stress; prostacyclin (PGI2) synthesis7 and nitric oxide (NO) production8 are enhanced in the presence of shear force, and the expression of NO synthase9 is also upregulated at the transcriptional level. The thrombomodulin (TM) gene is downregulated by shear force in bovine aortic endothelial cells (BAECs),10 whereas shear stress upregulates TM at the mRNA level in human umbilical vein endothelial cells (HUVECs).11In addition, the production of fibrinolytic mediators is also under the control of shear forces. Diamond et al12,13 reported that shear force increases the production of tissue plasminogen activator (t-PA) at the mRNA level. We previously reported that physiological fluid shear stress increased t-PA release and decreased plasminogen activator inhibitor-1 (PAI-1) secretion, and that shear forces further altered the effects of cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α), enhancing t-PA secretion and attenuating the PAI-1 response induced by these cytokines.14 Thus, the interacting effects of shear forces and cytokines can enhance fibrinolytic activity at the transcriptional level in endothelial cells to preserve antithrombogenicity.
Tissue factor (TF) is a single-chain integral membrane protein that binds factor VII, and the complex in the presence of membrane phospholipids cleaves factor X, thus initiating coagulation.15 Under normal conditions, endothelial cells do not express TF and present a nonthrombogenic surface. But when injured or exposed to stimuli such as cytokines, they rapidly become capable of initiating blood coagulation. In this procoagulant state, they synthesize TF as a transmembrane glycoprotein, essential for the initiation of the extrinsic coagulation pathway.16-19Although cytokines induce TF expression on cultured endothelium readily, the expression of TF mRNA and proteins in endothelial cells was reported to be absent in vivo even in the atherosclerotic plaque.20 Only restricted expression of TF in endothelial cells in vivo has been detected under unusual conditions, such as lethal sepsis21 or invasive breast cancer.22This discrepancy between observations in vitro and in vivo suggests the existence of endogenous inhibitory factor(s) for TF expression in vivo.
Grabowski et al23 first reported the effect of flow conditions on the functional expression of TF and showed that flow directly augments the functional activity of TF by monolayers of fibroblasts, but not endothelial cells, in which TF activity is normally limited even after activation by cytokines, in large part because of TF pathway inhibitors (TFPI). Recently, Lin et al24 reported that application of shear stress induced a transient increase of TF procoagulant activity in HUVECs, which was accompanied by a rapid and transient induction of mRNA. However, TF expression on cytokine-stimulated endothelium under shear conditions has been poorly understood. To investigate whether TF expression is altered by shear forces in cultured HUVECs in the presence of cytokines, we analyzed the effect of shear stress on TNF-α–induced TF expression in these cells using a modified cone-plate viscometer. We found that TNF-α–induced expression of TF antigen, activity, and mRNA is suppressed by shear stress, suggesting that shear force acts as an intrinsic modulator of TF expression in endothelium.
MATERIALS AND METHODS
Cell culture.
HUVECs were isolated by the method previously described.14Briefly, cells from two or three umbilical cords were pooled and cultured in M199 medium (GIBCO BRL, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS; Seromex, Vilshofen, Germany), 30 μg/mL of endothelial cell growth supplement (ECGS; Collaborative Research Inc, Bedford, MA) and 6 U/mL of heparin sodium (Shimizu Pharmaceutical, Inc, Shizuoka, Japan), and penicillin/streptomycin (GIBCO-BRL). Cells were incubated at 37°C in a humidified atmosphere of 5% CO2/95% air. For all experiments, confluent monolayers at second passage were grown in 35-mm culture dishes coated with type IV collagen (200 μg/mL; Iwaki, Inc, Chiba, Japan). TF expression was induced by treatment with 100 U/mL of TNF-α (Genzyme Inc, Cambridge, MA).
Shear stress apparatus.
A modified cone-plate type viscometer, originally developed for measurement of shear stress-induced platelet aggregation,25 26 was used. The cone-plate chamber was composed of a rotating cone (30 mm in diameter and 1 degree in cone angle) made of polymethylmethacrylate and a base plate into which a 35-mm culture dish could be fitted. The distance from the cone apex to the bottom of the culture dish was adjusted to 0.004 cm by a micrometer screw. Shear rate (γ) was calculated according to the formula γ = 6N/θ, where N was the rotational speed of the cone and θ was the cone angle. Shear stress was calculated by multiplying the shear rate by the viscosity of the fluid, assumed to be 0.01 poise for the culture media we used. The cone was rotated at 1.67, 3.33, 5.0, and 6.67 revolutions per second to generate shear stresses of 6, 12, 18, and 24 dynes/cm2, respectively. For the experiments where HUVECs were stimulated with TNF-α after preexposure to shear stress, shear application was briefly stopped for the TNF-α addition and then restarted.
TF mRNA semiquantification.
TF mRNA levels were semiquantified by a reverse transcriptase-polymerase chain reaction (RT-PCR) Southern blotting method. The primers used for amplification of TF mRNA were: 5′ATTCAGTGGGGAGTTCTCCTTCCAGCTCTG3′ (antisense primer), corresponding to nucleotides 925 to 954; and 5′ACTACTGTTTCAGTGTTCAAGCAGTGATTC3′ (sense primer), corresponding to nucleotides 722 to 751, according to Scarpati et al.27 Primers used for amplification of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA, used as an internal standard, were: 5′CAAAGTTGTCATGGATGACC3′ (antisense primer), corresponding to nucleotides 480 to 499; and 5′CCATGGAGAAGGCTGGGG3′ (sense primer), corresponding to nucleotides 305-322, according to Tso et al.28 After the HUVECs were washed with ice-cold phosphate-buffered saline without calcium and magnesium ions (PBS[−]), total cellular RNA was extracted by the acid guanidium thiocyanate-phenol-chloroform method.29 Reverse transcription of mRNA for TF and GAPDH was performed in a 50-μL reaction mixture containing 1.5 μg of total RNA, 500 nmol/L antisense primers for TF and GAPDH, four deoxynucleotide triphosphates (500 μmol/L each), and 500 U of Moloney murine leukemia virus (MMLV) reverse transcriptase in a reaction buffer of 50 mmol/L Tris-HCl (pH 8.3), 75 mmol/L KCl, 3 mmol/L MgCl 2, and 4 mmol/L dithiothreitol, for 1 hour at 37°C. The resulting cDNAs for TF and GAPDH were coamplified by PCR for 25 cycles using a DNA thermal cycler (Perkin Elmer Cetus, Norwalk, CT). PCR was performed in a 100-μL reaction volume containing 500 nmol/L sense primers, 50 μg/mL of bovine serum albumin (BSA) and 2 U of Taq DNA polymerase. Each cycle consisted of 1.5 minutes of denaturing at 94°C, 1.5 minutes of annealing at 50°C, and 3 minutes of primer extension at 72°C. After amplification, each reaction mixture was separated by agarose gel electrophoresis and blotted to nitrocellulose membranes (0.2-μm pore size). Prehybridization and hybridization were performed at 65°C as described previously.30 Random-primed32P-labeled 660-bp NcoI/HindIII fragments of human TF cDNA and 800-bp XbaI/PstI fragments of human GAPDH cDNA (provided by the American Type Culture Collection, Manassas, VA) were used as probes. After hybridization, blots were washed twice in 15 mmol/L NaCl, 1.5 mmol/L sodium citrate, and 0.1% sodium dodecyl sulfate (SDS) at 65°C for 30 minutes and exposed to x-ray film. Autoradiograms were scanned by a densitometer (DM-303; Advantec Toyo co.ltd., Tokyo, Japan), and the signal strength of each TF mRNA was normalized to the corresponding GAPDH mRNA.
TF antigen measurement.
After being washed with ice-cold PBS(−), HUVECs were lysed in lysis buffer (50 mmol/L Tris-HCl [pH 8.6], 0.5% Triton X-100, 5 mmol/L EDTA, and 6 mmol/L N-ethylmaleimide). Cell lysates were centrifuged to remove nuclei and cytoskeletons, and supernatant was used for TF antigen determination. TF antigen levels were determined by a highly sensitive sandwich enzyme-linked immunosorbent assay (ELISA).31 32 First, 96-well plates were coated with a monoclonal antihuman TF antibody, HTF-K14, and blocked with BSA. After TF sample incubation, a second monoclonal antihuman TF antibody, HTF-K180, conjugated with horseradish peroxidase (HRP), was added to each well. Then, the sensitivity of the assay was amplified by the addition of biotinyl-tyramide/H2O2 followed by incubation with HRP-conjugated streptavidin. After washing, 2,2′-azino-bis(3-ethylbenz-thiazoline-6-sulfonic acid; ABTS)/H2O2 was added as a substrate. The reaction was stopped with 0.01% NaN3/0.1 mol/L citric acid and absorbance at 420 nm was measured with the reference absorbance set at 630 nm.
Flow cytometry.
After being washed with ice-cold washing buffer (PBS[−] containing 0.1% NaN3, 1 mmol/L EDTA and 0.5% BSA), HUVECs were incubated with ice-cold PBS(−) containing 10 mmol/L EDTA and 0.5% BSA, and detached by gentle pipetting on ice. After centrifugation, cells were resuspended with ice-cold washing buffer and dispersed into single cells using a syringe connected to a 26G needle. Then, cells were incubated with aggregated human IgG for Fc receptor blocking, treated with HTF-K180, and washed twice with washing buffer. After incubation with fluorescein isothiocyanate (FITC)-labeled antimouse IgG (Tago, Inc, Burlingame, CA), cells were washed twice, fixed with 2% paraformaldehyde, and washed again. Flow cytometric analysis was performed by FACscan (Becton Dickinson, San Jose, CA).
Measurement of TF functional activity.
TF activities of the static, sheared, TNF-stimulated, or TNF-stimulated and sheared HUVECs were assayed by measuring the enzymatic activation of factor X by the TF/factor VIIa complex. Monolayers of HUVECs were washed with Hanks' Balanced Salt Solution, followed by addition of a reaction buffer containing 2 mmol/L Ca2+, 20 nmol/L factor VIIa, and 200 nmol/L factor X (Enzymatic Research Laboratories, South Bend, IN). The reaction time was 20 minutes before the addition of a stopping buffer containing 7.5 mmol/L EDTA. With the addition of the chromogen, S-2222 (Chromogenix AB, Mölndal, Sweden), aliquots of samples were assayed for their absorbance at 405 nm with the reference absorbance set at 492 nm. The formation rates of factor Xa in various samples were calculated, based on a standard curve, which had been established by using known amounts of factor Xa (Enzymatic Research Laboratories). The production of free chromophore in femtomoles per square centimeter of monolayer per minute was calculated. To confirm the specificity of the process for TF, HUVECs were preincubated for 30 minutes with antihuman TF monoclonal antibody (10 μg/mL of HTF-K14) or goat antihuman TF polyclonal antibody (15 μg/mL; American Diagnostic Inc, Greenwich, CT). In some experiments, factor Xa generation was measured in a cell-free system, in which the reaction was performed in 35-mm culture dishes without HUVECs.
Nuclear and cytoplasmic protein extraction.
After being washed with ice-cold PBS(−), HUVECs were incubated in 200 μL of lysis buffer (10 mmol/L Tris-HCl [pH 8.0], 60 mmol/L KCl, 1 mmol/L EDTA, 1 mmol/L dithiothreitol, 0.5% Nonidet P-40 [NP-40], and 1 mmol/L phenylmethylsulfonyl fluoride) on ice for 5 minutes. Then, lysates were scraped and spun at 400g at 4°C for 4 minutes. The supernatant was removed and respun at 13,000g for 5 minutes. The supernatants were used as cytoplasmic extracts. The pelleted nuclei from the first spin were briefly washed in lysis buffer without NP-40. The nuclear pellet was then resuspended in 50 μL of nuclear extract buffer (20 mmol/L Tris-HCl [pH 8.0], 420 mmol/L NaCl, 1.5 mmol/L MgCl2, 0.2 mmol/L EDTA, and 25% glycerol). After a 10-minute incubation at 4°C, the nuclei were briefly vortexed and spun at 13,000gfor 5 minutes. The supernatant was used as the nuclear extract.
Western blotting of p65 subunit of NFκB and IκBα.
Aliquots of cytoplasmic and nuclear extracts were separated by SDS-polyacrylamide gel electrophoresis with 10% running gels. The separated proteins were transferred to polyvinylidene difluoride (PVDF) membranes (Clear Blot Membrane-P; Atto, Tokyo, Japan). The membranes were blocked in 5% bovine albumin for 1 hour, and then incubated with 1 μg/mL detecting antibody for 1 hour. The antibodies used were rabbit anti-NFκB p65 subunit polyclonal antibody and anti-IκBα polyclonal antibody (Chemicon International Inc, Temecula, CA) for nuclear and cytoplasmic extracts, respectively. The membranes were washed with Tris-buffered saline (TBS; 10 mmol/L Tris-HCl [pH 8.0], 150 mmol/L NaCl) for 1 hour and further incubated with alkaline phosphatase-conjugated goat antirabbit IgG (1:1000; Dako, Glostrup, Denmark) for 1 hour. After the membranes were washed with TBS for 1 hour, the substrate nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate (GIBCO BRL) was used to develop the color.
Data analysis.
For comparisons between two groups, statistical analysis was performed using the Student's t-test. For multiple comparisons, analysis of variance (ANOVA) followed by the Dunnett test was used. Differences were considered significant at P < .05.
RESULTS
Effect of shear stress on the TNF-α–induced increase in TF mRNA.
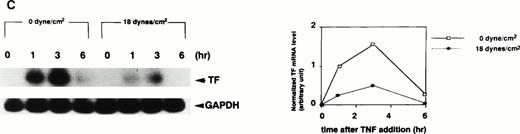
TF mRNA was undetectable in static, nonstimulated HUVECs by RT-PCR Southern blotting. However, stimulation of HUVECs with TNF-α (100 U/mL) under static conditions resulted in a rapid and transient increase in TF mRNA, with peak levels at 1 to 3 hours after TNF-α addition (Fig 1). Application of shear stress to HUVECs at 18 dynes/cm2, a physiological level in arteries,3 12 attenuated TNF-α–induced elevation of TF mRNA in an exposure time-dependent manner. When shear application started concomitantly with the addition of TNF-α, only a marginal decrease in TF mRNA was noted (Fig 1A). In this case, the normalized levels of TF mRNA at 1 or 3 hours after TNF-α addition under shear stress were decreased by 9% or 38%, respectively. However, the longer the length of preexposure time, the greater the decrease in TF mRNA expression. When shear application was started at 3 hours before TNF-α addition and continued until RNA sampling, the normalized levels of TF mRNA at 1 or 3 hours after TNF-α addition were decreased by 15% or 46%, respectively (Fig 1B). When shear stress was applied for 15 hours before TNF-α addition and continued until RNA sampling, the normalized levels of TF mRNA at 1 or 3 hours after TNF-α addition, were decreased by 76% or 69%, respectively (Fig 1C). In contrast, application of shear stress itself to HUVECs at 18 dynes/cm2 for 0.5, 1, 2, and 3 hours did not induce any detectable TF mRNA as assessed by RT-PCR Southern blotting.
Effect of preexposure time of shear stress on TNF-α–induced increase in TF mRNA in HUVECs. (A) HUVECs were exposed to shear stress (18 dynes/cm2) or left static for 1, 3, 6, 9, 15, and 24 hours after TNF-α (100 U/mL) addition, and then lysed and processed for RT-PCR Southern blot analysis using32P-labeled TF cDNA. (B) HUVECs were exposed to shear stress (18 dynes/cm2) or left static for 3 hours before and 1, 3, and 6 hours after TNF-α (100 U/mL) addition, and then processed as in (A). (C) HUVECs were exposed to shear stress (18 dynes/cm2) or left static for 15 hours before and 1, 3, and 6 hours after TNF-α (100 U/mL) addition, and then processed as in (A). (Left) Autoradiograms of TF and GAPDH RT-PCR products; (right) densitometric analysis of TF RT-PCR products normalized with respect to corresponding GAPDH RT-PCR product levels.
Effect of preexposure time of shear stress on TNF-α–induced increase in TF mRNA in HUVECs. (A) HUVECs were exposed to shear stress (18 dynes/cm2) or left static for 1, 3, 6, 9, 15, and 24 hours after TNF-α (100 U/mL) addition, and then lysed and processed for RT-PCR Southern blot analysis using32P-labeled TF cDNA. (B) HUVECs were exposed to shear stress (18 dynes/cm2) or left static for 3 hours before and 1, 3, and 6 hours after TNF-α (100 U/mL) addition, and then processed as in (A). (C) HUVECs were exposed to shear stress (18 dynes/cm2) or left static for 15 hours before and 1, 3, and 6 hours after TNF-α (100 U/mL) addition, and then processed as in (A). (Left) Autoradiograms of TF and GAPDH RT-PCR products; (right) densitometric analysis of TF RT-PCR products normalized with respect to corresponding GAPDH RT-PCR product levels.
Effect of shear stress on stability of TF mRNA.
To investigate whether the attenuation of TF mRNA by shear force is dependent on changes in the stability of mRNA, we examined the stability of TF mRNA in the presence or absence of shear stress. After treatment of HUVECs with TNF-α for 3 hours to induce maximum levels of TF mRNA, the cells were either exposed to shear stress (18 dynes/cm2) or left static, with the addition of actinomycin D (10 μg/mL) to the culture medium. Then, total RNA was collected at 30-minute intervals and processed for RT-PCR Southern blotting. As shown in Fig 2A, a slight increase in mRNA stability was noted in the presence of shear force. Because the attenuation of TF mRNA by shear stress is dependent on the length of preexposure time (Fig 1), we also examined TF mRNA stability under conditions where shear application of 18 dynes/cm2 was started 3 hours before TNF-α stimulation and continued until RNA sampling. In this measurement, actinomycin D was added 3 hours after TNF-α addition and total RNA was collected at 0, 15, 30, 60, and 120 minutes after actinomycin D addition. As expected from the result of Fig 1B, the normalized TF mRNA level in sheared cells at time 0 (when actinomycin D was added to the medium) was nearly half of that in control cells (Fig 2B). However, the declining pattern of TF mRNA levels in sheared cells was similar to that of control cells. Thus, the stability of TF mRNA after TNF-α stimulation was not lowered in the presence of shear stress.
Effect of shear stress on stability of TF mRNA in HUVECs. (A) After treatment of HUVECs with TNF-α (100 U/mL) for 3 hours, cells were exposed to shear stress (18 dynes/cm2) or left static for 0.5, 1, 1.5, and 2 hours in the presence of actinomycin D (10 μg/mL), and then processed as in Fig 1. (B) HUVECs were exposed to shear stress (18 dynes/cm2) or left static for 3 hours before and 3 hours after TNF-α (100 U/mL) addition and further 0, 0.25, 0.5, 1, and 2 hours in the presence of actinomycin D (10 μg/mL), and then processed as in Fig 1. Data represent mean ± standard deviation (SD) of two separate experiments. (Left) The representative data from autoradiogram of TF and GAPDH RT-PCR products; (right) densitometric analysis of TF RT-PCR products normalized with respect to corresponding GAPDH RT-PCR product levels.
Effect of shear stress on stability of TF mRNA in HUVECs. (A) After treatment of HUVECs with TNF-α (100 U/mL) for 3 hours, cells were exposed to shear stress (18 dynes/cm2) or left static for 0.5, 1, 1.5, and 2 hours in the presence of actinomycin D (10 μg/mL), and then processed as in Fig 1. (B) HUVECs were exposed to shear stress (18 dynes/cm2) or left static for 3 hours before and 3 hours after TNF-α (100 U/mL) addition and further 0, 0.25, 0.5, 1, and 2 hours in the presence of actinomycin D (10 μg/mL), and then processed as in Fig 1. Data represent mean ± standard deviation (SD) of two separate experiments. (Left) The representative data from autoradiogram of TF and GAPDH RT-PCR products; (right) densitometric analysis of TF RT-PCR products normalized with respect to corresponding GAPDH RT-PCR product levels.
Effect of shear stress on TF antigen levels in cell lysates.
Under static (0 dynes/cm2) conditions, the TF antigen level in nontreated cells was very low (137.6 ± 34.3 pg/106cells), TF antigen increased to 236.0 ± 47.1 pg/106cells at 1 hour after TNF-α stimulation, and reached a maximum of 1,660.9 ± 276.2 pg/106 cells after 6 hours of TNF-α stimulation. Thus, stimulation of HUVECs with TNF-α resulted in a drastic increase in the TF antigen level in the cell lysate, which could be detected as early as 1 hour after TNF-α stimulation (Fig 3A). Application of shear stress, at 18 dynes/cm2 started 15 hours before TNF-α addition and continued until sampling, attenuated the increase in TF antigen level in a statistically significant manner (236.0 ± 47.1 pg/106 cells v 99.6 ± 41.6 pg/106cells at 1 hour, 1,660.9 ± 276.2 pg/106 cells v782.7 ± 122.7 pg/106 cells at 6 hours after TNF-α addition; P < .05 at all the times examined here). Furthermore, when shear stress was applied to HUVECs with increasing intensity from 15 hours before to 6 hours after the addition of TNF-α, shear-intensity–dependent attenuation of the TF antigen level was observed (Fig 3B). This inhibition was also statistically significant (1,482.5 ± 99.3 pg/106 cells at 0 dynes/cm2v 350.1 ± 94.1 pg/106cells at 18 dynes/cm2; P< .01 at all shear intensities). In contrast, the application of shear stress itself at 18 dynes/cm2 did not increase TF antigen levels in cell lysates (data not shown).
Effect of shear stress on TF antigen levels in cell lysate. (A) HUVECs were exposed to shear stress (18 dynes/cm2) or left static for 15 hours before and 1, 3, 6, and 9 hours after TNF-α (100 U/mL) addition, and TF antigen levels in cell lysate were measured by ELISA methods. Data represent mean ± SD of three separate experiments. *, Significantly different from static control at the same time points (P < .05, Student's t-test). **, Significantly different from static control at the same time points (P < .01, Student'st-test). (□), 0 dyne/cm2; (•), 18 dynes/cm2. (B) HUVECs were exposed to shear stress (6, 12, 18, and 24 dynes/cm2) or left static for 15 hours before and 6 hours after TNF-α (100 U/mL) addition, and TF antigen levels in cell lysate were measured by ELISA methods. Data represent mean ± SD of three separate experiments. ***, Significantly different from static control treated with TNF-α (P < .01, ANOVA-Dunnett test).
Effect of shear stress on TF antigen levels in cell lysate. (A) HUVECs were exposed to shear stress (18 dynes/cm2) or left static for 15 hours before and 1, 3, 6, and 9 hours after TNF-α (100 U/mL) addition, and TF antigen levels in cell lysate were measured by ELISA methods. Data represent mean ± SD of three separate experiments. *, Significantly different from static control at the same time points (P < .05, Student's t-test). **, Significantly different from static control at the same time points (P < .01, Student'st-test). (□), 0 dyne/cm2; (•), 18 dynes/cm2. (B) HUVECs were exposed to shear stress (6, 12, 18, and 24 dynes/cm2) or left static for 15 hours before and 6 hours after TNF-α (100 U/mL) addition, and TF antigen levels in cell lysate were measured by ELISA methods. Data represent mean ± SD of three separate experiments. ***, Significantly different from static control treated with TNF-α (P < .01, ANOVA-Dunnett test).
Effect of shear stress on TF antigen levels on the cell surface.
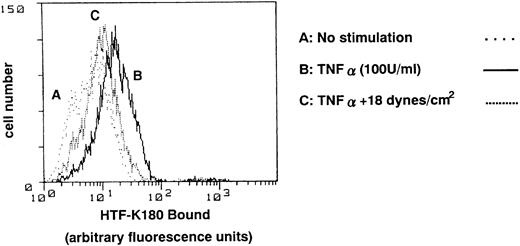
To investigate whether TF antigen is expressed on the cell surface, flow cytometric analysis was performed three times, using the monoclonal antibody, HTF-K180. Representative data is shown in Fig 4. Under static (0 dynes/cm2) conditions, stimulation of HUVECs with TNF-α (6 hours) resulted in an increase in TF antigen on the cell surface with a mean fluorescence ratio of 20.03 (Fig 4, profile B), as compared with 8.28 in nontreated cells (Fig 4, profile A). Application of shear stress, at 18 dynes/cm2 started 15 hours before TNF-α addition and continued until sampling, attenuated the increase in TF antigen levels, with a mean fluorescence ratio of 11.73 (Fig 4, profile C). Application of shear stress at 18 dynes/cm2 itself did not increase TF antigen on the cell surface, with a mean fluorescence ratio of 8.08, the same as that seen in nontreated cells.
Effect of shear stress on TF antigen levels on cell surface of HUVECs. HUVECs were exposed to shear stress (18 dynes/cm2) or left static for 15 hours before and 6 hours after TNF-α (100 U/mL) addition, and TF antigen levels on the cell surface were analyzed by flow cytometry using antihuman TF monoclonal antibody, HTF-K180. Representative plots from three separate experiments with essentially the same results. Profile A, nontreated cells; profile B, TNF-α–treated cells; profile C, presheared and sheared TNF-α–treated cells.
Effect of shear stress on TF antigen levels on cell surface of HUVECs. HUVECs were exposed to shear stress (18 dynes/cm2) or left static for 15 hours before and 6 hours after TNF-α (100 U/mL) addition, and TF antigen levels on the cell surface were analyzed by flow cytometry using antihuman TF monoclonal antibody, HTF-K180. Representative plots from three separate experiments with essentially the same results. Profile A, nontreated cells; profile B, TNF-α–treated cells; profile C, presheared and sheared TNF-α–treated cells.
Effect of shear stress on TF procoagulant activity.
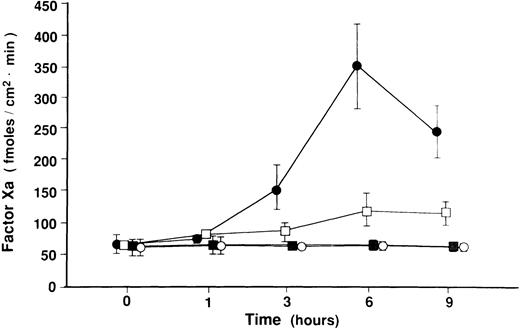
To investigate whether shear stress affects TF functional activity, HUVECs were loaded with shear stress alone at 18 dynes/cm2, or treated with TNF-α in the presence or absence of steady shear stress for various periods of time, followed by chromogenic assays, in which we assayed the conversion of factor X to Xa, as a measure of TF procoagulant activity, on the surface of HUVECs. This factor Xa generation rate reflects TF activity, as the factor Xa generation was inhibited by 80% in HUVECs preincubated with 10 μg/mL of HTF-K14 or 15 μg/mL of goat antihuman TF polyclonal antibody. Factor Xa generation in cell-free system without HUVECs was 68.9 ± 22.7 fmoles/cm2minute in the reaction buffer containing Ca2+, factor VIIa, and factor X, and 27.5 ± 9.2 fmoles/cm2minute in the buffer in which factor VIIa was omitted. Figure 5 indicates that the application of shear stress itself did not induce procoagulant activity on the cell surface (61.4 ± 27.6 fmoles/cm2minute at static cells v 65.1 ± 14.6 fmoles/cm2minute at 6 hours after shear stress applied). When HUVECs were stimulated with TNF-α, factor Xa generation increased sharply to 157.5 ± 61.2 fmoles/cm2minute at 3 hours, reached a maximum of 350.3 ± 116.3 fmoles/cm2minute at 6 hours, and then declined to 244.8 ± 71.9 fmoles/cm2minute after 9 hours of TNF-α stimulation. In contrast, application of shear stress, at 18 dynes/cm2, started 15 hours before TNF-α addition and continued until sampling, attenuated the increase in factor Xa generation in a statistically significant manner (350.3 ± 116.3 fmoles/cm2minute at 0 dynes/cm2v 122.8 ± 46.5 fmoles/cm2minute at 18 dynes/cm2after 6 hours of TNF-α stimulation; P < .01 at 3, 6, 9 hours).
Effect of shear stress on TF procoagulant activity of HUVECs. HUVECs were exposed to shear stress (18 dynes/cm2) or left static for 15 hours before and 1, 3, 6, and 9 hours after TNF-α (100 U/mL) addition. HUVECs were also exposed to shear stress itself (18 dynes/cm2) for 1, 3, 6, and 9 hours or left static. In four different conditions, TF procoagulant activity was measured by chromogenic assay as assessed by factor Xa generation on monolayers of HUVECs. Data represent mean ± standard error (SE) of three separate experiments from TNF-α (100 U/mL)–stimulated cells and four separate experiments from nonstimulated cells. (•), TNF-stimulated cells; (□), presheared and sheared TNF-stimulated cells; (▪), sheared, nonstimulated cells; (○), static, nonstimulated cells.
Effect of shear stress on TF procoagulant activity of HUVECs. HUVECs were exposed to shear stress (18 dynes/cm2) or left static for 15 hours before and 1, 3, 6, and 9 hours after TNF-α (100 U/mL) addition. HUVECs were also exposed to shear stress itself (18 dynes/cm2) for 1, 3, 6, and 9 hours or left static. In four different conditions, TF procoagulant activity was measured by chromogenic assay as assessed by factor Xa generation on monolayers of HUVECs. Data represent mean ± standard error (SE) of three separate experiments from TNF-α (100 U/mL)–stimulated cells and four separate experiments from nonstimulated cells. (•), TNF-stimulated cells; (□), presheared and sheared TNF-stimulated cells; (▪), sheared, nonstimulated cells; (○), static, nonstimulated cells.
Effects of acetyl salicyclic acid (ASA) and Nw-nitro-L-arginine methyl ester (L-NAME) on shear-induced TF mRNA attenuation.
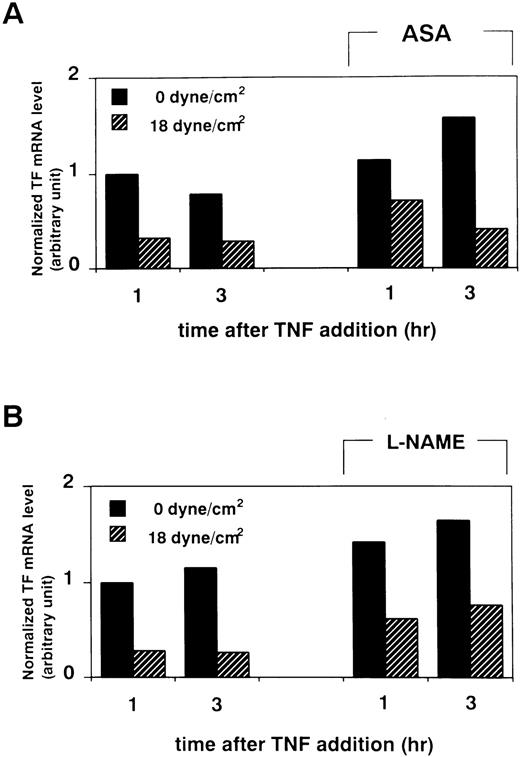
It has been shown that shear application increases the production of PGI27 and NO8 by endothelial cells. Therefore, it is possible that these autacoids participate indirectly in the shear-induced reduction of TF expression. To investigate this issue, we examined the effects of ASA (Sigma, St Louis, MO), an inhibitor of cyclooxygenase, and L-NAME (Sigma), an inhibitor of nitric oxide synthase, on shear stress-induced attenuation of TF expression. When we treated HUVECs with ASA (1 mmol/L) for 30 minutes before shear application (at 18 dynes/cm2, from 15 hours before to 6 hours after TNF-α addition), the attenuating effect of shear stress on TF mRNA elevation was not abolished (Fig 6A). Similarly, when HUVECs were exposed to shear stress in the presence of 100 μmol/L L-NAME, the attenuating effect of shear stress on TF mRNA elevation was still observed (Fig 6B).
Effect of treatment with ASA or L-NAME on shear-induced attenuation in TF mRNA level of HUVECs. (A) After pretreatment with ASA (1 mmol/L) for 30 minutes, HUVECs were exposed to shear stress (18 dynes/cm2) or left static for 15 hours before and 1 and 3 hours after TNF-α (100 U/mL) addition, and then processed as in Fig1. (B) HUVECs were exposed to shear stress (18 dynes/cm2) or left static for 15 hours before and 1 and 3 hours after TNF-α (100 U/mL) addition in the presence of L-NAME (100 μmol/L), and then processed as in Fig 1. Results of densitometric analysis of TF RT-PCR products normalized with respect to corresponding GAPDH RT-PCR product levels are shown.
Effect of treatment with ASA or L-NAME on shear-induced attenuation in TF mRNA level of HUVECs. (A) After pretreatment with ASA (1 mmol/L) for 30 minutes, HUVECs were exposed to shear stress (18 dynes/cm2) or left static for 15 hours before and 1 and 3 hours after TNF-α (100 U/mL) addition, and then processed as in Fig1. (B) HUVECs were exposed to shear stress (18 dynes/cm2) or left static for 15 hours before and 1 and 3 hours after TNF-α (100 U/mL) addition in the presence of L-NAME (100 μmol/L), and then processed as in Fig 1. Results of densitometric analysis of TF RT-PCR products normalized with respect to corresponding GAPDH RT-PCR product levels are shown.
Effect of shear stress on nuclear translocation of NFκB.
A transcription factor, NFκB, has been shown to play an important role in the induction of TF expression in many cells. In cytokine-stimulated endothelial cells, c-Rel/p65 heterodimer translocates into the nucleus after dissociation from its cytoplasmic inhibitor, IκB, and induces TF gene expression. Therefore, we examined whether shear forces affect this nuclear translocation of NFκB, using Western blotting. TNF-α stimulation of HUVECs resulted in nuclear translocation of p65 subunit in 30 minutes (Fig 7A, lanes 1 and 2). However, shear application at 18 dynes/cm2 started 15 hours before TNF-α addition and continued until protein extraction had no effects on this TNF-α–induced translocation of p65 subunits (Fig 7A, lanes 3 and 4). In addition, short-term (30 minutes) exposure of HUVECs to shear stress alone did not stimulate translocation of the p65 subunit into the nucleus of HUVECs (Fig 7A, lane 5). In cytoplasmic extracts, a corresponding reciprocal degradation pattern of IκBα was noticed (Fig 7B).
Effect of shear stress on nuclear translocation of NFκB. (A) Nuclear extract of HUVECs was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to PVDF membrane by Western blotting. Then, p65 subunit of NFκB was detected with rabbit anti-p65 polyclonal antibody. (B) Cytoplasmic extract of HUVECs was separated by SDS-PAGE and transferred to PVDF membrane by Western blotting. Then, IκBα was detected with rabbit anti-IκBα polyclonal antibody. Lane 1, static control; lane 2, cells stimulated with TNF-α (100 U/mL) for 30 minutes; lane 3, cells exposed to shear stress at 18 dynes/cm2 for 15.5 hours; lane 4, cells exposed to shear stress at 18 dynes/cm2 for 15 hours and then stimulated with TNF-α (100 U/mL) and sheared for a further 30 minutes; lane 5, cells exposed to shear stress at 18 dynes/cm2 for 30 minutes.
Effect of shear stress on nuclear translocation of NFκB. (A) Nuclear extract of HUVECs was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to PVDF membrane by Western blotting. Then, p65 subunit of NFκB was detected with rabbit anti-p65 polyclonal antibody. (B) Cytoplasmic extract of HUVECs was separated by SDS-PAGE and transferred to PVDF membrane by Western blotting. Then, IκBα was detected with rabbit anti-IκBα polyclonal antibody. Lane 1, static control; lane 2, cells stimulated with TNF-α (100 U/mL) for 30 minutes; lane 3, cells exposed to shear stress at 18 dynes/cm2 for 15.5 hours; lane 4, cells exposed to shear stress at 18 dynes/cm2 for 15 hours and then stimulated with TNF-α (100 U/mL) and sheared for a further 30 minutes; lane 5, cells exposed to shear stress at 18 dynes/cm2 for 30 minutes.
DISCUSSION
It has been shown that TF expression can be induced in cultured endothelial cells with many kinds of agents, such as inflammatory cytokines,17 lipopolysaccharide (LPS),18,19 or phorbol esters.19 However, Wilcox et al20reported that TF expression at the mRNA and antigen levels was not detected in endothelial cells in vivo, even in atherosclerotic plaques, whereas macrophages/monocytes overtly expressed TF, according to in situ and in vivo immunohistochemistry studies. The expression of TF in endothelial cells in vivo has been shown in very restricted vascular beds under unusual conditions, such as in the splenic microvasculature of baboon lethal sepsis model21 and in the endothelial cells of malignant infiltrating intraductal breast cancer in humans.22 The factor responsible for this discrepancy between in vitro experimental condition and in vivo observations was not known. Our results clearly show that shear forces act as an inhibitory regulator for TNF-α–induced TF expression of both protein and mRNA levels. The longer the preexposure time to constant shear stress, the greater the decrease in TF mRNA expression, suggesting that endothelial cells continuously exposed to steady laminar flow in vivo could be resistant to induction of TF expression by cytokines. In addition, flow immunocytometric analysis confirmed that cell-surface TF antigen levels induced by TNF-α were attenuated by shear forces and declined in parallel with TF mRNA levels. Furthermore, the increase in the procoagulant activity of TF, measured as the conversion rate of factor X to factor Xa in the presence of factor VIIa and Ca2+, was also attenuated by shear application, indicating the physiological significance of the shear-induced suppression of TF expression. Although the effect of shear force on coagulation activity of preexisting TF was investigated previously,23 this is the first study to examine the effect of shear stress on cytokine-induced TF expression per se in endothelial cells.
The effects of shear stress alone on TF procoagulant activity have been investigated in vitro. Gemmell et al33 have observed increased production of factor Xa under shear forces in a nonbiologic system that incorporated TF in a lipid bilayer immobilized on the inner surface of a glass capillary tube. Grabowski et al23 showed that although flow directly augments the Xa production, as a functional expression of TF, by monolayers of fibroblasts, it has little effect on Xa production by HUVECs. They found, however, that Xa generation was augmented under applied shear stress, in the presence of an antibody directed against TFPI. Recently, Lin et al24reported that shear stress at 12 dynes/cm2 induced a transient increase in TF procoagulant activity in HUVECs, which was accompanied by a rapid and transient induction of TF mRNA. However, in our study, shear stress at 18 dynes/cm2 did not induce the expression of TF mRNA, antigen, or procoagulant activity in HUVECs. This difference might be attributed to the differences in the experimental systems, such as culture medium or the shear-loading apparatus. Further studies are needed to clarify the regulation of TF procoagulant activities and the role of TFPI under shear forces, because endothelial cells appear to be the major site of synthesis of TFPI.34
The relationship between the stability of TF mRNA and TF expression in monocytes and HUVECs is controversial. Crossmen et al19showed that the rapid accumulation of TF mRNA in HUVECs stimulated by LPS is largely a result of an increase in mRNA stability, although Brand et al35 showed that the LPS-induced accumulation of TF mRNA levels in monocytic cells is accompanied by both transcriptional and posttranscriptional control mechanisms. We observed that the stability of TF mRNA in TNF-α–stimulated HUVECs was not decreased in the presence of shear stress, even when shear exposure was started at 3 hours before TNF stimulation, suggesting that the suppression might occur at the transcriptional level.
It has been reported that shear stress increases the production of PGI27 and NO8 by endothelial cells, and Crutchley et al36 reported that the prostacyclin analogs can inhibit TF expression with no apparent effect on TF mRNA stability. To investigate the possibility that these autacoids increased by shear forces could participate in attenuation of TF expression indirectly, we conducted the experiments using ASA as a cyclooxygenase inhibitor or L-NAME as an NO synthase inhibitor. Although the baseline levels of TF mRNA slightly increased in the presence of ASA (1 mmol/L) or L-NAME (100 μmol/L), the attenuating effect of shear stress on TF mRNA elevation was maintained. Thus, the inhibitory effect of shear stress on TF expression appears to be independent of PGI2 or NO.
Recent studies regarding the regulation of TF gene expression in cytokine-stimulated endothelial cells have suggested that NFκB plays a central role as a transcriptional factor, in that c-Rel/p65 can translocate into the nucleus after its dissociation from IκB.37-39 In addition, other transcriptional factors, AP-1 and Sp1, are also shown to be involved in TF gene expression. Interestingly, these transcriptional factors have been reported to mediate shear-induced gene expression. NFκB is shown to participate in shear stress-induced transcriptional activation of the PDGF-B chain gene in BAECs, as one of the nuclear proteins binding to the cis-acting shear stress responsive element within the PDGF-B chain promoter is NFκB.40 Shyy et al6 reported that phorbol ester TPA-responsive elements (TRE), to which AP-1 binds, are responsible for bovine MCP-1 gene expression. Moreover, Lan et al41 showed that shear stress stimulates the DNA binding activities of NFκB and AP-1 in BAECs. In contrast, Ando et al42 reported that TRE are responsible for shear-induced decreases in vascular cell adhesion molecule (VCAM)-1 gene expression in mouse endothelial cells. These observations suggest that one element could be either a positive or a negative responsive element for gene expression under different conditions. However, in our study shear stress neither stimulated translocation of NFκB into the nucleus in HUVECs nor inhibited TNF-α–induced translocation of NFκB. The molecular mechanism responsible for shear-induced attenuation of TF expression in HUVECs needs to be elucidated in further studies.
Previously, we have shown the interacting effects of shear force and cytokines on fibrinolytic systems; specifically, shear force alters the effects of cytokines, enhancing t-PA secretion and attenuating PAI-1 response induced by cytokines.14 The present study also showed that cytokine-induced TF expression can be attenuated by shear force. Taken together, these observations suggest that hemodynamic forces can modulate the action of inflammatory cytokines on endothelial properties. It is interesting that this modulation can be bidirectional, ie, shear stress attenuates cytokine-induced expression of PAI-1 and TF, whereas it synergistically enhances t-PA synthesis in endothelial cells. This suggests the existence of complex mutual cross-talk between the signaling pathways used by shear forces and inflammatory cytokines. It is likely that laminar shear stress in the physiological range acts on endothelial cells to render numerous functions antithrombotic in a cooperative way. The idea that constant laminar shear stress is indispensable for antithrombotic status in the endothelium might be supported by the observation that thrombosis/atherosclerosis-prone regions in blood vessels are located exclusively at flow dividers or curvatures, where shear stress is supposed to be small and irregular.1 Therefore, hemodynamic forces borne by blood flow might play a pivotal role, via modulation of endothelial functions, in maintaining antithrombogenicity.
Supported in part by Grants-in-Aid for Scientific Research (No. 07672500 to Y.K. and No. 08457641 to K.W.) from the Ministry of Education, Science, and Culture of Japan, and National Grant-in-Aid for the Establishment of High-Tech Research Center in a Private University of Japan, and Research Foundation for Traffic-Preventive Medicine in Japan.
Address reprint requests to Yohko Kawai, MD, Department of Laboratory Medicine, Keio University School of Medicine, 35 Shinanomachi, Shinjuku-ku, Tokyo 160, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal