Abstract
Moderate elevation of plasma total homocysteine (tHcy) is a strong and independent risk factor for coronary artery disease (CAD). It can result from genetic or nutrient-related disturbances in the transsulfuration or remethylation pathways for Hcy metabolism. A point mutation (C677T; Ala-to-Val) in the gene encoding the 5,10-methylenetetrahydrofolate reductase (MTHFR) has been recently reported to render the enzyme thermolabile and less active. Studies on the role of this mutation as a risk factor for CAD have given conflicting results. We studied a total of 415 subjects, 278 with angiographically documented multivessel CAD and 137 with angiographically documented normal coronary arteries. The overall frequency of the MTHFR V/V homozygous genotype was 15.7% (with 52.5% heterozygous and 31.8% normal). Subgroup analysis showed no significant differences between CAD and CAD-free subjects. A genotype/phenotype correlation study showed a marked effect of folate on the association between MTHFR genotypes and tHcy. Among individuals with folate levels below the median (11.5 nmol/L), fasting tHcy was significantly increased not only in V/V homozygotes (by 59%) but also, at intermediate values, in A/V heterozygotes (by 21% on average). Conversely, the mutation resulted neutral with respect to tHcy levels in subjects with adequate folate levels. We conclude that, in our population, the MTHFR C677T mutation is rather common, but it does not appear to be associated per se to CAD. A genetic-environmental interaction may contribute to the vascular risk by elevating tHcy when folate status is low.
HOMOCYSTEINE is a non–protein-forming, thiol-containing amino acid that results from the demethylation of the essential amino acid methionine.1 Elevated levels of plasma total homocysteine (tHcy) can result from genetic or nutrient-related disturbances in the two pathways for homocysteine metabolism, remethylation or transsulfuration. In remethylation, the primary methyl donor for the vitamin B12-dependent conversion of Hcy to methionine is 5-methyltetrahydrofolate, which in turn is formed from 5,10 methylenetetrahydrofolate by means of the enzyme methylenetetrahydrofolate reductase (MTHFR). In transsulfuration, Hcy is irreversibly catabolized to cystathionine by means of the vitamin B6-dependent enzyme cystathionine β-synthase (CBS) and eventually excreted as inorganic sulphate. Fasting levels of tHcy reflect mainly the remethylation pathway, whereas transsulfuration is thought to be reflected by the increase in tHcy above fasting levels after an oral methionine load.
In accordance with pioneer investigations,2,3 several recent studies have established that an elevated plasma level of tHcy is an independent risk factor for vascular disease, both in the coronary circulation and elsewhere (reviewed in Ueland et al,4 D'Angelo and Selhub,5 Boers,6and Boushey et al7). With respect to coronary atherosclerotic disease (CAD), the evidence for a detrimental role of tHcy is striking. Based on a meta-analysis considering both case-control and prospective studies, it has been calculated that 10% of the population's risk for CAD may be attributable to elevated tHcy.7 As with cholesterol levels, the risk gradually increases with increasing tHcy levels, and the enhanced risk associated with a 5 μmol/L elevation of tHcy was estimated to be the same as that associated with a 0.5 mmol/L increase in total cholesterol. More recently, a prospective study found a strong, graded association between tHcy and mortality in 587 patients with angiographically confirmed CAD.8 By virtue of this background, investigation of the determinants of even moderate hyperHcy has intensified. Early biochemical studies by Kang et al9,10 identified a thermolabile variant of MTHFR with reduced enzymatic activity; this defect was reported to be associated with both elevated tHcy levels and increased CAD risk. In 1995, the defect responsible for the thermolabile MTHFR was characterized, consisting of a missense mutation (C677T) that results in a valine substitution for an alanine.11 Given that homozygous mutants were reported to have increased plasma tHcy levels, the C677T mutation was proposed as a candidate genetic risk factor for CAD. However, subsequent studies (reviewed in Rozen12) have given remarkably conflicting results. These discrepancies might relate to both population-specific factors (ie, differences in the geographical distribution of the mutation and/or in the nutritional status) and to heterogeneity of study conditions (ie, differences in the selection of cases and controls and, sometimes, lack of adequate information on folate and tHcy levels).
In this report, we investigated the frequency of the C677T genotype and its association with tHcy levels before and after methionine loading in Italian patients with angiographically documented severe CAD compared with subjects with angiographically documented normal coronaries. Because an adequate folate status may counterbalance the defective MTHFR activity,13 we also examined the influence of plasma folate concentration on the relation between the MTHFR thermolabile polymorphism and plasma tHcy concentrations.
MATERIALS AND METHODS
Study population.
Between May 1996 and May 1997, we studied 415 consecutive unrelated adult patients of both sexes recruited from those referred to the Institute of Cardiovascular Surgery of Verona (Verona, Italy). Of these, 278 were candidate to coronary artery bypass grafting (CABG), having angiographically documented severe multivessel CAD. One hundred thirty-seven subjects examined for reasons other than suspected CAD (mainly valvular heart disease) and having angiographically documented normal coronary arteries were considered as the control group. Both CAD patients and controls came from the same geographical area (Northern Italy), with a similar socioeconomic background. At the time of the blood sampling, a complete clinical history including cardiovascular risk factors such as smoking and hypertension was collected in all participating subjects. We excluded only subjects with conditions known to influence homocysteine levels (thus interfering with genotype/phenotype correlation study), such as current or recent use of a folate or vitamin B12 supplement or of any multivitamin preparation; current or recent use of drugs interfering with homocysteine levels (ie, anticonvulsivants, methotrexate, and penicillamine); any major systemic acute illness (including myocardial infarction in the CAD group) in the last 3 months; serum creatinine level ≥1.8 mg/dL. Moreover, control subjects were enrolled providing that they had not only a normal coronary angiogram at cardiac catheterization, but also neither history nor clinical or instrumental evidence of atherosclerosis in vascular districts other than the coronary bed. Hypertension was defined as systolic blood pressure ≥160 mmHg or diastolic blood pressure ≥95 mmHg or on the basis of medical history and the use of antihypertensive medication. Among the CAD patients, the diagnosis of previous myocardial infarction (in 66% of them) was based on the medical history, previous ECG, and enzyme documentation and/or based on the finding of typical sequelae of infarction on ventricular angiography. The severity of CAD was determined by the number of significantly stenosed coronary arteries, ie, lesions with greater than 50% luminal stenosis. The angiograms were assessed by two cardiologists who were unaware that the patients were to be included in the study. Most of the CAD patients (76%) had severe CAD involving all the three major coronary arteries, 19% had two stenosed vessels and 5% had one. The majority of the women enrolled (n = 93) were in the menopausal status (88%), and none of them assumed hormone replacement therapy. Informed consent was obtained from every subject after a full explanation of the study.
Biochemical analysis.
Samples of venous blood were drawn from each subject in the free-living state, at scheduled ambulatory evaluation few days before surgery.
For tHcy (which refers to the sum of homocysteine, homocystine, and homocysteine-cysteine mixed disulfide, free and protein bound), blood was collected after an overnight fast into EDTA-containing vacuum tubes and kept on ice and in the dark; plasma was separated within 90 minutes; tHcy levels were determined by high-performance liquid chromatography (HPLC) with fluorescent detection, according to Araki and Sako.14 After the first blood sampling, a standardized methionine-loading test was performed by administering orally L-methionine (100 mg/kg) mixed with 200 mL of orange juice, together with a standardized low-protein breakfast; blood was then collected 6 hours later for the determination of the postmethionine loading (PML) tHcy level. Plasma folate and vitamin B12 concentrations were measured by an automated chemiluminescence method (Chiron Diagnostics, East Walpole, MA). Plasma triglycerides and total and high-density lipoprotein (HDL) cholesterol were determined by using a Technicon DAX 96 automated analyzer (Technicon Instruments, Tarrytown, NY); low-density lipoprotein (LDL) cholesterol levels were calculated using the Friedewald formula.
Mutation analysis.
DNA was extracted from peripheral lymphocytes using phenol/chloroform protocol and the analysis of the C677T mutation in the MTHFR gene was performed by PCR and HinfI digestion.11 Because the C677T mutation results in a valine (V) substitution for an alanine (A), the three genotypes were defined as follows: A/A, normal homozygous; A/V, heterozygous; and V/V, mutant homozygous.
Both biochemical and mutation analysis were conducted blind as to whether a sample came from a CAD or CAD-free subject.
Statistical analysis.
Statistical analysis was performed by the Systat 5.2.1 package (Systat Inc, Evanston, IL) working on Macintosh Performa 5300 (Apple Computer Inc, Cupertino, CA). Because of the skewness of the distributions of values for tHcy, folate, and vitamin B12, analyses were performed using natural log-transformed data; thus, geometric means of such variables are presented. Quantitative data were analyzed using the Student's t-test or by AN(C)OVA with Tukey's post hoc comparison of the means when appropriate. Qualitative data were analyzed using a χ2 test. A value of P < .05 was considered significant.
RESULTS
The relevant characteristics of the population studied are summarized in Table 1. As expected, CAD patients had more conventional risk factors (higher body mass index [BMI], total and LDL cholesterol, a higher prevalence of hypertension and smoking, and lower HDL cholesterol) as compared with CAD-free controls. The two groups were matched for age, but there were more women in the control group compared with the patient group. Geometric mean fasting tHcy levels were significantly higher in CAD than in CAD-free subjects, also after adjusting for both sex and age (Table 1). Geometric mean PML increase in tHcy (absolute PML value minus fasting) levels were not significantly different between the two groups. Although the measures of fasting and PML tHcy were highly correlated (r = .68; P < .001), different persons with elevated homocysteinemia were identified by each measure. By analyzing tHcy level as a categorical variable, the prevalence of hyperhomocysteinemia (defined as at least 1 of the 2 values in the top fifth of the control distribution, ie, fasting tHcy ≥19 μmol/L and PML increase in tHcy ≥31.7 μmol/L) was higher in CAD than in CAD-free subjects (41% v 28.8%, respectively; P < .05). There was no statistically significant difference in either plasma folate or in vitamin B12 concentrations between CAD and CAD-free subjects, although both the values tended to be lower in CAD subjects. Both in the whole population and in each of the two subgroups, fasting tHcy levels were inversely correlated with the concentrations of folate and vitamin B12 (r = −.38 and −.25, respectively, in the whole population, n = 415; P < .001 for both). Among the CAD patients, there was no difference in either tHcy values (fasting and PML increase) or in the concentrations of vitamins when subjects with or without previous myocardial infarction were compared (data not shown).
Biological Parameters of the Two Groups Studied
| . | CAD-Free (n = 137) . | CAD (n = 278) . |
|---|---|---|
| Age (yr) | 59.3 ± 11.8 | 61.1 ± 8.9 |
| Male/female | 79/58 | 243/35 |
| Menopausal women (%) | 83 | 97 |
| BMI (kg/m2) | 24.9 ± 3.2 | 26.3 ± 3-150 |
| Smoking (%) | 42.5 | 70-151 |
| Hypertension (%) | 29.8 | 57.4-151 |
| Total cholesterol (mmol/L) | 5.6 ± 1.1 | 6.2 ± 1.1-150 |
| LDL cholesterol (mmol/L) | 3.7 ± 0.9 | 4.2 ± 1-150 |
| HDL cholesterol (mmol/L) | 1.5 ± 0.4 | 1.2 ± 0.3-150 |
| Triglycerides (mmol/L) | 1.5 ± 0.7 | 2.1 ± 1.7-150 |
| Ascertained previous MI (%) | — | 66 |
| GM fasting tHcy (μmol/L) | 14.2 | 15.7-150 |
| GM PML tHcy increase (μmol/L) | 23.5 | 24.1 |
| Hyperhomocysteinemia-152(%) | 28.8 | 41-151 |
| GM folate (nmol/L) | 12.4 | 11.3 |
| GM vitamin B12 (ng/L) | 443 | 411 |
| . | CAD-Free (n = 137) . | CAD (n = 278) . |
|---|---|---|
| Age (yr) | 59.3 ± 11.8 | 61.1 ± 8.9 |
| Male/female | 79/58 | 243/35 |
| Menopausal women (%) | 83 | 97 |
| BMI (kg/m2) | 24.9 ± 3.2 | 26.3 ± 3-150 |
| Smoking (%) | 42.5 | 70-151 |
| Hypertension (%) | 29.8 | 57.4-151 |
| Total cholesterol (mmol/L) | 5.6 ± 1.1 | 6.2 ± 1.1-150 |
| LDL cholesterol (mmol/L) | 3.7 ± 0.9 | 4.2 ± 1-150 |
| HDL cholesterol (mmol/L) | 1.5 ± 0.4 | 1.2 ± 0.3-150 |
| Triglycerides (mmol/L) | 1.5 ± 0.7 | 2.1 ± 1.7-150 |
| Ascertained previous MI (%) | — | 66 |
| GM fasting tHcy (μmol/L) | 14.2 | 15.7-150 |
| GM PML tHcy increase (μmol/L) | 23.5 | 24.1 |
| Hyperhomocysteinemia-152(%) | 28.8 | 41-151 |
| GM folate (nmol/L) | 12.4 | 11.3 |
| GM vitamin B12 (ng/L) | 443 | 411 |
CAD-free subjects were those with documented normal coronary angiogram (n = 137). CAD subjects were those with angiographically documented coronary artery disease (n = 278).
Abbreviations: GM, geometric mean; tHcy, total homocysteine, adjusted for age and sex; MI, myocardial infarction.
P < .05 by using the t-test (on log-transformed data for skewed variables).
P < .05 by using the χ2 test.
Defined as at least one of the two values in the top fifth of the control distribution (ie, fasting tHcy ≥19 μmol/L and PML tHcy increase ≥31.7 μmol/L).
The distribution of the MTHFR genotypes in the whole population was compatible with the Hardy-Weinberg equilibrium. Allele and genotype frequencies were as follows: V allele frequency, 41.9%; A/A, 31.8%; A/V, 52.5%; and V/V, 15.7%. There was no sex-dependent variation in the prevalence of the V allele frequency or V/V genotype. Table 2 indicates the allele frequencies and genotype distributions in CAD and CAD-free subjects. The frequency distribution of the genotypes between CAD and CAD-free subjects was not significantly different (χ2 = 1.04, df = 2,P = .59). It was also not significantly different between CAD subjects with or without previous myocardial infarction (A/A 33.3%, A/V 55.1%, and V/V 11.6% in CAD without myocardial infarction [MI]; A/A 32.9%, A/V 51%, and V/V 16.1% in CAD with MI; χ2 = .9, df = 2, P = .63).
Frequencies of MTHFR Normal (Alanine) and Mutant (Valine) Alleles and of the Three Genotypes (A/A, A/V, and V/V) in CAD-Free (n = 137) and CAD (n = 278) Subjects
| MTHFR . | CAD-Free . | CAD . |
|---|---|---|
| Normal allele A | 154/274 (56.2%) | 328/556 (59%) |
| Mutant allele V | 120/274 (43.8%) | 228/556 (41%) |
| A/A | 42/137 (30.66%) | 90/278 (32.4%) |
| A/V | 70/137 (51.09%) | 148/278 (53.2%) |
| V/V | 25/137 (18.25%) | 40/278 (14.4%) |
| MTHFR . | CAD-Free . | CAD . |
|---|---|---|
| Normal allele A | 154/274 (56.2%) | 328/556 (59%) |
| Mutant allele V | 120/274 (43.8%) | 228/556 (41%) |
| A/A | 42/137 (30.66%) | 90/278 (32.4%) |
| A/V | 70/137 (51.09%) | 148/278 (53.2%) |
| V/V | 25/137 (18.25%) | 40/278 (14.4%) |
χ2 = 1.04, df = 2, P = .6.
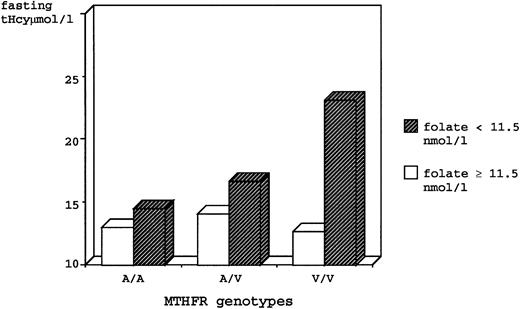
A genotype/phenotype correlation study focused on the effect of the MTHFR mutation on tHcy levels was performed using combined data of the entire study population. Because a mutation in the MTHFR gene might alter folic acid requirements in mutant individuals, we divided the sample into two groups on the basis of plasma folate levels, using the median value (11.5 nmol/L, which was similar in both groups) as the cutoff. Results are summarized in Table 3. Regardless of the folate levels, V/V homozygotes had significantly higher fasting tHcy as compared with the A/A and the A/V genotypes. However, the latter genotype had intermediate values that were not statistically different from the A/A group. After stratification by folate levels, it became evident that there was a marked effect of folate on the association between genotype and fasting tHcy. In individuals with folate levels above the median, there was no significant difference between genotypes. Conversely, the low-folate V/V subjects had fasting tHcy levels 59% greater than low-folate A/A subjects and 38% greater than the intermediate levels of low-folate A/V subjects. Noteworthy, after exclusion of the low-folate V/V group with extremely high fasting tHcy values, low-folate A/V heterozygotes had fasting tHcy levels significantly higher (by 21% on average) as compared with all the other genotype/phenotype categories (Table 3 and Fig 1).
Plasma Hcy Levels in the Whole Population as a Function of MTHFR Genotype and Folate Levels (Stratification by Folate Values Above and Below the Median)
| MTHFR Genotype . | A/A . | A/V . | V/V . |
|---|---|---|---|
| Fasting tHcy (μmol/L) | 13.9* | 15.2* | 18.4 |
| Folate ≥11.5 nmol/L | 13 | 14.1 | 12.7 |
| Folate <11.5 nmol/L | 14.5* | 16.7*,† | 23.1 |
| PML tHcy increase (μmol/L) | 23.6 | 23.6 | 25.5 |
| Folate ≥11.5 nmol/L | 22.9 | 23.5 | 21.4 |
| Folate <11.5 nmol/L | 24.9 | 25.1 | 28.8 |
| MTHFR Genotype . | A/A . | A/V . | V/V . |
|---|---|---|---|
| Fasting tHcy (μmol/L) | 13.9* | 15.2* | 18.4 |
| Folate ≥11.5 nmol/L | 13 | 14.1 | 12.7 |
| Folate <11.5 nmol/L | 14.5* | 16.7*,† | 23.1 |
| PML tHcy increase (μmol/L) | 23.6 | 23.6 | 25.5 |
| Folate ≥11.5 nmol/L | 22.9 | 23.5 | 21.4 |
| Folate <11.5 nmol/L | 24.9 | 25.1 | 28.8 |
tHcy data are expressed as geometric means.
Significantly different from V/V by ANOVA and Tukey's post-hoc multiple comparison of the means of log-transformed data (P < .001).
Significantly different from all the other categories (A/A with low and high folate, A/V and V/V with high folate) after exclusion of the V/V with low folate group from the analysis (ANOVA and Tukey's post-hoc multiple comparison of the means of log-transformed data;P < .001).
Fasting tHcy for genotypes of Ala-to-Val (C677T) MTHFR mutation, stratified by low and high plasma folate levels, in the total study population.
Fasting tHcy for genotypes of Ala-to-Val (C677T) MTHFR mutation, stratified by low and high plasma folate levels, in the total study population.
The PML increase in tHcy concentration was unrelated to MTHFR genotype, irrespective of plasma folate level (Table 3).
Table 4 summarizes the biological parameters in CAD subjects with different MTHFR genotypes. With respect to the conventional risk factors, there was no significant difference among patients with different genotypes for any of the listed variables except a lower prevalence of hypertensives in the heterozygous group. Differences in tHcy levels were similar to those observed in the entire population group. Folate levels were not significantly different between genotypes, either in this group or when considering the entire population study or the CAD-free subjects (data not shown).
Biological Parameters in CAD Subjects With Different MTHFR Genotypes
| MTHFR Genotype . | A/A . | A/V . | V/V . |
|---|---|---|---|
| Age (yr) | 60.2 ± 9.6 | 61.2 ± 8.4 | 62.7 ± 9.4 |
| BMI (kg/m2) | 26.2 ± 3.1 | 26.4 ± 2.9 | 26.1 ± 3.3 |
| Total cholesterol (mmol/L) | 6.19 ± 1.2 | 6.19 ± 1.1 | 5.99 ± 1.0 |
| LDL cholesterol (mmol/L) | 4.28 ± 1.1 | 4.18 ± 1.0 | 4.0 ± 0.9 |
| HDL cholesterol (mmol/L) | 1.27 ± 0.4 | 1.24 ± 0.3 | 1.29 ± 0.4 |
| Triglycerides (mmol/L) | 2.02 ± 1.5 | 2.26 ± 2.0 | 1.97 ± 1.2 |
| Hypertension (%) | 66 | 503-150 | 64 |
| Smoking (%) | 64 | 72 | 75 |
| Ascertained previous MI (%) | 65 | 64 | 72 |
| GM fasting tHcy (μmol/L) | 14†‡ | 15.63-151 | 21.1 |
| GM PML tHcy increase (μmol/L) | 23.8 | 23.8 | 25.6 |
| GM folate (nmol/L) | 11.7 | 11.3 | 10.3 |
| MTHFR Genotype . | A/A . | A/V . | V/V . |
|---|---|---|---|
| Age (yr) | 60.2 ± 9.6 | 61.2 ± 8.4 | 62.7 ± 9.4 |
| BMI (kg/m2) | 26.2 ± 3.1 | 26.4 ± 2.9 | 26.1 ± 3.3 |
| Total cholesterol (mmol/L) | 6.19 ± 1.2 | 6.19 ± 1.1 | 5.99 ± 1.0 |
| LDL cholesterol (mmol/L) | 4.28 ± 1.1 | 4.18 ± 1.0 | 4.0 ± 0.9 |
| HDL cholesterol (mmol/L) | 1.27 ± 0.4 | 1.24 ± 0.3 | 1.29 ± 0.4 |
| Triglycerides (mmol/L) | 2.02 ± 1.5 | 2.26 ± 2.0 | 1.97 ± 1.2 |
| Hypertension (%) | 66 | 503-150 | 64 |
| Smoking (%) | 64 | 72 | 75 |
| Ascertained previous MI (%) | 65 | 64 | 72 |
| GM fasting tHcy (μmol/L) | 14†‡ | 15.63-151 | 21.1 |
| GM PML tHcy increase (μmol/L) | 23.8 | 23.8 | 25.6 |
| GM folate (nmol/L) | 11.7 | 11.3 | 10.3 |
Abbreviation: GM, expressed as geometric means.
Significantly different from V/V and A/A by χ2(df = 2, P = .04).
Significantly different from V/V by ANOVA and Tukey's post-hoc multiple comparison of the means of log-transformed data (P < .001).
Significantly different from A/V by ANOVA and Tukey's post-hoc multiple comparison of the means of log-transformed data (P < .05).
DISCUSSION
The primary objectives of this study were (1) to assess the frequency distribution of the MTHFR C677T mutation in a large population of patients with angiographically documented severe CAD, in comparison to subjects with angiographically documented absence of CAD; and (2) an attempt to clarify the relative contribution of this genetic factor to plasma tHcy level, with particular reference to the interaction with an environmental factor potentially modifiable, such as folate status.
Since the original report from Frosst et al,11 the MTHFR C677T mutation has been object of intensive investigation as a possible genetic risk factor for vascular disease. An early study, including a mixture of subjects with premature cerebrovascular, peripheral, and coronary disease, reported a frequency of V/V homozygosity significantly higher in cases as compared with selected controls without conventional risk factors.15 To the best of our knowledge, the studies restricted to CAD patients reported so far are somewhat conflicting: some found a higher prevalence of the mutation than in controls,16,17 whereas others did not.18-26 However, these studies were remarkably heterogeneous with respect to several important conditions that could influence the results. First, most of them used subjects from the base population as control group, without objective angiographic information about their coronary arteries.18-24 Using this approach, one is never sure about the extent of the coronary narrowing, because those controls might have substantial (although not clinically manifest) coronary atherosclerosis, which in turn might contribute to attenuate the association between the MTHFR polymorphism and CAD. Second, some studies included only MI survivors as patients.18-21 Such a phenotype may be too selective, leading to possible underrepresentation of the V/V genotype in CAD patients, if the mutation is related to early mortality after MI. Third, several studies lacked adequate information on the levels of homocysteine,24 folate,17 or both.18,22,25 Finally, it has become clear that the prevalence of the C677T mutation is specific to populations. Reported frequencies of the V/V MTHFR genotype in control populations varied from 1.4% in African Americans27; to 5% in Dutch15,28; to about 10% in Japanese17and in Australians24,25; to 12% to 13% in French-Canadians,11 in people in the United States,19,21 and in people in the United Kingdom.18 In Italy, the frequencies of V/V homozygotes in two series of apparently healthy subjects from the Southern29 and the Northern30 part of the country were 15.1% and 21%, respectively. Our data confirm that the C677T mutation in our country is quite common (homozygosity in the entire population study and in controls was 15.7% and 18.2%, respectively), with a prevalence that is one of the highest reported so far. Such a high frequency argues per se against a possible role of this mutation as a single factor involved in the pathogenesis of CAD, a potentially lethal disease that is known to be multifactorial. In line with this view, in our population, the distribution of the MTHFR genotypes was not statistically different between patients with angiographically documented severe CAD and controls (Table 2). An important peculiarity that strengthens the negative results of our study relies on the fact that it included only subjects with objective angiographic information. Noteworthy, most of our CAD patients, recruited among those who were candidates to have CABG, had severe triple-vessel disease (76%). Only one of the two studies that found a positive association between MTHFR polymorphism and CAD reported angiographical data of CAD patients.17 In this study on a large sample of Japanese CAD patients, the frequency of the V/V genotype was correlated with the angiographical severity of CAD, and most of this association was due to patients with triple-vessel disease. On the other hand, in our study, the control population was chosen to provide a contrasting population with angiographically documented absence of CAD. In this way, having considered extreme and objective conditions, we feel confident to reduce the chance of spurious results, an inherent problem of allelic association studies.31 In agreement with previous data18-21 and keeping in mind the above-mentioned limitations, our study also excluded any relationship between the MTHFR C677T mutation and myocardial infarction, the major thrombotic complication of CAD.
Whereas it is now becoming clear that, at least in most of the populations studied so far, the C677T mutation cannot be considered as a single genetic risk factor for CAD, this polymorphism remains of great potential interest because of its influence on plasma homocysteine concentrations. This is particularly relevant to CAD patients, by virtue of the striking results of a recent prospective trial that found a strong graded and independent association between plasma tHcy and mortality in patients with angiographically confirmed CAD,8 confirming and extending a previous meta-analysis of observational studies.7 Actually, Nygård et al,8 after a median follow-up of 4.6 years, found that the mortality ranged linearly from 24.7% in patients with high tHcy levels to 3.8% in patients with low tHcy levels. The genotype/phenotype correlation study in our population confirms that the MTHFR polymorphism is a major determinant of plasma tHcy levels, but also clearly shows that it is not important as a single factor.
Early studies by Kang et al9,32 reported that not all the individuals with biochemically demonstrated thermolabile MTHFR had increased tHcy levels and that, among those who had it, normalization was seen after folate supplementation. Consistent with these observations, the interaction between MTHFR thermolabile genotype and folate status was first pointed out by Jacques et al13 on 356 individuals from the NHLBI Family Heart Study. They found that tHcy increased only in those V/V homozygous individuals who had concomitant plasma folate levels below the median (15.4 nmol/L in that population). Similar data were then obtained in other US19,20 and French-Canadian23 populations by several, but not all,21 investigators. Sequence homology studies suggest that the region in MTHFR relating to the C677T mutation is involved in folate binding and that the enzyme may be stabilized in the presence of adequate folate levels.33 Our data confirm that the mutation is neutral with respect to tHcy levels when folate status is adequate. Moreover, we found that in subjects with low folate status tHcy increased significantly not only in V/V homozygotes, but also, at intermediate values, in subjects with only one copy of the mutant allele (Fig 1). To our knowledge, this is the first study reporting increased plasma tHcy levels also in MTHFR C677T heterozygotes with concomitant low plasma folate levels. Our results are consistent with previous studies that correlated the MTHFR polymorphism to the biochemical phenotype, by means of in vitro evaluation of the enzyme activity and thermolability in lymphocytes.11,15,23,28 These studies demonstrated that the effects of the two alleles on MTHFR activity are codominant, with A/V heterozygotes having an enzyme activity intermediate between the two homozygote groups. Interestingly, it has been calculated that a plasma folate level of 15 nmol/L is that which ensure adequate tissue folate.34,35 Our data are also consistent with a longitudinal observational study that found that, at plasma folate levels markedly low (< 3.7 nmol/L), even the heterozygous A/V subjects have an increased likelihood of being significantly hyperhomocysteinemic.36 The discrepancies with the above-mentioned genotype/phenotype correlation studies may relate to different folate status in our population as compared with North Americans, in which the use of vitamin supplements and/or the consumption of folate fortified cereals is more common.35Indeed, the median folate level in our population (11.5 nmol/L) was lower than that reported, although this extrapolation needs to be followed with caution because of the different biochemical methods used for the determination of folate. It is noteworthy that interventional studies have clearly demonstrated that folic acid supplementation is effective in reducing plasma tHcy levels, particularly in subjects carrying the C677T mutation.36,37 Because of the high prevalence of the mutation (allele frequency of 42% in our population) and the linear association between tHcy and CAD from prospective studies,7,8 our results point out that a substantial proportion of people may have increased folate needs to maintain a low plasma level of tHcy. We add evidence to the view that both the low normal threshold values for plasma folate levels and the recommended dietary allowance (RDA) should be reconsidered,38especially in populations with a substantial number of mutants. It should be stressed that we are talking about a nutritional deficiency that has to be intended as marginal and limited to subjects with a genetically determined enzyme isoform, who may require an increased folate intake to stabilize the enzyme and/or to counteract the folate consumption needed to compensate the reduced MTHFR activity. In our series, the 65 V/V homozygotes had average plasma folate levels that tended to be lower, although not statistically significant, than the other genotypes (Table 4). However, 38% of them had plasma folate levels above the median and low plasma tHcy (Table 3), probably due to adequate dietary intake, because none of our subjects assumed any vitamin supplement.
It has been suggested that the determination of PML tHcy is also a useful tool to assess the risk of vascular disease attributable to moderate hyperhomocysteinemia.39,40 Only two studies13,26 have investigated also the relationship of the C677T mutation with PML tHcy, giving conflicting results. The increase in plasma tHcy levels after PML is generally thought to primarily reflect abnormalities in the transsulfuration pathway, both genetically determined (heterozygous CBS deficiency) or nutrient-related (inadequate B6 status).41 According to this hypothesis and to Jacques et al,13 we found that the thermolabile MTHFR genotype is not associated with the PML increase (Table 3).
Finally, according to the majority of studies,17,18,21,24we cannot confirm the association between the MTHFR genotype and other conventional risk factors, such as BMI and/or hypertension, reported by some investigators.25
In conclusion, our findings are against a role of the V/V MTHFR genotype as an independent genetic risk factor for CAD in our population. However, they are consistent with an important genetic-environmental interaction between folic acid and MTHFR, which may have detrimental effects, especially in subjects with angiographically documented CAD.8
ACKNOWLEDGMENT
The authors thank Maria Luisa Zenari and Diego Minguzzi for their excellent technical assistance and Mirella Chesini for helpful assistance in collecting data.
Supported by grants from the Veneto Region, from the Cariverona Foundation, from MURST 60%, and from CNR Project Biotechnologies.
Address reprint requests to Domenico Girelli, MD, PhD, Institute of Medical Pathology, Chair of Internal Medicine, Policlinico Borgo Roma, 37134 Verona, Italy.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal