Abstract
Investigations of VH gene mutational patterns in B-cell tumors are often performed at an arbitrary time point of disease. To assess the effects of disease progression, tumor-derived VHgenes have been monitored from presentation through treatment and relapse in one patient with follicle center lymphoma (FCL), and two patients with primary diffuse large B-cell lymphoma (DLCL). The patient with FCL and one patient with DLCL both achieved clinical remission, although this was only partial in the FCL. However, both subsequently relapsed, and the second patient with DLCL was refractory to radiotherapy and chemotherapy. In each case, the tumor-derived VH sequence was identified, and the CDR3 “clonal signature” was used to track tumor cell sequences in subsequent biopsies. All cases showed somatic mutations, with intraclonal heterogeneity evident at presentation, and some sequences were aberrant. The VH sequences of the DLCL which responded to treatment became homogeneous at relapse. The sequences of both the FCL and the refractory DLCL remained heterogeneous. In all cases, transcripts of multiple Ig isotypes could be identified, and there was immunophenotypic evidence for expression of several Ig isotypes. The case of refractory DLCL had identifiable transcripts from IgM, IgD, IgA, IgG, and IgE, but appeared to lose the ability to produce alternative isotype transcripts and protein at the late stage of disease. These cases indicate that VH gene analysis can be used to probe tumor cell behavior in cases of lymphoma and that perturbations caused by therapy and disease progression can occur.
THE ABILITY TO identify and sequence Ig variable region genes is providing information relevant for understanding the behavior of B-cell tumors. Recombination of the three genetic components of the heavy chain to create the VH-DH-JH transcriptional unit is a unique feature of B cells. Subsequent events of VL-JL recombination, somatic mutation, isotype switching, and antigen selection all leave evidence in the V-gene sequences of the stage of differentiation reached by the B cell of origin. This clonal history is maintained in the neoplastic B cell and is providing insight into B-cell differentiation and tumor development.1 2
VH gene analysis has shown that chronic lymphocytic leukemia (CLL) may be heterogeneous, with one subset derived from naive B cells with unmutated sequences, and another from cells that have acquired somatic mutations.3 Because the mutational machinery is likely to be activated in the germinal center of the lymphoid follicle,4 this indicates that, in some cases, the cell of origin may have traversed this site. Similar analysis has shown that VH genes from cases of follicle center lymphoma (FCL) have undergone somatic mutation and continue to accumulate mutations after malignant transformation.5,6 This gives rise to VH sequences with a clear, common “clonal signature” in the recombination site (CDR3), but with nucleotide differences between the sequences generating intraclonal heterogeneity.6 Cases of endemic or sporadic Burkitt's lymphoma have a similar pattern.7 In contrast, cases of putatively more mature lymphomas such as splenic lymphoma with villous lymphocytes8 or multiple myeloma,9,10 although somatically mutated, show no intraclonal heterogeneity, indicating that the final neoplastic event may have occurred at a postfollicular stage.10
Primary diffuse large B-cell lymphomas (DLCL) are aggressive lymphomas and represent 30% to 40% of B-cell malignancies.11Typically they present with rapid growth at the site of involvement and require combination chemotherapy. Primary DLCL can be distinguished from cases of FCL which have undergone blastic transformation, in having no preceding low-grade phase or accompanying histological features of low-grade disease. Typically, DLCL are composed of sheets of centroblastic or immunoblastic cells.11 In a study of VH genes in tumors from 17 patients with primary DLCL, the most notable finding was of biased VH gene usage, with the V4-34 gene involved in 11 of 17 cases.12Significant levels of somatic mutation were present, but no intraclonal variations. This suggested that DLCL was distinct from FCL in that mutations were not accumulating following neoplastic transformation. However, it was pointed out in the study that these patients were mainly at a late stage of relapse after treatment.12
After somatic mutation, a normal B cell either undergoes selection by antigen or dies by apoptosis.13 It remains possible that antigen may play a role in driving growth of neoplastic B cells, but tumor cells have various devices to escape apoptotic death.6 The question of whether a tumor cell can also differentiate further after neoplastic transformation, which is suggested in tumors such as lymphoplasmacytoid lymphoma by morphology,11 is being investigated at the genetic level. Phenotypic analysis shows that a few tumors can undergo isotype switch.14 Analysis of transcripts defines this ability more clearly and has shown that, in CLL, alternative isotype production may be quite common.15-19
Deductions concerning the cell of origin are usually made from VH gene analysis of a single biopsy specimen. However, we20 and others5 have shown that the genetic profile of lymphoma can change with disease progression. This has been observed in FCL which, after intensive chemotherapy, can show a narrowing of intraclonal heterogeneity toward homogeneity.20 One possibility is that this reflects a second event in one cell which allows escape from chemotherapy.21 To analyze this more closely, we have studied three cases of lymphoma from presentation through disease progression. We have shown that changes in VH gene mutational pattern may occur during treatment and that intraclonal variation is present in DLCL in early disease. We have also found multiple alternative isotype transcripts in all three cases, and have suggestive evidence that the range of isotypes may diminish with progression.
MATERIALS AND METHODS
Patients and histological assessment.
Cases were chosen randomly based on the availability of tissue from the time of diagnosis and at least one further sample at a relapse or disease progression. Clinical information was obtained from case records in the CRC Wessex Oncology Unit (Southampton, UK), and the course of disease in the three patients (BA, EJ, and BR) is summarized in Table 1. The slides of all samples were reassessed by an experienced lymphoma pathologist (B.S.W.). Archival biopsy material was available either as frozen tissue stored in liquid nitrogen (F biopsies), or as paraffin-embedded tissue (P biopsies). Stage at presentation and treatment outcome was assessed using standard criteria (clinical examination, chest radiograph, ultrasound/computer tomography of the abdomen ± chest and bone marrow [BM] aspirate + trephine, full blood count [FBC], and serum biochemistry). Patient BA with marrow-positive FCL was treated with chlorambucil and achieved a partial remission with one residual submandibular node. A frozen tissue biopsy (BA-1F) was available from presentation, and a paraffin-embedded biopsy (BA-2P) from a second site at relapse. Patient EJ with DLCL also only achieved partial responses to two standard regimens of combination chemotherapy.22,23 Frozen tissue biopsy specimens were obtained at presentation (EJ-1F), and from a tonsil (EJ-2F) at first progression. A third frozen tonsillar biopsy (EJ-3F) was available after second relapse. Patient BR with testicular DLCL achieved complete clinical remission but relapsed after 14 months. In this case, a paraffin-embedded biopsy (BR-1P) only was available at presentation, but a frozen biopsy (BR-2F) from a cervical lymphnode was obtained at relapse.24
To confirm the findings in the cases of DLCL a group of seven more presentation biopsy specimens from primary nodal B-DLCL have since been studied.
Immunohistochemistry.
For analysis of Ig light- and heavy-chain expression, cryostat sections were cut from frozen samples and stained using an established indirect immunoperoxidase method.25 The primary monoclonal antibodies (MoAbs) used were anti-IgD (DAKO, High Wycombe, UK), IgM, IgG, and IgA (kind gift from Dr M. Glennie, Tenovus Laboratory). Negative controls were performed by omitting the primary antibody.
Paraffin section immunohistochemistry was also performed to assess the expression of B-cell–associated (CD20, CD79a) and T-cell–associated (CD3, CD45RO) antigens (all from DAKO) plus Ki67 antigen as an estimator of the proliferating fraction of tumor cells. An established streptavidin-biotin complex immunoperoxidase method was used with appropriate antigen retrieval.26
Preparation of cDNA from frozen biopsy samples.
Frozen tissue was transferred into the cold chamber of the cryostat and embedded in OCT compound (R. Lamb, London, UK). Three to four 5-μm sections were cut and transferred to sterile Eppendorf vials. Extraction of RNA was performed in a laminar flow hood using 800 μL RNAzol (Cinna Biotecx Labs, Inc, Houston, TX). Twenty microliters of solution was reverse transcribed using an oligo-d(T) primer and a first-strand cDNA synthesis kit (Pharmacia, Uppsala, Sweden).
Preparation of genomic DNA from paraffin-embedded biopsy specimens.
Paraffin-embedded tissue was cut using a microtome with a thoroughly cleaned blade and blockholder. Five to six 5-μm sections from each sample were transferred to a sterile 1.5-mL Eppendorf vial using a sterile needle. Paraffin was removed by two separate additions of 0.5 mL of xylene, with incubation at room temperature for 5 minutes, followed by three washes with stepwise increments of ethanol to a final wash in 100% ethanol. Protein was digested with proteinase K (400 ng/mL; Sigma, Poole, UK) in a final volume of 400 μL for 6 hours at 45°C. The enzyme was inactivated at 90°C for 5 minutes, and the vial centrifuged at 15,000 rpm for 20 minutes. The supernatant was used directly in the subsequent polymerase chain reaction (PCR).
Amplification of VH genes.
For identification of the tumor VH genes, 50-μL PCR reactions were performed using 2 μL of cDNA or genomic DNA (gDNA). Mixed 5′-oligonucleotide primers specific for the VH leader or framework region 1 (FWR1) sequences27,28 were used together with a consensus 3′-JH primer,29 with conditions as described.28 Seminested FWR2 PCR amplifications were performed with a consensus 5′-FWR2 primer and an outer (30 cycles) and inner (20 cycles) consensus 3′-JH primer.29 At least two separate PCR amplifications were performed and analyzed for each sample.
Amplification of constant region genes.
Nested PCRs were performed for identification of constant regions and tumor-derived isotype variants. The initial amplification from cDNA was performed with the family-specific VH leader 5′-primer, together with an outer 3′-constant region primer30,31 (and Table 2). Thirty cycles of amplification were used, with annealing at 60°C and a final extension at 72°C for 20 minutes. For the second reaction, a patient-specific 5′-primer was designed based on the 3′-end of the VH gene segment and the first half of the CDR3 (Table 2). These primers were used together with an inner 3′-primer30 31 (and Table 2). Primer pairs were used at 20 pmol/50 μL per reaction, and 20 cycles of amplification performed under the same conditions as the outer PCR. Each of the five constant regions was amplified separately, and at least two separate PCR amplifications were performed for each constant region and biopsy.
Cloning and sequencing of PCR products.
Amplified products were gel-purified, cloned by ligation into pGEM-T vector, and transfected into JM109 competent bacteria (Promega, Southampton, UK). Some products from the constant-region second-round PCRs were ligated directly without purification. Nucleotide sequences were determined by the dideoxy chain termination method using the M13-20 and reverse primers. Alignment was made to Entrez and V-BASE32 databases, using MacVector 4.5.3 software (Kodak IBI, New Haven, CT).
RESULTS
Histologic and immunophenotypic analysis of patients' biopsy specimens.
The clinical and biopsy information is summarized in Table 1. Stained sections showed extensive infiltration by tumor cells in all cases, with follicular architecture in the FCL from BA, but sheets of tumor cells without any follicularity in both cases of DLCL. There was no significant change in histologic appearance in the different tissues from each patient during the course of the disease and, specifically, the presenting biopsy samples of the two DLCL showed no evidence of transformation from low-grade lymphomas. All tumor cells expressed the B-cell markers CD20 and CD79a, and were negative for the T-cell markers CD3 and CD45RO. The proliferative fraction of cells expressing the Ki67 antigen was approximately 20% in the FCL and 60% in the DLCL (Table3). Expression of Ig by tumor cells could only be assessed from the frozen sections, and results are shown in Table 3. All cases expressed monotypic light chains in all tumor cells in at least one biopsy. However, in the last biopsy of patient EJ (EJ-3F), light chains were not detected, possibly because of a technical problem. Monotypic λ light chain and dual expression of IgA (100%) and IgG (>80%) was seen on tumor cells at presentation for patient BA (BA-1F). However, there were also single IgM+cells localized within and without the neoplastic follicles, which could not definitively be assigned to the tumor cell population. For patient EJ, the presentation biopsy (EJ-1F) showed all tumor cells expressing IgM, with a proportion also expressing IgD, and some positive for IgG. This IgG was not detectable in the subsequent two biopsy samples (Table 3). For patient BR, the tumor cells in the biopsy specimen at relapse (BR-2F) expressed monotypic κ light chains and both IgM (100%) and IgG (90%) (Table 3). This strongly suggests that IgM and IgG were expressed on the same cells.
Identification of tumor-derived VH genes.
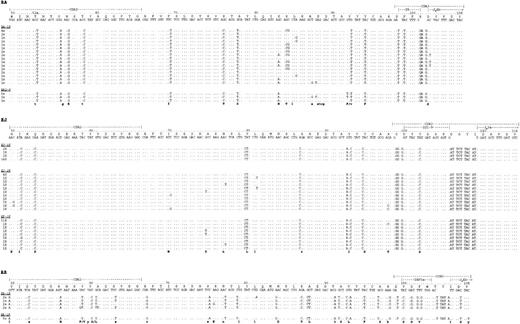
Predominant repeated VH-DH-JHsequences with a clonally related CDR3 “clonal signature” were identified in all cases (Table 4). The finding of only one group of repeated sequences with identical CDR3 strongly indicates their derivation from the tumor cells.28Other sequences detected were individually distinct and were likely to be derived from normal B cells. The same clone was detected at presentation and in subsequent biopsy specimens for all patients (Table4). The human VH repertoire is known33-35 and polymorphic variation in human VH genes generally arises by insertional or deletional changes.34 Therefore, the closest germline sequences for each case could be identified by comparison with the database. VH genes were derived from VH1 (BA) and VH3 families (EJ and BR) and showed a significant level of somatic mutation in all cases (Table 4). BR showed the highest frequency of mutations with 84.6% (BR-1P) and 86.7% (BR-2F) sequence homology to the closest germline match. Comparison with the closest germline D-segment genes showed evidence for N-additions in all cases, and JH3a and JH4b genes were used (Fig1). Nucleotide sequences have been deposited in the EBML database (accession nos. AJ001404to AJ001413).
Nucleotide sequence comparison of the tumor-derived sequences to the closest germline VH, D-segment and JH-gene shown from CDR2. The numbering indicated above each case refers to the codons counted from the beginning of VH. Sequence identity to germline is indicated by dots in each sequence, each mutation by the appropriate nucleotide. The bottom line in each instance indicates whether a mutation is silent (lowercase) or has led to a replacement amino acid (uppercase). N-additions between VH and D, as well as between D and JH, are shown in each sequence. Note the stopcodons in position 89 in BA.
Nucleotide sequence comparison of the tumor-derived sequences to the closest germline VH, D-segment and JH-gene shown from CDR2. The numbering indicated above each case refers to the codons counted from the beginning of VH. Sequence identity to germline is indicated by dots in each sequence, each mutation by the appropriate nucleotide. The bottom line in each instance indicates whether a mutation is silent (lowercase) or has led to a replacement amino acid (uppercase). N-additions between VH and D, as well as between D and JH, are shown in each sequence. Note the stopcodons in position 89 in BA.
Pattern of somatic mutations and intraclonal sequence variation.
In all cases, somatic mutations were scattered throughout the VH sequence and, although there were many mutations common to all clones of each case, there was clear evidence for intraclonal heterogeneity, indicating ongoing mutational activity. The patterns from the three patients are illustrated by the partial sequences (CDR2-CDR3) in Fig 1 (partial sequences are shown to simplify the data presentation). Each identified clone had a degree of mutational variation, although in some cases the variant nucleotides cannot be seen in the figure as they lie outside the CDR2-CDR3 region. The nucleotide variation observed was greater than expected from the error rate of Taq DNA-polymerase (∼1/5,000 bp in this laboratory). To be confident of the reality of mutational changes and to remove the possibility of their being caused by PCR error, only those changes which occurred in more than one sequence have been considered for inclusion in Fig 1. However, in both biopsy samples of BA, in all three from EJ, and in the sample at presentation of BR we found additional mutations that were not repeated in other sequences. Their frequency also by far exceeded Taq error rate (BA: ≈1:500, EJ: ≈1:900, BR-1P: ≈1:250) and this greatly increases (over twofold) the number of unique sequences for these biopsy specimens.
Similar nucleotide variations were seen in the later biopsy samples from patients BA and EJ (Fig 1), who failed to achieve complete remissions. For patient BA, an identical stop codon was detected at residue 88 (GCT → TCT) in a subclone from both the presentation biopsy sample and that taken at relapse, 10 months later. Because both RNA and DNA analysis identified only one VH-DH-JH rearrangement in this patient and its sequences are therefore likely to be functional, the nucleotide change in position 88 renders the potentially functional VH gene nonfunctional. Detection at two timepoints could be due either to a repeated mutation in this codon, or might indicate that neoplastic B cells with nonfunctional VH sequences can survive. In contrast, the second biopsy specimen from patient BR, who had achieved complete clinical remission after chemotherapy, contained only a single sequence in 10 of 10 clones, showing a homogeneous pattern of mutation with no apparent intraclonal variation (Fig 1). The homogeneity in the BR-2F sequence underlines the reality of the intraclonal variation observed in the initial biopsy sample from BR and in the other patients.
To confirm the finding of intraclonal heterogeneity in DLCL, seven further cases of B-cell DLCL have been studied. As for cases BR and EJ the samples were presentation biopsies of primary nodal lymphomas without clinical or histological evidence of transformation from low-grade lymphoma. Intraclonal heterogeneity was evident in 6 of 7 lymhomas; however, one case was homogeneous with 10 of 10 identical sequences (unpublished data, December 1997).
The predominant VH sequences of each patient were analyzed for statistically significant clustering of replacement mutations which would be indicative of a role for antigen selection.36 No significant clustering was observed in the CDRs. Evidence for conservation of sequence could be shown in some samples in the FW regions. In BA the statistical analysis yielded a P value of borderline significance at diagnosis (BA-1F) and in BR P values for sequence conservation reached statistical significance in both samples (Table 5).
Transcripts of alternative isotypes.
Analysis of transcripts of tumor-derived VH-DH-JH clonal sequences linked to Cμ, Cδ, Cγ, Cα, or Cε was restricted to frozen material from which RNA could be isolated. For this, patient-specific 5′-primers complementary to sequences spanning the 3′ end of VH to the beginning of CDR3 were used together with 3′-constant region primers for the selected isotype. In all cases, a product was obtained from the major isotype expected by immunophenotypic analysis (IgA for BA, IgM for BR and EJ) (Fig 2). However, products from additional isotypes were identified for all patients (Table6). These contained the same CDR3 as observed in the full sequences obtained from VH to JH (Figs 1 and 2).
Identification of clonally related multiple isotype transcripts in the three tumors. Aligned are the tumor-derived sequences from the patient-specific primer sequence through CDR3 to the constant regions. Sequence identity to germline is indicated by dots in each sequence, each mutation by the appropriate nucleotide. The underlined sequences show the patient specific 5′ primers constructed for each case separately.
Identification of clonally related multiple isotype transcripts in the three tumors. Aligned are the tumor-derived sequences from the patient-specific primer sequence through CDR3 to the constant regions. Sequence identity to germline is indicated by dots in each sequence, each mutation by the appropriate nucleotide. The underlined sequences show the patient specific 5′ primers constructed for each case separately.
For patient BA (Fig 2), there was a mutation in JH (codon position 102, GAC → GAT) that was present in only some of the clones in Fig 1 and is, therefore, part of the intraclonal variation. Although it occurred in only one sequence from IgA, it was also found in the IgM and IgG transcripts. In the IgM-derived sequences (Fig 2) there was identity with those from IgA across the CDR3. Also, the same mutation in JH (codon position 111, ACC → GCC), as well as intraclonal variation at codon position 102, were observed, confirming derivation from the same B-cell clone. In the constant region of the IgM, there was a deletion of nine nucleotides at the J-C junction observed in all of the IgM-derived clones, obtained from three separate PCRs. Other B cells from the same patient did not show this deletion, suggesting that it reflects a tumor-specific lesion, possibly in RNA processing. Isotype transcripts of VH-Cγ (IgG2 subclass) were also detected in two of the clones sequenced from a separate PCR, while the other clones were derived from B cells with unrelated CDR3s. This indicates the presence of other IgG+B cells. In the CDR3 a nucleotide change in codon position 102, as well as changes in codon 108 (GGC → GAC) and 111, documented intraclonal variation. No transcripts could be identified from IgD or IgE in BA.
All three biopsy samples from patient EJ had tumor-derived transcripts from IgM. These were readily matched to the original VHsequence due to the long CDR3 sequence, and to two mutations in JH (Fig 2, codon positions 120 and 121). In addition, the presentation biopsy specimen yielded transcripts from IgD, IgG (subclass IgG3) and IgA (Fig 2), all of which had these features. Transcripts from IgE were also detected in the second biopsy sample (EJ-2F). The third biopsy specimen (EJ-3F) taken at relapse yielded abundant VH-Cμ sequences, but no alternative transcripts were detected (Table 6).
For patient BR, transcripts of VH-Cμ were obtained as expected from the phenotype. The only alternative isotype detectable was VH-Cγ (IgG3 subclass), found in 5 of 8 clones from the PCR. Detection of IgG-derived sequence is consistent with IgG protein expression, and no IgD, IgA, or IgE transcripts were found. Identity of the IgG3 sequences with the IgM-derived clones was clear from the CDR3 sequence, and confirmed by finding the same five mutations in the JH sequence of both isotypes (Fig 2). Two of these mutations were also documented in all sequences from leader to JH (codon positions 107, GAC → GAT and 108, TAC → TAT).
In an attempt to assess the generality of the presence of alternative isotype transcripts, the presentation biopsy specimen of a further typical primary nodal DLCL, with a reported phenotype IgMκ, was investigated. Transcripts with a clonally related VH-Cμ, VH-Cδ, and VH-Cγ were identified (unpublished data, December 1997), indicating that our three patients were not exceptional.
DISCUSSION
Analysis of VH gene sequences is being used increasingly to provide information about the clonal history of the cell of origin of B-cell lymphomas. Patterns of somatic mutations within the tumor cell population can also reveal whether or not the tumor cells are continuing to mutate after neoplastic transformation.1,5,20,21 The consequent intraclonal heterogeneity is commonly found in FCL and might fit with the location of these tumors in the germinal center where the mutation mechanism is likely to be activated.4,37 A similar pattern has been reported in some,7,38 but not all,39,40 cases of endemic and sporadic Burkitt's lymphoma. However, the majority of B-cell tumors, including CLL,3 lymphoplasmacytoid tumors,8 and multiple myeloma10,30 41 show stable homogeneous VH sequences.
Diffuse large cell lymphoma can develop as a primary tumor or after blastic transformation of FCL.42 In the latter cases, it has been found that the intraclonal heterogeneous VHsequences in the FCL can be narrowed to a single sequence, suggesting that transformation occurred in a single cell.5 Fewer studies have been made of primary DLCL but, in one study of 17 cases, no intraclonal heterogeneity was observed, leading to the conclusion that there was a fundamental difference between FCL and DLCL.12 Although little clinical information was given, it was noted that many of the cases were obtained after patients had relapsed from treatment.12 Therefore, it is possible that those cases were similar to the second biopsy specimen of our case BR, who had obtained a clinical complete remission after treatment but who later relapsed. We found intraclonal heterogeneity only in the presenting biopsy sample, and a single sequence in all clones at relapse. The presence of intraclonal heterogeneity in both DLCL at diagnosis suggests that there is no real difference in mutational pattern between FCL and DLCL. Furthermore, we have been able to detect intraclonal heterogeneity in a consecutive series of a further 6 of 7 cases of primary nodal DLCL, confirming that this is a common feature in DLCL (unpublished data, December 1997). Because it is ethically inappropriate to rebiopsy untreated patients, it is difficult to assess whether duration of disease has an influence on intraclonal variation. However, we can compare successfully treated patients with those who fail to achieve remission. The latter appear to retain intraclonal heterogeneity, and in the case of patient EJ, that heterogeneity was maintained in two biopsy specimens taken at different timepoints from the same tonsillar site. For patient BR, who relapsed after complete remission, the primary site had been removed, and the homogeneous sequence that emerged was from a different tissue site. Although it is likely that this reflects the selective pressure of chemotherapy, there remains a possibility that heterogeneity may not be present at all tumor sites. To avoid possible pertubations introduced by treatment we would argue that deductions of the clonal history of the cell of origin of a B-cell tumor should ideally be made from material at presentation. In our case of FCL and in case EJ, chemotherapy did not achieve clinical remission, and therefore the heterogeneous clone continued to exist throughout the course of disease in both cases.
The availability of the full VH sequences from the tumor cells allowed us to seek transcripts from constant regions of Ig that were not obvious from immunophenotypic analysis. In fact the tumors had been assigned by initial immunophenotyping as IgAλ (BA) and IgMκ (BR and EJ). The additional presence of IgG had been ignored because of the possibility of contamination from serum IgG in the tissue section, and this may be a common conclusion. It may have been influenced by early analyses of CLL, where dual expression of IgM and IgG was often observed, but was usually due to bound serum IgG.43However, probing of transcripts is now showing multiple isotypes in the majority of cases of CLL.15-19 There is a suspicion that these transcripts are produced by minor cell populations which have undergone deletional isotype-switching events.19 However, there is the possibility that alternative transcripts can be generated by trans-splicing of RNA,44,45 and this is a likely mechanism in the rare cases of CLL where the cells clearly express both isotypes.21 Even here, caution in deductions from phenotype are required, particularly if there is a high level of secreted IgG. In a recent analysis of IgG-secreting lymphoplasmacytoid tumors, cells were phenotypically IgM+IgG+, but the clonally related transcripts from each of the two isotypes had different patterns of somatic mutations and must, therefore, be from different cells.46
We randomly chose our three lymphomas based on the availability of frozen material. With no paraproteins to complicate phenotypic analysis, a more detailed Ig profile was largely consistent with transcript identification, and suggested that for BA (sIgA+IgG+) and BR (sIgM+IgG+) a single cell population was producing at least two isotypes. This points to RNA processing as a possible mechanism. Even if deletional recombination has occurred on the productive chromosome, processing could involve constant region gene transcripts from the allelic chromosome.45 The situation is less clear for the IgM population in BA which phenotypically appeared as a minor subset. It is also difficult to unravel the findings in patient EJ, where transcripts from all isotypes were evident in the second biopsy specimen, but the cells were immunophenotypically IgM+ with some (20%) IgD+only. If subsets synthesizing other isotypes were present, they must have been so in very small numbers. Interestingly, the alternative transcripts disappeared after combination chemotherapy.
Although the patient samples were taken randomly for investigation, the common finding of alternative isotype transcripts raises the question of whether these cases were typical. However, the analysis of a further cases of DLCL with a reported IgM+ phenotype gave similar results, indicating that alternative transcripts may occur frequently. Our data do not show that all transcripts are functional templates for protein synthesis. Immunohistochemistry is not sufficiently discriminating to demonstrate expression of alternative Ig isotypes at low levels or in minor subpopulations, particularly as serum Ig may interfere. To detect subpopulations of tumor cells that may have undergone deletional isotype switch, with expression of downstream isotypes, sorting of cell suspensions will be required. This approach will also allow separation into single cells, which can then be studied for potential expression of multiple isotypes.
In summary, immunogenetic analysis of these three cases of lymphoma taken randomly from our clinical material has revealed two new findings: the first is that VH gene mutational patterns may be influenced by disease status. Our limited cases suggest that DLCL at presentation may still be influenced by the mutation mechanism. The second finding is that alternative isotype transcripts are not confined to CLL. Lymphomas of both FCL and DLCL categories also appear to show signs of isotype-switching events. Whether these represent real differences in differentiation status or reflect unexpected products of RNA processing will have to be answered at the single-cell level.
Supported by the Cancer Research Campaign, UK.
Address reprint requests to Christian H. Ottensmeier, MD, Molecular Immunology Group, Tenovus Laboratory, Southampton University Hospitals, Tremona Rd, Southampton SO16 6YD, UK.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.