Abstract
Thymidylate synthase (TS) inhibition causes cell death, and this enzyme is the target for the important chemotherapy regime 5-fluorouracil/leucovorin. GW1843 (1843U89) is a potent and specific folate analog TS inhibitor in clinical development. Because of the importance of TS as a chemotherapy target, we are studying the mechanism of TS inhibition-induced cell death by GW1843. Ceramide is a regulatory lipid generated by the action of sphingomyelinase and is believed to signal apoptosis. The role of the ceramide in apoptotic signaling was studied in Molt-4 human T-cell leukemia cells undergoing cell death after treatment with GW1843. In response to GW1843, Molt-4 cells undergo apoptosis with both acidic pH, Mg2+-independent sphingomyelinase (ASMase) and neutral pH, Mg2+-dependent sphingomyelinase (NSMase) activities elevated as early steps in the initiation of apoptosis before Molt-4 commitment to death. These activities lead to ceramide production with kinetics consistent with a role as an effector molecule signaling the initiation of apoptosis in Molt-4 cells. These changes were found to be independent of caspase 3–like (CPP32/apopain) activity and DNA degradation, but were not separable from membrane blebbing or cell lysis in this cell line. In this report, kinetic evidence is provided for a role of ceramide in initiating GW1843-induced cell death of Molt-4 T-cell leukemia cells.
THYMIDYLATE SYNTHASE (TS) catalyzes the synthesis of thymidylate from deoxyuridylate plus 5,10-methylenetetrahydrofolate and as such is critical for DNA synthesis and cell growth. Inhibition of this enzyme produces cell death.1-4 This enzyme is the target for the important chemotherapy regime 5-fluorouracil/leucovorin. A number of new folate analog TS inhibitors are now in clinical development.5-7One of these agents is GW1843 (GW1843 has previously been reported in the literature as 1843U89), a 3-methyl-substituted benzoquinazoline folate analog very specific for thymidylate synthase.8 9
Because of the importance of TS as a chemotherapy target, we are studying the mechanism of TS inhibition-induced cell death by GW1843. In this context, we are interested in determining the means by which the cell translates a TS inhibition event into commitment to cell death. A more thorough understanding of these downstream elements may facilitate development of novel agents and regimes to enhance important TS-based therapies and to overcome natural and developed resistance mechanisms. The goal of the current study was to investigate the mechanism of possible apoptotic signaling pathways initiated in Molt-4 human T-cell leukemia cells in response to GW1843 therapy.
Apoptosis describes an orderly and regulated process by which cells die. It has been known for some time that most cytotoxic anticancer compounds kill by inducing apoptosis in susceptible cells.10 The effector molecules that couple the drug stimulus to cell death are now beginning to be elucidated. One strong candidate for such an effector molecule is ceramide. This lipid molecule is the second messenger in the sphingomyelin (SM) signaling pathway or “sphingomyelin cycle” as it has become known.11 In this pathway, SM is metabolized by a sphingomyelinase (SMase), yielding ceramide and phosphocholine. Ceramide has been shown to be increased under a diverse array of conditions that cause apoptosis12,13; however, it is not known if ceramide is the actual intracellular mediator of the extracellular death stimuli. The evidence that extracellularly applied short chain synthetic analogs of ceramide and ceramide itself can mimic the effects of various external apoptotic stimuli suggests that ceramide is at least an integral component of the apoptotic signaling pathway in a number of experimental systems.14 Two possible effector molecules that are regulated by ceramide include the ceramide-activated protein kinase15 and ceramide-activated protein phosphatase.16 It is not known for certain what role, if any, the substrates for the kinase or phosphatase may play in apoptotic signaling.
Several different types of SMase isoforms have been described including a membrane bound, Mg2+-dependent form (NSMase) active at neutral pH,17 a lysosomal, Mg2+-independent form (ASMase) maximally active at acidic pH,18 a secreted Zn2+-dependent, acidic pH form produced from the same gene as the lysosomal form,19 and a cytosolic, Mg2+-independent, neutral pH form.20 The most well characterized enzyme is ASMase, which is present in all cells and is involved in the normal, lysosomal turnover of SM in the plasma membrane. Patients with Niemann-Pick disease type A or B have either a complete or partial loss of ASMase activity resulting in accumulation of SM. NSMase has not been as well characterized and has not yet been cloned. Both SMase enzymes have been reported to be activated in response to tumor necrosis factor-α (TNF-α), and it has been suggested that ceramide generated by NSMase activates a proline-directed serine/threonine protein kinase15 and ceramide from ASMase activates NF-κB.21 Interleukin-1β has also been reported to signal through a SMase enzyme,22,23 which appears to be NSMase.24 It has also been reported that cell death signaling through Fas/APO-1, a receptor structurally related to p55 TNF-R, is mediated by the SM signaling pathway.25 It is clear that the SM signaling pathway is important for these processes, but it is not clear which SMase enzyme is the most important for the particular cellular responses induced by these cytokines. The determination of whether or not there is a role for the SM signaling pathway as a general mediator of apoptotic stimuli will require further research.
We report here that in response to GW1843, both ASMase and NSMase activities are elevated as early steps in the initiation of apoptosis before Molt-4 commitment to death. These activities lead to ceramide production with kinetics consistent with a role as an effector molecule signaling the initiation of apoptosis in Molt-4 cells. These changes were found to be independent of caspase 3–like (CPP32/apopain) activity and DNA degradation, but were not separable from membrane blebbing or cell lysis in this cell line. The implications for the involvement of ceramide as a mediator of apoptosis in this cell line are discussed.
MATERIALS AND METHODS
Reagents.
GW1843 was synthesized and used as described.26 Histopaque 1077 and 1119 were from Sigma (St Louis, MO). RPMI 1640 was from GIBCO (Grand Island, NY). Fetal calf serum (FCS) was from JRH Biosciences (Lenexa, KS). Molecular weight markers were from BioRad (Richmond, CA). Propidium iodide was from Molecular Probes (Eugene, OR). Acetyl-asp-glu-val-asp-aldehyde (Ac-DEVD-CHO), acetyl-asp-glu-val-asp-aminomethylcoumarin (Ac-DEVD-AMC), acetyl-tyr-val-ala-asp-aldehyde (Ac-YVAD-CHO), and acetyl-tyr-val-ala-asp-aminomethylcoumarin (Ac-YVAD-AMC) were from BACHEM Bioscience, Inc (King of Prussia, PA). The caspase 3/interleukin-1–β-converting enzyme (ICE) assay kit was a generous gift of Promega Corporation (Madison, WI) and was used according to the manufacturer's instructions. Annexin-V coupled to fluorescein (Annexin-V-Fluos) was from Boehringer Manheim Corporation (Indianapolis, IN). All other reagents were supplied from standard commercial sources.
Histopaque preparation of Molt-4 cell fractions.
Molt-4 cells were maintained in RPMI 1640 containing 10% FCS. Cells were grown to log phase and seeded in 35 mL of RPMI 1640 with 10% FCS in 75 cm2 flasks at 1 × 106/mL for treatment with 8 nmol/L GW1843 and to 2.5 × 105/mL for control studies. Cells from each flask were grown for 24 hours and transferred to a 50-mL conical bottom centrifuge tube. A total of 10 mL of a 1:1.8 mix of RPMI 1640:histopaque 1077 was underlayed beneath the cells and 10 mL of histopaque 1119 was underlayed beneath that layer. The tube was centrifuged at room temperature at 700g for 30 minutes. The cells were resolved into three fractions, the live appearing cells form a band at the interface between the two histopaque layers, the blebbed cells form a band at the interface between the culture media and the 1:1.8 mix of RPMI 1640:histopaque 1077 and the dead cells form a pellet. Cell fractions were harvested by aspirating the media until approximately 1 cm remained above the top band of cells. The cells were removed with a pasteur pipette, diluted 1:1 with media, and pelleted at 1,000g for 5 minutes. The media and histopaque were aspirated and the cell pellet was washed with RPMI 1640 and resuspended in a suitable volume of media.
Analysis of DNA laddering by agarose gel electrophoresis.
DNA from Molt-4 cells was isolated by a modification of previously published methods.27 28 Cells (1 × 106) were pelleted by centrifugation at 1,500g and washed with phosphate buffered saline. Cells were lysed with 100 μL of a solution containing 50 mmol/L Tris-HCl pH 8.0, 10 mmol/L EDTA, 0.5% sodium lauryl sarcosine, and 0.5 mg/mL proteinase K. After an overnight incubation at 50°C, RNase A was added to 0.5 mg/mL and the sample incubated at room temperature for 2 hours. Samples were extracted with an equal volume (100 μL) of phenol:chloroform:isoamyl alcohol (25:24:1) using Phase Lock Gel 1 Heavy extraction tubes (5 prime-3 prime, Boulder, CO) following the manufacturer's instructions. Fragments of DNA from approximately 5 × 105 cells were separated by electrophoresis on 1% agarose gels and visualized by ultraviolet (UV)-transillumination.
Analysis of poly(ADP-ribose) polymerase (PARP) cleavage by Western blotting.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed as described29 with minor modification. Molt-4 cell aliquots (2 × 106 cells) were pelleted in a microfuge tube and lysed in 50 μL of Laemlli sample buffer.29 The samples were sonicated for 5 minutes in a bath sonicator and incubated at 37°C for 1 hour. Samples were electrophoresed in 7.5% polyacrylamide SDS gels at 200 V (constant voltage) and blotted onto nitrocellulose using a Genie electrophoretic blotter as instructed by the manufacturer (Idea Scientific Co, Minneapolis, MN). Blots were blocked in 10% (wt/vol) nonfat dry milk in Tris-buffered saline (TBS) overnight at room temperature. The blots were incubated for 16 hours at room temperature in 5% (wt/vol) nonfat dry milk in TBS containing 1:1,000 dilution of a monoclonal antibody against PARP (kindly provided by Dr S.H. Kaufmann, Mayo Clinic, Rochester, MN). Blots were washed and incubated with horse radish peroxidase-conjugated secondary antibody for 1 hour at room temperature. Resolved PARP was visualized using the ECL Western blotting analysis system (Amersham, Arlington Heights, IL) as instructed by the manufacturer.
Sphingomyelinase activity determination.
ASMase and NSMase activity was assayed using a trichloroacetic acid (TCA) precipitation method in 96-well format. Assay buffer for NSMase was 20 mmol/L Tris-HCl pH 7.4, 10 mmol/L EGTA, 5 mmol/L EDTA, and for ASMase, it was 25 mmol/L NaOAc pH 5.0, 10 mmol/L EGTA, 5 mmol/L EDTA. Both SMase activities were assayed in the presence and absence of 20 mmol/L MgCl2 to determine the Mg2+ dependence of the SMase activity being assayed. Cells were treated and homogenized at 2 × 107 cells/mL at 4°C in 20 mmol/L Tris-HCl pH 7.4, 250 mmol/L sucrose, 10 mmol/L EGTA, 5 mmol/L EDTA, 0.3% Triton X-100, 1 mmol/L benzamidine, 0.2 mmol/L [4-(2-aminoethyl)benzenesulfonylfluoride, HCl] (AEBSF), 10 μmol/L leupeptin, 10 μmol/L antipain, 1 μmol/L E-64, 1 μg/mL aprotinin, and 1 μmol/L pepstatin A. Reactions (50 μL) contained 10 μL of total Molt-4 homogenate (approximately 30 μg protein) in the appropriate assay buffer and 1 μL of [14C]sphingomyelin (New England Nuclear, Boston, MA) in methanol (54.5 Ci/mol, 7.4 μmol/L final concentration). Reactions were performed for 1 hour at 37°C and stopped with 5.6 μL of 10% (wt/vol) aqueous fatty acid free bovine serum albumin (BSA) followed by 11.1 μL 50% (wt/vol) aqueous TCA (reactions were found to be linear with time and protein under these conditions). The 96-well plates were mixed well and centrifuged at room temperature at 2,000g for 10 minutes. A total of 40 μL of each supernatant was transferred to the corresponding well of a Millipore (Bedford, MA) 96-well filtration plate (MAHV N45) and filtered under vacuum. Each well was washed with 10 μL of ice cold 10% (wt/vol) aqueous TCA. The filtrate was collected in a clear-bottom, opaque 96-well plate containing 200 μL of OptiPhase “Supermix” scintillant (Wallac, Gaithersburg, MD). Plates were covered with mylar film, mixed until homogenous, and counted on a Wallac Trilux μBeta 96-well plate counter.
Caspase activity measurements.
ICE-like and caspase 3–like activity was analyzed in cell homogenates prepared as for sphingomyelinase activity measurements. ICE-like activity was determined against 50 μmol/L Ac-YVAD-AMC in the presence or absence of the same concentration of Ac-YVAD-CHO. Caspase 3 activity was determined against 50 μmol/L Ac-DEVD-AMC in the presence or absence of the same concentration of Ac-DEVD-CHO. Assays were run at 30°C in 100 μL total volume containing 32 μL of assay buffer (from supplier), 53 μL of water, and 10 μL of 0.1 mol/L dithiothreitol, and initiated with 5 μL of Molt-4 homogenate. Reaction progress was monitored in 96-well plates with a Perseptive Biosystems (Cambridge, MA) Cytofluor 4000 with excitation and emission wavelengths of 360 and 460 nm, respectively. Activities were taken from the slope of the linear portion of the progress curve. No activity was detectable when 50 μmol/L Ac-DEVD-CHO was included in reactions where 50 μmol/L Ac-DEVD-AMC was used as the assay substrate.
Lipid measurements.
Lipids were extracted from cells via the method of Bligh and Dyer.30 Half of the extracted lipid was set aside for phosphate determination,31 and the remaining half was used in the diacylglycerol kinase assay as previously described.32 Ceramide phosphate and phosphatidic acid spots on thin-layer chromatography (TLC) plates were quantitated using a Phosphorimager (Molecular Dynamics, Sunnyvale, CA). External standards were run concomitantly to quantitate ceramide and diacylglycerol levels and lipid levels were normalized to total lipid phosphate.
Annexin-V binding.
Surface phosphatidylserine (PS) was measured by fluorescence microscopy with the calcium-dependent PS binding protein Annexin-V coupled to fluorescein isothiocyanate. Molt-4 cells at 1 × 106cells/mL were exposed to 15 nmol/L GW1843 for 24 hours. This produced a population of 75% live cells, 21% blebbed cells, and 4% lysed cells. One million cells from this population were then washed twice with Dulbecco's phosphate-buffered saline and resuspended in 100 μL of 10 mmol/L HEPES, 140 mmol/L NaCl, 5 mmol/L CaCl2. Annexin-V binding was determined by fluorescence microscopy as fluorescein green fluorescence.
Fluorescence-activated cell sorting (FACS) analysis.
Molt-4 cells were treated with 0, 8, and 16 nmol/L GW1843 and timepoints taken at 24, 48, and 72 hours. Cells were fixed in 2 mL ice cold 70% ethanol and stored at −20°C. Cells were pelleted at 1,500g for 5 minutes and resuspended at 1 × 106 cells/40 μL phosphate/citrate buffer containing 0.192 mol/L Na2HPO4 and 4 mmol/L citric acid pH 7.8. Cells were incubated for 30 minutes with gentle agitation and pelleted as above. The supernatant was transferred to a fresh tube and the pellets were resuspended in 1 mL propidium iodide (PI) buffer (3.4 mmol/L citric acid, 10 mmol/L NaCl, 50 μg/PI, 0.6% NP-40, and 37 μg/mL RNase). PI-stained DNA from the pellets was analyzed using a Becton Dickinson (Franklin Lakes, NJ) FACSort instrument.
RESULTS
GW1843 induces cell death in Molt-4 cells.
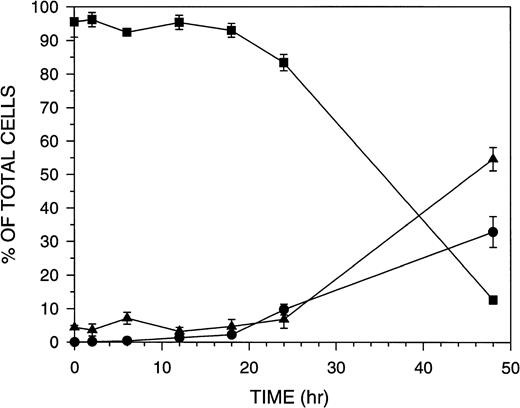
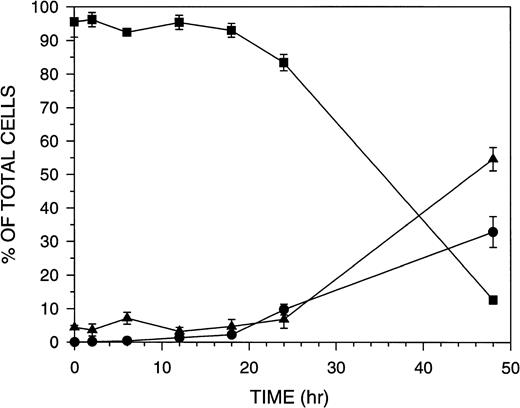
The kinetics of Molt-4 cell death in response to 8 nmol/L GW1843 (IC50 for Molt-4 growth inhibition is 0.7 nmol/L) were studied. Dead cells began to appear approximately 24 hours after treatment. Three Molt-4 morphologic populations were observed: (1) live cells which exclude trypan blue and appear normal; (2) blebbed cells which also exclude trypan blue and therefore apparently have intact membranes and have the nucleus marginated and compacted near the membrane; and (3) dead cells, which do not have intact membranes and thus take up trypan blue.29 The time course for these changes (Fig 1) indicates that at 24 hours the Molt-4 cells are beginning to undergo death and by 48 hours 90% of the cells are either blebbed or dead. At 18 hours, there was no significant change in the number of cells in the blebbed or dead fractions indicating that actual cell death begins to occur between 18 and 24 hours of GW1843 treatment.
Time course for Molt-4 morphologic fractions formed after treatment with 8 nmol/L GW1843. Molt-4 cells in log phase were treated with GW1843 and aliquots taken at the indicated times. Phase contrast microscopy was used to score cells as live (▪), blebbed (•), or dead (▴) based on trypan blue exclusion. The graph represents the average of three separate experiments (mean ± SEM).
Time course for Molt-4 morphologic fractions formed after treatment with 8 nmol/L GW1843. Molt-4 cells in log phase were treated with GW1843 and aliquots taken at the indicated times. Phase contrast microscopy was used to score cells as live (▪), blebbed (•), or dead (▴) based on trypan blue exclusion. The graph represents the average of three separate experiments (mean ± SEM).
GW1843 induces apoptosis in Molt-4 cells as measured by morphology, endonucleosomal DNA hydrolysis, specific PARP cleavage, and PS externalization.
The GW1843-induced morphologic changes of Molt-4 cells observed with phase contrast microscopy was also detected using transmission electron microscopy.29 Morphologic hallmarks typical of apoptosis were clearly evident in electron micrographs including marginated chromatin and invaginated nuclei, micronuclei, and vacuolization of the cytoplasm (data not shown).
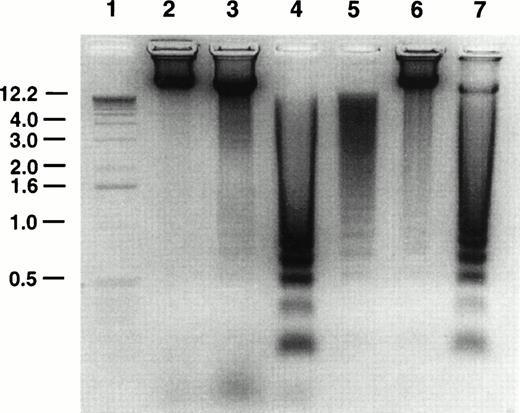
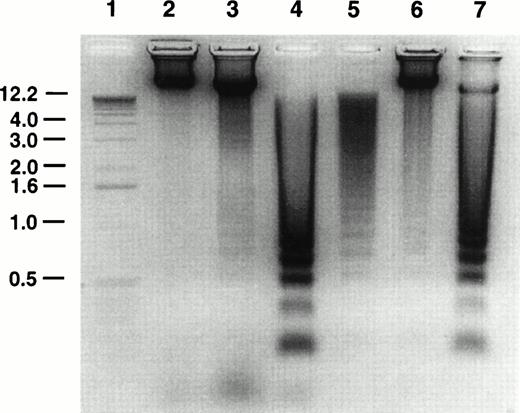
Oligonucleosomal DNA laddering to approximately 180-bp fragments is considered to be characteristic of apoptosis.33 The effect of GW1843 treatment on Molt-4 cell DNA was examined using agarose gel electrophoresis. A representative DNA analysis gel is shown in Fig 2. Untreated control cells (lane 6) contained approximately 7% dead cells as determined by trypan blue exclusion. The DNA from these cells showed a faint ladder; however, most of the DNA was intact. Cells treated for 48 hours with GW1843 contained 26% normal-appearing cells, 52% blebbed cells, and 22% dead cells. The DNA from these cells (lane 7) was mostly laddered with only a small fraction migrating in the region of intact DNA. The DNA from the treated cells clearly showed the approximately 180-bp oligonucleosomal laddering indicating that the cells undergo apoptosis.
Agarose gel electrophoresis of Molt-4 DNA. Molt-4 cells were untreated or treated with 8 nmol/L GW1843 for 48 hours and purified live, blebbed, and dead cell populations isolated on histopaque gradients as described in Materials and Methods. Cellular DNA was prepared and resolved by electrophoresis in 1% agarose gels gel as described in Materials and Methods. Lane 1, one kb ladder; lane 2, live fraction from untreated control cells after resolution by histopaque gradients; lane 3, GW1843-treated, normal-appearing cells after histopaque gradients (fraction contains approximately 6% dead cells); lane 4, GW1843-treated, blebbed cells after gradients; lane 5, GW1843-treated, dead cells after gradients; lane 6, untreated cells before gradients (fraction contains 93% normal cells and 7% dead cells); lane 7, GW1843-treated cells before gradients (26% normal cells, 52% blebbed, and 22% dead). The gel is from a representative experiment from three separate determinations.
Agarose gel electrophoresis of Molt-4 DNA. Molt-4 cells were untreated or treated with 8 nmol/L GW1843 for 48 hours and purified live, blebbed, and dead cell populations isolated on histopaque gradients as described in Materials and Methods. Cellular DNA was prepared and resolved by electrophoresis in 1% agarose gels gel as described in Materials and Methods. Lane 1, one kb ladder; lane 2, live fraction from untreated control cells after resolution by histopaque gradients; lane 3, GW1843-treated, normal-appearing cells after histopaque gradients (fraction contains approximately 6% dead cells); lane 4, GW1843-treated, blebbed cells after gradients; lane 5, GW1843-treated, dead cells after gradients; lane 6, untreated cells before gradients (fraction contains 93% normal cells and 7% dead cells); lane 7, GW1843-treated cells before gradients (26% normal cells, 52% blebbed, and 22% dead). The gel is from a representative experiment from three separate determinations.
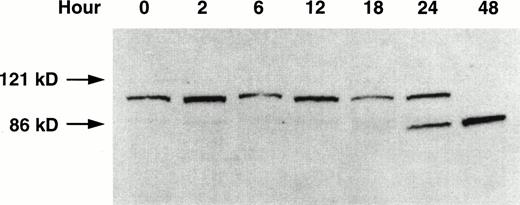
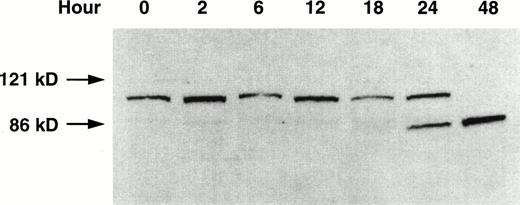
The specific proteolytic cleavage of PARP, a nuclear protein involved in DNA repair,34 is a marker of apoptosis.27 29The effect of GW1843 on the integrity of PARP in the Molt-4 cells was investigated using Western blot analysis (Fig 3). At 24 hours of treatment, degradation of the Mr 116,000 PARP to theMr 85,000 fragment was clearly shown, and only theMr 85,000 fragment was present at 48 hours. The cleavage of PARP in GW1843-treated Molt-4 cells is further evidence that GW1843 induces Molt-4 cells to die through an apoptotic process.
Western blot of PARP proteolysis on treatment of Molt-4 cells with GW1843. Molt-4 cells in log-phase growth were treated with 8 nmol/L GW1843 and aliquots of 2 × 106 cells were taken at the indicated times. Cells were prepared for electrophoresis, electrophoresed on 7.5% polyacrylamide SDS gels,29 and blotted onto nitrocellulose as described in Materials and Methods. The blot is an x-ray film image of PARP protein detected with a horseradish peroxidase-conjugated secondary antibody visualized with chemiluminescent substrate (Amersham) blotted as described in Materials and Methods. The Mr values were determined by running prestained Mr markers (BioRad). Intact PARP (upper band) is Mr 116,000 and the proteolytic fragment is Mr 85,000. This blot is from a representative experiment from four separate determinations.
Western blot of PARP proteolysis on treatment of Molt-4 cells with GW1843. Molt-4 cells in log-phase growth were treated with 8 nmol/L GW1843 and aliquots of 2 × 106 cells were taken at the indicated times. Cells were prepared for electrophoresis, electrophoresed on 7.5% polyacrylamide SDS gels,29 and blotted onto nitrocellulose as described in Materials and Methods. The blot is an x-ray film image of PARP protein detected with a horseradish peroxidase-conjugated secondary antibody visualized with chemiluminescent substrate (Amersham) blotted as described in Materials and Methods. The Mr values were determined by running prestained Mr markers (BioRad). Intact PARP (upper band) is Mr 116,000 and the proteolytic fragment is Mr 85,000. This blot is from a representative experiment from four separate determinations.
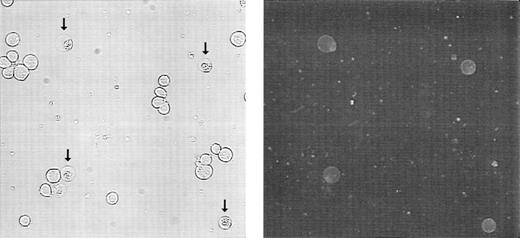
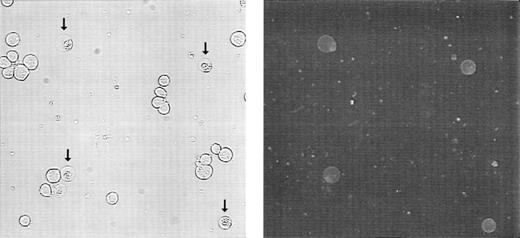
PS normally resides on the inner leaflet of the plasma membrane and is externalized to the outer leaflet during apoptosis.35 36Molt-4 cells were treated with 15 nmol/L GW1843 for 24 hours resulting in a cell population of 75% live cells, 21% blebbed cells, and 4% lysed cells. Externalized PS was detected by treating with fluorescein isothiocyanate (FITC)-labeled Annexin-V in the presence of calcium. Figure 4 shows phase contrast (left) and fluorescence microscopy (right) of these cells. In the phase contrast photomicrograph, normal-appearing cells and blebbed cells with apoptotic nuclei (arrows) were observed (in some fields, apoptotic, lysed cells were also observed). However, only the blebbed and lysed cells with apoptotic nuclei were found to bind Annexin-V, indicating that PS appearance on the outer leaflet corresponded with the apoptotic morphology in the Molt-4 cells.
Externalization of phosphatidylserine on 24 hours treatment of Molt-4 cells with GW1843. Cells were collected, washed, treated with FITC-Annexin-V, and viewed by phase contrast (left) or fluorescein fluorescence (right) microscopy. Arrows indicate blebbed cells. These photomicrographs are from a representative experiment from four separate determinations.
Externalization of phosphatidylserine on 24 hours treatment of Molt-4 cells with GW1843. Cells were collected, washed, treated with FITC-Annexin-V, and viewed by phase contrast (left) or fluorescein fluorescence (right) microscopy. Arrows indicate blebbed cells. These photomicrographs are from a representative experiment from four separate determinations.
Purification and characterization of Molt-4 morphologic populations on histopaque gradients.
A histopaque centrifugation method was developed to separate the Molt-4 cell populations after treatment with GW1843 for 24 hours. Table 1 indicates that at 24 hours, the populations are highly enriched for the cell type of interest. The DNA gel in Fig 2 shows the enrichment of the cell fractions after histopaque gradient centrifugation. When untreated Molt-4 cells were subjected to the centrifugation, the 7% dead cells in the population from lane 6 were removed from the live cells as evidenced by the absence of laddered DNA in the live fraction (lane 2). When the treated cells from lane 7 were purified on the gradients, the cell populations were resolved. The DNA from the live appearing cells (lane 3) was largely intact; this cell population still contained 6% dead cells (as determined by trypan blue staining) and the modest amount of laddered DNA was consistent with this. The DNA from the blebbed and lysed cell fractions (lanes 4 and 5, respectively) was completely laddered indicative of cells having undergone apoptosis.
The viability of the three histopaque Molt-4 cell fractions was investigated by determining their outgrowth potential. We have noted previously that these cells are difficult to clone in agar, especially after chemotherapy29; thus, viability was determined in liquid culture. Cell fractions generated from histopaque centrifugation after 48 hours treatment with GW1843 were placed back into culture (0.05 × 106 cells/mL) and examined for outgrowth. In standard media, control cells underwent five doublings in 96 hours (doubling time is usually 21 hours under our conditions), while the live cells underwent less than one doubling, presumably because of persistent thymidylate synthase inhibition by GW1843 (Table 2). However, when 20 μmol/L thymidine was included in the media to bypass the TS block,9 the live cell fraction proceeded through two doublings in 48 hours and 3.9 doublings in 96 hours, one doubling less than untreated cells, indicating that the cells in this fraction were viable. The blebbed cells did not grow in culture with or without added thymidine (Table 2) indicating that they are committed to die.
GW1843-induced ceramide and SMase changes in Molt-4 cells during apoptosis.
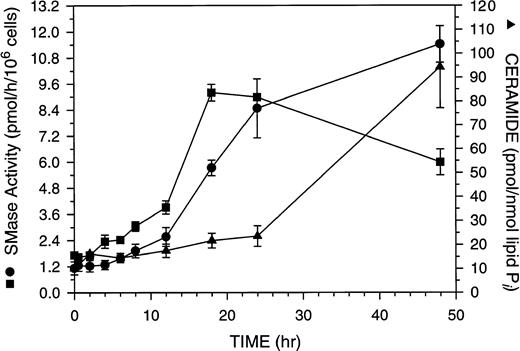
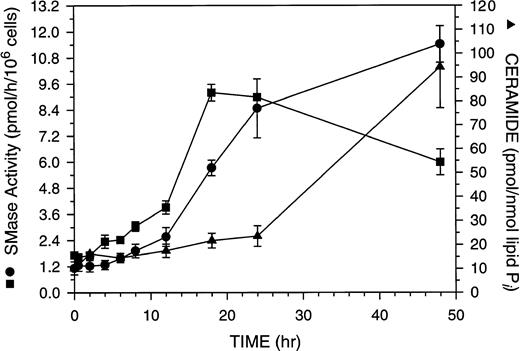
ASMase or NSMase have been implicated as components of the apoptotic signaling cascade,37 38 although data on the true kinetic competence of these enzymes in this signaling pathway is limited. The kinetics of NSMase, ASMase, and ceramide changes during GW1843-induced cell death are shown in Fig 5. Both ASMase and NSMase were elevated approximately fivefold over control after 18 hours of GW1843 treatment (Fig 5), a time when there was no reproducible increase in the number of blebbed or dead cells in the population (Fig 1). The time course for ASMase activation was slightly delayed compared with that for NSMase. At 18 hours, NSMase activity peaked at 5.4-fold over control, whereas ASMase was 5.3-fold over control, approximately 50% of its activity at 48 hours. By 48 hours, NSMase activity had declined to 3.5-fold over control, but the ASMase activity was further elevated to 10-fold over control in parallel with the accumulation of dead cells.
Time course of ceramide production and acidic pH, Mg2+-independent and neutral pH, Mg2+-dependent sphingomyelinase (ASMase and NSMase, respectively) activation in total populations of Molt-4 cells treated with 8 nmol/L GW1843. Log-phase Molt-4 cells were treated with 8 nmol/L GW1843, aliquots taken at the indicated times, and assayed for ASMase activity (•), NSMase activity (▪), and ceramide levels (▴) as described in Materials and Methods. The graph represents the average of four separate experiments (mean ± SEM).
Time course of ceramide production and acidic pH, Mg2+-independent and neutral pH, Mg2+-dependent sphingomyelinase (ASMase and NSMase, respectively) activation in total populations of Molt-4 cells treated with 8 nmol/L GW1843. Log-phase Molt-4 cells were treated with 8 nmol/L GW1843, aliquots taken at the indicated times, and assayed for ASMase activity (•), NSMase activity (▪), and ceramide levels (▴) as described in Materials and Methods. The graph represents the average of four separate experiments (mean ± SEM).
Ceramide is produced from the action of SMase on SM and has been reported to be a regulatory molecule for apoptosis.11 We used diacylglycerol (DAG) kinase to assay ceramide levels in GW1843-treated Molt-4 cells.32 Ceramide levels were modestly elevated at 18 to 24 hours (2.2-fold over control, Fig 5). At longer times, ceramide increases paralleled ASMase activity and continued to increase to 48 hours, at which time ASMase was elevated 10-fold over control and ceramide levels were 7.7-fold over control. The increases in ceramide are not due to an autocrine action of TNF-α39 in response to GW1843, as we were unable to detect any increase in TNF-α using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (data not shown). In contrast to the large changes in ceramide, we found no reproducible change in the level of DAG at any time point (data not shown).
Increases in SMase activity and ceramide levels in purified populations of Molt-4 cells undergoing apoptosis.
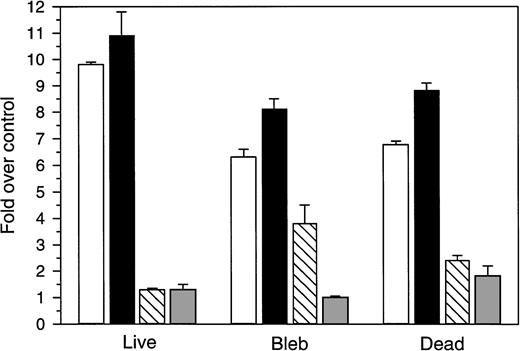
To provide additional insight on the relationship of the SMases and ceramide to GW1843-induced cell death, we determined their levels in the resolved histopaque cell subpopulations. Molt-4 cells were treated for 24 hours with 8 nmol/L GW1843, fractionated on histopaque, and the fractions assayed for both ASMase and NSMase activity, as well as ceramide levels. Interestingly, both ASMase and NSMase activities were dramatically elevated in all three cell types, including the normal-appearing cells with outgrowth potential (Fig 6). Despite the elevated SMase activity in all three cell types, the ceramide levels in the live cell fraction were unchanged (1.3-fold over control). Ceramide levels were substantially increased over control in the blebbed and dead cell fractions, 3.8- and 2.4-fold, respectively; thus, ceramide was only elevated in cells that were committed to apoptosis. These results, as with the kinetics, support an initiation role for the SMases with commitment to apoptosis occurring with ceramide accumulation.
Ceramide, DAG, and acidic pH, Mg2+-independent and neutral pH, Mg2+-dependent sphingomyelinase (ASMase and NSMase, respectively) activity in purified populations of Molt-4 cells treated with GW1843. Log-phase Molt-4 cells were treated with 8 nmol/L GW1843 for 24 hours, cell fractions were resolved on histopaque step gradients, and assayed for ASMase activity (open), NSMase activity (closed), ceramide levels (cross-hatched), and DAG (shaded) as described in Materials and Methods. Control activity of NSMase (mean ± SEM) was 3.3 ± 0.48 pmol/h/106 cells, control activity of ASMase was 3.1 ± 0.18 pmol/h/106cells and control levels of ceramide and DAG were 4.0 ± 1.8 and 29.2 ± 7.0 pmol/nmol lipid phosphate, respectively. The graph represents the average of four separate experiments (mean ± SEM).
Ceramide, DAG, and acidic pH, Mg2+-independent and neutral pH, Mg2+-dependent sphingomyelinase (ASMase and NSMase, respectively) activity in purified populations of Molt-4 cells treated with GW1843. Log-phase Molt-4 cells were treated with 8 nmol/L GW1843 for 24 hours, cell fractions were resolved on histopaque step gradients, and assayed for ASMase activity (open), NSMase activity (closed), ceramide levels (cross-hatched), and DAG (shaded) as described in Materials and Methods. Control activity of NSMase (mean ± SEM) was 3.3 ± 0.48 pmol/h/106 cells, control activity of ASMase was 3.1 ± 0.18 pmol/h/106cells and control levels of ceramide and DAG were 4.0 ± 1.8 and 29.2 ± 7.0 pmol/nmol lipid phosphate, respectively. The graph represents the average of four separate experiments (mean ± SEM).
Inhibition of caspase 3 does not affect activation of SMases or accumulation of ceramide by GW1843.
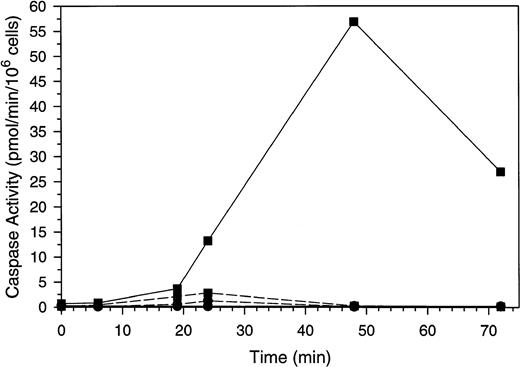
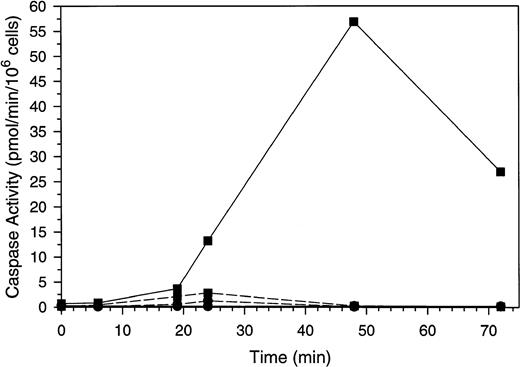
To exclude the possibility that the activation of SMase and the corresponding increase in ceramide is a consequence of apoptosis rather than a signal for this process, we sought to inhibit apoptosis in the Molt-4 cells in the presence of GW1843 and determine if increases in ceramide still occurred. Caspase 3 is activated late in apoptosis to cleave PARP and other proteins40 and can be inhibited in vitro and in cells with Ac-DEVD-CHO.41 Others have shown that inhibition of caspase 3 with this compound blocks apoptotic PARP cleavage and DNA degradation.42 Changes in caspase 3–like and ICE-like caspase activity in response to GW1843 are shown in Fig 7. Treatment with GW1843 alone resulted in a maximal 35-fold increase in caspase 3–like activity over the first 48 hours of treatment (paralleling cell death), which declined at 72 hours. Inclusion of the caspase 3 inhibitor Ac-DEVD-CHO in the enzyme assay completely blocked the activity confirming that this was caspase 3–like activity (data not shown).
Caspase activation by GW1843 in the presence and absence of Ac-DEVD-CHO. Cells were treated for the indicated times with 8 nmol/L GW1843 alone (▪) or in combination with 50 μmol/L Ac-DEVD-CHO (•). Aliquots were collected and assayed for caspase 3–like activity with Ac-DEVD-AMC as the substrate (solid lines) or ICE-like activity using Ac-YVAD-AMC as the substrate (dashed lines). Assays were performed as described in Materials and Methods. The data in these plots are from a representative experiment from three separate determinations.
Caspase activation by GW1843 in the presence and absence of Ac-DEVD-CHO. Cells were treated for the indicated times with 8 nmol/L GW1843 alone (▪) or in combination with 50 μmol/L Ac-DEVD-CHO (•). Aliquots were collected and assayed for caspase 3–like activity with Ac-DEVD-AMC as the substrate (solid lines) or ICE-like activity using Ac-YVAD-AMC as the substrate (dashed lines). Assays were performed as described in Materials and Methods. The data in these plots are from a representative experiment from three separate determinations.
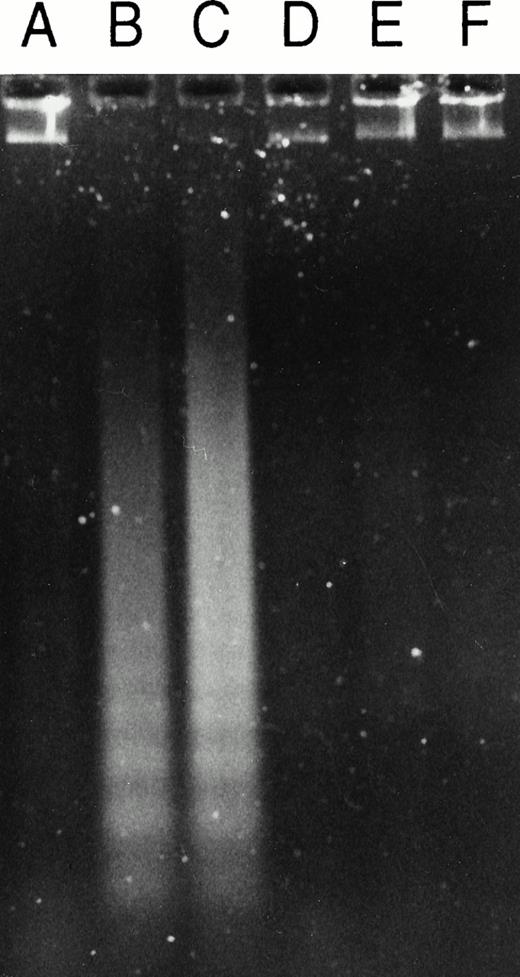
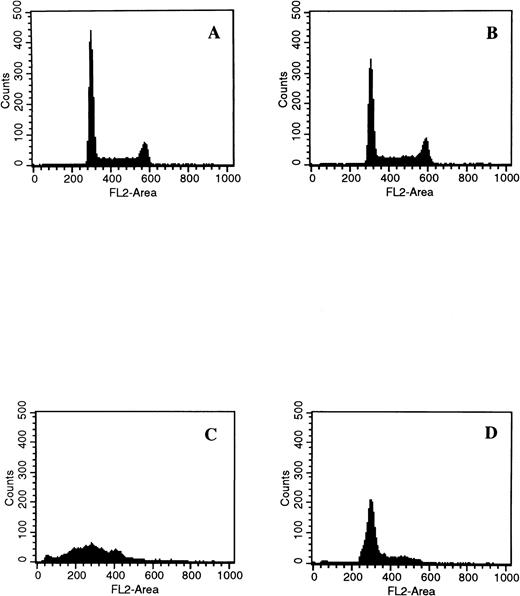
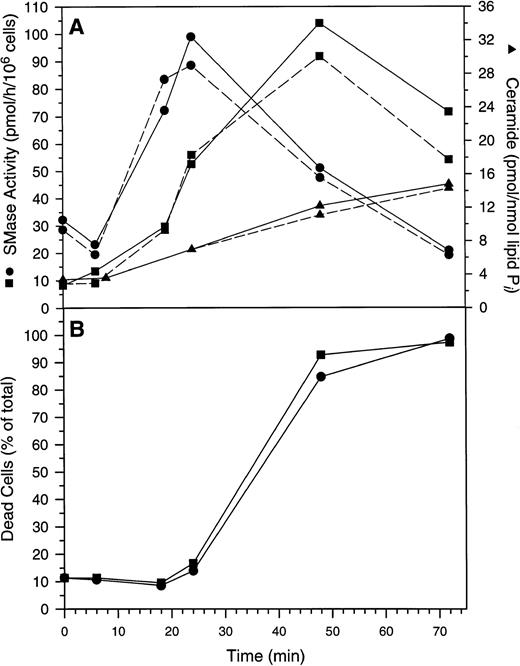
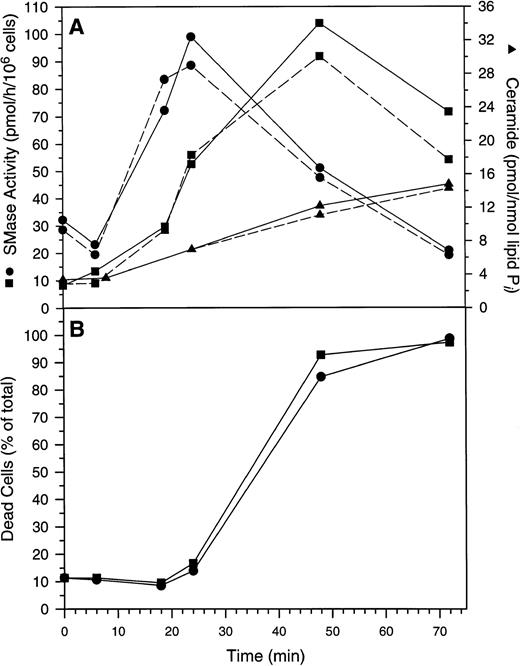
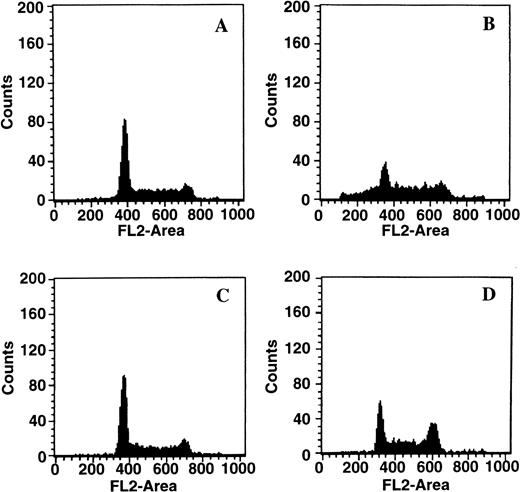
When cells were treated with 50 μmol/L Ac-DEVD-CHO plus GW1843, caspase 3–like activity was completely suppressed. This concentration of Ac-DEVD-CHO was found to be nontoxic to cells for at least 72 hours (data not shown). That caspase 3 was inhibited in vivo by 50 μmol/L Ac-DEVD-CHO was demonstrated by the inhibition of GW1843-induced DNA oligonucleosomal laddering determined with agarose gel electrophoresis (Fig 8, left) and the reduced formation of cells with <2N DNA per cell determined with FACS analysis (Fig 8, right). Thus, the observed apoptotic DNA degradation was dependent on caspase 3–like activity consistent with our observation of PARP degradation. By contrast, 50 μmol/L Ac-DEVD-CHO had no effect on the increases in ASMase or NSMase activities or ceramide levels observed on GW1843 treatment (Fig 9A).
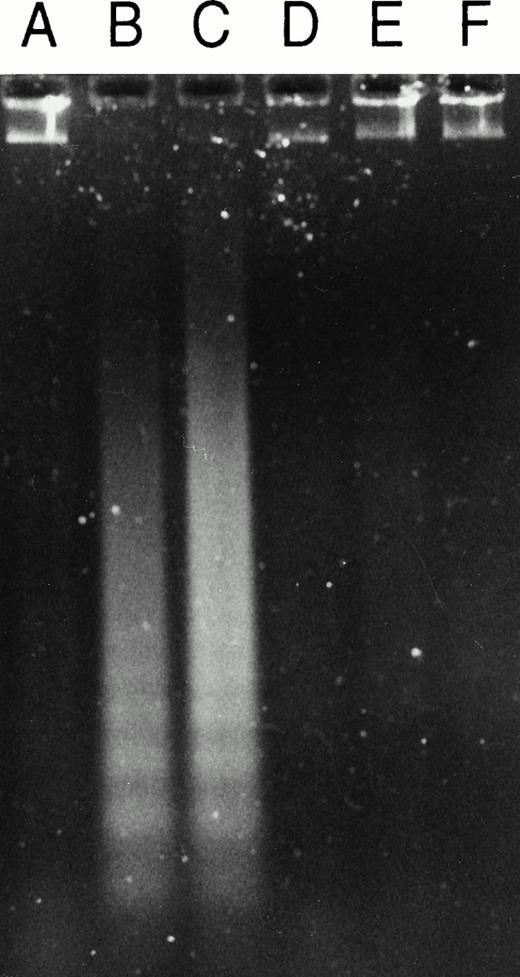
Effect of the caspase 3 inhibitor Ac-DEVD-CHO on GW1843-generated apoptotic DNA. (Left) Log-phase Molt-4 cells were treated for 48 hours with GW1843 alone or in combination with 50 μmol/L Ac-DEVD-CHO. Cells were harvested, DNA fragments isolated, and resolved using agarose gel electrophoresis as described in Materials and Methods. Lanes A, B, and C were treated with 0, 8, and 16 nmol/L GW1843, respectively. Lanes D, E, and F were treated with 0, 8, and 16 nmol/L GW1843, respectively, in the presence of 50 μmol/L Ac-DEVD-CHO. This gel is from a representative experiment from three separate determinations. (Right) Log-phase Molt-4 cells were treated for 48 hours as described below and prepared for FACS analysis as described in Materials and Methods. Cells were treated as follows: (A), mock treated cells; (B), 50 μmol/L Ac-DEVD-CHO; (C), 8 nmol/L GW1843; and (D), 8 nmol/L GW1843 with 50 μmol/L Ac-DEVD-CHO.
Effect of the caspase 3 inhibitor Ac-DEVD-CHO on GW1843-generated apoptotic DNA. (Left) Log-phase Molt-4 cells were treated for 48 hours with GW1843 alone or in combination with 50 μmol/L Ac-DEVD-CHO. Cells were harvested, DNA fragments isolated, and resolved using agarose gel electrophoresis as described in Materials and Methods. Lanes A, B, and C were treated with 0, 8, and 16 nmol/L GW1843, respectively. Lanes D, E, and F were treated with 0, 8, and 16 nmol/L GW1843, respectively, in the presence of 50 μmol/L Ac-DEVD-CHO. This gel is from a representative experiment from three separate determinations. (Right) Log-phase Molt-4 cells were treated for 48 hours as described below and prepared for FACS analysis as described in Materials and Methods. Cells were treated as follows: (A), mock treated cells; (B), 50 μmol/L Ac-DEVD-CHO; (C), 8 nmol/L GW1843; and (D), 8 nmol/L GW1843 with 50 μmol/L Ac-DEVD-CHO.
Changes in Molt-4 cells after treatment with GW1843. (A) Changes in acidic pH, Mg2+-independent sphingomyelinase activity (ASMase), neutral pH, Mg2+-dependent sphingomyelinase activity (NSMase), and ceramide. Log-phase Molt-4 cells were treated for the indicated times with 8 nmol/L GW1843 alone (solid lines) or in combination with 50 μmol/L Ac-DEVD-CHO (dashed lines). Cell aliquots were assayed for ASMase activity (▪), NSMase activity (•), or ceramide levels (▴) as described in Materials and Methods. (B) Quantitation of dead cells (both blebbed and dead fractions). Log-phase Molt-4 cells were treated for the indicated times with 8 nmol/L GW1843 alone (▪) or in combination with 50 μmol/L Ac-DEVD-CHO (•). Cells aliquots assayed for the number of blebbed and dead cells after trypan blue staining as described in Materials and Methods. This data is from a representative experiment from three separate determinations.
Changes in Molt-4 cells after treatment with GW1843. (A) Changes in acidic pH, Mg2+-independent sphingomyelinase activity (ASMase), neutral pH, Mg2+-dependent sphingomyelinase activity (NSMase), and ceramide. Log-phase Molt-4 cells were treated for the indicated times with 8 nmol/L GW1843 alone (solid lines) or in combination with 50 μmol/L Ac-DEVD-CHO (dashed lines). Cell aliquots were assayed for ASMase activity (▪), NSMase activity (•), or ceramide levels (▴) as described in Materials and Methods. (B) Quantitation of dead cells (both blebbed and dead fractions). Log-phase Molt-4 cells were treated for the indicated times with 8 nmol/L GW1843 alone (▪) or in combination with 50 μmol/L Ac-DEVD-CHO (•). Cells aliquots assayed for the number of blebbed and dead cells after trypan blue staining as described in Materials and Methods. This data is from a representative experiment from three separate determinations.
ICE-like activity was also quantitated during GW1843-induced apoptosis of Molt-4 cells (Fig 7). This activity was not detectable under basal conditions, but was detected maximally at 24 hours after GW1843 treatment. We estimate the increase over control activity to be approximately twofold and at this level, was at least 25-fold less than the maximal activation of caspase 3–like activity.
Quite surprisingly, while Ac-DEVD-CHO blocked DNA degradation, it had no effect on the morphologic changes that occur during GW1843-induced Molt-4 cell death (blebs, lysis, or nuclear margination). This is shown in Fig 9B where it can be seen that kinetics for blebbed and lysed cell accumulation were equal with and without Ac-DEVD-CHO.
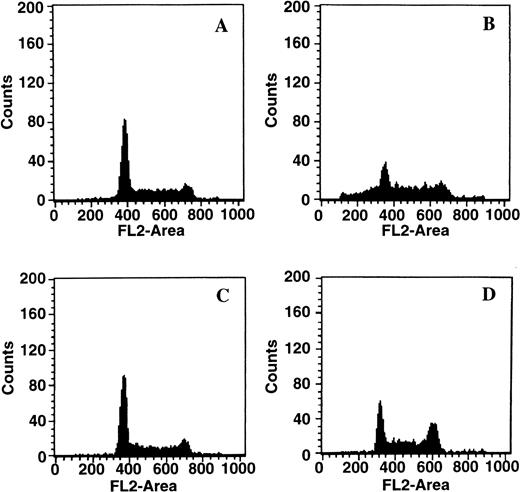
To determine whether C2-ceramide could mimic the action of the GW1843-induced intracellular ceramide increases, Molt-4 cells were treated with 20 μmol/L C2-ceramide. This treatment resulted in cell death and fragmentation of DNA, confirming previous reports that ceramide can activate apoptosis.11,13,14,25 43-47 Representative data are shown in Fig 10. Treatment of Molt-4 cells for 17 hours with 20 μmol/L C2-ceramide led to accumulation of cells with less than 2N DNA content (Fig 10B), similar to results shown in Fig 8 for GW1843. Incubation of cells with 50 μmol/L Ac-DEVD-CHO alone was without effect (Fig 10C); however, incubation of cells with both 50 μmol/L Ac-DEVD-CHO and 20 μmol/L C2-ceramide completely prevented the ceramide-induced degradation of DNA (Fig 10D), as shown for GW1843 in Fig 8.
Effect of caspase 3 inhibition on apoptotic DNA fragmentation in response to C2-ceramide. Log-phase Molt-4 cells were treated as follows: (A) 0.2% dimethyl sulfoxide (DMSO) for 17 hours; (B) 20 μmol/L C2-ceramide for 17 hours; (C) 50 μmol/L Ac-DEVD-CHO for 35 hours; (D) 18-hour preincubation with 50 μmol/L Ac-DEVD-CHO followed by 17 hours coincubation with 20 μmol/L C2-ceramide. Cells were harvested and prepared for FACS analysis as described in Materials and Methods. This data is a representative experiment from three separate determinations.
Effect of caspase 3 inhibition on apoptotic DNA fragmentation in response to C2-ceramide. Log-phase Molt-4 cells were treated as follows: (A) 0.2% dimethyl sulfoxide (DMSO) for 17 hours; (B) 20 μmol/L C2-ceramide for 17 hours; (C) 50 μmol/L Ac-DEVD-CHO for 35 hours; (D) 18-hour preincubation with 50 μmol/L Ac-DEVD-CHO followed by 17 hours coincubation with 20 μmol/L C2-ceramide. Cells were harvested and prepared for FACS analysis as described in Materials and Methods. This data is a representative experiment from three separate determinations.
As with GW1843, Ac-DEVD-CHO had no effect on C2-ceramide–dependent plasma membrane breakdown. After 8 hours, 76% of the cells were viable and 24% were dead in the presence of C2-ceramide alone, and 79% cells were viable and 21% were dead in the presence of the combination of C2-ceramide and Ac-DEVD-CHO. After 17 hours, 22% of the cells were viable and 78% were dead in the presence of C2-ceramide alone and 28% of the cells were viable and 72% were dead in the presence of the combination. Thus, as with GW1843-induced membrane changes in Molt-4 cells, C2-ceramide–induced membrane changes were also independent of caspase 3–like activity.
DISCUSSION
The goal of this study was to investigate the role of ceramide and the SMases in the apoptosis initiated by GW1843, a highly specific TS inhibitor. The current data provide strong, novel evidence for their intimate role in the process at a phase temporally preceding caspase 3 activation. Induction of apoptosis by GW1843 in the Molt-4 cells was demonstrated using a number of criteria including morphologic changes characteristic of apoptosis determined with phase contrast and transmission electron microscopy, DNA degradation to the characteristic incremental approximately 180-bp ladder, caspase 3 activation, proteolytic cleavage of PARP from the intact Mr116,000 form to the Mr 85,000 fragment, and flipping of PS from the inner to the outer leaflet of the plasma membrane. All of these changes were found to accumulate coordinately as normal-appearing live cells became blebbed and lysed cells. Consistent with this, the blebbed cells were found to be committed to cell death, as thymidine, which bypasses the GW1843 block of TS, was not able to rescue them, but was able to rescue a large proportion of the normal-appearing, live cells.
The data in Figs 5, 6, and 9A show that ceramide accumulated in parallel with the accumulation of dead cells. Thus at 24 hours, the ceramide level in the live cell population was 1.3-fold over control, whereas in the blebbed population, it was 3.8-fold over control. The kinetics of ceramide accumulation in the total population (Figs 5 and9A) were also consistent with increased ceramide levels signaling commitment to apoptosis. Thus, only modest ceramide elevations ≤ twofold over control, were found before 24 hours, the time at which the blebbed and lysed cells began to appear. After this time, ceramide levels increased along with the number of blebbed and lysed cells to approximately eightfold over control by 48 hours.
Activation of NSMase and ASMase clearly preceded commitment to apoptosis as shown in Figs 5, 6, and 9A. Thus, these enzymes (and caspase 3) showed significant elevations in enzymatic activity by 18 hours, when few cells were committed to apoptosis. Indeed, NSMase was fully activated by 18 hours. Activation of ASMase and caspase 3 lagged behind NSMase; activity of both of these enzymes increased through 48 hours. Because NSMase activation and increased ceramide generation precede caspase 3 activation, it is feasible that NSMase-derived ceramide provides the caspase 3 activation signal. That activation of the SMases preceded commitment was also shown using the histopaque purified cell fractions. Thus, both enzymes were elevated about 10-fold in the purified live cell fraction, and no further increase was observed in either activity in the apoptosis-committed blebbed cells.
PS has been shown to externalize during apoptosis.35 36 We showed here that this is also observed during GW1843-induced cell death, and the PS externalization observed here occurred after the morphologic change from the normal-appearing viable cells to blebbed cells (Fig 4). An important aspect of our observed activation of the SMases in the viable cell fraction, before conversion to the blebbed cells, is that SMase activation cannot, therefore, be a result of the PS flip. Thus, SMase activation is upstream of the PS flip. Whether the PS flip is a result of SMase activity cannot be resolved from our data, however.
Activation of the SMases before commitment, as shown from the activity in the live cell fraction, is a necessary condition for a role of these enzymes in initiating commitment to apoptosis. To further elucidate the sequence of events, Molt-4 cells were treated with a combination of GW1843 to initiate the apoptotic process, plus Ac-DEVD-CHO to block cellular caspase 3–like activity. In these experiments, we asked whether this caspase 3 inhibition would prevent SMase activation and ceramide accumulation. Neither SMase nor ceramide levels were affected by the addition of the caspase 3 inhibitor that completely blocked caspase 3–like activity and DNA degradation (Figs 7 and 8). The results clearly show that SMase activation is not dependent on prior caspase 3 activation. Rather, the data show that SMase activation and ceramide accumulation are either upstream of caspase 3 activation or on parallel, independent paths. Consideration that ceramide itself can lead to caspase 3 activation and/or DNA degradation as shown here and elsewhere,11,13,14,25 43-47 strongly suggests that the ceramide generated via SMase activity in response to GW1843 is indeed upstream of caspase 3.
The observation that Ac-DEVD-CHO was able to block GW1843-induced DNA degradation, but not bleb formation or lysis of Molt-4 cells, was unexpected, and we do not yet completely understand its significance. Nonetheless, it clearly shows that membrane events including SMase activation, ceramide accumulation, and cell lysis can proceed in the absence of caspase 3–like activity or DNA breakdown, which may be a chemotherapeutic advantage by circumventing some cellular resistance mechanisms. The mechanism by which apoptosis is initiated with a membrane-generated signal in the absence of a DNA degradation signal awaits further investigation. This observation is consistent with another recent report on the effect of a caspase inhibitor on apoptosis.48 These investigators observed that ZVAD-FMK completely blocked cleavage of PARP and DNA on induced BAX expression. This treatment, however, had no effect on the fall in mitochondrial membrane potential, production of reactive oxygen, or membrane blebbing induced by BAX, indicating that this latter set of changes was caspase independent. Taken together, the data reported here and previously indicate that molecules like BAX and GW1843 activate lethal membrane events and DNA degradation likely in parallel. In the case of GW1843, ceramide may be involved in signaling because ceramide can be shown to activate caspase 3–like activity,46 47 and, as we show here, ceramide can produce cell lysis in the presence of blocked caspase 3 activation and DNA degradation.
Which SMase is the relevant enzyme for the production of ceramide involved in the membrane or DNA degradation events reported here is unclear. While the NSMase was activated earlier in the time course of GW1843 treatment, a role for the ASMase cannot be ruled out; indeed activation of ASMase more closely paralleled accumulation of ceramide and apoptotic cells. It is feasible that both enzymes are involved. Others have reported that fibroblasts from ASMase-deficient Niemann-Pick patients or some tissues from ASMase knockout mice that do not express ASMase are resistant to apoptosis.49 Similarly, it has been reported that loss of NSMase activity is associated with resistance to apoptosis.50 51 Experiments with specific inhibitors of each enzyme will be required to dissect the roles of the individual SMases in the process of GW1843-induced cell death in Molt-4 cells.
An important, but nascent area of investigation, is the mechanism of the SMase activation observed in the many conditions and cell types studied. It is not known whether the increases in SMase activities reported here and elsewhere are due to induction of enzyme synthesis or to activation of preexisting enzyme. Because the time course for activation of the SMases in our system is rather long, it is conceivable that both processes could be at work. The activation of NSMase in response to TNF-α has been proposed to occur via indirect coupling of the enzyme to p55 TNF-R through the coupling protein, FAN, without the need for protein synthesis.21,38,52 Similarly, the activation of ASMase in response to TNF-α has been proposed to occur via coupling to another region of the TNFR1 intracellular domain.21 Whether, the apoptotic signal generated by GW1843 is transduced via a cell surface receptor is unknown, but we found no detectable Molt-4 synthesis of TNF-α in response to GW1843 treatment, and Molt-4 cells do not respond to an anti-Fas–induced apoptotic signal53 (C.J.S., unpublished observations).
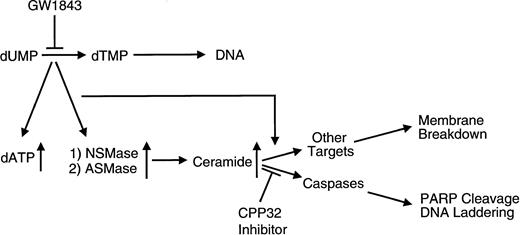
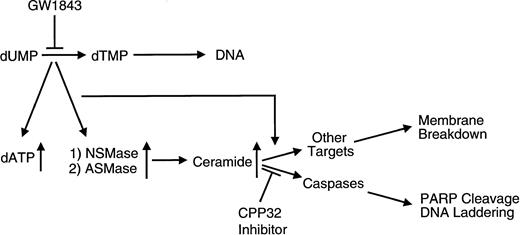
In summary, we have shown that GW1843 induces apoptosis in Molt-4 cells. Prior to commitment to apoptosis, both NSMase and ASMase are activated with a parallel increase in ceramide levels. Caspase 3 inhibition, which completely blocks DNA degradation, does not influence the activation of either SMase or the accumulation of ceramide. Further, the caspase inhibitor does not influence membrane breakdown due to either GW1843 or ceramide in this cell line. The results support a role for the SMase(s) and ceramide in the initiation of apoptosis at both the membrane and nuclear level. The order of events in GW1843-induced apoptosis of Molt-4 cells consistent with our observations is shown in Fig 11. The main processes include inhibition of TS, followed by loss of thymidine triphosphate and accumulation of deoxyadenosine triphosphate (dATP);54 activation of NSMase is followed by activation of ASMase resulting in the generation of ceramide from SM. There are two distinct pathways at this point, in one activation of caspase 3 (perhaps by a signal initiating from ceramide) leads to PARP degradation and DNA laddering. In the other pathway, ceramide increases lead to plasma membrane blebbing and lysis.
Schematic diagram representing the major processes involved in GW1843-induced apoptosis of Molt-4 cells. GW1843 inhibits the production of thymidine monophosphate (TMP), which is essential to DNA replication, resulting in accumulation of dATP. An uncharacterized signal causes the activation of both acidic pH, Mg2+-independent sphingomyelinase activity (ASMase) and neutral pH, Mg2+-dependent sphingomyelinase activity (NSMase). An increase in intracellular ceramide levels as a consequence of the increased SMase activity causes plasma membrane breakdown and may also activate caspases. The caspase pathway is sensitive to CPP32 inhibition and is characterized by events recognized as hallmarks of apoptosis such as PARP cleavage and DNA laddering.
Schematic diagram representing the major processes involved in GW1843-induced apoptosis of Molt-4 cells. GW1843 inhibits the production of thymidine monophosphate (TMP), which is essential to DNA replication, resulting in accumulation of dATP. An uncharacterized signal causes the activation of both acidic pH, Mg2+-independent sphingomyelinase activity (ASMase) and neutral pH, Mg2+-dependent sphingomyelinase activity (NSMase). An increase in intracellular ceramide levels as a consequence of the increased SMase activity causes plasma membrane breakdown and may also activate caspases. The caspase pathway is sensitive to CPP32 inhibition and is characterized by events recognized as hallmarks of apoptosis such as PARP cleavage and DNA laddering.
Address reprint requests to Ronald M. Laethem, PhD, Glaxo Wellcome, Inc, 5 Moore Dr, Research Triangle Park, NC 27709.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.