Abstract
Three chimpanzees experimentally infected with human immunodeficiency virus (HIV) developed significant chronic thrombocytopenia after 5, 4, and 2 years, with peripheral platelet counts averaging 64 ± 19 × 103/μL (P = .004 compared with 228 ± 92 × 103/μL in 44 normal control animals), mean platelet volumes of 11.2 ± 1.8 fL (P > .5 compared with 10.9 ± 0.7 fL in normal controls), endogenous thrombopoietin (TPO) levels of 926 ± 364 pg/mL (P < .001 compared with 324 ± 256 pg/mL in normal controls), uniformly elevated platelet anti-glycoprotein (GP) IIIa49-66 antibodies, and corresponding viral loads of 534, 260, and 15 × 103 RNA viral copies/mL. Pegylated recombinant human megakaryocyte growth and development factor (PEG-rHuMGDF) was administered subcutaneously (25 μg/kg twice weekly for 3 doses) to determine the effects of stimulating platelet production on peripheral platelet concentrations in this cohort of thrombocytopenic HIV-infected chimpanzees. PEG-rHuMGDF therapy increased (1) peripheral platelet counts 10-fold (from 64 ± 19 to 599 ± 260 × 103 platelets/μL;P = .02); (2) marrow megakaryocyte numbers 30-fold (from 11.7 ± 6.5 × 106/kg to 353 ± 255 × 106/kg;P = .04); (3) marrow megakaryocyte progenitor cells fourfold (from a mean of 3.6 ± 0.6 to 14.1 × 103 CFU-Meg/1,000 CD34+ marrow cells); and (4) serum levels of Mpl ligand from 926 ± 364 pg/mL (endogenous TPO) to predosing trough levels of 1,840 ± 353 pg/mL PEG-rHuMGDF (P = .02). The peripheral neutrophil counts were also transiently increased from 5.2 ± 2.6 × 103/μL to 9.9 ± 5.0 × 103/μL (P= .01), but neither the erythrocyte counts nor the reticulocyte counts were altered significantly (P > .1). The serum levels of antiplatelet GPIIIa49-66 antibodies exhibited reciprocal reductions during periods of thrombocytosis (P < .07). PEG-rHuMGDF therapy did not increase viral loads significantly (395, 189, and 53 × 103 RNA viral copies/mL; P > .5 compared with baseline values). The striking increase in peripheral platelet counts produced by PEG-rHuMGDF therapy implies that thrombocytopenia in HIV-infected chimpanzees is attributable to insufficient compensatory expansion in platelet production resulting from HIV-impaired delivery of platelets despite stimulated megakaryocytopoiesis. These data suggest that PEG-rHuMGDF therapy may similarly correct peripheral platelet counts in thrombocytopenic HIV-infected patients.
CHIMPANZEES ARE reproducibly infected with human immunodeficiency virus type 1 (HIV) by injecting cell-free virus, infected peripheral blood mononuclear leukocytes, or homogenates of infected tissues from HIV-infected donors.1-3 Infected chimpanzees develop humoral responses similar to HIV-infected patients, and viral loads gradually decrease during the first year of infection due to immunologic and cellular clearance mechanisms, analogous to asymptomatic human HIV carriers.4,5 Chronic thrombocytopenia in 1 HIV-infected chimpanzee has been reported6 and was associated with chronic lymphocytopenia affecting both CD4+ and CD8+ T-cell counts, an eightfold increase in HIV-specific antibody titer, and the presence of cell-free virus in plasma.
Three additional chimpanzees have now developed chronic thrombocytopenia and lymphocytopenia at Yerkes Regional Primate Research Center (Atlanta, GA). The present study evaluates the effects of stimulating megakaryocytopoiesis in this cohort of HIV-infected thrombocytopenic chimpanzees by administering pegylated recombinant human megakaryocyte growth and development factor (PEG-rHuMGDF) and measuring (1) peripheral platelet counts, mean platelet volumes (MPVs), and platelet thrombopoietin (TPO) receptor numbers; (2) marrow megakaryocyte numbers, volumes, ploidy distributions, and CD34+ megakaryocyte progenitor cells; (3) trough levels of PEG-rHuMGDF; (4) antiplatelet glycoprotein (GP) IIIa49-66 antibodies7; and (5) viral loads.
MATERIALS AND METHODS
Animals studied.
Three chimpanzees (Pan troglodytes) previously infected with HIV and maintained at Yerkes Regional Primate Research Center were included in this study.6 All procedures were approved by the Institutional Animal Care and Use Committee and conducted in accordance with federal guidelines (Guide for the Care and Use of Laboratory Animals, National Institutes of Health, Bethesda, MD, NIH Publication No. 86-23). Ketamine hydrochloride (5 to 20 mg/kg intramuscularly) was administered to achieve short-term immobilization for obtaining blood samples, bone marrow aspirates, and biopsies.
Study design.
The effects of administering PEG-rHuMGDF (25 μg/kg subcutaneously twice weekly on Monday and Thursday) were assessed in 3 thrombocytopenic HIV-infected chimpanzees. A twice weekly dose regimen was selected because animal accession by darting was practically limited to once every few days, and every other day administration of PEG-rHuMGDF had previously been shown to stimulate megakaryocytopoiesis adequately.8 9 Before the initial dose of PEG-rHuMGDF, baseline blood and marrow samples were obtained. Before each subsequent dose of PEG-rHuMGDF, blood was obtained for complete blood counts (including leukocyte counts with differential counts and platelet counts), serum antiplatelet GPIIIa49-66 antibodies, and serum PEG-rHuMGDF levels. When the platelet counts reached normal values, dosing was discontinued and blood samples were obtained for lymphocyte counts, antiplatelet GPIIIa49-66 antibodies, serum PEG-rHuMGDF levels, and viral loads. In addition, bone marrow cells were obtained for repeat morphologic evaluation, flow cytometric quantitation of megakaryocytopoiesis, and in vitro cell culture assessment of megakaryocytic progenitor responsiveness to PEG-rHuMGDF. Therapy was discontinued when the peripheral platelet counts reached normal levels.
PEG-rHuMGDF reagent.
PEG-rHuMGDF, a gift from Amgen Inc (Thousand Oaks, CA), is a nonglycosylated polypeptide produced in Escherichia coli-transfected with a plasmid containing cDNA that encodes for the 1- to 163-residue aminoterminus of human Mpl ligand. The resulting polypeptide is covalently coupled to poly ethylene glycol.10 11 After extraction, refolding, and purification, this truncated protein was supplied as a sterile, clear, aqueous solution.
Laboratory procedures.
Peripheral platelet counts, mean platelet volumes, red blood cell counts, and total white blood cell counts were determined in whole blood collected in Na2EDTA (2 mg/mL) using Serono/Baker model 9000 whole blood analyzer (Allentown, PA).12-14
HIV serologic status was determined using a commercially available enzyme-linked immunosorbent assay (ELISA) and confirmed with Western blot assay.15
Plasma viral load was assayed by means of a reverse transcription polymerase chain reaction (PCR).3,6,16 The quantitative HIV RNA PCR assay was performed according to the manufacturer's instructions (Amplicor HIV-1 Monitor Test; Roche Diagnostic Systems, Branchburg, NJ). RNA was extracted from heparinized samples using silica.17 Fifty microliters of each prepared RNA sample was used for the PCR assay. After amplification and detection of the PCR product, the initial HIV RNA load in each sample was calculated by comparing it with the internal quantitation standard, and the results were expressed as HIV RNA copies per milliliter of plasma.
Antiplatelet GPIIIa49-66 antibody profile was performed as recently described.7 In prior studies, the strong correlation between antiplatelet GP IIIa49-66 antibody levels and thrombocytopenia has been interpreted to reflect direct binding of these antiplatelet GPIIIa49-66 antibodies to platelet GP IIIa49-66 epitope7 as well as contributing to the binding of immune complexes to platelets.18
Serum levels of endogenous TPO and PEG-rHuMGDF were determined using ELISAs involving initial polyclonal antibody capture procedure followed by enzyme product formation and determination.19 20
The baseline number of platelet receptors was obtained before and 10 days after initiating PEG-rHuMGDF therapy using purified rHu-TPO (a gift from Amgen Inc) radiolabeled by Iodo-beads iodination reagent (Pierce, Rockford, IL). rHu-TPO was incubated with 50 mmol/L sodium phosphate buffer, pH 7.2, and 125I for 15 minutes. Platelets were obtained from blood drawn in acid-citrate-dextrose (ACD) anticoagulant, pelleting platelets from platelet-rich plasma by centrifuging at 500g for 15 minutes, and resuspension in Tyrode's buffer containing 1:7 vol/vol ACD, pH 6.2, 1% bovine serum albumin (BSA), and 0.01% Tween. Binding isotherms were determined by incubating platelets in plasma-free Tyrode's buffer, 1:7 vol/vol ACD, 1% BSA, 0.01% Tween, pH 6.2, and various amounts of125I-TPO (40 to 640 ng/mL final concentrations) for 1 hour at room temperature. Nonspecific binding was assessed comparing the effects of adding 100-fold excess unlabeled TPO 30 minutes before the addition of125I-TPO. Nonspecific binding ranged from 10% to 20%. Binding isotherms were analyzed using the Biosoft Ligand Program (Cambridge, UK) to compute the number of binding classes, the number of molecules bound per platelet, and the dissociation constant (Kd).
The appearance of activated platelets in the peripheral blood was evaluated by flow cytometry using fluoresceinated monoclonal antibodies (MoAbs) against neoantigen(s) comprising conformationally altered activated GPIIb/IIIa, ligand-induced binding sites (LIBS; a gift from Dr E. Plow, La Jolla, CA).21,22 In addition, enhanced binding to platelets by fluoresceinated annexin V (a gift from Dr T. Yokoyama, Tokyo, Japan) was also examined using flow cytometry.23-25 Flow cytometric analysis was performed using FACScan (Becton Dickinson, San Jose CA).
Measurements of platelet production.
Megakaryocyte number, size, and ploidy were measured by flow cytometry using a previously reported method for multiparameter correlative marrow analysis with a single-argon ion laser FACScan analyzer (Becton Dickinson).26-31 Cell DNA in aspirated marrow was stained with propidium iodide, and surface membrane receptors were analyzed using specific MoAbs labeled with fluorescein. Megakaryocytes expressing GPIIb/IIIa were enumerated in relation to the nucleated erythroid precursors expressing glycophorin A.29,32Measurements of megakaryocyte diameters were based on the time of flight principle, ie, time required for a cell in suspension to pass through a focused light beam.26,27,29 31 Megakaryocytes were selected on the basis of their distinct immunofluorescence at levels above that of control cells labeled with an unrelated MoAb. In each sample, 2,000 to 3,000 megakaryocytes were analyzed. Bone marrow aspirates were obtained baseline and after peaking of the platelet counts after the administration of PEG-rHuMGDF.
Estimates of marrow megakaryocyte mass were used to represent the marrow substrate giving rise to circulating platelets and were calculated as the product of megakaryocyte numbers and mean megakaryocyte volumes.33 34 Normal chimpanzee marrow values (n = 10) averaged megakaryocyte diameter of 39 μg (range, 21 μg for 2N to 56 μg for 64N cells), volume of 28 ± 4.5 × 103 fL, and megakaryocyte number of 11 ± 2.1 × 106/kg, giving a total megakaryocyte mass of 31 ± 5.3 × 1010 fL/kg. The normal modal ploidy was 16N.
Marrow megakaryocyte progenitors.
The assays for megakaryocyte colony-forming units (CFU-Meg) was based on a plasma clot matrix formed from human citrated AB plasma.35 Aliquots of 5 to 10 mL of bone marrow were collected in heparin. Cells were diluted in modified Hank's buffered saline solution (HBSS) layered over Ficoll-Hypaque and centrifuged at 2,000 rpm in a Sorvall RT6000 at room temperature for 30 minutes. The mononuclear layer was collected, diluted with HBSS, washed twice by centrifugation at 1,500 rpm for 5 min/wash, and then counted. CD34+ cells used in the megakaryocyte assay were selected using the Miltenyi Biotech MiniMACS magnetic cell separation kit (Miltenyi Biotech, Sunnyvale, CA). Postcolumn purity of the CD34+ cell fraction was determined by staining an aliquot of cells with phycoerythrin-conjugated HPCA-2 MoAb (Becton Dickinson Immunocytometry Systems, San Jose, CA) and subsequent FACS analysis. PEG-rHuMGDF was used at a final concentration of 10 ng/mL and cells were plated in a modified Iscove's modified Dulbecco's medium (IMDM) at 2 × 104 cells/mL in 15% human AB plasma. Cells were cultured in a 24-well microtiter plate with 300 μL/well volumes in triplicate for 8 days in a 37°C incubator with 5% CO2 humidity. Cultures were fixed with methanol:acetone (1:2) and stained with antiplatelet CD41/42 (GPIIb/IIIa) MoAbs, followed by goat antimouse fluorescein isothiocyanate (FITC). Nuclei were stained with propidium iodide. A CFU-Meg colony was defined as ≤3 brightly fluorescent cells by inverted fluorescence microscopy.
Morphology.
Marrow biopsies were obtained from the posterior superior iliac spine before and immediately after the last dose of PEG-rHuMGDF therapy. The biopsies were fixed in 10% buffered formalin solution, embedded in paraffin, sectioned, and stained with polychromatophilic dyes for examination at the light level.
Data analysis.
Data were analyzed using SIGMA STAT (Jandel Scientific Software, San Rafael, CA). Comparisons between two groups were performed using the two-tailed Student's t-test, unless the data were not distributed randomly, in which case nonparametric analysis was performed. Analysis of variance was used to compare values for a particular group at various time points.36 Unless otherwise stated, variance about the mean is given as ±1 SD.
RESULTS
Three chimpanzees, 2 males and 1 female, experimentally infected with HIV developed significant chronic thrombocytopenia 5, 4, and 2 years after infection. Peripheral platelet counts averaged 64 ± 19 × 103/μL (P = .004 compared with 228 ± 92 × 103/μL in 44 normal control animals; Table 1), and the mean platelet volume was 11.2 ± 1.8 fL (P > .5 compared with 10.9 ± 0.7 fL in controls). Endogenous TPO levels were substantially increased, averaging 926 ± 364 pg/mL (P < .001 compared with 324 ± 256 pg/mL in normal controls; Table 1). The circulating load of HIV was 534, 260, and 15 × 103 RNA viral copies/mL, respectively (Table 1). Antiplatelet GPIIIa49-66 antibodies were readily detected in the sera of all 3 animals (Table 1).
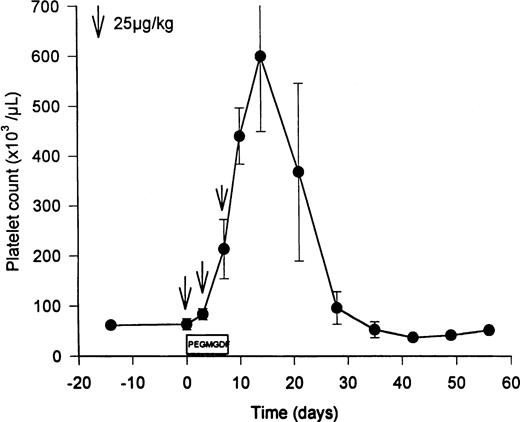
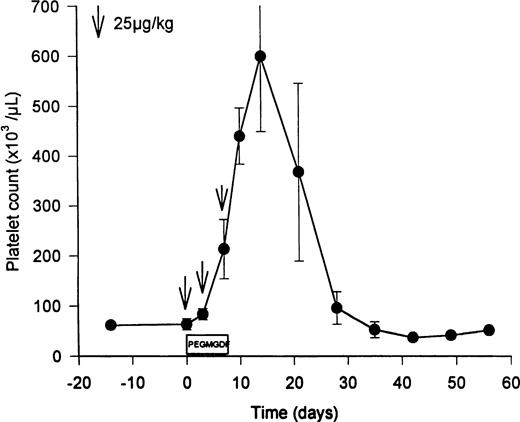
PEG-rHuMGDF (3 doses of twice weekly of 25 μg/kg) increased the levels of Mpl ligand from the basal levels of 926 ± 364 pg/mL (endogenous TPO) to predosing trough levels of 1,840 ± 353 pg/mL PEG-rHuMGDF (Table 1; P = .02). Three doses of PEG-rHuMGDF amplified peripheral platelet counts 10-fold (Fig 1), peaking at 599 ± 260 × 103 platelets/μL on day 12 (P = .02 compared with 64 ± 19 × 103 platelets/μL pretreatment; Table1), and gradually returned to baseline values over the subsequent 2 weeks (Fig 1). During thrombocytosis, the mean platelet volume was reciprocally reduced to 9.5 ± 0.9 fL (P = .1 compared with 11.2 ± 1.8 fL pretreatment), analogous to the decrease observed in baboons after dosing with PEG-rHuMGDF.37,38 Resting unstimulated platelets did not express membrane activation markers during PEG-rHuMGDF therapy (ie, LIBS were 290 ± 17 v 429 ± 146 LIBS/platelet baseline, and annexin V binding sites were 3,900 ± 786 v2,570 ± 1,040 annexin V binding sites/platelet baseline; P> .3 in both cases), concordant with the absence of platelet activation in baboons receiving PEG-rHuMGDF.37 38Similarly, PEG-rHuMGDF therapy did not change platelet TPO receptor numbers significantly (99 ± 43 v 152 ± 74 receptors/platelet baseline; P = .11).
Elevation of peripheral platelet counts in thrombocytopenic HIV-infected chimpanzees by PEG-rHuMGDF. Three doses of PEG-rHuMGDF (25 μg/kg on days 1, 4, and 7) produced peripheral platelet counts that peaked at 599 ± 260 × 103platelets/μL (P = .02 compared with pretreatment value of 64 ± 19) 3 days after discontinuing therapy. The timing of the 3 doses of PEG-rHuMGDF is indicated by the downward arrows. Platelet counts returned to baseline thrombocytopenic levels during the subsequent 2 weeks.
Elevation of peripheral platelet counts in thrombocytopenic HIV-infected chimpanzees by PEG-rHuMGDF. Three doses of PEG-rHuMGDF (25 μg/kg on days 1, 4, and 7) produced peripheral platelet counts that peaked at 599 ± 260 × 103platelets/μL (P = .02 compared with pretreatment value of 64 ± 19) 3 days after discontinuing therapy. The timing of the 3 doses of PEG-rHuMGDF is indicated by the downward arrows. Platelet counts returned to baseline thrombocytopenic levels during the subsequent 2 weeks.
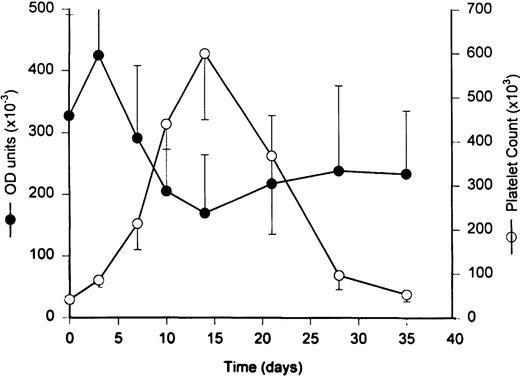
The serum levels of antiplatelet GPIIIa49-66 antibodies decreased reciprocally during the period of thrombocytosis (baseline of 333 ± 278 v nadir of 169 ± 164 arbitrary OD units;P < .07; Fig 2 and Table 1). Presumably, the decline in circulating levels of antiplatelet GPIIIa49-66 antibodies represented transient depletion from plasma by high-affinity binding to the 10-fold increase in the circulating concentration of platelets during thrombocytosis.
Effects of PEG-rHuMGDF therapy on circulating levels of antiplatelet GP IIIa49-66 antibodies. Stimulation of megakaryocytopoiesis by PEG-rHuMGDF (3 subcutaneous twice weekly doses of 25 μg/kg) increased the peripheral concentration of platelets 10-fold (○), peaking at day 12 and followed by a gradual return to baseline values. The mean concentration of circulating antiplatelet GP IIIa49-66 antibodies exhibited a reciprocal reduction (•) during the period when the peripheral platelet counts were elevated. This transient decrease in antibody levels is attributable to depletion resulting from high-affinity binding to the greatly expanded pool of platelets in the circulation.
Effects of PEG-rHuMGDF therapy on circulating levels of antiplatelet GP IIIa49-66 antibodies. Stimulation of megakaryocytopoiesis by PEG-rHuMGDF (3 subcutaneous twice weekly doses of 25 μg/kg) increased the peripheral concentration of platelets 10-fold (○), peaking at day 12 and followed by a gradual return to baseline values. The mean concentration of circulating antiplatelet GP IIIa49-66 antibodies exhibited a reciprocal reduction (•) during the period when the peripheral platelet counts were elevated. This transient decrease in antibody levels is attributable to depletion resulting from high-affinity binding to the greatly expanded pool of platelets in the circulation.
The administration of PEG-rHuMGDF did not significantly alter viral loads (395, 189, and 53 × 103 RNA viral copies/mL;P > .5 compared with baseline values; Table 1) or CD4+ T-cell counts (Table 1).
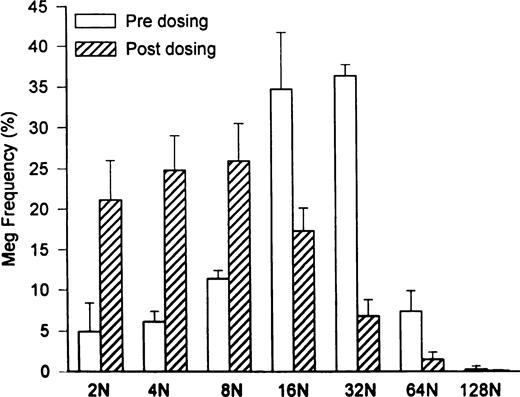
Marrow megakaryocyte numbers expanded 30-fold to 353 ± 255 × 106/kg (P = .04 compared with 11.7 ± 6.5 × 106/kg pretreatment; Table 1), and megakaryocyte progenitor cell numbers increased more than fourfold to 14.1 × 103 CFU-Meg/1,000 CD34+ marrow cells (compared with 3.6 ± 0.6 × 103 CFU-Meg/1,000 CD34+ marrow cells pretreatment; Table 1). However, when peripheral platelet counts peaked, mean megakaryocyte volumes were significantly decreased to 18.7 ± 2.1 × 103 fL (P = .014 compared with 38 ± 1.9 × 103 fL pretreatment; Table 1) and megakaryocyte ploidy was reduced in concert (Fig 3; P = .013). The calculated overall megakaryocyte mass was expanded 15-fold to 624 ± 373 × 1010 fL/kg (P = .04 compared with 45.2 ± 27.2 × 1010 fL/kg pretreatment; Table 1).
Shift in megakaryocyte ploidy distribution induced by PEG-rHuMGDF treatment of HIV-infected thrombocytopenic chimpanzees. Baseline megakaryocyte ploidy distribution (□) was shifted to a modal ploidy of 32N (P = .5 compared with a modal ploidy of 16N in normal controls). By contrast, 2 weeks after initiating high-dose PEG-rHuMGDF therapy (subcutaneous injections of 25 μg/kg for 3 doses administered twice weekly), there was a 30-fold expansion of megakaryocyte numbers with a modal ploidy of 8N (P = .01).
Shift in megakaryocyte ploidy distribution induced by PEG-rHuMGDF treatment of HIV-infected thrombocytopenic chimpanzees. Baseline megakaryocyte ploidy distribution (□) was shifted to a modal ploidy of 32N (P = .5 compared with a modal ploidy of 16N in normal controls). By contrast, 2 weeks after initiating high-dose PEG-rHuMGDF therapy (subcutaneous injections of 25 μg/kg for 3 doses administered twice weekly), there was a 30-fold expansion of megakaryocyte numbers with a modal ploidy of 8N (P = .01).
DISCUSSION
HIV-infected chimpanzees exhibit many of the features observed in HIV-infected patients, including (1) reproducible infection with HIV (type 1) by injecting cell-free virus, infected peripheral blood mononuclear leukocytes, or homogenates of infected tissues from HIV-infected donors; (2) comparable viral loads; (3) analogous patterns of cellular and humoral immunologic responses; and (4) similar hematologic complications.1-6 The present study confirms chronic thrombocytopenia among the hematologic alterations to be expected.
Chronic thrombocytopenia develops in approximately one third of humans infected with HIV at some time during the course of acquired immunodeficiency syndrome (AIDS).39-41 The pathophysiologic basis for the development of thrombocytopenia in HIV-patients has been ascribed to variable and changing proportions of accelerated platelet destruction, enhanced splenic platelet sequestration, and impaired platelet production.18,39,40,42-45 Kinetic studies have demonstrated shortened platelet lifespan in thrombocytopenic HIV patients, indicating that platelet production was not sufficiently expanded to compensate for antibody-mediated platelet destruction in those patients.43,46 In a recent study of HIV-infected patients, thrombocytopenia was due to a combination of three factors: accelerated platelet removal from the systemic circulation, enhanced platelet sequestration in the splenic circulation, and inadequate compensatory increases in platelet formation despite a threefold expansion in marrow megakaryocyte mass.47 This threefold disparity between marrow platelet substrate and circulating platelet product is attributed to ineffective platelet formation by HIV-infected megakaryocytes or HIV-induced inhibitory cytokines. The possibility of HIV having detrimental effects on platelet formation by megakaryocytes is supported by observations that thrombocytopenia in HIV-infected patients responds to antiviral therapy.48,49 In addition, in situ hybridization studies have demonstrated HIV infection in marrow megakaryocytes from thrombocytopenic HIV patients50 and morphologic abnormalities in megakaryocytes from thrombocytopenic HIV patients, including naked nuclei and broad peripheral cytoplasmic rims. Moreover, accelerated apoptosis has been documented in megakaryocytes obtained from thrombocytopenic HIV patients, and the degree of programmed cell death was inversely proportional to the severity of thrombocytopenia.51 Thus, impaired platelet production in thrombocytopenic HIV patients may be due to premature apoptosis developing in HIV-infected megakaryocytes before the formation of platelets by the dying megakaryocytes.
Platelet production in HIV chimpanzees with chronic thrombocytopenia (Table 1) resembles platelet production in thrombocytopenic HIV-infected patients in several important respects.47First, antiplatelet GPIIIa49-66 antibodies form and bind to circulating platelets, producing accelerated platelet removal and enhanced splenic sequestration.7 Second, circulating levels of endogenous TPO are increased threefold over normal controls. Third, marrow megakaryocytes are significantly increased in number, volume, and ploidy distribution compared with normal controls; enhanced megakaryocyte volume and ploidy are particularly characteristic of TPO-driven augmentation of megakaryocytopoiesis.38
Three doses of PEG-rHuMGDF in thrombocytopenic HIV-infected chimpanzees increased peripheral platelet counts 10-fold by amplifying marrow megakaryocyte progenitors fourfold and marrow megakaryocyte numbers 30-fold, thereby expanding overall megakaryocyte mass 15-fold without mobilizing HIV reservoirs. The reciprocal decrease in circulating levels of antiplatelet GPIIIa49-66 antibodies during the period of thrombocytosis is attributable to binding-depletion resulting from the 10-fold increase in the concentration of peripheral platelets (Table 1). Surprisingly, PEG-rHuMGDF therapy was associated with a reduction in megakaryocyte volume and ploidy in these animals. Previous studies in nonhuman primates and patients receiving PEG-rHuMGDF therapy showed enlargement of megakaryocyte volume and ploidy in a log-linear dose-dependent manner.9,37,38,52 53 One possible explanation for the 30-fold increase in megakaryocyte number (Table 1) with a reduction in megakaryocyte ploidy (Fig 2) may be that high-dose PEG-rHuMGDF stimulation of chronic pre-existing TPO-stimulated megakaryocytopoiesis may favor the proliferation of megakaryocytes from progenitors, rather than fostering endoproliferation of already formed megakaryocytes. Alternatively, the matured megakaryocytes may have been selectively lost by cytoplasmic fragmentation into circulating platelets and nuclear processing without a sustained stimulus for high ploidy replacement during the 3 days between final dosing and marrow sampling.
The striking elevation of the peripheral platelet count after administering PEG-rHuMGDF therapy (3 doses of 25 μg/kg over 7 days) to thrombocytopenic HIV chimpanzees constitutes convincing evidence of therapeutic benefit. Based on PEG-rHuMGDF's pharmacokinetics and log-linear dose-response38 and presumed platelet kinetic profile,47 it seems likely that a single 25 μg/kg dose of PEG-rHuMGDF would have transiently normalized peripheral platelet counts and that twice weekly injections of 5 μg/kg would have maintained the peripheral platelet concentrations within the normal range.
Thus, the extraordinary increase in peripheral platelet counts produced by PEG-rHuMGDF therapy in thrombocytopenic chimpanzees infected with HIV implies that the thrombocytopenia is largely due to insufficient compensatory expansion in platelet production due to HIV-dependent impairment in the delivery of platelets, despite stimulated megakaryocytopoiesis. These findings suggest that PEG-rHuMGDF therapy may be similarly corrective of peripheral platelet counts in thrombocytopenic HIV-infected patients.
Supported in part by a grant from the National Institutes of Health to Yerkes Regional Primate Research Center (RR-00165) and a Research Grant from Amgen Inc.
Address reprint requests to Laurence A. Harker, MD, Division of Hematology and Oncology, Emory University, 1003 Woodruff Memorial Bldg, 1639 Pierce Dr, Atlanta, GA 30322.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.