Abstract
Tissue factor (TF)-induced coagulation was compared in contact pathway suppressed human blood from normal, factor VIII-deficient, and factor XI-deficient donors. The progress of the reaction was analyzed in quenched samples by immunoassay and immunoblotting for fibrinopeptide A (FPA), thrombin-antithrombin (TAT), factor V activation, and osteonectin. In hemophilia A blood (factor VIII:C <1%) treated with 25 pmol/L TF, clotting was significantly delayed versus normal, whereas replacement with recombinant factor VIII (1 U/mL) restored the clot time near normal values. Fibrinopeptide A release was slower over the course of the experiment than in normal blood or hemophilic blood with factor VIII replaced, but significant release was observed by the end of the experiment. Factor V activation was significantly impaired, with both the heavy and light chains presenting more slowly than in the normal or replacement cases. Differences in platelet activation (osteonectin release) between normal and factor VIII-deficient blood were small, with the midpoint of the profiles observed within 1 minute of each other. Thrombin generation during the propagation phase (subsequent to clotting) was greatly impaired in factor VIII deficiency, being depressed to less than 1/29 (<1.9 nmol TAT/L/min) the rate in normal blood (55 nmol TAT/L/min). Replacement with recombinant factor VIII normalized the rate of TAT generation. Thus, coagulation in hemophilia A blood at 25 pmol/L TF is impaired, with significantly slower thrombin generation than normal during the propagation phase; this reduced thrombin appears to affect FPA production and factor V activation more profoundly than platelet activation. At the same level of TF in factor XI-deficient blood (XI:C <2%), only minor differences in clotting or product formation (FPA, osteonectin, and factor Va) were observed. Using reduced levels of initiator (5 pmol/L TF), the reaction was more strongly influenced by factor XI deficiency. Clot formation was delayed from 11.1 to 15.7 minutes, which shortened to 9.7 minutes with factor XI replacement. The maximum thrombin generation rate observed (∼37 nmol TAT/L/min) was approximately one third that for normal (110 nmol/L TAT/min) or with factor XI replacement (119 nmol TAT/L/min). FPA release, factor V activation, and release of platelet osteonectin were slower in factor XI-deficient blood than in normal blood. The data demonstrate that factor XI deficiency results in significantly delayed clot formation only at sufficiently low TF concentrations. However, even at these low TF concentrations, significant thrombin is generated in the propagation phase after formation of the initial clot in hemophilia C blood.
IN VITRO, DISTINCT PLASMA coagulation pathways lead to the generation of thrombin: the intrinsic (or contact) pathway and the extrinsic (or tissue factor [TF]) pathway.1 In recent years, the TF pathway has been considered to be central to thrombin generation in normal hemorrhage control in vivo.2 The TF pathway is thought to proceed by assembly of three distinct surface-dependent complexes.3,4The initiating complex is the extrinsic tenase (factor VIIa and cofactor TF on a membrane), which assembles when circulating plasma factor VIIa encounters TF at the site of injury.5 During an initiation phase, this complex activates a fraction of the circulating zymogens factors X and IX to their respective active forms, factors Xa and IXa.6-9 Factor Xa, in complex with factor V/Va,10 potentiates factor V and factor VIII activation indirectly via the generation of limited amounts of thrombin.11 12 Additional factor Xa is generated by the intrinsic tenase complex, composed of factor IXa assembled with factor VIIIa on the platelet surface. Factor Xa assembles with factor Va into the prothrombinase complex, the major activator of prothrombin to α-thrombin during a propagation phase. The importance of the extrinsic and intrinsic tenase complexes and prothrombinase in thrombin generation and coagulation is underscored by the observation that deficiencies in factor VII, factor VIII, factor IX, factor X, and factor V are associated with hemorrhagic tendencies.
Current theories of coagulation and hemostasis propose that, in the quiescent state, low-level thrombin generation is suppressed by endogenous inhibitory mechanisms, including antithrombin-III (AT-III),13,14 TF pathway inhibitor (TFPI),15,16 and the protein C pathway.17Ongoing generation of factor IXa and factor Xa by the factor VIIa/TF complex is limited due to inhibition by TFPI and AT-III.2,18-20 At high concentrations of TF, such as are used in a prothrombin time (PT) assay, levels of extrinsic tenase formed are sufficient to lead to thrombin in excess of the threshold levels necessary for hemostasis.21 However, at lower TF concentrations, the activity of the intrinsic tenase is essential for above-threshold levels of thrombin and hemostasis.8,9 22
Whereas the extrinsic and intrinsic tenases have distinct roles in factor Xa and thrombin generation in coagulation, evidence for the involvement of the initiating members of the contact pathway is limited. In factor XI deficiency (hemophilia C), severe spontaneous bleeding is rarely observed; affected patients typically exhibit significant hemorrhage only upon surgical challenge or extreme trauma.23-25 No associations are found between factor XII or prekallikrein deficiency and abnormal bleeding, a peculiar circumstance given that factor XIIa is a potent activator of factor XI.26-28 Recent work has sought to clarify the role of factor XI during coagulation in vivo. Slow activation of factor XI by thrombin has been demonstrated in vitro, and the reaction is greatly accelerated on negatively charged surfaces such as dextran sulfate.29,30 After its activation by limited amounts of thrombin, factor XIa may support TF-initiated thrombin generation by elevating levels of factor IXa and consequently the intrinsic tenase. Thus, substantial contributions to thrombin generation may be provided by both the intrinsic tenase and factor XIa. Whereas these observations explain the bleeding that may accompany factor XI deficiency, they have been challenged on the basis that the rate of factor XI activation by thrombin in vitro is extremely slow in the absence of exogenously added surfaces.31 Other than recent studies in plasma systems, little physiological evidence exists to support or refute this mechanism.32 33
The current study was undertaken to evaluate the established role of factor VIII and the potential contribution of factor XI during whole blood coagulation in vitro, using a model of the TF pathway in which factor XIIa is blocked.34 The advantage of this system is that the native state of all the blood components is preserved, providing a physiologically relevant platform to test theories of blood coagulation.
MATERIALS AND METHODS
Materials.
Recombinant human TF and recombinant factor VIII were provided as gifts by Drs Roger Lundblad and Shu-Len Liu (Hyland Division, Baxter Healthcare Corp, Duarte, CA) and human factor XI was a gift from Dr Richard Jenny (Hematologic Technologies, Inc, Essex Junction, VT). Trypsin inhibitor from corn was either obtained from Fluka (Ronkonkoma, NY) or prepared as described below. 1-Palmitoyl-2-oleoyl phosphatidylserine (PS) and 1-palmitoyl-2-oleoyl phosphatidylcholine (PC) were purchased from either Sigma Chemical Co (St Louis, MO) or Avanti Polar Lipids, Inc (Birmingham, AL). D-Phenylalanyl-L-prolyl-L-arginine chloromethyl ketone (FPRck35) was obtained as a gift from Hematologic Technologies, Inc (Essex Junction, VT) or purchased from Calbiochem (La Jolla, CA). Diisopropyl fluorophosphate (DFP) was obtained from Sigma Chemical Co, diluted to working concentration (1 mol/L) in anhydrous isopropanol, and stored at −20°C. Pooled standardized normal (FACT, lot no. D12S1) and factor XI-deficient (lot no. GK1122-N17P1) plasmas were obtained from George King Biomedical (Overland Park, KS). Thromboplastin (Simplastin Excel) and activated partial thromboplastin time (aPTT; Automated APTT) reagents were purchased from Organon Teknika (Durham, NC). The following analytes were estimated using enzyme-linked immunosorbent assay (ELISA) kits obtained from the manufacturers according to the instructions provided: thrombin-AT-III (Enzygnost TAT; Behring, Westwood, MA); fibrinopeptide A (Asserachrom FPA; Diagnostica Stago/American Bioproducts, Parsippany, NJ); and platelet osteonectin (a gift from Dr Richard Jenny, Hematologic Technologies, Inc).
A murine monoclonal antibody (αFVaHC#17; 5 to 10 μg/mL) that recognizes an epitope between residues 307 and 506 in the heavy chain (HC) of factor V/Va36 was prepared according to previously published procedures.37 The reactivity and specificity of this antibody in Western analyses are similar to another antibody described previously (αFVaHC#6).38 A second murine monoclonal antibody, directed against the light chain of the cofactor (αFVaLC#9; 5 to 10 μg/mL), was prepared as described elsewhere.39
Human donors.
All donors, normal and deficient, were recruited and advised according to a protocol approved by the University of Vermont Human Studies Committee. Normal individuals (age range, 22 to 36 years) were selected so as to exclude donors with a personal or familial history of thrombosis/hemorrhage or regular aspirin or drug use. All individuals exhibited values in the normal range for the PT (11.6 to 13.8 seconds), aPTT (27 to 36 seconds), and fibrinogen and platelet counts (172,000 to 376,000/μL). Subsequent to each control experiment, factor XI levels were assayed for the normal donors (range, 95 to 119 U/dL), falling within the accepted normal adult range (75 to 130 U/dL).23
Two hemophilia A donors have been examined to date. The first hemophilia A donor detailed in this report (patient A1) was a 46-year-old man with severe factor VIII deficiency (VIII:C <0.5%) who exhibited a life-long tendency toward bleeding. The propositus suffered recurrent hemarthroses in the elbows, knees, and ankles and has a limited range of motion with pain in the shoulders, elbows, and ankles; he had received no replacement therapy for 2.5 weeks before the experiment. As is common among hemophilia A patients transfused with human products, this individual had developed a CDC class A-III HIV infection. Treatment with indinevir resulted in thrombocytopenia, which partially resolved upon discontinuation of the drug. The platelet count on the day of the experiment was 97,000 platelets/μL, but has decreased since then (December 1995); there is no evidence of antiplatelet antibodies. Current medications are trimethoprim-sulfamethoxazole, zidovudine, lamivudine, and zalcitabine.
A second hemophilia A donor has been examined (patient A2) and is discussed briefly. This 18-year-old man has tested positive for hepatitis C. However, he exhibits normal fibrinogen levels, platelet count, and tissue thromboplastin time (prothrombin time), although he is severely factor VIII-deficient (prolonged aPTT; VIII:C <1%). This donor has a history of bleeding and joint pain and routinely self-administers recombinant factor VIII products when symptomatic. Before our study, he had not received recombinant factor VIII products for at least 4 days (50% replacement dose, >7 half-lives). There was no evidence of inhibitors (eg, anti-factor VIII antibodies).
Three hemophilia C (factor XI-deficient) donors have been studied; results with two of these patients are described here in detail. Patient C1 is a 53-year-old woman with no family history of bleeding. She had exhibited easy bruising as well as frequent nosebleeds, but no menorrhagia. At age 33, she had surgical correction of a deviated nasal septum because of the nosebleeds, but afterward continued to experience mild nose and gum bleeding. At age 48, she was found to have a prolonged aPTT during a preoperative evaluation for osteoarthritis of the left hip. The patient had a PT of 12.4 seconds and prolonged aPTT (factor XI:C = 2%). Factor XII, fibrinogen, and platelet counts are in the normal range; there was no evidence of an inhibitor. The patient underwent successful total hip replacement after replacement with fresh frozen plasma to normalize the aPTT and experienced no complications.
Patient C2 is a 49-year-old man with a personal history of episodic bleeding but no family history of hemorrhage. At age 8, he had a tonsillectomy that required an extra day of hospitalization due to excessive bleeding. At age 10, he experienced 2 days of oozing after a dental extraction. A fracture of the clavicle at age 25 was not accompanied by significant bleeding. After his 38th birthday, he was evaluated for a possible bleeding disorder, which yielded a PT of 11.1 seconds, a prolonged aPTT (factor XI:C = 10%), a bleeding time of 4.5 minutes, and levels of factors VIII, IX, and XII in the normal range (90% to 103%, with no evidence of an inhibitor). At the time of the experiment, patient C2 also presented with a platelet count (145,000/μL) slightly below the normal range (172,000 to 376,000/μL); this platelet count was observed for this individual on two separate occasions.
Preparation of corn trypsin inhibitor.
For repeat experiments with the second hemophilia A patient, corn trypsin inhibitor was prepared according to the procedure of Hojima et al,40 with a few minor modifications. Dry popcorn seed was obtained from a local grocery and was repeatedly extracted until no further prolongation of the aPTT in normal plasma was observed (see below). Acetone precipitation was performed as described,40and the resuspended material was applied to a column (2.5 × 60 cm) of DEAE-Sepharose. The major peak of inhibitory activity was pooled and applied to a second column (2.5 × 94 cm) of Sephadex G-50. This final pooled fraction was dialyzed (3,000 molecular weight cutoff) versus 10 mmol/L ammonium bicarbonate, pH 7.8, and then lyophilized to dryness. The isolated protein exhibited a single band at 14,000 molecular weight and was reconstituted in HBS (HEPES-buffered saline; 20 mmol/L HEPES, 150 mmol/L NaCl, pH 7.4). No attempt was made to further purify the isoforms of this inhibitor, all of which exhibit similar inhibitory potential with factor XIIa.40
Preparation of TF/lipid reagent.
TF (5 nmol/L) was relipidated into small unilammelar vesicles41 of 25 mol% PS/75 mol% PC (10 μmol/L total lipid) in HBS plus calcium (2 mmol/L) for 30 minutes at 37°C.8 Concentrated sucrose (60% wt/vol) was subsequently added to the relipidation mixture to 10% final to stabilize the vesicles for long-term freezer storage (up to 12 months). Aliquots of the reagent (200 μL) were stored at −20°C, which could be rehydrated 60 minutes before each experiment and used with reproducible results.
Factor XI preparations.
Using a factor Xa amplification assay, other investigators32,33 have shown that traces of contaminating factor XIa in factor XI preparations (picomoles down to femtomoles) can significantly affect levels of the intrinsic tenase, leading to artificially high levels of factor Xa generation. Factor XI preparations were routinely treated to remove traces of factor XIa. Concentrated factor XI stocks (∼300 to 350 U/mg, ∼3 to 5 mg/mL) were loaded into a Slide-A-Lyzer dialysis cassette (molecular weight cutoff 10,000; Pierce Chemical, Rockford, IL) and were treated initially with FPRck (10 μmol/L for 30 minutes at 25°C) in HBS. After dialysis versus 4 L HBS (2 changes for 2 hours each at 4°C), an additional treatment was performed with DFP (2 mmol/L) for a 15-minute interval. Final dialysis was performed versus 4 L HBS (2 changes for 2 hours each at 4°C), and the factor XI stock was aliquotted into capped nonstick microcentrifuge tubes (VWR Scientific, West Chester, PA), quick-frozen inside the capped tubes by immersion in a dry ice/methanol slurry, and stored at −70°C. aPTT clotting assays indicated that the specific activity of factor XI was unaffected by these treatments. After FPRck and DFP treatments, analysis of residual factor XIa activity was performed using an aminonaphthalenesulfonamide derivative12,42 of the tripeptide D-Leu-L-Pro-L-Arg, with kcat/Km = 7.10 × 105 mol/L−1s−1. In factor XI preparations (typically 200 nmol/L), factor XIa concentrations were below the limits of the assay (<100 fmol/L factor XIa activity). Therefore, at plasma concentrations of factor XI (25 to 30 nmol/L), potential contamination by factor XIa was less than 12.5 fmol/L. No clotting (>20 minutes) was observed in factor XI-deficient blood when factor XI and corn trypsin inhibitor were added in the absence of TF. However, as a final precaution for the replacement experiments using a very low TF concentration (5 pmol/L), human α1-protease inhibitor (0.5 mg/mL, 9.6 μmol/L) was added to the factor XI preparations (1.5 mg/mL, 9.4 μmol/L).43 The α1-protease inhibitor added to the blood with these factor XI preparations was insignificant (22 nmol/L) relative to the level plasma inhibitor already present (∼47 μmol/L).
Assay for TF dependence in factor XI-deficient plasma.
Relipidated TF reagent (see above) was rehydrated (200 μL) and diluted with HBS/calcium (2 mmol/L) to obtain a stock reagent containing 552 pmol/L TF and 1.10 μmol/L PCPS. This initial stock was further diluted with PCPS (1.10 μmol/L) in HBS/calcium (2 mmol/L) to obtain a set of working TF stock concentrations ranging from 17.25 to 552 pmol/L TF at constant lipid concentration. The assay was performed according to the following protocol. Into a polystyrene tube (12 × 75 mm) was added 200 μL human plasma (XI-deficient, <1% XI:C; or XI-deficient with factor XI replaced at 3.5 μg/mL, 100 U/dL) and 10 μL corn trypsin inhibitor (1.15 mg/mL in HBS). After 30 seconds of equilibration at 37°C, 10 μL of working TF stock was added, followed immediately by the addition of 10 μL of 390 mmol/L CaCl2 in water. Upon addition of the calcium, a timer was started and the tube was rocked in the 37°C water bath until strands of fibrin or a solid clot could be identified, at which point the time was noted.
PT and aPTT assays.
Assays were performed in plastic tubes (VWR Scientific, no. 60818-270) on 100-μL samples of fresh frozen, citrated human plasma using the manual tilt-tube approach described by the manufacturer of the PT or aPTT reagent. When testing the effect of buffer or inhibitor, no more than 10 μL of the additive was mixed with 90 μL of plasma to minimize dilution errors.
Coagulation in whole blood.
The protocol used is a modification of the protocol of Rand et al34 performed under the supervision of one of the authors (R.F.B.) at the Clinical Research Center, Fletcher Allen Health Care (Burlington, VT). Clotting in freshly drawn, nonanticoagulated whole blood was performed in 32 capped polystyrene culture tubes as described, except that two series were performed per experiment (16 tubes/series). Reagents were loaded in the following amounts: corn trypsin inhibitor (all tubes, to give 50 μg/mL blood); relipidated TF (lipid:protein = 2,000) in HBS with 5 mmol/L calcium (all tubes in each series except phlebotomy control tube, to give 25 or 5 pmol/L TF/mL blood); factor VIII or factor XI (all tubes, replacement series only, to give 1 U/mL final); and equivalent volume factor VIII/XI dilution buffer (HBS, pH 7.4, all tubes, deficiency series only). No more than 45 μL of reagent was loaded in each tube. The zero tube of each series was pretreated using 1 mL of inhibitor cocktail (containing 50 mmol/L EDTA and 20 mmol/L benzamidine-HCl in HBS, pH 7.4) and 10 μL of 10 mmol/L FPRck (diluted in 0.01 mol/L HCl).
Patient or normal donor blood was drawn by venipuncture under a protocol approved by the Human Studies Committee at the University of Vermont, as described.34 Clotting was initiated by delivery into the reagent-loaded tubes and with periodic quenching of the tubes with inhibitor cocktail and FPRck as described above. Two series of quenched samples were obtained after reaction progress up to 20 minutes after initiation; both reducing (1% β-mercaptoethanol) and nonreducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) samples were prepared from each tube (60 μL supernatant, 190 μL 2% SDS-PAGE sample solution,34 heated exactly 5 minutes at 98°C ± 2°C). An aliquot from each tube was filtered to remove cellular contaminants for osteonectin assays (200 μL; 0.2-μm AcroDisc; Gelman Sciences, Ann Arbor, MI). The remaining serum and cell pellets/clots were aliquotted to screw cap tubes, frozen, and stored at −20°C for immunoblot or immunoassay analysis.
Immunoassays and Western analysis.
Commercial ELISAs for fibrinopeptide A (FPA), thrombin-AT-III (TAT), and platelet α-granule release (osteonectin) were performed according to manufacturers protocols, with corrections for sample dilution by added quench solution (1.00 mL) and hematocrit (typically 40% of the total blood volume). Results were analyzed on a Vmax microtiter plate reader (Molecular Devices, Menlo Park, CA) equipped with SOFTMax ver. 2.0 software and an IBM Personal System 2 Model 30/286 PC (IBM Corp, Armonk, NY). In each assay, a minimum of 5 standard concentrations were run in duplicate, with duplicate sample determinations. The relationship between concentration of standard and optical density was established by fitting the data to either a four-parameter or log-logit fit, as described by the manufacturer (Molecular Devices). For analysis of factor Va, samples were separated on SDS-PAGE according to Laemmli44 as modified by our laboratory.34 Separate gels were run for heavy-chain and light-chain analysis. Each gel containing samples was loaded along with a prestained molecular weight standard mixture (14 to 200 kD) and dilute standards (3 to 4 samples) allowing comparison and quantitation of analyte amounts horizontally on the immunoblots. Transfer from the gel to nitrocellulose (BioRad, Hercules, CA) was performed for 1.5 to 3 hours via an SDS-free tank transfer procedure as described,45,46 with subsequent immunoblot analysis according to Rand et al.34
Transmittance scans of immunoblot images on Reflection film (product # NEF-496; NEN Life Science Products, Boston, MA) were performed on a Hewlett-Packard ScanJet 4C/T equipped with a transparency adapter for backlighting the x-ray film (Hewlett-Packard, Palo Alto, CA). Analysis of the .TIFF files was performed on a Power Macintosh 9500/200 computer (Apple Computers, Cupertino, CA) using the public domain NIH Image program (v. 1.60, Spring 1994, developed at the US National Institutes of Health and available from the internet by anonymous FTP from zippy.nimh.nih.gov or on floppy disk from the National Technical Information Service, Springfield, VA, part no. PB95-500195GEI). The recommendations of the software developers were followed to minimize nonlinearity of scanned data with respect to sample concentration (see addendum to the NIH Image manual, “Using Image for Densitometric Analysis of 1-D gels”). Concentrations were estimated by comparison of sample band density with a minimum of four serial dilutions of purified standard proteins loaded on the same gel (internal standard method). Standard curves were obtained from plots of the logarithm of the standard concentration versus scanned density, which are typically linear. From fits of these plots, a relationship between scanned density and concentration is obtained, allowing conversion of sample density to concentration. From these values, relative concentrations were determined by normalizing the data relative to the maximum. Limits of detectability are also estimated from the standard curve, typically 0.9 nmol/L or lower for factor VaHC and 0.3 nmol/L or lower for factor VaLC.
RESULTS
Blood coagulation in hemophilia A.
Coagulation in blood from a patient with severe hemophilia A (patient A1, <0.5% VIII:C) was compared with coagulation in blood from normal donors after initiation with 25 pmol/L TF (Fig 1). Depicted in Fig 1A are the time courses for TAT generation in normal and factor VIII-deficient blood, with and without factor VIII replacement. The normal profile (•) is constructed from averaged data (±SEM) from a series of 14 experiments conducted over a period of 18 months with 4 normal subjects. Little TAT is detected throughout the initiation phase; subsequently, the bulk of the TAT produced is generated explosively (55 nmol/L/min) during the propagation phase after clot time (4.0 ± 0.2 minutes, arrow a). In factor VIII-deficient blood (○), clot time occurred at 6.5 minutes (arrow c), representing an increase in the length of the initiation phase of 60% over the normal case. The explosive thrombin generation ordinarily observed in normal blood after clot time is greatly depressed (maximum rate, 1.9 nmol/L/min; 4% of normal). Replacement with recombinant factor VIII (□; 1 U/mL) shortened the clot time (4.1 minutes, arrow b) and increased TAT formation to 46 nmol/L/min between 5 and 16 minutes.
Coagulation in normal and hemophilia A blood, with and without replacement. Using 25 pmol/L TF, coagulation was initiated in normal and hemophilia A blood (see the Materials and Methods and Rand et al34) with and without recombinantfactor VIII replacement (1 U/mL blood). Smooth curves have been drawn by hand through the points to approximate the data. (A) Time courses for TAT in normal blood (•), hemophilia A blood (patient A1; ○), and hemophilia A blood with recombinant factor VIII (□). Each point on the normal curve represents an average TAT from 14 experiments on four normal subjects (with error bars, SEM). Average clot time for the composite normal curve is 4.0 ± 0.2 minutes (arrow a). Clotting in factor VIII-deficient blood occurred at 6.5 minutes (arrow c), which was shortened to 4.1 minutes with factor VIII replacement (arrow b); a control tube without TF in the factor VIII-deficient experiment did not clot (>20.6 minutes), and the replacement control clotted at 17.8 minutes. (B) FPA generation in normal blood, factor VIII-deficient blood, and factor VIII-deficient blood with replacement (symbols as in [A]). The normal curve represents the averaged results from nine experiments performed on two individuals, with error bars (SEM). The average clot time for the normal profile was 4.1 ± 0.2 (SEM) minutes (arrow a), with the other clot times as in (A). (C) Osteonectin release was measured to examine platelet activation (symbols as in [A]). The normal curve is constructed from blood taken from a single normal donor (clot time = 4.1 minutes, arrow a) drawn contemporaneously with the factor VIII-deficient patient. Other clot times and symbols are as in (A). (D) After analysis of factor V activation by immunoblotting, profiles were constructed by densitometric analysis as in the Materials and Methods. Time courses are given for formation of the heavy (▪, □) and light chains (•, ○) of factor Va (factor VaHCand VaLC), with factor VIII replacement (solid symbols) and without (open symbols). For clarity, the normal profile is omitted, but is similar to the profile with factor VIII replacement. Clot times are 4.1 minutes (hemophilia A with factor VIII replacement, arrow a) and 6.5 minutes (hemophilia A, arrow b).
Coagulation in normal and hemophilia A blood, with and without replacement. Using 25 pmol/L TF, coagulation was initiated in normal and hemophilia A blood (see the Materials and Methods and Rand et al34) with and without recombinantfactor VIII replacement (1 U/mL blood). Smooth curves have been drawn by hand through the points to approximate the data. (A) Time courses for TAT in normal blood (•), hemophilia A blood (patient A1; ○), and hemophilia A blood with recombinant factor VIII (□). Each point on the normal curve represents an average TAT from 14 experiments on four normal subjects (with error bars, SEM). Average clot time for the composite normal curve is 4.0 ± 0.2 minutes (arrow a). Clotting in factor VIII-deficient blood occurred at 6.5 minutes (arrow c), which was shortened to 4.1 minutes with factor VIII replacement (arrow b); a control tube without TF in the factor VIII-deficient experiment did not clot (>20.6 minutes), and the replacement control clotted at 17.8 minutes. (B) FPA generation in normal blood, factor VIII-deficient blood, and factor VIII-deficient blood with replacement (symbols as in [A]). The normal curve represents the averaged results from nine experiments performed on two individuals, with error bars (SEM). The average clot time for the normal profile was 4.1 ± 0.2 (SEM) minutes (arrow a), with the other clot times as in (A). (C) Osteonectin release was measured to examine platelet activation (symbols as in [A]). The normal curve is constructed from blood taken from a single normal donor (clot time = 4.1 minutes, arrow a) drawn contemporaneously with the factor VIII-deficient patient. Other clot times and symbols are as in (A). (D) After analysis of factor V activation by immunoblotting, profiles were constructed by densitometric analysis as in the Materials and Methods. Time courses are given for formation of the heavy (▪, □) and light chains (•, ○) of factor Va (factor VaHCand VaLC), with factor VIII replacement (solid symbols) and without (open symbols). For clarity, the normal profile is omitted, but is similar to the profile with factor VIII replacement. Clot times are 4.1 minutes (hemophilia A with factor VIII replacement, arrow a) and 6.5 minutes (hemophilia A, arrow b).
Progress curves for FPA release in these experiments are given in Fig1B. The normal profile is the average of a series of nine experiments on two individuals. FPA is liberated between 4 and 6 minutes at a maximum rate of 5.2 μmol/L/min, with about 4.8 μmol/L (30% maximum) observed at clot time (arrow a). In factor VIII deficiency, the maximum FPA release rate is 1.6 μmol/L/min (30% of normal), reflecting fibrin formation at a slower rate than normal over the course of the experiment (20 minutes). The extent of FPA release at clot time in the hemophilic case (arrow c) is the same as in the normal profile (∼30% maximum in both cases). Replacement of factor VIII increases the maximum FPA rate to 6.4 μmol/L/min, exceeding the normal rate by 23%. At clot time the extent of FPA release is 35% (7.4 μmol/L), similar to the estimates from normal and factor VIII-deficient blood.
Profiles for osteonectin release as a measure of platelet activation47 48 are given in Fig 1C. In the blood of a normal individual (contemporaneous control), osteonectin release is approximately 50% by clot time (arrow a) and is complete by 5 minutes. In factor VIII-deficient blood, the progress curve is slightly delayed versus normal. Complete osteonectin release is observed by 6 minutes, reaching maximum levels before clot time (6.5 minutes, arrow c). A curve similar to the control was obtained when factor VIII was replaced in the deficient blood. Taken together, the similarity of these profiles indicates that platelet activation at 25 pmol/L TF is only slightly affected by the absence of factor VIII.
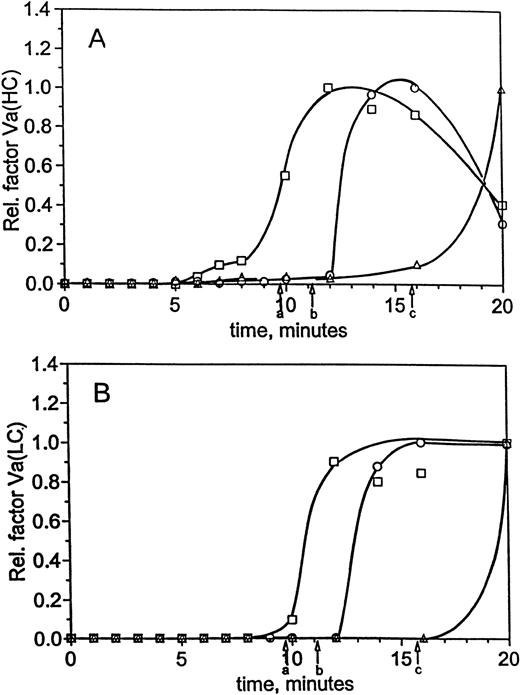
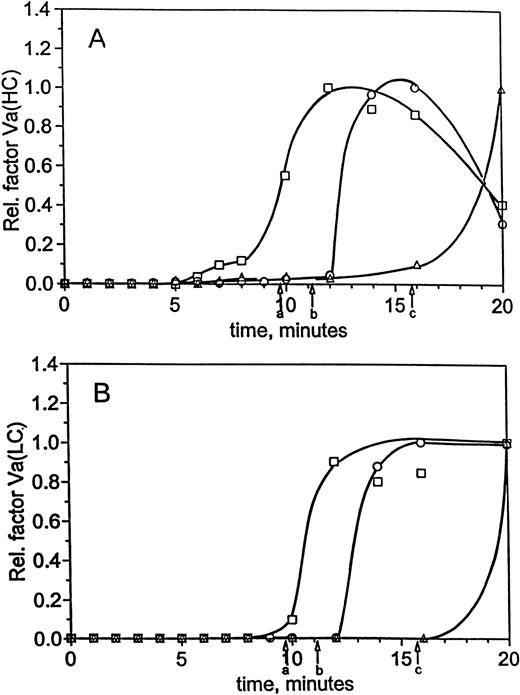
Time courses for factor VaHC generation in hemophilia A blood are shown in Fig 1D with and without factor VIII replacement. When factor VIII is present, significant generation of factor VaHC begins at 3 minutes and is complete by 6 minutes (▪). In the absence of factor VIII (□), generation of factor VaHC is slowed and does not reach a maximum until 10 to 12 minutes. At clot time in each experiment, approximately 50% to 55% of the heavy chain is generated. When factor VIII is present, formation of the light chain (LC; □, Fig 1D) is first detected at clot time (4.1 minutes, arrow a) and is complete at 6 minutes. In contrast, factor VaLC generation is dramatically delayed in factor VIII-deficient blood (○), with traces observed at 8 minutes and rapid generation after 9 minutes. Thus, LC production, the limiting step in expression of factor Va cofactor activity,34 49 is significantly delayed in the absence of factor VIII.
Impaired factor V activation may contribute to the limited prothrombin conversion observed in hemophiliacs. Earlier studies34 in normal blood have shown that, during the propagation phase of thrombin generation, prothrombinase concentration is estimated at 7 pmol/L at clot time, increasing rapidly to a maximum of 150 pmol/L 3 minutes later. As a result, factor Xa was proposed to be the limiting component of prothrombinase in normal blood, because factor Va heavy chain levels and platelet activation could be demonstrated at concentrations well in excess of 150 pmol/L.34 Calculations based on TAT data in the present study for the normal case indicate approximately 35 pmol/L prothrombinase at clot time from TAT measurements, which increases to 106 pmol/L before the reaction levels off (12 minutes into the reaction). In hemophilic blood with factor VIII replacement, prothrombinase estimates similar to the normal case were calculated from the TAT data (19 pmol/L at clot time, 136 pmol/L at maximum). Similar to the case in normal blood,34 the heavy chain of factor Va is present in excess of these concentrations (50% to 55% of maximum at clot time, ∼12 to 13 nmol/L). Light chain is not significantly converted until after clot time, but is rapidly converted once thrombin generation reaches explosive levels (quantitative by 3 minutes after clot time).
However, the present studies in factor VIII-deficient blood suggest approximately 1 pmol/L prothrombinase at clot time, which did not exceed 6 pmol/L during the experiment. Previous model studies have shown that factor Xa generation is impaired when either factor VIII or factor IX is present at an abnormally low levels8,9,50; therefore, the limited prothrombinase activity inferred for this patient probably results in part from limited factor Xa generation. Whereas factor Va heavy chain was observed at nearly 75% by clot time, light chain was virtually undetectable until after clot time, increasing only slowly thereafter. Therefore, the low levels of prothrombinase observed in hemophilia A blood throughout the course of the experiment are likely the result of reduced factor Xa generation in the absence of factor VIII, with a possible contribution from the limited levels of fully activated factor Va that are only slowly generated within the clot. The slowed formation of fully activated factor Va suggests an additional contribution to the impaired thrombin generation, because free factor Xa alone is virtually ineffective in converting prothrombin.3,4 Furthermore, free factor Xa lacks the relative protection against inactivation by TFPI and AT-III that fully formed factor Va provides in the prothrombinase complex.51 52
We have confirmed these results in whole blood from a second severe hemophilia A patient (patient A2, data not shown). Under conditions that induced normal blood clotting at 5.2 minutes (∼12.5 pmol/L TF), clotting in hemophilia A blood was observed at 9.8 minutes. Explosive thrombin generation was absent, as evidenced by low levels of TAT detected throughout the course of the experiment. Replacement with natural human factor VIII (Hemofil M, 1 U/mL blood) restored clotting to 5.8 minutes, as well as explosive TAT generation. As a result of the reduced thrombin levels, final levels of FPA generated were lower in the factor VIII-deficient case (∼15 μmol/L) than with replacement (nearly 20 μmol/L). Platelet activation was delayed approximately 3 minutes without factor VIII replacement, but nevertheless reached maximal levels by the end of each experiment, with identical fluid-phase osteonectin within 2 to 3 minutes after clot time. Therefore, reduced thrombin generation in hemophilia A blood was reflected in a lower final level of fibrin formation, whereas platelet activation was delayed but not significantly reduced.
Clotting in factor XI-deficient plasma as a function of TF.
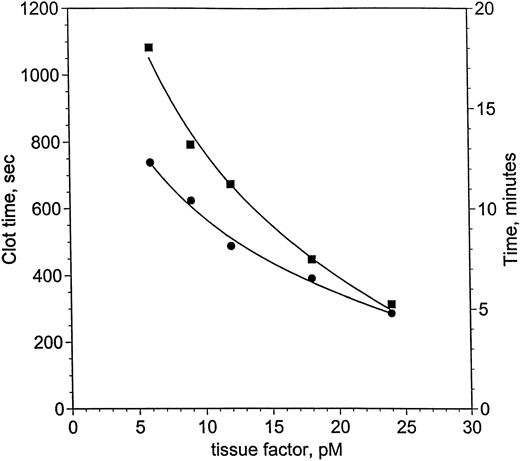
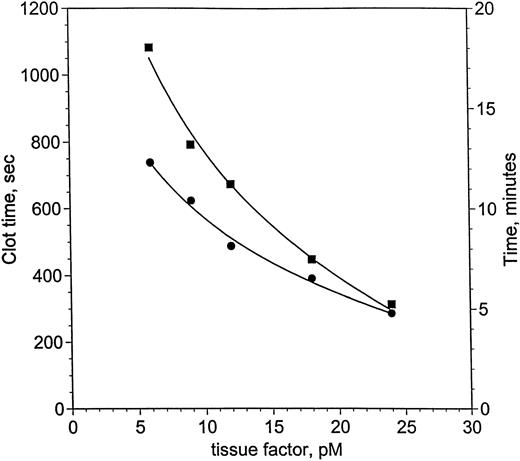
von dem Borne et al33 showed an effect of factor XI on fibrin formation in plasma at very low thromboplastin concentrations. Using decreasing concentrations of relipidated TF, a similar dependence on factor XI was demonstrated for clotting in factor XI-deficient plasma, measured with suppression of contact activation in the presence of corn trypsin inhibitor (Fig 2). Clot times with (•) and without (▪) factor XI lie along curves that intersect near 24 pmol/L TF, but diverge at concentrations of TF below 24 pmol/L. Clotting at 24 pmol/L is 28 seconds slower without factor XI, whereas by 6 pmol/L TF, the difference reaches 5.7 minutes. Based on these observations, blood coagulation in hemophilia C was investigated at 25 and 5 pmol/L.
Clotting in factor XI-deficient plasma as a function of TF and factor XI. Clot time in factor XI-deficient plasma (<1%) was measured as a function of TF and factor XI (as described in the Materials and Methods). The ordinate is shown in seconds (left-hand axis) and minutes (right-hand axis). Two curves are presented: one for factor XI-deficient plasma without factor XI replacement (<1%, ▪) and a second curve for the same plasma with 1 U/mL factor XI (25 nmol/L, •). Smooth curves following each set of data were drawn, meeting near 24 pmol/L TF. The curves become increasingly divergent as TF concentration is reduced to 6 pmol/L TF, where the difference is most pronounced.
Clotting in factor XI-deficient plasma as a function of TF and factor XI. Clot time in factor XI-deficient plasma (<1%) was measured as a function of TF and factor XI (as described in the Materials and Methods). The ordinate is shown in seconds (left-hand axis) and minutes (right-hand axis). Two curves are presented: one for factor XI-deficient plasma without factor XI replacement (<1%, ▪) and a second curve for the same plasma with 1 U/mL factor XI (25 nmol/L, •). Smooth curves following each set of data were drawn, meeting near 24 pmol/L TF. The curves become increasingly divergent as TF concentration is reduced to 6 pmol/L TF, where the difference is most pronounced.
Coagulation in factor XI-deficient and normal whole blood at 25 pmol/L TF.
Table 1 reports the clot times for coagulation in factor XI-deficient blood, deficient blood with replacement, and normal whole blood initiated at 25 and 5 pmol/L TF with suppression of contact activation by corn trypsin inhibitor. At 25 pmol/L TF, at which concentration deficiency of factor VIII results in impaired clot formation and suppression of the propagation phase of thrombin generation, factor XI deficiency has a negligible effect. Clot time is 3.5 minutes (contemporaneous normal control = 3.3 minutes) and is not shortened by factor XI replacement (3.7 minutes). In the control tubes (corn trypsin inhibitor present, no TF added), clotting is significantly prolonged or nonexistent, indicating that other sources of initiation contribute negligibly in these experiments. At 25 pmol/L TF, thrombin generation profiles for the normal and factor XI-deficient (patient C1) donors are almost identical, whereas the profile for factor XI replacement exhibits somewhat faster thrombin generation (Fig 3A). The maximum rates of thrombin generation are nearly identical in the normal (61 nmol/L/min) and factor XI-deficient (63 nmol/L/min) experiments, whereas factor XI replacement increased the rate of thrombin generation to 85 nmol/L/min. This increase leads to a 65% higher concentration of final TAT in the replacement case (950 nmol/L) than in the deficient case (575 nmol/L). These results indicate that factor XI is not required for explosive thrombin generation in blood initiated with 25 pmol/L, but modestly influenced the rate of thrombin generation after clot formation in this individual.
Coagulation in normal and hemophilia C blood at 25 pmol/L initiator, with and without replacement. Using 25 pmol/L TF, coagulation was initiated in normal and hemophilia C blood (see the Materials and Methods; Rand et al34), with and without factor XI replacement (1 U/mL blood). Time courses for TAT are provided (A) after immunoassay analysis of quenched samples from the normal blood (•), hemophilia C blood (patient C1, ○), and hemophilia C blood with factor XI replacement (□). Clot times for the experiments and control tubes are as given in Table 1 and are denoted by arrows for blood from normal (arrow a), hemophilia C (arrow c), and hemophilia C donors with factor XI replacement (arrow b, 1 U/mL). In addition to TAT, FPA (B) and platelet osteonectin (C) profiles are provided. (Symbols and clot times as in [A].)
Coagulation in normal and hemophilia C blood at 25 pmol/L initiator, with and without replacement. Using 25 pmol/L TF, coagulation was initiated in normal and hemophilia C blood (see the Materials and Methods; Rand et al34), with and without factor XI replacement (1 U/mL blood). Time courses for TAT are provided (A) after immunoassay analysis of quenched samples from the normal blood (•), hemophilia C blood (patient C1, ○), and hemophilia C blood with factor XI replacement (□). Clot times for the experiments and control tubes are as given in Table 1 and are denoted by arrows for blood from normal (arrow a), hemophilia C (arrow c), and hemophilia C donors with factor XI replacement (arrow b, 1 U/mL). In addition to TAT, FPA (B) and platelet osteonectin (C) profiles are provided. (Symbols and clot times as in [A].)
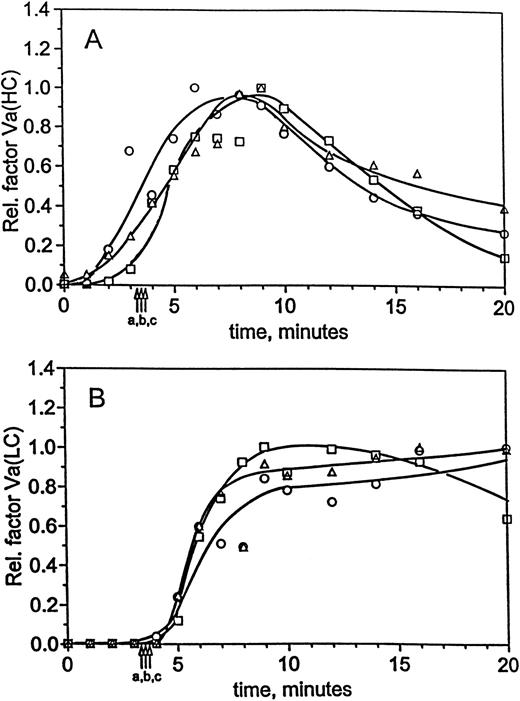
Figure 3B shows the FPA profiles obtained at 25 pmol/L TF. For each case, the reaction progress occurs over similar time scales and to similar extents. In factor XI-deficient blood, FPA is released at a maximum rate of 6.1 μmol/L/min (5.6 μmol/L/min in normal blood), whereas replacement of factor XI increased this rate to 7.3 μmol/L/min. Fibrinogen conversion at clot time was between 30% and 41% in all cases. In addition, osteonectin release profiles (Fig 3C) show that platelet activation is not strongly influenced by the presence or absence of factor XI when the reaction is initiated with 25 pmol/L TF. In all cases, the profiles were similar and exhibited maximal release by 5 minutes. Evaluation of factor Va generation in normal and factor XI-deficient blood showed identical activation profiles. Densitometric profiles of the heavy chain (Fig 4A) show that factor VaHCis detectable within 1 minute of initiation in all reactions, and by clot time approximately 33% to 50% is observed in each profile. Likewise, the profiles for light chain formation in the factor XI-deficient, replacement, and normal experiments are similar to each other (Fig 4B). Levels of factor VaLC are below the limits of detection throughout the initiation phase and only a small fraction is generated at clot time. After clot time, light chain is generated quantitatively within 1 to 2 minutes (4 and 5 minutes postinitiation). Together, the factor VaHC and VaLC profiles demonstrate that cofactor activation is unaffected by factor XI.
Factor Va generation during coagulation in normal and hemophilia C blood at 25 pmol/L initiator, with and without replacement. For the experiments described in Fig 3, analysis of factor V activation was performed by immunoblotting. Profiles following factor Va heavy chain (A) and light chain (B) were constructed by densitometric analysis as in the Materials and Methods. Time courses are given for formation of the heavy and light chains in normal blood (○) and hemophilia C blood (patient C1), with (□) and without (▵) factor XI replacement. Clot times are as in Table 1 and Fig 3, and curves have been drawn through the points by hand.
Factor Va generation during coagulation in normal and hemophilia C blood at 25 pmol/L initiator, with and without replacement. For the experiments described in Fig 3, analysis of factor V activation was performed by immunoblotting. Profiles following factor Va heavy chain (A) and light chain (B) were constructed by densitometric analysis as in the Materials and Methods. Time courses are given for formation of the heavy and light chains in normal blood (○) and hemophilia C blood (patient C1), with (□) and without (▵) factor XI replacement. Clot times are as in Table 1 and Fig 3, and curves have been drawn through the points by hand.
Coagulation in factor XI-deficient and normal blood at 5 pmol/L TF.
Coagulation in normal and factor XI-deficient blood (patient C2) after initiation at 5 pmol/L TF are summarized in Table 1 and Fig 5. Confirmation of the data has been obtained in a separate experiment with a third factor XI-deficient individual (data not shown). Clotting in factor XI-deficient blood at 5 pmol/L initiator (15.7 minutes, Table 1) was delayed 4.6 minutes relative to normal (11.1 minutes, contemporaneous donor). Factor XI replacement shortened the clot time in hemophilia C blood by 6 minutes (9.7 minutes), in agreement with the predictions of the plasma assay (Fig 2). In the absence of TF (corn trypsin inhibitor only), the controls for hemophilia C did not clot with or without factor XI replacement (over 20.5 minutes), confirming that factor XIa contamination was not significant in the factor XI preparations.
Coagulation in normal and hemophilia C blood at 5 pmol/L initiator, with and without replacement. Using 5 pmol/L TF, coagulation was initiated in normal and hemophilia C blood (see the Materials and Methods; Rand et al34), with and without factor XI replacement (1 U/mL blood). Time courses for TAT are provided (A) after immunoassay analysis of quenched samples from the normal blood (•), hemophilia C blood (patient C2, ○), and hemophilia C blood with factor XI replacement (□). Clot times for the experiments and control tubes are as given in Table 1 and are denoted by arrows for blood from normal (arrow a), hemophilia C (arrow c), and hemophilia C donors with factor XI replacement (arrow b, 1 U/mL). In addition to TAT, FPA (B) and platelet osteonectin (C) profiles are provided. (Symbols and clot times as in [A].) Clot times are as in Table 1 and (A), and curves have been drawn through the points by hand.
Coagulation in normal and hemophilia C blood at 5 pmol/L initiator, with and without replacement. Using 5 pmol/L TF, coagulation was initiated in normal and hemophilia C blood (see the Materials and Methods; Rand et al34), with and without factor XI replacement (1 U/mL blood). Time courses for TAT are provided (A) after immunoassay analysis of quenched samples from the normal blood (•), hemophilia C blood (patient C2, ○), and hemophilia C blood with factor XI replacement (□). Clot times for the experiments and control tubes are as given in Table 1 and are denoted by arrows for blood from normal (arrow a), hemophilia C (arrow c), and hemophilia C donors with factor XI replacement (arrow b, 1 U/mL). In addition to TAT, FPA (B) and platelet osteonectin (C) profiles are provided. (Symbols and clot times as in [A].) Clot times are as in Table 1 and (A), and curves have been drawn through the points by hand.
In the TAT profiles of Fig 5A, the bulk of the thrombin is formed after clot time, and factor XI replacement increases the rate of thrombin generation during the propagation phase in hemophilia C blood. In normal blood (Fig 5A), TAT is generated at 110 nmol/L/min after clot time (arrow b), compared with approximately 37 nmol/L/min after clot time (arrow c) in factor XI-deficient blood. Factor XI replacement increases the TAT rate to 119 nmol/L/min at clot time (arrow a). The result is that final levels of TAT are higher in the normal and replacement experiments (750 and 600 nmol/L), but only reached approximately 150 nmol/L when the factor XI-deficient experiment was terminated. But even at 5 pmol/L TF, thrombin production in hemophilia C blood is in excess of levels observed in hemophilia A blood at 25 pmol/L, consistent with the relative clinical severity of the two congenital diseases.
Figure 5B displays profiles for the release of fibrinopeptide A, which also becomes dependent on factor XI at 5 pmol/L TF. FPA release occurs more slowly in hemophilia C blood at 5 pmol/L initiator (maximum rate, 2.7 μmol/L/min) than in normal blood (15.5 μmol/L/min) and is incomplete by the end of the experimental period. Replacement of factor XI increases FPA formation to 7.4 μmol/L/min and provides complete fibrinogen conversion within 2 to 2.5 minutes of clot time.
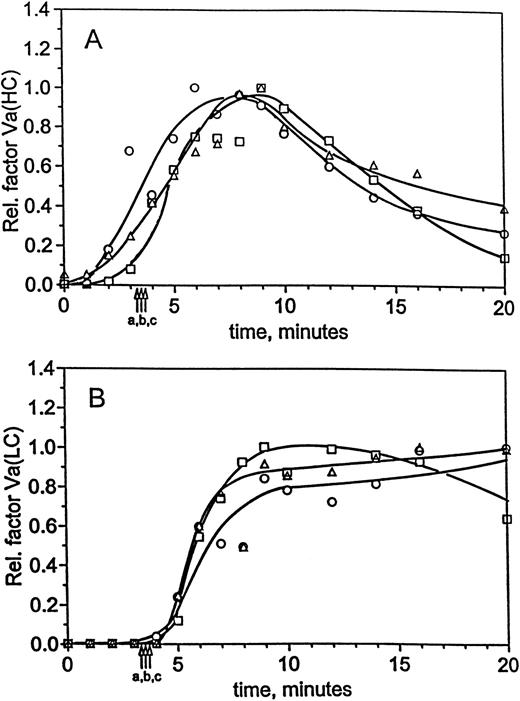
Factor Va generation during coagulation in normal and hemophilia C blood at 5 pmol/L initiator, with and without replacement. For the experiments described in Fig 5, analysis of factor V activation was performed by immunoblotting. Profiles following factor Va heavy chain (A) and light chain (B) were constructed by densitometric analysis as in the Materials and Methods. Time courses are given for formation of the heavy and light chains in normal blood (○) and hemophilia C blood (patient C2), with (□) and without (▵) factor XI replacement. Clot times are as in Table 1 and Fig 5, and curves have been drawn through the points by hand.
Factor Va generation during coagulation in normal and hemophilia C blood at 5 pmol/L initiator, with and without replacement. For the experiments described in Fig 5, analysis of factor V activation was performed by immunoblotting. Profiles following factor Va heavy chain (A) and light chain (B) were constructed by densitometric analysis as in the Materials and Methods. Time courses are given for formation of the heavy and light chains in normal blood (○) and hemophilia C blood (patient C2), with (□) and without (▵) factor XI replacement. Clot times are as in Table 1 and Fig 5, and curves have been drawn through the points by hand.
Platelet activation in blood initiated at 5 pmol/L TF was estimated by osteonectin release (Fig 5C) and contrasted starkly with the data at higher levels of initiator (Fig 3C). In factor XI-deficient blood, osteonectin release is very slow during the initiation phase, reaching approximately 65% by clot time (arrow c). During this period, aggregates were noted as grainy accumulations at the walls of the reaction tubes. With factor XI replacement, osteonectin again increases slowly over the initiation phase, reaching 67% by clot time (arrow a); the remaining osteonectin is released within 2 minutes. This was similar to the normal profile in which 42% fluid phase osteonectin is detected at clot time (arrow b) followed by rapid and immediate release of the remaining protein. In general, at 5 pmol/L TF, platelet activation is slower and less complete at clot time (42% to 67%), associated with limited thrombin generation during the initiation phase. After clot formation, the observed thrombin burst activates the platelets rapidly, ensuring maximal activation. Thus, unlike hemophilias A and C at 25 pmol/L TF, platelet activation at 5 pmol/L TF is significantly influenced by the absence of factor XI.
At 5 pmol/L TF, the generation of factor VaHC in hemophilia C blood, like FPA release and platelet activation, is slower than normal (Fig 6A). The bulk of the heavy chain does not appear until 20 minutes (▵), whereas in the replacement (□) and normal (○) profiles the maximum occurs after clot time in each case near 12 and 15 minutes, respectively. Only small amounts of factor VaHC appear before clot formation in these experiments. In contrast, factor VaLC (Fig 6B) is undetectable until after clot time in each of the three experiments and again appears to be the limiting step in cofactor activation. Thus, factor V activation is affected by the presence or absence of factor XI, with the bulk of factor V activation occurring after clot time in each case.
DISCUSSION
Previously, our laboratory had described coagulation in normal blood after initiation by TF under conditions in which contact activation was suppressed by corn trypsin inhibitor.34 In those studies, clotting was observed at the end of an initiation phase in which limited amounts of thrombin (∼15 nmol/L) had been produced. Immediately after clot formation, a period of explosive thrombin generation was observed (ie, the propagation phase) in which substantial thrombin (up to 360 nmol/L total) was produced within 8 minutes. Using this approach, we investigated coagulation in hemophilia A and hemophilia C blood. In severe hemophilia A at 25 pmol/L TF, clotting was delayed versus normal (∼2.4 minutes), evidence of modestly reduced levels of thrombin during the initiation phase leading to clot formation. A more striking observation was the severely depressed thrombin generation during the propagation phase (ie, after the clot had been detected), measuring less than 4% of the normal rate. Consequently, this impaired thrombin generation was reflected in a reduced rate of fibrinogen cleavage and drastically delayed factor Va generation. Replacement of factor VIII restored normal clotting and thrombin generation in the propagation phase after clotting. These results support earlier observations of reduced prothrombinase activity in the absence of functional intrinsic tenase.6,8,21,22 51In addition, our study in whole blood shows that reduced thrombin generation in the absence of factor VIII also leads to severely reduced factor Va generation, which further reduces the effectiveness of the limited factor Xa produced during coagulation in hemophilia A.
Although thrombin generation, fibrinogen cleavage, and platelet activation were retarded during coagulation in hemophilia A blood, we found only a relatively slight delay in platelet activation versus normal (∼1 minute). In a model system containing zymogens, cofactors, and platelets, Hoffman et al50 observed that factor IXa is not an effective initiator of platelet activation. Our results at 25 pmol/L TF parallel theirs, showing that the progress of platelet release is virtually unaffected in factor VIII deficiency, with 100% activation observed before clot time. Thus, platelet activation is largely independent of the intrinsic tenase. Together, the observations of complete platelet activation and slow fibrinogen formation correlate with the description of clotting in the bleeding time wounds of hemophiliacs,53 54 in which the primary platelet plug is devoid of normal fibrin stabilization, leading to a friable clot that is subject to rupture.
The effect of factor XI deficiency on fibrin formation in plasma coagulation has recently been shown to depend on the level of TF used.33 We have now extended these observations to include observations on thrombin, factor Va, and platelets in hemophilia C blood. In contrast with the results for hemophilia A at 25 pmol/L TF, coagulation was hardly affected in hemophilia C blood. Clotting was identical in hemophilia C and normal experiments, as was explosive thrombin generation in the propagation phase. Replacement of factor XI modestly increased thrombin generation after clot time, but all other products of the reaction (factor V activation, osteonectin release from activated platelets, and FPA) were unaffected by the presence or absence of factor XI. Reducing the initiator concentration to 5 pmol/L prolongs the initiation phase of hemophilia C blood coagulation by 4.6 minutes versus normal, and factor XI replacement shortens this clot time by nearly 6 minutes. The results indicate that impaired coagulation in hemophilia C will occur at lower initiator concentrations than those observed for impaired coagulation in hemophilia A. Furthermore, we observed that, in hemophilia C, maximum thrombin generation rates decrease as TF is reduced from 25 to 5 pmol/L. This agrees with results in a reconstituted model of the TF pathway without factor XI,21 in which the combination of TFPI and AT-III decrease the rate of thrombin generation as the initiating TF concentration was reduced. However, in normal blood as well as in hemophilia C blood with factor XI replacement, such a decrease in thrombin generation was not observed. In fact, an increase was detected: from 61 and 85 nmol/L TAT/min in the normal and replacement experiments using 25 pmol/L TF, respectively, to 110 and 119 nmol/L TAT/min with 5 pmol/L TF. These observations indicate that factor XI plays an increasingly significant role in supplementing prothrombinase levels as the initiator concentration is reduced.
Our results correlate well with the clinical severity of the disorders. At 25 pmol/L TF in hemophilia A, clotting and product formation are greatly affected by deficiency of factor VIII. Conversely, factor XI deficiency appears to have little consequence for coagulation at 25 pmol/L TF. Only when initiator concentration falls below 25 pmol/L does the reaction become sensitive to the presence of factor XI. But even at 5 pmol/L TF, thrombin generation is not ablated, as observed in factor VIII deficiency at 25 pmol/L TF, consistent with the clinical observation that hemophilia C is a less severe disease than hemophilia A.
The current studies complement the work of others33 who measured the dependence of fibrin formation on factor XI and thromboplastin in factor XII-deficient human plasma. In that investigation, fibrin formation in plasma with and without factor XI showed few differences when the length of the initiation phase was less than 6 minutes. FPA profiles in hemophilia C blood are consistent with this result, being virtually identical at 25 pmol/L with or without factor XI replacement. However, additional thrombin is detected late in the propagation phase in our studies, well after fibrinogen conversion is complete, indicating that thrombin levels are sensitive to factor XI even at this concentration of initiator. At lower TF (ie, at 5 pmol/L), at which the initiation phase is extended beyond 6 minutes, the procoagulant effect of factor XI is well-established.
The present investigation was performed in minimally altered whole human blood without the addition of cofactors for factor XI activation by thrombin such as dextran sulfate.29 30 The dependence on factor XI observed under these conditions suggests that blood contains the necessary components to produce biologically sufficient factor XIa activity on a physiologically relevant time scale. In addition, our investigations describe in detail not only thrombin generation and fibrin conversion, but also platelet and cofactor (factor V) activation, and are the only investigations performed to date in which platelets and other blood cells are available as the procoagulant surface. Compared with plasma coagulation studies on exogenously added lipids, these whole blood studies provide a description of the processes occurring on endogenous blood cells during the coagulation reaction.
As discussed above, results here and elsewhere have consequences for the observed instability of the hemophilic platelet-fibrin plug.54 Although rates of fibrin formation are reduced within the clot in hemophilia A, significant fibrin is detectable at clot time and continues to form thereafter. Thus, it may be necessary to consider factors in addition to impaired fibrin formation that may contribute to clot instability. Because the most striking observation made in hemophilia A blood is the severe attenuation of thrombin generation after clot time, the resulting pathology may be more associated with impaired thrombin generation within the clot rather than with initial fibrin formation. Evidence exists for thrombin-dependent mechanisms that regulate clot stability in normal blood, which may be severely affected by impaired thrombin generation in hemophilias. For example, factor XIIIa cross-linking of fibrin within the platelet-fibrin plug relies on fibrin-bound thrombin for its activation.55 At present, impaired cross-linking has not been examined as a possible source of plug instability in hemophilia. The fibrinolytic pathway may also play a major role in clot stability, as evidenced by the successful treatment of mild hemophilias with plasmin inhibitors such as ε-aminocaproic acid (see, for example, Walsh et al56). Recently, a thrombin-activatable fibrinolysis inhibitor (TAFI) has been described that slows the rate of clot lysis,57 and investigators have demonstrated that both TAFI activation and fibrinolysis are reduced in hemophilia A and C plasmas.58 Thus, normal coagulation and fibrinolysis are probably coregulated by prothrombin activation within the clot,59 which is impaired in the hemophilias.
By specifically inhibiting factor XI activity in blood, it may be possible to modulate the length of the initiation phase and subsequent thrombin generation at TF concentrations at which the involvement of factor XI is significant. In the therapeutic venue, such an approach may prove valuable by reducing thrombin generation within clots to make them more amenable to thrombolytic therapy. This approach appears feasible, because impaired thrombin generation in classic hemophilia and factor XI deficiency has been suggested to cause an increased tendency toward clot lysis.33,58 Thrombolysis after selective inhibition of factor XI has been reported most recently in an animal model system using a polyclonal antibody against rabbit factor XI60; however, attempts to correlate factor XI activity with fibrinolysis in humans have met with mixed results.61Whether modulation of factor XI in TF-induced coagulation can produce significant benefits remains to be seen, but additional work will certainly focus on a strategy of factor XI inhibition to increase the effectiveness of current thrombolytic techniques.
In the clinical setting, the routine use of cell-free plasma assays, with fibrin formation as an endpoint, has proven utility as a diagnostic tool in identifying severe coagulation abnormalities. But these approaches do not give a detailed picture of the entire coagulation reaction and do not reflect the role of blood cells and platelets in the process. Therefore, valuable insights are gained from detailed investigations directly in whole blood, allowing a more complete and biologically relevant description of the manifold processes occurring during the complex coagulation reaction.
ACKNOWLEDGMENT
The authors thank the following for their contributions: Richard Jenny, PhD (Hematologic Technologies, Inc) for gifts of FPRck, factor XI, and osteonectin ELISA kits; Roger Lundblad, PhD, and Shu-Len Liu, PhD, (Hyland Division, Baxter Healthcare Corp) for gifts of purified factor VIII and recombinant TF; Mollie D. Winfield and Jason J. Pennucci for their expert technical assistance with a few of the experiments and reagent preparation; and Miriam Husted for assistance with the phlebotomies.
Supported in part by GCRC Grant No. RR00109 from the National Institutes of Health, by Program Project Grant No. HL 46703 (Project 1) from the National Institutes of Health (K.G.M.), by Training Grant No. PHS T32 HL07594-12 from the US Public Health Service (K.M.C.), and by a TALENT stipendium of the Netherlands Organization of Scientific Research (C.v.V.).
Presented in part at the Thirty-eighth Annual Meeting of the American Society of Hematology, December 6-10, 1996, Orlando, FL (Blood 88:520a, 1996 [abstr 2067, suppl 1]).
Address reprint requests to Kenneth G. Mann, PhD, Department of Biochemistry, College of Medicine, University of Vermont, Burlington, VT 05405-0068.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 1. Coagulation in normal and hemophilia A blood, with and without replacement. Using 25 pmol/L TF, coagulation was initiated in normal and hemophilia A blood (see the Materials and Methods and Rand et al34) with and without recombinantfactor VIII replacement (1 U/mL blood). Smooth curves have been drawn by hand through the points to approximate the data. (A) Time courses for TAT in normal blood (•), hemophilia A blood (patient A1; ○), and hemophilia A blood with recombinant factor VIII (□). Each point on the normal curve represents an average TAT from 14 experiments on four normal subjects (with error bars, SEM). Average clot time for the composite normal curve is 4.0 ± 0.2 minutes (arrow a). Clotting in factor VIII-deficient blood occurred at 6.5 minutes (arrow c), which was shortened to 4.1 minutes with factor VIII replacement (arrow b); a control tube without TF in the factor VIII-deficient experiment did not clot (>20.6 minutes), and the replacement control clotted at 17.8 minutes. (B) FPA generation in normal blood, factor VIII-deficient blood, and factor VIII-deficient blood with replacement (symbols as in [A]). The normal curve represents the averaged results from nine experiments performed on two individuals, with error bars (SEM). The average clot time for the normal profile was 4.1 ± 0.2 (SEM) minutes (arrow a), with the other clot times as in (A). (C) Osteonectin release was measured to examine platelet activation (symbols as in [A]). The normal curve is constructed from blood taken from a single normal donor (clot time = 4.1 minutes, arrow a) drawn contemporaneously with the factor VIII-deficient patient. Other clot times and symbols are as in (A). (D) After analysis of factor V activation by immunoblotting, profiles were constructed by densitometric analysis as in the Materials and Methods. Time courses are given for formation of the heavy (▪, □) and light chains (•, ○) of factor Va (factor VaHCand VaLC), with factor VIII replacement (solid symbols) and without (open symbols). For clarity, the normal profile is omitted, but is similar to the profile with factor VIII replacement. Clot times are 4.1 minutes (hemophilia A with factor VIII replacement, arrow a) and 6.5 minutes (hemophilia A, arrow b).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/12/10.1182_blood.v91.12.4581/4/m_blod41212001x.jpeg?Expires=1767812444&Signature=fjyfafjOKaIYv-jLirpnP6ecWX57bsTX0p~uVh9eWpkv3qAhJzG~qlwC5IU4PJCvWnqaHyMbifWqCO55JIXRJP3oueV484eAmqc1TXKMPf1bx3SHi9jJ5u4GJHRAcO3-wx~-7Q1qo-e9H6LWG5JZ3YZa84OKI5SYrxkcQWL9kqJcy3pkG-lr2GeqHpFB3hOfQdmOkERJ3pIQcPHfu-94NEKSAwd8ahjOxH16pDihjwisydI-lxnrgZcTuwa5xRdTGNwJ29AxxpuZckPXTCLlW7YwWNnXlXpvhZ7-mCWTE5DTDBE59a7rEIh-GHCdujjwhTvVsa2rAS3GsXc1VipYbA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Coagulation in normal and hemophilia C blood at 25 pmol/L initiator, with and without replacement. Using 25 pmol/L TF, coagulation was initiated in normal and hemophilia C blood (see the Materials and Methods; Rand et al34), with and without factor XI replacement (1 U/mL blood). Time courses for TAT are provided (A) after immunoassay analysis of quenched samples from the normal blood (•), hemophilia C blood (patient C1, ○), and hemophilia C blood with factor XI replacement (□). Clot times for the experiments and control tubes are as given in Table 1 and are denoted by arrows for blood from normal (arrow a), hemophilia C (arrow c), and hemophilia C donors with factor XI replacement (arrow b, 1 U/mL). In addition to TAT, FPA (B) and platelet osteonectin (C) profiles are provided. (Symbols and clot times as in [A].)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/12/10.1182_blood.v91.12.4581/4/m_blod41212003x.jpeg?Expires=1767812444&Signature=Iqt-FPIKHopil9vGxoVdgFvEdgofQM2-Prtot645zp716Ki7Lbcgdm-SRfaHB~1XcjCDQlfhBgZ9AQUe4aA61ts7rQLmBItcnCWfSx3MJ8xyRe6T1ZUeQ4yulgtMd9SosMoJX2Yh0bjd8XERcqE3-p-61qQ59LtmaZS2CdwBzKTuHGHwcSYXC2~hxFW4IWffjm1iWwOuAbf7Qk3KhrK5vr14pt8yqNzgs8hVTaSty7H5Dq7JcNuynm6AlSaQNMV9D6Mf5IjcLQc8WPcwFJ~gqknv2hrVIPZc5WXpSmZdEgguJ8Vf5cjXArVRY~rVmhUd1HzNwDE2t8Y7L~BhzDVoJA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Coagulation in normal and hemophilia C blood at 5 pmol/L initiator, with and without replacement. Using 5 pmol/L TF, coagulation was initiated in normal and hemophilia C blood (see the Materials and Methods; Rand et al34), with and without factor XI replacement (1 U/mL blood). Time courses for TAT are provided (A) after immunoassay analysis of quenched samples from the normal blood (•), hemophilia C blood (patient C2, ○), and hemophilia C blood with factor XI replacement (□). Clot times for the experiments and control tubes are as given in Table 1 and are denoted by arrows for blood from normal (arrow a), hemophilia C (arrow c), and hemophilia C donors with factor XI replacement (arrow b, 1 U/mL). In addition to TAT, FPA (B) and platelet osteonectin (C) profiles are provided. (Symbols and clot times as in [A].) Clot times are as in Table 1 and (A), and curves have been drawn through the points by hand.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/12/10.1182_blood.v91.12.4581/4/m_blod41212005x.jpeg?Expires=1767812444&Signature=bUN1eTqmPPZ6h9fwgs~8IymBPJlsJpjCf1A~M97vYU2xPgRt7hfhGL09KnuxFI31Q0xpePHqXPHdvlJn46REa6drLwUS95pDQ-2XY5kAwma1HOcGZWJb9G61uQZXnRke95OJWeOknTN87F6b3VbMxyyVZLbnghvyvDg7D-2snaj2PYw2ylAB8j1UNrkK2gj84NPbZ6ak7ef2iygEAdo2pRu0tT4SL5D2QqUc-m~MzGtGLCEJv0ZhtK16R-w84OMJ9zNU~t0fW-gRd4edTyM7GiHQ57sI2-A797oHIxQWUZ9xbqOc1jeImy9FlPcGZIMOQoxpO9wUSIaESS8sxk8wNw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 1. Coagulation in normal and hemophilia A blood, with and without replacement. Using 25 pmol/L TF, coagulation was initiated in normal and hemophilia A blood (see the Materials and Methods and Rand et al34) with and without recombinantfactor VIII replacement (1 U/mL blood). Smooth curves have been drawn by hand through the points to approximate the data. (A) Time courses for TAT in normal blood (•), hemophilia A blood (patient A1; ○), and hemophilia A blood with recombinant factor VIII (□). Each point on the normal curve represents an average TAT from 14 experiments on four normal subjects (with error bars, SEM). Average clot time for the composite normal curve is 4.0 ± 0.2 minutes (arrow a). Clotting in factor VIII-deficient blood occurred at 6.5 minutes (arrow c), which was shortened to 4.1 minutes with factor VIII replacement (arrow b); a control tube without TF in the factor VIII-deficient experiment did not clot (>20.6 minutes), and the replacement control clotted at 17.8 minutes. (B) FPA generation in normal blood, factor VIII-deficient blood, and factor VIII-deficient blood with replacement (symbols as in [A]). The normal curve represents the averaged results from nine experiments performed on two individuals, with error bars (SEM). The average clot time for the normal profile was 4.1 ± 0.2 (SEM) minutes (arrow a), with the other clot times as in (A). (C) Osteonectin release was measured to examine platelet activation (symbols as in [A]). The normal curve is constructed from blood taken from a single normal donor (clot time = 4.1 minutes, arrow a) drawn contemporaneously with the factor VIII-deficient patient. Other clot times and symbols are as in (A). (D) After analysis of factor V activation by immunoblotting, profiles were constructed by densitometric analysis as in the Materials and Methods. Time courses are given for formation of the heavy (▪, □) and light chains (•, ○) of factor Va (factor VaHCand VaLC), with factor VIII replacement (solid symbols) and without (open symbols). For clarity, the normal profile is omitted, but is similar to the profile with factor VIII replacement. Clot times are 4.1 minutes (hemophilia A with factor VIII replacement, arrow a) and 6.5 minutes (hemophilia A, arrow b).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/12/10.1182_blood.v91.12.4581/4/m_blod41212001x.jpeg?Expires=1768048244&Signature=rK-MBIsYYq6myCuS2-zDa8V43a1YK7OcrzQzij49erf0IlS1xW1X8xbL0lxv1Dy6VwKlr73pwk7GwUq~RYAEeHV~zBPfXLcznKpsdPn9N3oGXxjCh7asJc0fZCkNF-CTPZUpznq5dcYGtVs8U8CIVgbgZFNITImILEKjN3wDVbisaGQQDCJ7V4waWnzuHetj2xdecpopgp9jbJMl8x4ivSTUB9gWEPDkBPkhNru~IaOhcN5R35a5GeE5nvyyHYkjCP3MTYnmahlX1PyYcu9yOmqvZoqTZhzY9xnmNWjFCOHCg4z2w-Y6DfjVFIRFjgNzxMZNeb~CKT-N8NuiayJssw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Coagulation in normal and hemophilia C blood at 25 pmol/L initiator, with and without replacement. Using 25 pmol/L TF, coagulation was initiated in normal and hemophilia C blood (see the Materials and Methods; Rand et al34), with and without factor XI replacement (1 U/mL blood). Time courses for TAT are provided (A) after immunoassay analysis of quenched samples from the normal blood (•), hemophilia C blood (patient C1, ○), and hemophilia C blood with factor XI replacement (□). Clot times for the experiments and control tubes are as given in Table 1 and are denoted by arrows for blood from normal (arrow a), hemophilia C (arrow c), and hemophilia C donors with factor XI replacement (arrow b, 1 U/mL). In addition to TAT, FPA (B) and platelet osteonectin (C) profiles are provided. (Symbols and clot times as in [A].)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/12/10.1182_blood.v91.12.4581/4/m_blod41212003x.jpeg?Expires=1768048244&Signature=4Q93CP3fKwA5ZJJrTjuTvwstVGNQF1~Lp~uqXN~RiyXgiPSvNlTEUqYU6GrCAgidQ-wrfIg15XhXPi3TnMlww2CYdiQF244sIOFWu6riylWM9Qj1bIH8sxKRBXw9~Kz5vGISWrv~W53ZgJsk2-0I-S6EmXFpzPm6uk0Agy~fKv7oO8UxoX02KJ-~QuNekFL1jputPvuLS~90N208JrNnA1iJQhS5sRxeojsng4oBsVpZo5oQkL4OfAK2gKwtj-0YRGscXoQYkmVW-h~DHcYpmD~QAxp4fFUFk8e4AKwhQff7UujTg6Z1HdwPmJnDsBBx-YfqZhQDJu-H56myHwi5Sw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Coagulation in normal and hemophilia C blood at 5 pmol/L initiator, with and without replacement. Using 5 pmol/L TF, coagulation was initiated in normal and hemophilia C blood (see the Materials and Methods; Rand et al34), with and without factor XI replacement (1 U/mL blood). Time courses for TAT are provided (A) after immunoassay analysis of quenched samples from the normal blood (•), hemophilia C blood (patient C2, ○), and hemophilia C blood with factor XI replacement (□). Clot times for the experiments and control tubes are as given in Table 1 and are denoted by arrows for blood from normal (arrow a), hemophilia C (arrow c), and hemophilia C donors with factor XI replacement (arrow b, 1 U/mL). In addition to TAT, FPA (B) and platelet osteonectin (C) profiles are provided. (Symbols and clot times as in [A].) Clot times are as in Table 1 and (A), and curves have been drawn through the points by hand.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/12/10.1182_blood.v91.12.4581/4/m_blod41212005x.jpeg?Expires=1768048244&Signature=aTakNZb5FViI3PbHEapO2YjFwuRUE3E7OHxRlJ6CkVA7M83xyeOYhMKy2IT6pweRVaE8hIOcfIPGMVvJ00wt~OUAasrYZV1IfAuCYrj0-czMEq4ZnqvtgtqObViT5vODb7QenCQEQ-2OlN2Mw5YzVseZ0xM9nXVG5eVBfCs0XUb36n5IQ9HQOeh7mFdzuneSNRmyh1pua1DR5brRplETi6~Etu-h7L5PoF1~1-UrfTvAsASo340Boe7FK9unVCvXFqbL2Omu-7fpvHdX54D6sIzOUzRMfuB4IIhmBAFhnx7Fhlgb~52RgM1NiU3KEq4ArNDuM-LWLEDy4gR7S-CFfA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)