Abstract
Chemokines as well as the signaling through the adhesion molecules intercellular adhesion molecule (ICAM)-3 and CD43 are able to induce in T lymphocytes their switching from a spherical to a polarized motile morphology, with the formation of a uropod at the rear of the cell. We investigated here the role of CD43 in the regulation of T-cell polarity, CD43-cytoskeletal interactions, and lymphocyte aggregation. Pro-activatory anti-CD43 monoclonal antibody (MoAb) induced polarization of T lymphocytes with redistribution of CD43 to the uropod and the CCR2 chemokine receptor to the leading edge of the cell. Immunofluorescence analysis showed that all three ezrin-radixin-moesin (ERM) actin-binding proteins localized in the uropod of both human T lymphoblasts stimulated with anti-CD43 MoAb and tumor-infiltrating T lymphocytes. Radixin localized at the uropod neck, whereas ezrin and moesin colocalized with CD43 in the uropod. Biochemical analyses showed that ezrin and moesin coimmunoprecipitated with CD43 in T lymphoblasts. Furthermore, in these cells, the CD43-associated moesin increased after stimulation through CD43. The interaction of moesin and ezrin with CD43 was specifically mediated by the cytoplasmic domain of CD43, as shown by precipitation of both ERM proteins with a GST-fusion protein containing the CD43 cytoplasmic tail. Videomicroscopy analysis of homotypic cell aggregation induced through CD43 showed that cellular uropods mediate cell-cell contacts and lymphocyte recruitment. Immunofluorescence microscopy performed in parallel showed that uropods enriched in CD43 and moesin localized at the cell-cell contact areas of cell aggregates. The polarization and homotypic cell aggregation induced through CD43 was prevented by butanedione monoxime, indicating the involvement of myosin cytoskeleton in these phenomena. Altogether, these data indicate that CD43 plays an important regulatory role in remodeling T-cell morphology, likely through its interaction with actin-binding proteins ezrin and moesin. In addition, the redistribution of CD43 to the uropod region of migrating lymphocytes and during the formation of cell aggregates together with the enhancing effect of anti-CD43 antibodies on lymphocyte cell recruitment suggest that CD43 plays a key role in the regulation of cell-cell interactions during lymphocyte traffic.
THE POLARIZATION OF cell surface and intracellular structures is important during differentiation for immune response induction and cognate interactions between immune and other cells.1 T lymphocytes migrate through lymphocyte organs and extravasate into inflammatory sites to accomplish effector functions by sequential engagement of adhesion and signaling receptors.2-4 Effector activity of these cells involves plasma membrane clustering of LFA-1 and CD4 and cytoskeletal reorganization of microfilaments, microtubules, and talin.5-7 Migrating cells also display other polarized features, such as the localization of chemokine receptors towards the leading edge of the cell,8,9 an area of the cell that undergoes dynamic changes in the organization of the actin cytoskeleton,10 and the uropod at the opposite pole where intercellular adhesion molecules (ICAMs), CD44, and CD43 molecules are clustered.11
CD43 (leukosialin, sialophorin) is a transmembrane glycoprotein that bears a heavily O-glycosylated extracellular N-terminal region and that is expressed by hematopoietic cells.12-15 In humans, low and high molecular weight (113 to 120 and 125 to 135 kD, respectively) glycoforms of CD43 are generated by differential O-glycosylation.16 Although its high negative charge may provide a repulsive barrier that interferes with cellular adhesion phenomena, this molecule was reported to be a counter-receptor for ICAM-1 and major histocompatibility complex (MHC) class I molecules.17,18 In this regard, CD43 has been described as promoting leukocyte aggregation.19,20 However, the functional analysis of T cells from CD43-deficient mice showed an in vitro increase in their proliferative response to mitogens and an enhanced homotypic cell aggregation capability, suggesting that CD43 could act as an antiadhesive molecule.21 In accordance, CD43 expression in target cells diminishes its susceptibility to cytolysis by T cytotoxic and natural killer cells.22,23Thus, the role of CD43 as an adhesion receptor still remains controversial. However, it is now clear that CD43 plays a role in the regulation of T-lymphocyte adhesiveness and cellular activation.24,25 In this regard, we previously described the regulatory role of CD43 on integrin-mediated T-cell adhesion to endothelium and proteins of extracellular matrix.26 On the other hand, the existence of a putative endothelial cell ligand for CD43 has recently been proposed, suggesting a role for this molecule in the selective traffic of T cells through lymphoid tissues.27
Interaction of cell adhesion receptors with cytoskeletal proteins may propagate signals for cellular reorganization.1 Previous studies have demonstrated that CD43 colocalized with the ezrin-radixin-moesin (ERM) family of plasma membrane-cytoskeleton cross-linkers implicated in the formation of membrane dynamic structures such as filopodia, microvilli, and microspicules.28-30 During cytokinesis of cell division, these proteins interact directly or indirectly with the cytoplasmic domain of CD43 in the cleavage furrow.31 In this report, we explored the cellular localization of ERM proteins and CD43 in polarized motile lymphocytes as well as in cell aggregates induced by anti-CD43 antibodies. In addition, we studied the possible participation of CD43 in the formation of cell-cell contacts. Evidence is presented for the association of moesin and ezrin with CD43 and its coredistribution to the cellular uropods during T-cell aggregation and cell recruitment induced through CD43.
MATERIALS AND METHODS
Antibodies and reagents.
The anti-ICAM-3 HP2/19 (IgG2a), anti-CD43 HP2/21 (IgM) and TP1/36 (IgG1), anti-CD44 HP2/9 (IgG1), anti-CD45 RP2/21 (IgG1), anti-CCR2 MCP-1R03 (IgG1), antimoesin/radixin 38/87 (IgG1), and anti-HLA-A,B W6/32 (IgG1) monoclonal antibodies (MoAbs) have been described.9,32-36 The fluorescein isothiocyanate (FITC)-conjugated 2D1 anti-CD45 (IgG1) MoAb was purchased from Becton Dickinson (San Jose, CA). The moesin-specific polyclonal antiserum (pAb) 95/2 was raised in rabbits by immunization with recombinant human moesin and purified by affinity chromatography.37 The ezrin-specific pAb 90/3 was also raised in rabbits by immunization with purified human ezrin.38 The affinity-purified polyclonal antibodies 464 and 457, raised against unique peptides from murine ezrin and radixin, respectively,39 were kindly provided by Dr F. Solomon (Department of Biology and Center for Cancer Research, MIT, Cambridge, MA). Recombinant human (rh) moesin was obtained as described.38 Butanedione monoxime was purchased from Sigma Chemical Co (St Louis, MO). The 80-kD fibronectin fragment (FN80) was a generous gift of Dr A. Garcı́a-Pardo (Centro de Investigaciones Biológicas, Madrid, Spain). The chimeric ICAM-1-Fc, consisting of the extracellular domains of ICAM-1 and the Fc region of IgG1, was obtained as described.11
Cells.
Human T lymphoblasts were prepared from peripheral blood mononuclear cells by treatment with phytohemagglutinin (PHA) 0.5% (Pharmacia, Uppsala, Sweden) for 48 hours. Cells were then washed and cultured in RPMI 1640 (Flow Laboratories, Irvine, Scotland) containing 10% fetal calf serum (FCS; Flow Laboratories) and 50 U/mL interleukin-2 (IL-2) kindly provided by Eurocetus (Madrid, Spain). T lymphoblasts cultured by 10 to 15 days were used in all experiments. These cells were analyzed by flow cytometry and their phenotype was 98% CD3 and 99% CD45RO; also, they show a heterogeneous expression of chemokine receptors CCR2 and CCR5.9,40 Melanoma cells and CD8+tumor-infiltrating lymphocytes (TIL) were isolated from melanoma specimens from patients with metastatic melanoma (Department of Pathology, Hospital General Universitario Gregorio Marañón, Madrid, Spain). These cells were cultured in AIM-V medium (Flow Laboratories) containing 10% HY-ultroser serum (Pharmacia) and 5,000 U/mL IL-2.40 Jurkat cells were grown in complete medium without the addition of IL-2.
Immunofluorescence microscopy.
Immunofluorescence experiments were performed as described.11 Briefly, 1 × 106 T lymphoblasts were incubated in flat-bottommed, 24-well plates (Costar Corp, Cambridge, MA) in a final volume of 400 μL complete medium on coverslips coated with FN80 at 20 μg/mL. The polarization-inducing anti-CD43 HP2/21 (supernatant) and TP1/36 at 4 μg/mL were added and cells were allowed to remain in a cell incubator at 37°C and 5% CO2 atmosphere. After 30 minutes, cells were fixed with 3.7% (wt/vol) paraformaldehyde in phosphate-buffered saline (PBS), pH 7.4, at room temperature and then rinsed in TBS. Fixed cells were incubated with specific MoAb or pAb. After washing, cells were stained with an FITC-labeled rabbit F(ab)′2 antimouse IgG, donkey antirabbit IgG (Pierce, Rockford, IL), or Cy3-tagged goat antimouse IgG (Amersham, Pittsburgh, PA). For double immunofluorescence analysis, cells were saturated with 10% normal mouse serum in TBS. Coverslips were then incubated with biotinylated MoAb, followed by washing and labeling with either FITC-extrAvidin (Sigma) or Cy3-streptavidin.
Cell aggregation assays.
Homotypic cell aggregation was performed as previously described.33 Briefly, T lymphoblasts were incubated in flat-bottommed, 24-well microtiter plates (Costar Corp) at 4 × 106/mL in a final volume of 0.5 mL of complete medium. Then, 1:20 dilution of anti-CD43 HP2/21 supernatant was added and cells were incubated at 37°C and 5% CO2 atmosphere. Aggregation was then determined at different periods of time by direct visualization of the plate with an inverted microscope and counting the free cells of at least five randomly chosen areas of 0.025 mm2. Results were expressed as a percentage of aggregated cells.
For immunofluorescence staining, after either 10 minutes (small cell aggregates) or 30 minutes (large cell aggregates) of incubation, cells were fixed with 3.7% paraformaldehyde in PBS for 10 minutes at room temperature and rinsed in TBS. To directly visualize CD43, cells were stained with a 1:50 dilution of an FITC-labeled rabbit F(ab)′2 antimouse IgG (Pierce). To visualize moesin, after cell permeabilization with 0.1% Triton X-100, cell aggregates were incubated with the biotinylated antimoesin 38/87 MoAb followed by washing and labeling with 1:1,000 dilution of Cy3-streptavidin. Cells were observed using a Nikon Eclipse-800 photomicroscope with 60× and 100× oil immersion objectives. Preparations were photographed with Ektachrome 400 film (Eastman Kodak Co, Rochester, NY).
Time-lapse videomicroscopy.
Video microscopy analysis was performed using a Nikon Diaphot 300 inverted microscopy equipped with a Sony SSC-M350CE CCD black and white videocamara coupled to a Sony SVT-5000P time lapse videocassette recorder and a Sony PVM-1453MD video monitor (Japan).
Cell recruitment assays.
T lymphoblasts were allowed to attach for 20 minutes at 37°C to 35-mm plastic petri dishes (Costar Corp) previously coated with ICAM-1Fc (10 μg/mL) in the presence of anti-CD43 HP2/21. After the addition of a second layer of T lymphoblasts, cell-cell interactions were recorded for 1 hour under phase contrast using a 20× objective. Images were acquired every 3.2 seconds, and sequential frames were digitalized by using Optimas software (Optimas Corp, Bothell, WA).
Immunoprecipitation and Western blot analysis.
T lymphoblasts either untreated or treated with the anti-CD43 HP2/21 MoAb adhered to FN80-coated dishes were lysed by incubation for 20 minutes in 1.5 mL RIPA buffer containing 0.1% sodium dodecyl sulfate (SDS), 0.5% deoxycholate, 1% Nonidet P-40, 150 mmol/L NaCl, 50 mmol/L Tris (pH 8), 1 mmol/L p-amidino phenylmethyl sulfonyl fluoride (PMSF), and 15 μg/mL leupeptin. Cell lysate was removed from the dish with a rubber policeman and then precleared by centrifugation at 10,000g for 20 minutes. Immunoprecipitations were performed with 45 μL of TP1/36 anti-CD43 or RP2/21 anti-CD45 (control) MoAb directly coupled to Sepharose 4B beads (Pharmacia) at 1 mg/mL. Proteins bound to Sepharose beads were eluted by boiling in sample buffer, subjected to SDS 7.5% polyacrylamide gel electrophoresis (PAGE) under reducing conditions, and transferred onto a nitrocellulose membrane (Millipore, Bedford, MA) in Tris-Glycine-Methanol buffer during 25 minutes at 17 V using a Transfer-Blot SD Semi-Dry Transfer Cell (Bio-Rad Laboratories, Hercules, CA). To detect moesin, membranes were soaked overnight in TBS containing 3% bovine serum albumin (BSA) and washed three times with TBS-0.1% Tween 20 during 15 minutes, followed by 1 hour of incubation with a 1:1,000 dilution of rabbit antimoesin 95/2 pAb. After three washes, blots were incubated with a peroxidase-conjugated goat antirabbit IgG (Pierce) and developed using an enhanced chemiluminiscence system (Amersham Corp). To detect the presence of ezrin coprecipitated with CD43, the 95/2 and secondary antibodies were removed from the membrane by treating it with a stripping buffer (100 mmol/L 2-mercaptoetanol, 2% SDS, 62.5 mmol/L Tris-HCl, pH 6.7) at 50°C for 30 minutes. Then, blots were reprobed with the antiezrin 90/3 pAb.
Construction, expression, and purification of GST-CyD7CD43 fusion protein.
The cytoplasmic region of CD43 without the nucleotides coding for the first seven residues plasma membrane-proximal was amplified by polymerase chain reaction (PCR) and cloned as Sal I-NotI fragments into pGEX-4T (Pharmacia LKB Biotechnology, Uppsala, Sweden). For amplification of the CD43 cytoplasmic region (from residues T294 to P400), the primers SalI43T8 (5′-CAATTTGTCGACAACTGGGGCCCTCGTGCTGAGC-3′) and NotCD43 (5′-ATAAGAATGCGGCCGCCGACACTTAAGGGGCAGCC-3′) were used.
Expression of GST-fusion proteins in DH1OB cells and purification were performed following the manufacturer's instructions. The proteins were stored at 4°C on glutathione Sepharose 4B beads as a 50% slurry in 10 mmol/L Tris, pH 7.4, 140 mmol/L NaCl, 0.5% Triton X-100, 0.02% azide, and protease inhibitors (Complete Protease Inhibitors; Boehringer Mannheim Corp, Indianapolis, IN). The amount of fusion protein was stimated by coomassie blue staining of SDS-PAGE.
In vitro binding of GST-CyD7CD43 fusion protein to cell extracts.
Jurkat cells (2 × 107 cells/mL) were labeled overnight with a mixture of [35S]methionine/cysteine (500 μCi) in methionine/cysteine-free RPMI 1640 medium supplemented with 10% dialyzed fetal bovine serum. 35S-labeled Jurkat cells were disrupted in 1 mL lysis buffer (10 mmol/L Tris/HCl, pH 7.6, 150 mmol/L NaCl, 1% Nonidet P-40, 5 mmol/L EDTA, 50 mmol/L sodium fluoride, 0.4 mmol/L sodium orthovanadate, 10 mmol/L iodoacetamide, 5 mmol/L sodium pyrophosphate, 1 mmol/L penoxymethyl sulphofluoride, and 10 mg/mL aprotinin, leupeptin, pepstatin A, chymostatin, and α1-antitrypsin) on ice for 10 minutes, followed by 10 minutes of microcentrifugation. The supernatant was mixed with approximately 50 μg of GST-fusion proteins attached to glutathione Sepharose 4B beads for 12 hours at 4°C. The beads were collected by centrifugation and washed twice with lysis buffer alone, twice with lysis buffer plus 0.1% SDS, twice with lysis buffer plus 0.65 mol/L NaCl, and twice with lysis buffer alone. The beads were then subjected to SDS-PAGE 8% under reducing conditions.
RESULTS
Engagement of CD43 induces T-cell polarization with redistribution of membrane and cytoskeletal proteins.
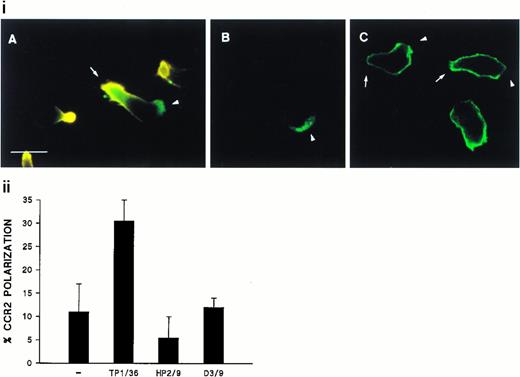
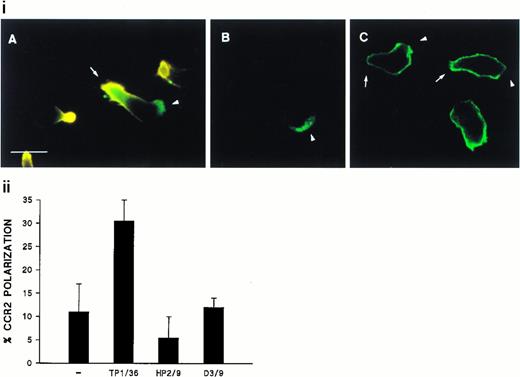
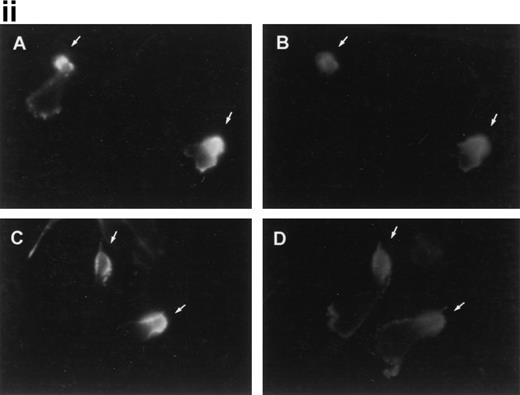
We have previously reported the regulatory role of CD43 in cell morphology and its redistribution towards the uropods of polarized T lymphocytes.26 Herein, we investigated whether the engagement of CD43 is also capable to redistribute other membrane receptors as well as cytoskeletal components to different specialized compartments of the cell. Fluorescence microscopy analysis of T lymphoblasts adhered to FN80 and stimulated with anti-CD43 MoAb showed that, during cell polarization, the chemokine receptor CCR2 redistributed to the leading edge of the cell (Fig 1i, A and B, and 1ii), whereas CD43 was concentrated at the cellular uropod (Fig 1i, A). In contrast, CD45 did not redistribute during CD43-induced cell polarization (Fig 1i, C).
Polarized distribution of CD43 and CCR2 on migrating T lymphoblasts. (i) T lymphoblasts adhered to FN80 were stimulated with the TP1/36 anti-CD43 MoAb for 30 minutes at 37°C and then fixed as described under the Materials and Methods. In (A), cells were double-stained with anti-CD43 (orange) and biotinylated-anti-CCR2 chemotactic receptor MoAb (green), and both fluorescences were photographed on the same frame by double exposure. In (B) and (C), cells were stained for CCR2 and CD45 (FITC-conjugated anti-CD45), respectively. Arrows point to the cellular uropods, whereas arrowheads point to the leading edge. Bar = 10 μm. (ii) T lymphoblasts were allowed to adhere to FN80-coated coverslips and then were stimulated with the TP1/36 anti-CD43, HP2/9 anti-CD44, or D3/9 anti-CD45 MoAb. Cells were then stained for CCR2 and the percentage of cells on which the chemokine receptor was redistributed calculated by random choice of 10 fields for each condition and direct cell counting (400 to 500). The arithmetic mean ± SD of three independent experiments is shown.
Polarized distribution of CD43 and CCR2 on migrating T lymphoblasts. (i) T lymphoblasts adhered to FN80 were stimulated with the TP1/36 anti-CD43 MoAb for 30 minutes at 37°C and then fixed as described under the Materials and Methods. In (A), cells were double-stained with anti-CD43 (orange) and biotinylated-anti-CCR2 chemotactic receptor MoAb (green), and both fluorescences were photographed on the same frame by double exposure. In (B) and (C), cells were stained for CCR2 and CD45 (FITC-conjugated anti-CD45), respectively. Arrows point to the cellular uropods, whereas arrowheads point to the leading edge. Bar = 10 μm. (ii) T lymphoblasts were allowed to adhere to FN80-coated coverslips and then were stimulated with the TP1/36 anti-CD43, HP2/9 anti-CD44, or D3/9 anti-CD45 MoAb. Cells were then stained for CCR2 and the percentage of cells on which the chemokine receptor was redistributed calculated by random choice of 10 fields for each condition and direct cell counting (400 to 500). The arithmetic mean ± SD of three independent experiments is shown.
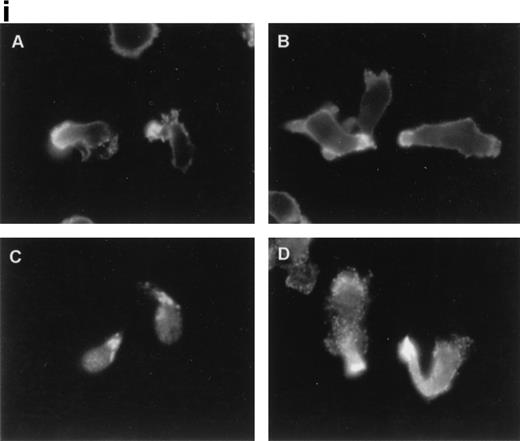
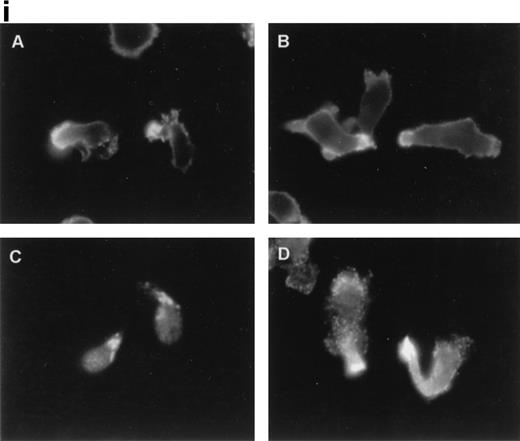
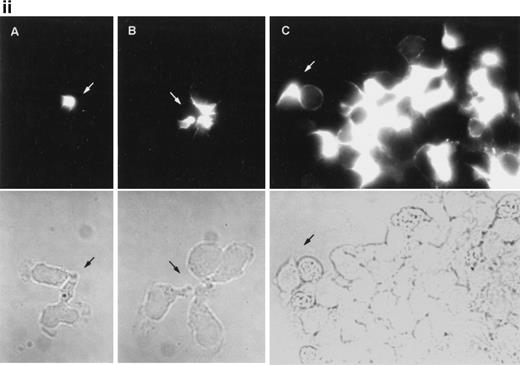
Immunofluorescence staining of ERM cytoskeletal proteins using specific monoclonal and polyclonal antibodies showed that, in anti-CD43–treated T lymphocytes, CD43, ezrin, and moesin (Fig2i, A, B, and D, respectively) redistributed to the cellular uropods, whereas radixin showed a staining pattern at the uropod neck (Fig 2i, C). Furthermore, double immunofluorescence staining showed that CD43 (Fig 2ii, A and C) colocalized with moesin (Fig 2ii, B) and ezrin (Fig2ii, D) at the uropod tips. In addition, a weak signal of ezrin was also detected at the leading edge (Fig 2ii, D).
Ezrin and moesin colocalize with CD43 at the uropods of T lymphoblasts stimulated with anti-CD43 MoAb. T lymphoblasts were allowed to adhere to coverslips coated with FN-80 and then stimulated with the anti-CD43 HP2/21 MoAb. (i) Cells were fixed and stained for CD43 (A), ezrin (90/3 pAb) (B), radixin (C), and moesin (38/87 MoAb) (D). Note the redistribution of all three ERM proteins as well as CD43 to cellular uropods. (ii) Cells were double-stained for CD43 (HP2/21 MoAb) (A and C, orange) and for moesin (38/87 MoAb) (B, green) or ezrin (90/3 pAb) (D, green), as described under the Materials and Methods. Arrows point to cellular uropods.
Ezrin and moesin colocalize with CD43 at the uropods of T lymphoblasts stimulated with anti-CD43 MoAb. T lymphoblasts were allowed to adhere to coverslips coated with FN-80 and then stimulated with the anti-CD43 HP2/21 MoAb. (i) Cells were fixed and stained for CD43 (A), ezrin (90/3 pAb) (B), radixin (C), and moesin (38/87 MoAb) (D). Note the redistribution of all three ERM proteins as well as CD43 to cellular uropods. (ii) Cells were double-stained for CD43 (HP2/21 MoAb) (A and C, orange) and for moesin (38/87 MoAb) (B, green) or ezrin (90/3 pAb) (D, green), as described under the Materials and Methods. Arrows point to cellular uropods.
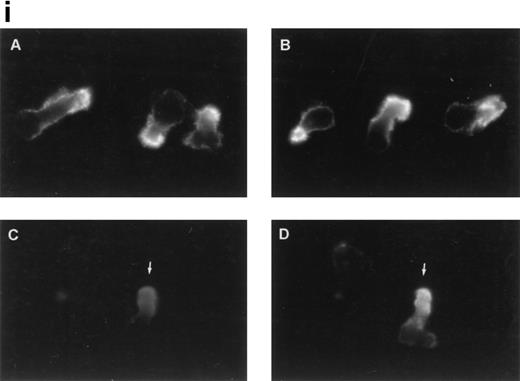
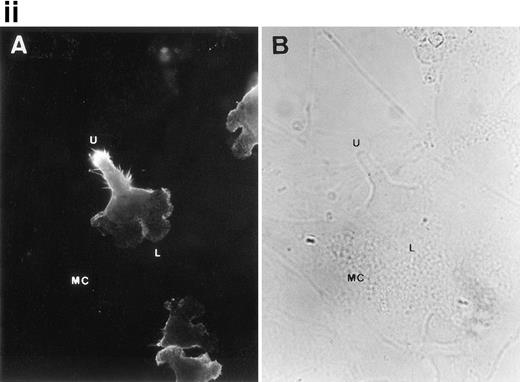
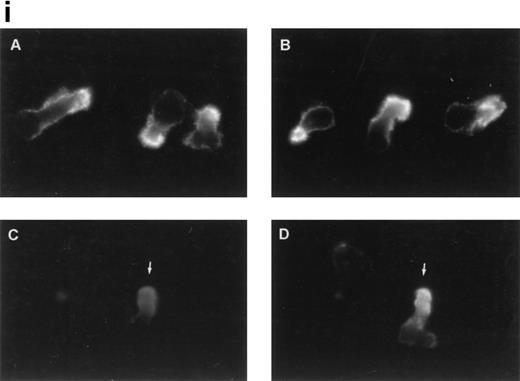
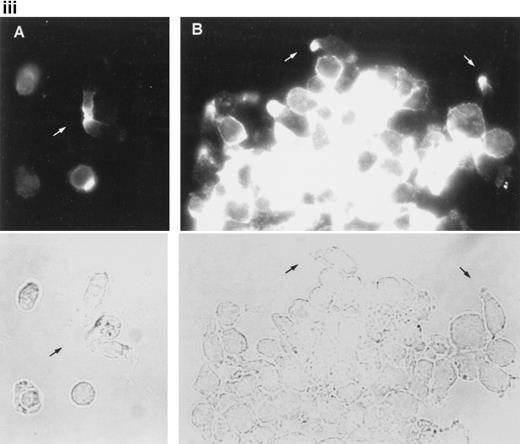
To further analyze the spatial codistribution of CD43 and moesin, we performed immunofluorescence analysis on CD8+ TIL adhered to FN80. These in vivo activated cells (CD45RO+, CD69+) were isolated from patients with melanoma. In these cells, CD43 and moesin were also colocalized at its pronounced uropods (Fig 3i). When TIL were allowed to adhere to melanoma cells, they displayed the polarized morphology typical of motile cells with CD43 clustered in the cellular uropods (Fig 3ii).
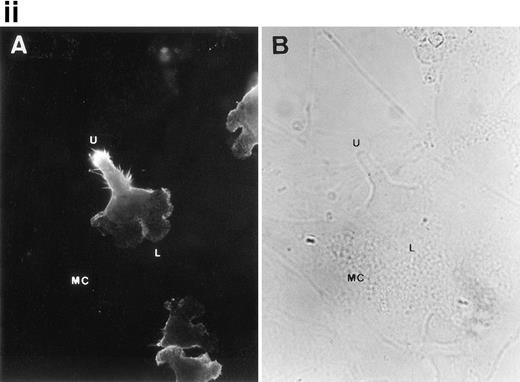
CD43 clusters at the uropod of polarized TILs and colocalizes with moesin. (i) TILs were allowed to adhere to coverslips coated with FN80. Then, single (A and B) or double (C and D) immunostainings were performed as described under the Materials and Methods. (A and C) Staining with the anti-CD43 HP2/21 MoAb. (B and D) Staining with the antimoesin 38/87 MoAb. Arrows point to cellular uropods. (ii) TILs were cocultured on a monolayer of autologous melanoma cells for 1 hour at 37°C. Fixed cells were then stained with the anti-CD43 TP1/36 MoAb (A). In (B), the same field was photographed under bright field conditions. Note in (A) the typical migratory morphology of the TIL on melanoma cell (MC), showing the frontal leading edge (L) and the trailing uropod (U).
CD43 clusters at the uropod of polarized TILs and colocalizes with moesin. (i) TILs were allowed to adhere to coverslips coated with FN80. Then, single (A and B) or double (C and D) immunostainings were performed as described under the Materials and Methods. (A and C) Staining with the anti-CD43 HP2/21 MoAb. (B and D) Staining with the antimoesin 38/87 MoAb. Arrows point to cellular uropods. (ii) TILs were cocultured on a monolayer of autologous melanoma cells for 1 hour at 37°C. Fixed cells were then stained with the anti-CD43 TP1/36 MoAb (A). In (B), the same field was photographed under bright field conditions. Note in (A) the typical migratory morphology of the TIL on melanoma cell (MC), showing the frontal leading edge (L) and the trailing uropod (U).
Interaction of CD43 with ERM proteins in T lymphoblasts.
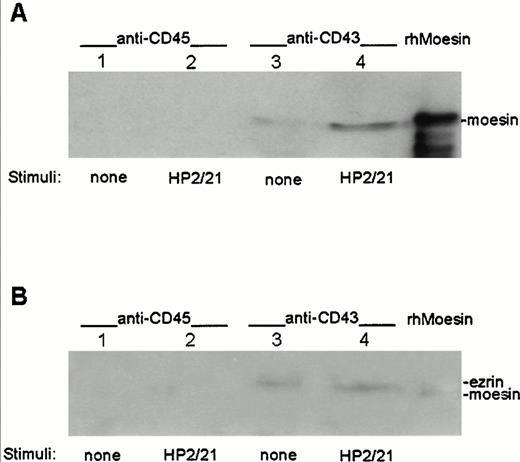
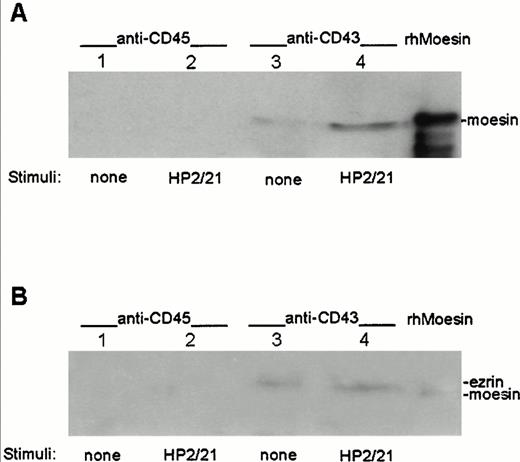
To determine whether moesin and ezrin physically interact with CD43, cell lysates from T lymphoblasts either nonstimulated or stimulated with the anti-CD43 MoAb HP2/21 were immunoprecipitated with the TP1/36 anti-CD43 MoAb or with the RP2/21 anti-CD45 MoAb as control, transferred to nitrocellulose membranes, and blotted with pAb against either moesin (Fig 4A) or ezrin (Fig 4B). Coprecipitation of moesin and ezrin was observed in immunoprecipitates from CD43 (Fig 4A and B, lanes 3 and 4, respectively), whereas neither moesin nor ezrin were detected in the CD45 immunoprecipitates (Fig 4A and B, lanes 1 and 2). An increase in the amount of moesin associated to CD43 was detected in immunoprecipitates from cells pretreated with the polarization-inducing HP2/21 anti-CD43 MoAb compared with untreated cells (Fig 4A, lanes 3 and 4). In contrast, comparable amounts of ezrin were found associated to CD43 in treated and untreated cells (Fig 4B, lanes 3 and 4).
Association of ezrin and moesin with CD43 in polarized T lymphoblasts. T cells either unstimulated (lanes 1 and 3) or stimulated with anti-CD43 HP2/21 MoAb (lanes 2 and 4) were allowed to adhere to FN80. Cells were then lysed and the soluble fraction was immunoprecipitated with the anti-CD45 RP2/21 (lanes 1 and 2) and anti-CD43 TP1/36 MoAb (lanes 3 and 4). A human recombinant moesin standard was run in the right lanes. Each immunoprecipitate as well as standards were immunoblotted with the antimoesin 95/2 pAb after SDS 7.5% PAGE (A) and reprobed with the anti-ezrin 90/3 pAb (B) as described under the Materials and Methods.
Association of ezrin and moesin with CD43 in polarized T lymphoblasts. T cells either unstimulated (lanes 1 and 3) or stimulated with anti-CD43 HP2/21 MoAb (lanes 2 and 4) were allowed to adhere to FN80. Cells were then lysed and the soluble fraction was immunoprecipitated with the anti-CD45 RP2/21 (lanes 1 and 2) and anti-CD43 TP1/36 MoAb (lanes 3 and 4). A human recombinant moesin standard was run in the right lanes. Each immunoprecipitate as well as standards were immunoblotted with the antimoesin 95/2 pAb after SDS 7.5% PAGE (A) and reprobed with the anti-ezrin 90/3 pAb (B) as described under the Materials and Methods.
Interaction of the cytoplasmic tail of CD43 with moesin and ezrin.
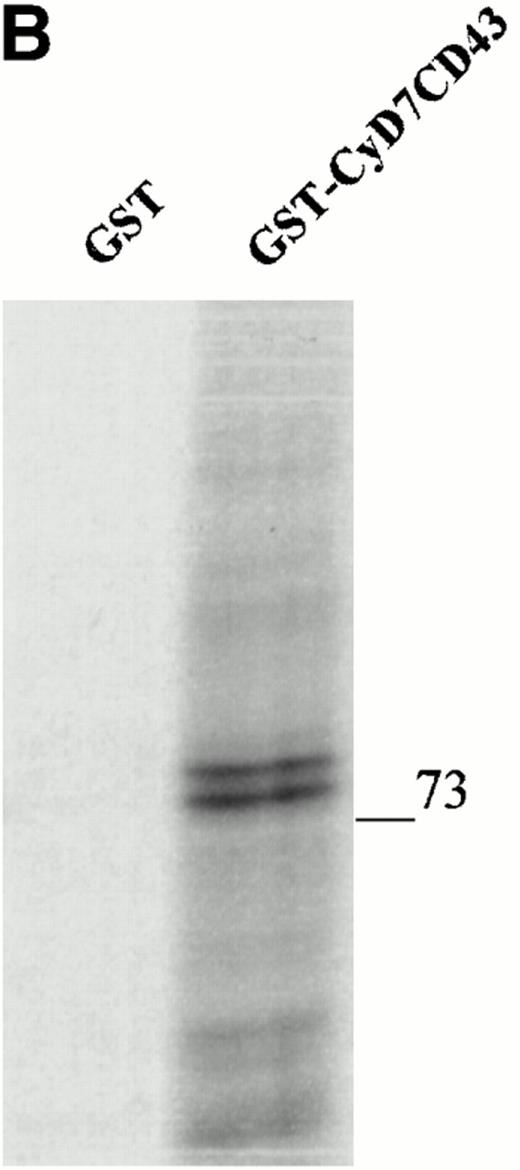
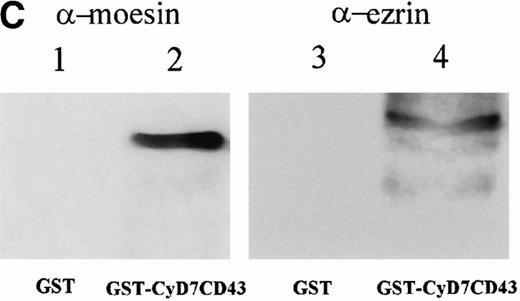
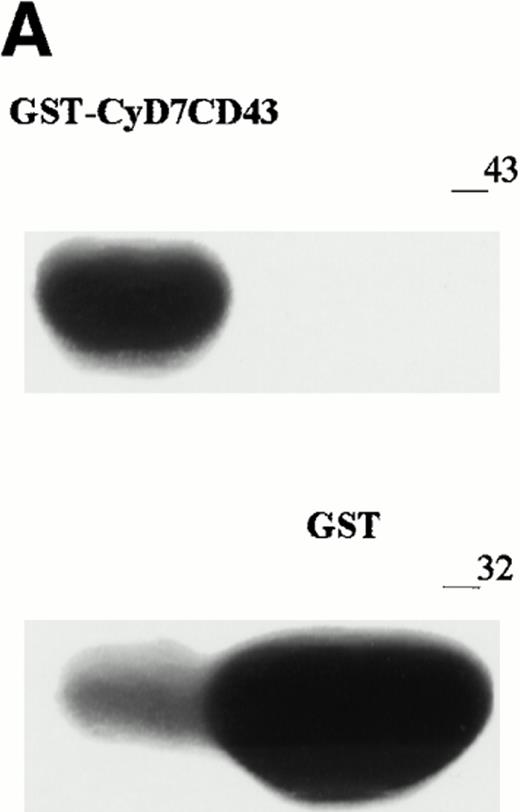
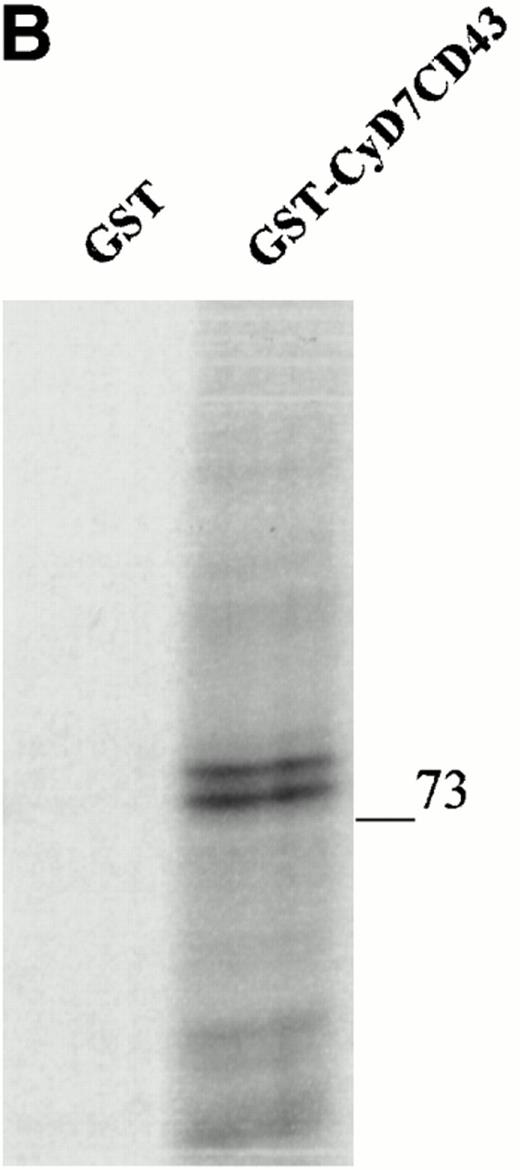
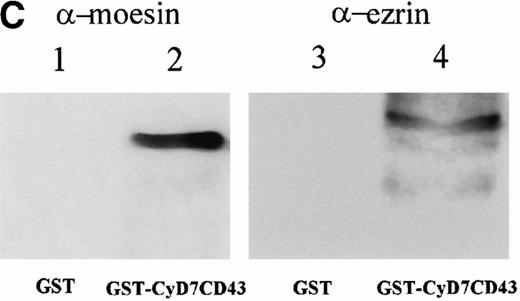
To further confirm the interaction of CD43 with moesin and ezrin, we performed precipitation studies with a GST fusion protein containing the CD43 cytoplasmic region (Fig 5). All attempts to induce the expression in DH1OB cells of a GST-fusion protein containing the full-length cytoplasmic region of CD43 failed. However, we were able to induce the expression of a GST-fusion protein bearing the cytoplasmic region of CD43 lacking the first seven N-terminal residues, designated as GST-CyD7CD43 (Fig 5A). We found that the GST-CyD7CD43 fusion protein specifically precipitated two bands of 78 and 80 kD from metabolically labeled Jurkat T-cell lysates, whereas no protein band was observed in precipitates from GST control protein (Fig 5B). Western blot analysis performed in parallel showed that the 78- and 80-kD proteins bound to GST-CyD7CD43 corresponded to moesin and ezrin, respectively (Fig 5C). These results further indicate that the cytoplasmic tail of CD43 interacts with moesin and ezrin.
Interaction of the cytoplasmic region of CD43 with moesin and ezrin from Jurkat cell lysates. (A) Coomassie blue-stained SDS-PAGE of GST-CyD7CD43 fusion protein purified with glutathione Sepharose 4B beads. (B) Specific association of two proteins of 78 and 80 kD with GST-CyD7CD43. Jurkat cells were metabolically labeled and lysed. After discarding cell debris and nuclei, supernatants were collected and allowed to bind to equivalent amounts of GST and GST-CyD7CD43 proteins bound to glutathione Sepharose 4B beads by overnight incubation at 4°C. Bound proteins were sequentially washed with lysis buffer containing 0.1% SDS and 0.65 mol/L NaCl and then subjected to 8% SDS-PAGE. Before drying, the gel was incubated for 30 minutes in Amplify solution (Amersham Corp). (C) Precipitates from unlabeled Jurkat cells were performed as in (B), SDS-PAGE separated, and immunoblotted with the antimoesin 95/2 (lanes 1 and 2) or the antiezrin 90/3 (lanes 3 and 4) pAb. Molecular weights in kilodaltons are indicated at the right.
Interaction of the cytoplasmic region of CD43 with moesin and ezrin from Jurkat cell lysates. (A) Coomassie blue-stained SDS-PAGE of GST-CyD7CD43 fusion protein purified with glutathione Sepharose 4B beads. (B) Specific association of two proteins of 78 and 80 kD with GST-CyD7CD43. Jurkat cells were metabolically labeled and lysed. After discarding cell debris and nuclei, supernatants were collected and allowed to bind to equivalent amounts of GST and GST-CyD7CD43 proteins bound to glutathione Sepharose 4B beads by overnight incubation at 4°C. Bound proteins were sequentially washed with lysis buffer containing 0.1% SDS and 0.65 mol/L NaCl and then subjected to 8% SDS-PAGE. Before drying, the gel was incubated for 30 minutes in Amplify solution (Amersham Corp). (C) Precipitates from unlabeled Jurkat cells were performed as in (B), SDS-PAGE separated, and immunoblotted with the antimoesin 95/2 (lanes 1 and 2) or the antiezrin 90/3 (lanes 3 and 4) pAb. Molecular weights in kilodaltons are indicated at the right.
CD43 and moesin are redistributed to cell-cell contacts during homotypic T-cell aggregation induced by anti-CD43 MoAb.
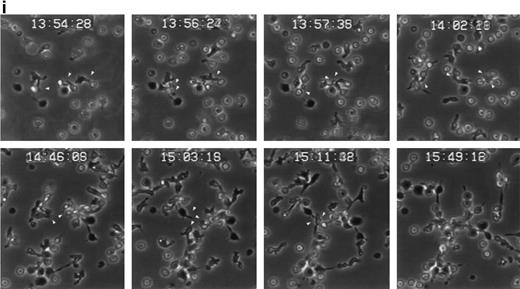
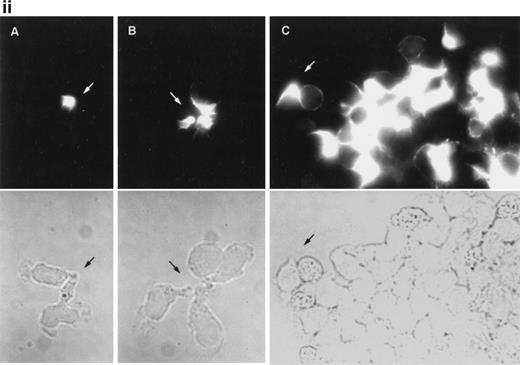
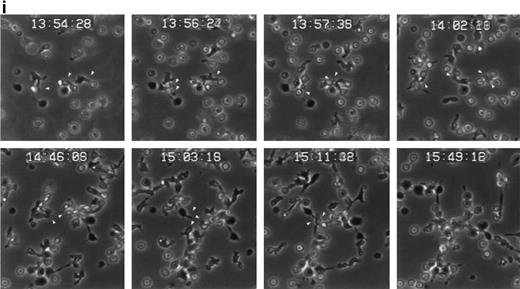
The induction of homotypic leukocyte aggregation by anti-CD43 MoAb has been described.20,41,42 In this regard, we have previously selected a number of anti-CD43 MoAb for their ability to promote rapid and strong homotypic aggregation of normal T lymphoblasts and U937 myelomonocytic cells.26 However, little is known about the dynamics of aggregation and the nature of cell-cell interactions established during this phenomenon. In an attempt to understand this issue, we performed time-lapse videomicroscopy studies of homotypic T-lymphoblast aggregate formation induced by the anti-CD43 MoAb HP2/21 (Fig 6i). Five minutes after addition of anti-CD43, T cells initiated locomotion on the substratum, displayed a polarized morphology, and established early cell-cell interactions with other polarized and spherical cells, mainly through their uropods, forming motile small aggregates (Fig 6i). One hour later, motile small cell aggregates contacted with other small aggregates through externally exposed cell uropods generating large cellular aggregates (Fig 6i). Immunofluorescence studies performed in parallel showed that CD43 as well as moesin were concentrated at the uropods of small cell aggregates that were mediating cell-cell interactions (Fig 6ii, A and B, and iii, A, respectively). On the other hand, in large cell aggregates, CD43 and moesin were located in the uropods projected towards the outer milieu (Fig 6iii, C, and iii, B, respectively) as well as in cell-cell contacts. These results strongly suggested that the clusters of CD43 projected by the prominent uropods could facilitate the interaction of this molecule with a putative ligand to allow formation of cell aggregates.
Dynamics of CD43 and moesin during cell aggregation. (i) T lymphoblasts adhered to FN80 were treated with the proaggregatory anti-CD43 HP2/21 MoAb. Time-lapse videomicroscopy analysis was then performed as described in the Materials and Methods. Sequential time frames are shown. White arrowheads point to uropods of cells participating in the formation of small and large aggregates. (ii) T lymphoblast aggregation was induced by using the anti-CD43 HP2/21 MoAb, and upon cell incubation at 37°C for 5 (A), 10 (B), and 30 (C) minutes, cells were fixed and stained for CD43. (iii) Similarly, 5 (A) and 30 (B) minutes after the triggering of cell aggregation with the anti-CD43 HP2/21 MoAb, cells were fixed and stained for moesin by using the biotinylated antimoesin 38/87 MoAb. The same cells were photographed under epifluorescent (above) and bright field (below) conditions. Arrows point to cellular uropods.
Dynamics of CD43 and moesin during cell aggregation. (i) T lymphoblasts adhered to FN80 were treated with the proaggregatory anti-CD43 HP2/21 MoAb. Time-lapse videomicroscopy analysis was then performed as described in the Materials and Methods. Sequential time frames are shown. White arrowheads point to uropods of cells participating in the formation of small and large aggregates. (ii) T lymphoblast aggregation was induced by using the anti-CD43 HP2/21 MoAb, and upon cell incubation at 37°C for 5 (A), 10 (B), and 30 (C) minutes, cells were fixed and stained for CD43. (iii) Similarly, 5 (A) and 30 (B) minutes after the triggering of cell aggregation with the anti-CD43 HP2/21 MoAb, cells were fixed and stained for moesin by using the biotinylated antimoesin 38/87 MoAb. The same cells were photographed under epifluorescent (above) and bright field (below) conditions. Arrows point to cellular uropods.
Myosin disruption prevents CD43-induced homotypic T-cell aggregation.
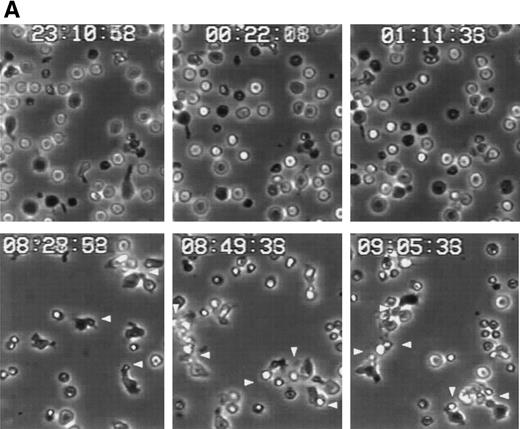
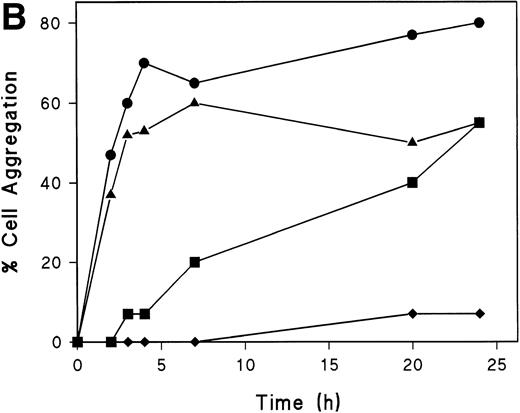
Polarized T cells express linear arrays of myosin at the uropod neck,43 and we have described that the disruption of this structure by the butanedione monoxime prevents uropod formation and redistribution of both CD43 and moesin.26 44 To investigate the role of myosin in the induction of T lymphoblast aggregation triggered through CD43, we performed time-lapse videomicroscopy of T-cell aggregation in the presence of butanedione monoxime. At short times, although cell aggregation was observed in the absence of this agent (Fig 6i), the addition of this drug prevented the acquisition of polarized morphology of T cells adhered to FN80 and the formation of cell clusters (Fig 7A). At longer times, when the butanedione effect start to vanish, some cells become polarized participating in small aggregates through its uropods (Fig 7A and B). We have also tested the role of colchicine and cytochalasin D on cell aggregation induced by the anti-CD43 HP2/21 MoAb. Interestingly, whereas cytochalasin D inhibit cell aggregation, no significant inhibition was observed under colchicine treatment (Fig7B). These results indicate that myosin plays a central role in the establishment of cell-cell interactions required for aggregate formation induced by CD43.
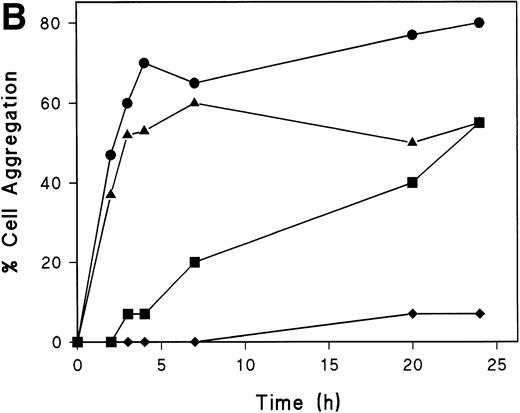
Myosin-disruption prevents CD43-mediated cell aggregation. (A) T lymphoblasts were pretreated during 30 minutes with 20 mmol/L of butanedione monoxime. Cells were then allowed to adhere to FN80 in the presence of the proaggregatory anti-CD43 HP2/21 MoAb. Cells were filmed with a time-lapse videocassete recorder for 10 hours. Note the lack of cell polarization and aggregation in the first 2 hours recorded. White arrowheads point to cellular uropods displayed by cells after 9 hours of anti-CD43 treatment. (B) T lymphoblasts were incubated for 30 minutes at 37°C in the presence of 10 mmol/L butanedione monoxime (▪), 20 μmol/L colchicine (▴), or 20 μmol/L cytochalasin D (⧫) or in the absence of any cytoskeletal drug (•), before the addition of anti-CD43 HP2/21 MoAb. The percentage of aggregation was calculated at different times as described in the Materials and Methods.
Myosin-disruption prevents CD43-mediated cell aggregation. (A) T lymphoblasts were pretreated during 30 minutes with 20 mmol/L of butanedione monoxime. Cells were then allowed to adhere to FN80 in the presence of the proaggregatory anti-CD43 HP2/21 MoAb. Cells were filmed with a time-lapse videocassete recorder for 10 hours. Note the lack of cell polarization and aggregation in the first 2 hours recorded. White arrowheads point to cellular uropods displayed by cells after 9 hours of anti-CD43 treatment. (B) T lymphoblasts were incubated for 30 minutes at 37°C in the presence of 10 mmol/L butanedione monoxime (▪), 20 μmol/L colchicine (▴), or 20 μmol/L cytochalasin D (⧫) or in the absence of any cytoskeletal drug (•), before the addition of anti-CD43 HP2/21 MoAb. The percentage of aggregation was calculated at different times as described in the Materials and Methods.
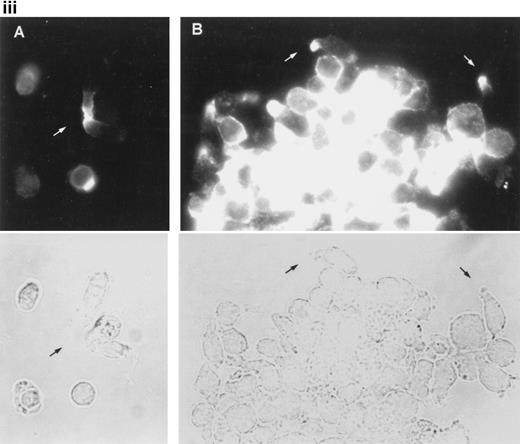
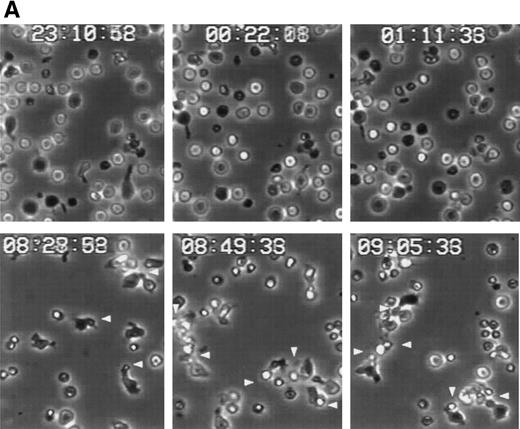
Redistribution of CD43 to the uropods enhance recruitment and transport of T cells.
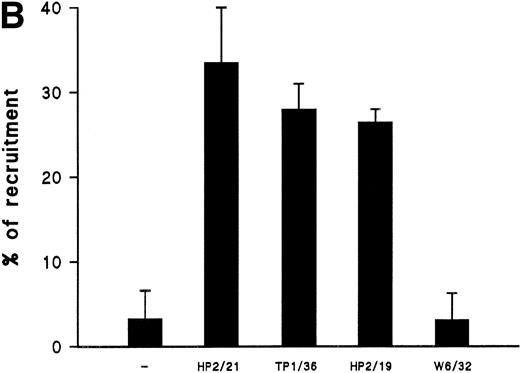
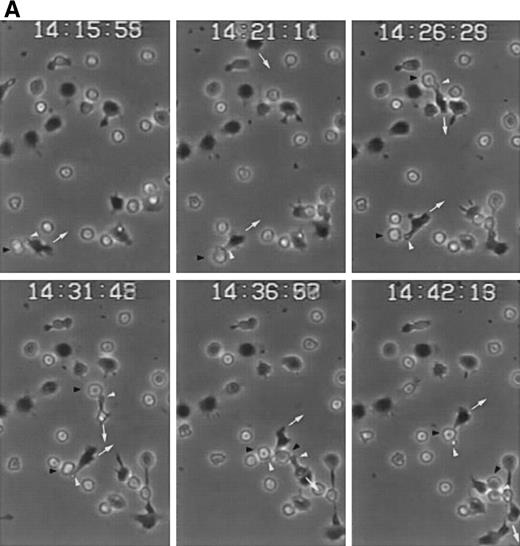
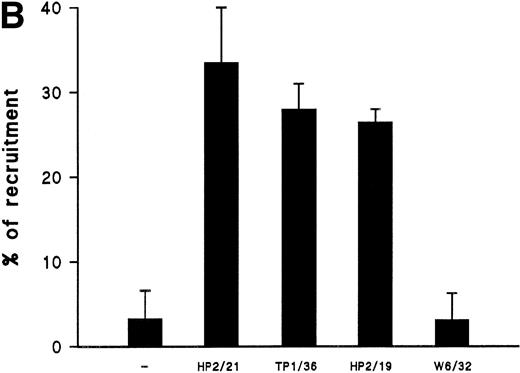
We have recently described the involvement of T-cell uropods in the transport and recruitment of other lymphocytes as well as the role of ICAM-3 and chemokines in the induction of this phenomenon.40 To investigate whether similar phenomena take place upon CD43 engagement, we studied this issue by time-lapse videomicroscopy. A first layer of T cells was allowed to attach to ICAM-1-Fc–coated surface plates and then induced to develop uropods with the HP2/21 anti-CD43 MoAb. A second layer of T cells was then added and cellular interactions were filmed. Interestingly, cells of the second layer (phase-bright cells) were contacted, trapped, and transported by cells of the first layer (phase-dark cells) locomoting on the ICAM-1-Fc–coated surface (Fig 8A). Quantification of cells of the second layer recruited and transported by the adhered cells of the first layer showed that the treatment with anti-CD43 induced a 10-fold increase in cell recruitment compared with unstimulated cells (Fig 8B).
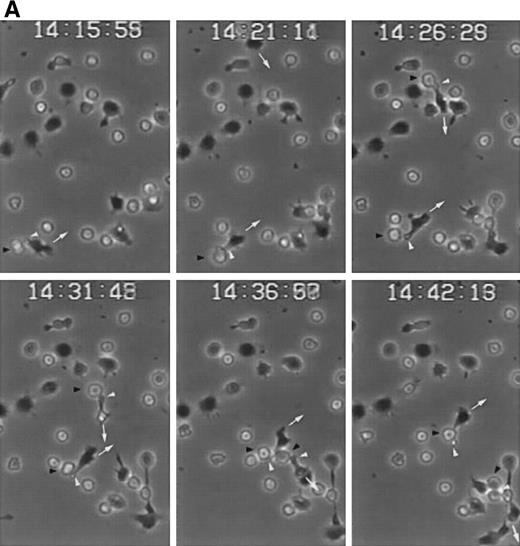
CD43-stimulated T lymphocytes recruit additional cells through the uropods. (A) T lymphoblasts adhered to petri dishes coated with ICAM-1-Fc were treated with the anti-CD43 HP2/21 MoAb. A second layer of untreated T lymphoblasts (bright cells) was then added and cell-cell interactions were recorded by videomicroscopy for 30 minutes. Black arrowheads point to transported cells; white arrowheads point to cellular uropods of cells from the first cohort, whereas white arrows point from cellular leading edges. (B) Quantitation of T-cell lymphocyte recruitment mediated by anti-CD43 polarized cells. T lymphoblasts were allowed to adhere to plastic petri dishes coated with ICAM-1-Fc for 30 minutes at 37°C in the presence of the proaggregatory anti-CD43 (HP2/21 and TP1/36) and anti-ICAM-3 (HP2/19) MoAb or the control anti-HLA-A,B W6/32 MoAb. After the addition of a second layer of cells, cell-cell interactions were recorded for 1 hour, and the recruitment index was calculated as the number of cells of the second layer contacted per cell number adhered to the substrate.
CD43-stimulated T lymphocytes recruit additional cells through the uropods. (A) T lymphoblasts adhered to petri dishes coated with ICAM-1-Fc were treated with the anti-CD43 HP2/21 MoAb. A second layer of untreated T lymphoblasts (bright cells) was then added and cell-cell interactions were recorded by videomicroscopy for 30 minutes. Black arrowheads point to transported cells; white arrowheads point to cellular uropods of cells from the first cohort, whereas white arrows point from cellular leading edges. (B) Quantitation of T-cell lymphocyte recruitment mediated by anti-CD43 polarized cells. T lymphoblasts were allowed to adhere to plastic petri dishes coated with ICAM-1-Fc for 30 minutes at 37°C in the presence of the proaggregatory anti-CD43 (HP2/21 and TP1/36) and anti-ICAM-3 (HP2/19) MoAb or the control anti-HLA-A,B W6/32 MoAb. After the addition of a second layer of cells, cell-cell interactions were recorded for 1 hour, and the recruitment index was calculated as the number of cells of the second layer contacted per cell number adhered to the substrate.
DISCUSSION
CD43 is a heavily charged transmembrane sialomucin that provides stimulatory signals inducing lymphocyte proliferation and IL-2 secretion24,45 and costimulates T lymphoblasts in a CD28-independent manner.25 In contrast, a negative regulation of T-cell activation and adhesion through CD43 has also been reported.21,22 However, it has been described that anti-CD43 MoAb are able to trigger homotypic leukocyte aggregation in both CD18-dependent and CD18-independent manner.19,20,41 46Because cell aggregation and polarization studies have become useful assays for analyzing leukocyte motility and activation in vitro, we decided to study the role of CD43 in these assays to further understand the biological function of this molecule.
Previously, we have reported that proaggregatory and activating anti-CD43 MoAbs enhance β1 and β2 integrin-mediated T-cell adhesion to both endothelial and ECM proteins and induce T-cell polarization with redistribution of CD43 to cellular uropods.26 We demonstrate herein that CD43 plays an important regulatory role in T-cell polarization, inducing the appearance of specialized cellular domains with redistribution of the CCR2 chemokine receptor to the leading edge and ERM actin-binding proteins to the uropod of polarized T lymphocytes. For the ERM proteins analyzed, ezrin and moesin colocalized with CD43 at the uropod tips, whereas radixin was detected at the uropod neck. It is worth mentioning that the data regarding redistribution of CD43 and ezrin contrast with our previous results showing no colocalization of ezrin with ICAM-3 at the uropods of T cells treated with chemokines.44 This discrepancy seems to be due to the different polyclonal antibodies used in both studies; whereas 90/3 pAb was generated against purified human ezrin, the affinity-purified pAb 464 was raised against an unique peptide of mouse ezrin.44 Therefore, it is evident that the bright immunostaining of cell uropods produced by the 90/3 pAb indeed corresponds to the redistribution of ezrin to this structure. Furthermore, the CD43/ezrin codistribution found by us agrees with previous data from other investigators regarding the presence of ezrin in the uropods of mouse T cells and with the colocalization of ERM proteins and CD43 in the cleavage furrows during cytokinesis of mouse thymocytes in division.47,48 Interestingly, our data demonstrate that ezrin and moesin physically interact with CD43 in unstimulated T lymphocytes, yet only moesin/CD43 interaction was increased after CD43 stimulation. Moreover, the coprecipitation studies with the GST-CyD7CD43 fusion protein demonstrate a direct interaction of moesin and ezrin with the cytoplasmic region of CD43. In this regard, other adhesion molecules, such as CD44 and ICAM-3, have been reported to interact with moesin in motile T cells, and this association is increased under conditions that induce cell polarization such as the treatment with chemokines.44 Thus, it appears that the process of moving membrane molecules towards the trailing edge of the cell is directed by the submembranous cytoskeleton.49 By linking adhesion molecules to submembranous cytoskeleton, moesin could promote the redistribution of membrane complexes towards the cellular uropod.
It has been described that cell aggregation can be induced in T lymphoblasts upon engagement of a large array of cell adhesion molecules, including CD43.33,50,51 We have undertaken a dynamic assessment of cell aggregation by time-lapse video recording of CD43-stimulated T lymphocytes. Upon treatment with anti-CD43 MoAb, the formation of large cell aggregates takes place by congregation of small ones. In small aggregates, cells contact each other mainly through their uropods, where moesin and CD43 colocalize, suggesting that moesin/CD43 interaction may have a role in the initial phase of the establishment of cell-cell contacts, as it has been described for ICAM-3.33 In accordance, a similar mechanism of cell-cell interactions has been described in NK-resistant cells, which after ezrin transfection, display an uropod where ICAM-2 is redistributed, allowing cellular interactions and becoming NK-sensitive.47Similarly, uropod formation, moesin/CD43 interaction, and CD43 redistribution during T-cell polarization induced through CD43 would increase molecular density and accessibility of CD43 in the cellular uropod enabling interactions with putative ligands, facilitating homotypic cell aggregation. In this regard, the existence of cellular ligands for CD43 involved in leukocyte endothelial cell interactions has recently been postulated on the basis of in vivo inhibition of lymphoid cell homing with an anti-CD43 MoAb.27
In previous studies using the myosin-disrupting drug butanedione monoxime, we found that myosin is involved in uropod formation, cell aggregation induced by anti-ICAM-3 MoAb, and adhesion receptors and moesin redistribution to the uropod.11,26,43 44 Our data indicate that myosin is also necessary for the cell-cell interactions that occur during the homotypic T-cell aggregation induced through CD43, reinforcing the issue that uropod formation is required to optimize cell-cell interactions.
We previously reported that cellular uropods induced by both chemokines and activating anti-ICAM-3 MoAb are able to mediate the recruitment of lymphocytes and transports them through extracellular matrix layers or endothelial cells specialized in lymphocyte extravasation.40 Likewise, CD43-mediated cell polarization and CD43 and moesin redistribution towards uropods are involved in the migration of T lymphoblasts on ICAM-1 as well as in the capture and transport of additional bystander cells. Because the phenotype of T lymphoblasts used in these studies is similar to memory and effector T cells (95% CD45RO, 98% CD3, and 96% CD43high), our work agrees with a previous report showing that the high expression of CD43 by memory T cells facilitate the extravasation of these cells.20 In this regard, it is conceivable that the highly polarized morphology of TIL, with a pronounced uropod where most of CD43 molecules are redistributed, facilitates the motility of these lymphocytes through cellular layers amplifying the recruitment of leukocytes as well as its interaction with tumoral cells. Altogether, our findings on cell aggregation induced through CD43 suggest that this molecule is a proadhesive rather than antiadhesive molecule and provide evidence for the role of CD43 as a membrane receptor able to interact with putative counter-receptor(s), mediating cell-cell contacts through uropods in leukocyte intercellular interactions.
Recently, it has been postulated the involvement of CD43 in the selective binding and recruitment of T lymphocytes to lymphoid organs as well as in the existence of an unidentified vascular ligand for CD43.27 In neutrophils, the interaction of P-selectin glycoprotein ligand-1 (PSGL-1) with P-selectin is uncoupled upon cell activation with the concomitant redistribution of PSGL-1 towards the cellular uropod.52 On the other hand, PSGL-1 redistribution towards the uropod of neutrophils after chemotactic activation could be responsible for cell-cell interactions between neutrophils and activated platelets.53 Because CD43, as PSGL-1, is a sialylated mucin, it is feasible that, in T lymphocytes, the redistribution of CD43 to the cellular uropod by its interaction with ERM-mediated cytoskeleton would facilitate transendothelial migration and recruitment of other T lymphocytes by interaction through their uropods.
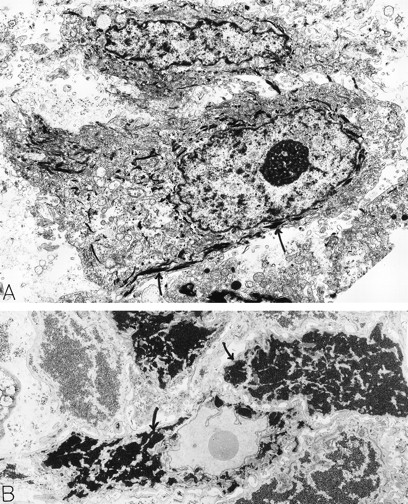
Squamous cell carcinoma. An 80-year-old man had a scalp lesion excised, which had been evident for three months. By light microscopy, this was a squamous cell carcinoma with an extensive clear cell component. Electron microscopy revealed typical squamous epithelial carcinoma cells attached by desmosomes and filled with bundles of electron-dense tonofilaments (arrows, A) in the cytoplasm. The clear cell component was comprised of glycogen-rich (B, arrows) squamous epithelial cells. The material for electron microscopy in (A) was processed by a standard method; in (B), the sample was postfixed in potassium ferrocyanide-reduced osmium to highlight glycogen stores which are electron dense (B, arrows). A: ×8,000; B: ×6,000. Original magnifications: (A), ×8,000; (B), ×6,000. (Courtesy of Ann M. Dvorak, MD, Beth Israel Deaconess Medical Center, 330 Brookline Ave, Boston, MA 02215.)
Squamous cell carcinoma. An 80-year-old man had a scalp lesion excised, which had been evident for three months. By light microscopy, this was a squamous cell carcinoma with an extensive clear cell component. Electron microscopy revealed typical squamous epithelial carcinoma cells attached by desmosomes and filled with bundles of electron-dense tonofilaments (arrows, A) in the cytoplasm. The clear cell component was comprised of glycogen-rich (B, arrows) squamous epithelial cells. The material for electron microscopy in (A) was processed by a standard method; in (B), the sample was postfixed in potassium ferrocyanide-reduced osmium to highlight glycogen stores which are electron dense (B, arrows). A: ×8,000; B: ×6,000. Original magnifications: (A), ×8,000; (B), ×6,000. (Courtesy of Ann M. Dvorak, MD, Beth Israel Deaconess Medical Center, 330 Brookline Ave, Boston, MA 02215.)
ACKNOWLEDGMENT
The authors thank Dr P. Lauzurica for critical readings of the manuscript. We are greatfully indebted to M.C. Montoya and E. Fernández-Villareal for their help and advice with different techniques and to R. Tejedor for preparing recombinant ICAM-1-Fc.
Supported by Grant No. SAF96/0039 from Plan Nacional de Investigación y Desarrollo, Grant No. 07/44/96 from Comunidad Autónoma de Madrid, a grant from Fundación Cientı́fica de la Asociación Española contra el Cancer (to F.S.M.), by fellowship BAE FIS 97/5089 to M.A.P., and by Grant No. AR41045 from the US Public Health Service (to H.F.)
Address reprint requests to Francisco Sánchez-Madrid, PhD, Servicio de Inmunologı́a, Hospital de la Princesa, Universidad Autónoma de Madrid, C/ Diego de Leon, 62, Madrid, Spain; e-mail:fsmadrid/princesa@hup.es.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.