To the Editor:
The endothelial protein C receptor (EPCR) is a recently described member of the protein C anticoagulant pathway.1,2 The membrane form of EPCR works in concert with thrombomodulin (TM) to augment protein C activation on endothelial cells,3 whereas a soluble form of EPCR from normal human plasma inhibits the generation and function of activated protein C.4 The protein C pathway plays a critical role in both anticoagulant processes and in the host response to inflammation, especially bacterial sepsis.5 TM is a well-known endothelial receptor of the protein C activation complex and soluble TM fragments have been detected in the plasma of patients. In various disease states, increased levels of soluble TM reflect endothelial cell damage that is often correlated with disease severity.6-8
We examined whether soluble plasma EPCR may serve as an additional marker of endothelial integrity in patients with systemic lupus erythematosus (SLE; American College of Rheumatology classification criteria9) or sepsis (ACCP/SCCM concensus conference10). These patients have inflammatory processes involving the vasculature, often complicated by thrombotic tendency. Plasma samples from these patient groups were assayed for soluble TM by enzyme-linked immunosorbent assay (ELISA).11Soluble EPCR levels were determined by ELISA essentially as described,4 except that the coating and detecting antibodies were reversed and the chromogenic substrate was BluePhos (KPL, Gaithersburg, MD). Citrated plasma samples from the lupus patients were obtained at the time of a scheduled outpatient visit to the clinic. Samples from the sepsis patients were collected into Bauer's anticoagulant and obtained upon admission to the intensive care unit. The septic patients required hemodynamic support, had [ATIII] less than 70%, no previous liver disease, and no hematologic disease. All samples were obtained with informed consent.
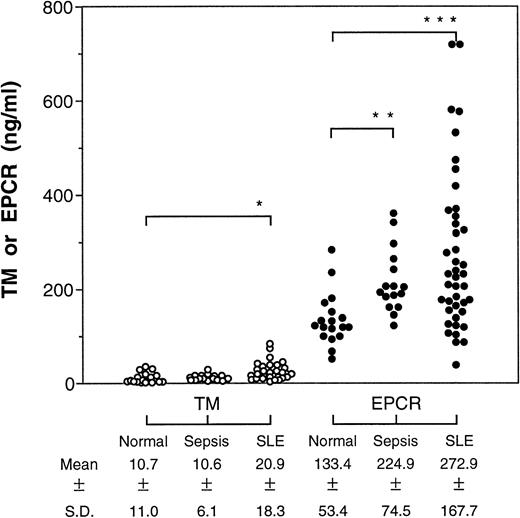
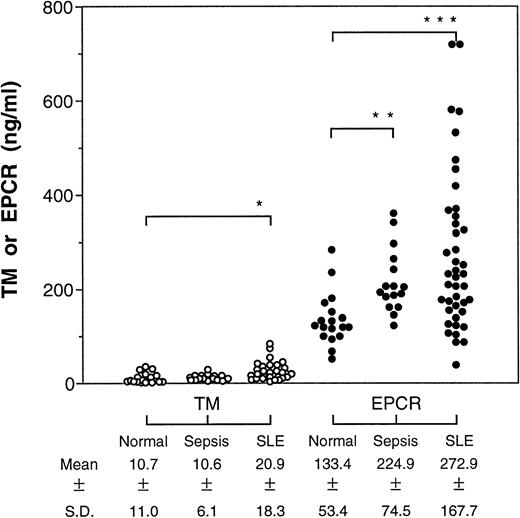
In both patient groups, we found significant elevations in soluble EPCR levels relative to normal volunteers (Fig1), ranging up to a fivefold increase over the normal average in the SLE group. The soluble TM levels were higher in the SLE patients as reported by others,7 but, unlike earlier studies,8 we did not find any difference in soluble TM levels in the septic patients. In the septic patients, there was no correlation between the soluble EPCR levels and multiple organ failure score, survival, or presence of septic shock.
Levels of soluble TM and EPCR in patients with sepsis or SLE. Plasma from patients with sepsis (n = 16) or SLE (n = 42) were assayed for the soluble receptors by ELISA and the means were compared by Student's t-test with receptor levels found in normal plasma (n = 18). *P < .05; **P < .001; ***P < .0001.
Levels of soluble TM and EPCR in patients with sepsis or SLE. Plasma from patients with sepsis (n = 16) or SLE (n = 42) were assayed for the soluble receptors by ELISA and the means were compared by Student's t-test with receptor levels found in normal plasma (n = 18). *P < .05; **P < .001; ***P < .0001.
Interestingly, the soluble TM and EPCR levels did not correlate in either the SLE patients (r2 = .018) or in the septic patients (r2 = .013), despite the fact that both proteins are found primarily on endothelium. Immunohistochemistry studies have shown that membrane-bound EPCR is expressed almost exclusively on the large vessels along with TM but is largely absent in the microcirculation where TM is abundant.12 Thus, the lack of correlation between the soluble plasma EPCR and TM levels may result from differences in the site of injury, reflecting large-vessel versus small-vessel disease processes. Currently, there are no candidates for an endothelium-specific marker that can show large-vessel disease processes. Clinical studies with defined patient groups will be required to establish the utility of soluble plasma EPCR as a marker of large-vessel disease processes.