Abstract
The cellular isoform of the prion protein (PrPC) is a small glycoprotein attached to the outer leaflet of the plasma membrane by a glycosylphosphatidylinositol anchor. This molecule is involved in the pathogenesis of prion diseases in both humans and animals. We have characterized the expression patterns of PrPC during human leukocyte maturation by flow cytometry with monoclonal antibodies to PrPC, the glycan moiety CD15, and the stem cell marker CD34. We observe that prion protein is present on CD34+bone marrow (BM) stem cells. Although lymphocytes and monocytes maintain PrPC expression throughout their differentiation, PrPC is downregulated upon differentiation along the granulocyte lineage. In vitro retinoic acid–induced differentiation of the premyeloid line HL-60 into granulocyte-like cells mimics the suppression of PrPC in granulocyte differentiation, as both PrPC mRNA and protein are downregulated. These data suggest that selected BM cells and peripheral mononuclear cells may support prion agent replication, because this process is dependent on availability of PrPC. Additionally, retinoic acid–induced extinction of PrPC expression in HL-60 cells provides a potential model to study PrP gene regulation and protein function. Finally, these data suggest the existence of cell-specific glycoforms of PrPC that may determine cellular susceptibility to infection by the prion agent.
THE PRION PROTEIN is best known for its involvement in the transmissible spongiform encephalopathies (TSE) or prion diseases, a group of human and animal neurodegenerative diseases which include Creutzfeldt-Jakob disease in humans, scrapie in sheep and goats, and bovine spongiform encephalopathy in cattle. The pathology of the TSEs is characterized by neuronal loss, spongiform change, gliosis, and accumulation of an abnormal protease-resistant protein designated PrPSc in both brain and peripheral tissues, including organs of the lymphoreticular system. The prevalent “protein-only” hypothesis of prion disease proposes that PrPSc is indeed the infectious agent, and that this modified, pathogenic form of the normal cellular protein replicates by converting the normal isoform to the disease isoform.1
The normal cellular isoform of the prion protein PrPC is a small glycosylphosphatidylinositol (GPI)-anchored cell-surface protein expressed predominantly in the brain, but also in a wide variety of peripheral tissues, including peripheral mononuclear blood cells.2,3 PrPC is present in all vertebrates examined to date, is widely expressed during embryogenesis, and has been strongly conserved throughout evolution, suggesting a vital function for the protein.4-6 Surprisingly, PrP null mice have shown that the protein is apparently not necessary for normal murine development or mature survival because these mice are viable, do not show any obvious developmental phenotype, and show no behavioral abnormalities up to 93 weeks of age.7,8 Ablation of the prion gene renders these mice resistant to experimental scrapie and obviates accumulation of PrPSc.8 More target-directed assays have shown subtle abnormalities in null mice such as defective GABAA receptor-mediated fast inhibition and impaired long-term potentiation, altered circadian rhythms, and loss of cerebellar Purkinje cells.9-11 These phenotypes are reminiscent of certain features of TSEs and suggest that loss of the normal function of this protein may contribute to disease.12
Clues to the function of PrPC may be gleaned by examination of cell-specific expression patterns. In peripheral blood of humans and rodents, PrPC is detected at the cell surface of lymphocytes and monocytes, but is absent from erythrocytes and granulocytes.2,3 Our laboratory has previously determined that PrPC expressed by mononuclear cells may play a role in cell activation.2 In this study, we further characterized the expression patterns and gene regulation of the protein in cells of the immune system. We now show that PrPC is expressed very early in hematopoiesis, indicated by its presence on CD34+stem cells. Differentiation along the lymphocyte or monocyte lineages supports the expression of surface PrPC, while differentiation along the granulocyte lineage progresses with concomitant downregulation of PrPC surface protein. This cell-type specific extinction of PrPC in granulocyte differentiation can be recapitulated in vitro by retinoic acid induction of HL-60 cells.
These results allow us to better define the regulation of the expression of PrPC in human leukocytes, which has potential implications for human and animal prion diseases. In addition, our studies provide a novel in vitro system with which to study PrP gene regulation and protein function.
MATERIALS AND METHODS
Cells.
Human peripheral blood leukocytes were isolated from whole blood from healthy volunteer donors and separated using Lymphocyte-poly (Cedarlane, Hornby, Ontario, Canada) density centrifugation. The two resulting layers were pooled, washed three times with phosphate-buffered saline (PBS), and stained for flow cytometry analysis as described in the following section.
Human low-density bone marrow (BM) cells were isolated by Ficoll-Paque (Pharmacia, Baie D'Urfé, Québec, Canada) density centrifugation of BM aspirates from patients undergoing diagnostic BM evaluation (kindly provided by Dr Alan Brox, Royal Victoria Hospital, McGill University). Cells were washed extensively and stained for flow cytometry analysis.
The human premyeloid line HL-60 (ATCC CCL-240) was maintained in RPMI 1640 medium (GIBCO-BRL, Burlington, Ontario, Canada) supplemented with 10% fetal bovine serum (FBS), 2.5 mg/mL penicillin, 2.5 mg/mL streptomycin, and 2 mmol/L glutamine. Differentiation along the monocytoid lineage was induced by culture in 100 nmol/L of phorbol 12-myristate 13-acetate (PMA; Sigma, St Louis, MO) for 3 days. Differentiation along the granulocyte lineage was induced by culture in 1 mol/L all-trans retinoic acid (Sigma) for 0, 1, 3, and 6 days. Cells were then stained for flow cytometry analysis or procured for isolation of total RNA.
Monoclonal antibodies (MoAbs) and flow cytometry.
The MoAb 3F4, which uniquely recognizes PrPC at the cell surface2 13 (and N.R.C., unpublished data), was prepared as ascites fluid in pristane-primed Balb/c mice, and used unconjugated at a 1:5,000 dilution. 3F4 was followed by either a goat anti-mouse Fab fluorescein isothiocyanate (FITC) conjugate or a donkey anti-mouse phycoerhythrin (PE) conjugate (both from Jackson Immunoresearch, West Grove, PA). Anti–CD15-FITC and anti–CD34-PE MoAb direct conjugates were obtained from Becton Dickinson (Saint-Laurent, Québec, Canada). FITC-conjugated, PE-conjugated, and unconjugated isotype-matched irrelevant control MoAbs were included in all experiments (Becton Dickinson and Sigma).
For immunofluorescence staining, cells were first incubated on ice for 30 minutes in PBS supplemented with 10% normal goat serum to block nonspecific binding. Cells were then stained with the appropriate MoAbs, each for 30 minutes on ice. Samples were analyzed on a FACSCAN flow cytometer using LYSYS II software (Becton Dickinson).
Semiquantitative polymerase chain reaction (PCR) analysis.
HL-60 cells were differentiated with all-trans retinoic acid as described. Cells were harvested at days 0, 1, and 3 and total RNA was isolated using TRIzol (GIBCO-BRL). Total RNA was then treated with RQ1 RNase free DNase (Promega, Madison, WI) and re-extracted twice with phenol-chloroform. Total RNA was reverse-transcribed to cDNA using Superscript reverse transcriptase (GIBCO-BRL) according to the manufacturer's instructions. This cDNA was then used for both prion and β-actin amplification by PCR. Primers used for the PCR reactions had the following sequences: human prion forward 5′-AAGCCTGGAGGATGGAACACT-3′, reverse 5′-GTTGCTGTACTCATCCATGGG-3′, and β-actin forward 5′-ATGCCATCCTGCGTCTGGACCTGGC-3′, reverse 5′-AGCATTTGCGGTGCACGATGGAGGG-3′. The primers for human prion and β-actin were designed to generate fragments of 434 and 606 bp, respectively. Two hundred nanograms of cDNA was added to the reaction mixture containing PCR buffer, 0.5 mmol/L dNTPs (Pharmacia), 50 pmol of either primer set, and 0.5 μL Taq polymerase (GIBCO). Samples were placed in a thermocycler (Perkin Elmer Cetus Corp, Norwalk, CT) for 25 cycles of 94°C for 1 minute, 60°C for 2 minutes, and 72°C for 3 minutes. The PCR products were separated on a 1% 1× Tris-acetate EDTA (TAE) agarose gel, transferred to a nylon membrane overnight by capillary action, and the Southern blots were probed with the appropriate random primed [32]P-labeled probes for 16 hours at 42°C in Rapid-Hyb buffer (Amersham, Burlington, Ontario, Canada). Blots were washed, and exposed to Phosphor Screen and analyzed on a PhosphorImager (Molecular Dynamics, Sunnyvale, CA).
RESULTS
Prion protein expression in human peripheral blood cells.
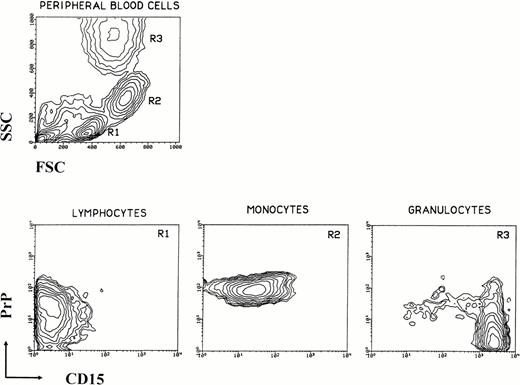
Human peripheral blood leukocytes were analyzed for PrPCsurface immunoreactivity by flow cytometry (Fig 1). Three main populations can be defined on the basis of size (forward scatter [FSC]) and granularity (side scatter [SSC]). When these three populations are examined for PrPC and CD15 immunoreactivity, each presents a unique staining profile. CD15 provides a convenient secondary marker because of its differential distribution among leukocytes.14-16 Lymphocytes have low to intermediate levels of PrPC and are CD15−, monocytes are PrPC high and CD15 low, and granulocytes are PrPC− and CD15 high. Erythrocytes are negative for both markers (data not shown). The absence of PrPC on granulocytes suggests at least two possibilities: lymphocytes and monocytes acquire PrPC during differentiation, or granulocytes lose PrPC as they mature.
Expression of prion protein versus CD15 by human peripheral blood leukocytes. Leukocytes were isolated from whole blood of normal donors, washed, stained for PrPC and CD15, and analyzed by flow cytometry. A typical light scatter contour plot of normal peripheral human blood mononuclear cells is shown in the upper left. Analysis gates R1-R3 are set on forward and side scatter alone and define three major subpopulations: lymphocytes (R1), monocytes (R2), and granulocytes (R3). Contour plots of surface prion protein versus CD15 for each of the three subpopulations are shown. Lymphocytes are (PrP+, CD15−), monocytes are (PrP+, CD15lo-med), and granulocytes are (PrP−, CD15hi). The small amount of PrP+ cells in panel R3 are due to contaminating monocytes.
Expression of prion protein versus CD15 by human peripheral blood leukocytes. Leukocytes were isolated from whole blood of normal donors, washed, stained for PrPC and CD15, and analyzed by flow cytometry. A typical light scatter contour plot of normal peripheral human blood mononuclear cells is shown in the upper left. Analysis gates R1-R3 are set on forward and side scatter alone and define three major subpopulations: lymphocytes (R1), monocytes (R2), and granulocytes (R3). Contour plots of surface prion protein versus CD15 for each of the three subpopulations are shown. Lymphocytes are (PrP+, CD15−), monocytes are (PrP+, CD15lo-med), and granulocytes are (PrP−, CD15hi). The small amount of PrP+ cells in panel R3 are due to contaminating monocytes.
Prion protein expression in human BM cells.
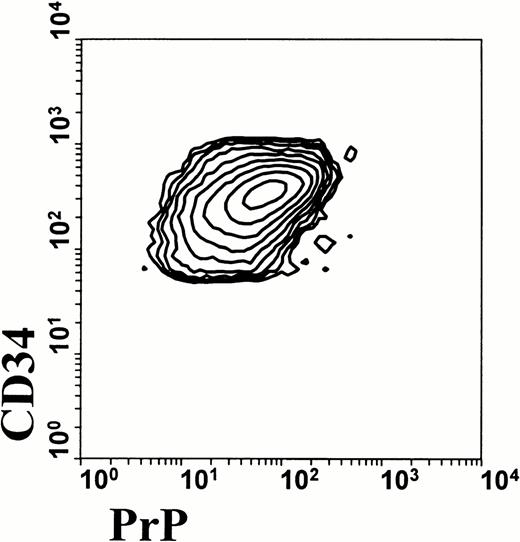
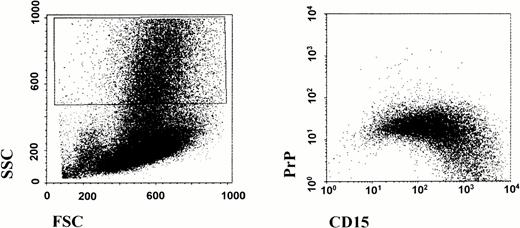
To examine the question of acquisition or suppression of PrPC in leukocyte differentiation, we first examined PrPC expression in CD34+ multipotential stem cells. As shown in Fig 2, cells that express CD34 coexpress surface prion protein. This suggests that prion protein may indeed be specifically downregulated in the granulocyte and erythroid lineage, and that other lineages maintain their expression of prion protein. We further investigated this possibility by analyzing low density human BM cells by flow cytometry (Fig 3). Cells that are differentiating along the granulocyte lineage show a dramatic increase in CD15 staining in the maturation process. These highly granular cells show a progressive loss of PrPC surface immunoreactivity as they gain CD15 staining. These data demonstrate that prion protein is present on pluripotential stem cells, and that it is downregulated during subsequent differentiation along the granulocyte lineage.
Surface prion protein expression by human CD34+ BM cells. Human BM aspirates were fractionated for low-density BM cells to enrich for multipotential stem cells. Cells were stained for CD34 and PrPC and analyzed by flow cytometry. Contour plot of low-density human BM cells shows that all CD34+ cells also express PrPC. Data were analyzed using gates for both high side scatter and CD34 expression.
Surface prion protein expression by human CD34+ BM cells. Human BM aspirates were fractionated for low-density BM cells to enrich for multipotential stem cells. Cells were stained for CD34 and PrPC and analyzed by flow cytometry. Contour plot of low-density human BM cells shows that all CD34+ cells also express PrPC. Data were analyzed using gates for both high side scatter and CD34 expression.
Downregulation of surface prion protein during in vivo granulocyte maturation. Human BM aspirates were fractionated for low-density BM cells as described. Cells were washed and stained for flow cytometry analysis as described in the legend to Fig 1. Differentiating granulocytes displaying high side scatter were gated as shown on left. As these cells mature along the granulocyte lineage and become CD15hi, there is a progressive loss of surface PrPC staining.
Downregulation of surface prion protein during in vivo granulocyte maturation. Human BM aspirates were fractionated for low-density BM cells as described. Cells were washed and stained for flow cytometry analysis as described in the legend to Fig 1. Differentiating granulocytes displaying high side scatter were gated as shown on left. As these cells mature along the granulocyte lineage and become CD15hi, there is a progressive loss of surface PrPC staining.
Regulation of prion protein expression in differentiating HL-60 cells.
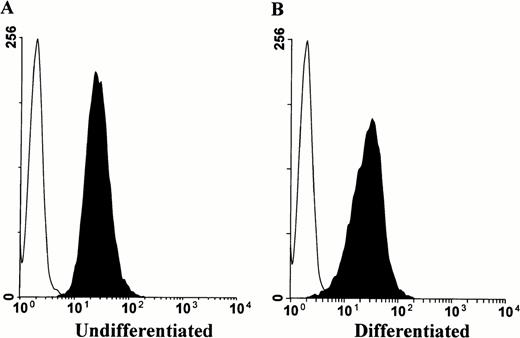
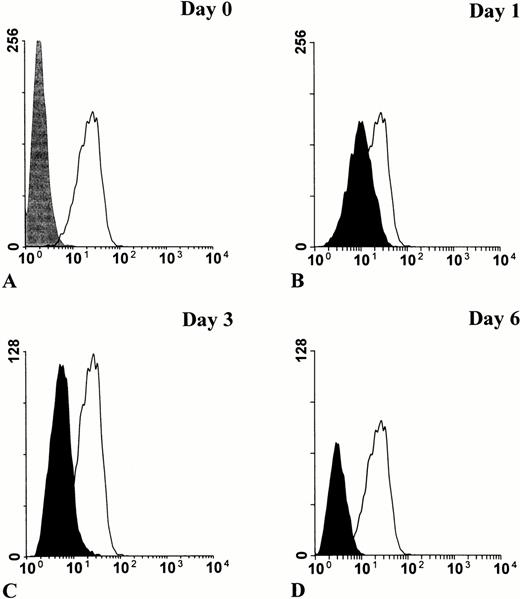
To provide a more convenient model of myeloid cellular differentiation, we examined the premyeloid cell line HL-60, which can be induced to differentiate along either the granulocyte or monocyte lineages by retinoic acid or phorbol esters, respectively.17-20 HL-60 cells express PrPC, which persists upon phorbol ester induced differentiation into macrophage-like cells (Fig 4). Conversely, induction of HL-60 cells into granulocyte differentiation by retinoic acid was associated with progressive downregulation of surface PrPC(Fig 5). The cells were observed over a 6-day period, with the proportionally largest decline in surface prion protein immunoreactivity occurring after the first 24 hours (Fig 5B).
Surface prion protein expression by HL-60 cells persists upon induction of monocyte lineage differentiation. HL-60 cells were cultured in the presence or absence of 100 nmol/L PMA for 3 days, then stained for flow cytometry analysis with MoAb 3F4. Histograms show PrPC surface immunoreactivity relative to an IgG2b isotype control. Minimal change is observed in PrPC staining intensity between undifferentiated (A) and differentiated cells (B).
Surface prion protein expression by HL-60 cells persists upon induction of monocyte lineage differentiation. HL-60 cells were cultured in the presence or absence of 100 nmol/L PMA for 3 days, then stained for flow cytometry analysis with MoAb 3F4. Histograms show PrPC surface immunoreactivity relative to an IgG2b isotype control. Minimal change is observed in PrPC staining intensity between undifferentiated (A) and differentiated cells (B).
Downregulation of surface PrPC in retinoic acid–induced granulocytic differentiation of HL-60 cells. HL-60 cells were cultured in the presence of 1 mol/L all-trans retinoic acid for 0, 1, 3, and 6 days, then stained for flow cytometry analysis with MoAb 3F4. Basal level of PrPC staining in undifferentiated cells (solid line) is shown relative to an IgG2b isotype control (in gray) (A). The progressive decrease of PrPC staining during differentiation (in black) is shown relative to basal level (B through D).
Downregulation of surface PrPC in retinoic acid–induced granulocytic differentiation of HL-60 cells. HL-60 cells were cultured in the presence of 1 mol/L all-trans retinoic acid for 0, 1, 3, and 6 days, then stained for flow cytometry analysis with MoAb 3F4. Basal level of PrPC staining in undifferentiated cells (solid line) is shown relative to an IgG2b isotype control (in gray) (A). The progressive decrease of PrPC staining during differentiation (in black) is shown relative to basal level (B through D).
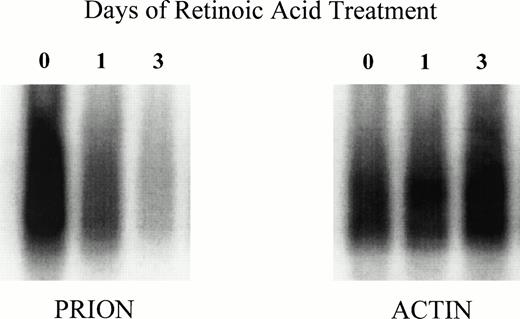
To investigate the role of gene transcription in the loss of granulocyte PrPC immunoreactivity, we examined the level of mRNA for the prion protein by semiquantitative PCR. To exclude potential contamination by PrP genomic sequences, the total RNA extract was treated with RNase-free DNase before PCR amplification. HL-60 cells were differentiated with retinoic acid as described and harvested for RNA extraction after 24 and 72 hours. A progressive decrease in the level of prion mRNA can be observed, while the level of β-actin mRNA remains approximately constant (Fig 6). This shows a strong association between decreased surface PrPC immunoreactivity and decreased PrP mRNA, most probably the result of decreased transcription triggered by retinoic acid treatment.
Downregulation of PrP mRNA in retinoic acid–induced differentiation of HL-60 cells. HL-60 cells were cultured in the presence of 1 mol/L all-trans retinoic and obtained at days 0, 1, and 3. Total RNA was isolated and reverse-transcribed to cDNA, which was then used for both prion and β-actin amplification by PCR. The PCR products were then Southern blotted with radioactively labeled PrP and β-actin probes. Blots were analyzed by PhosphorImager scanning.
Downregulation of PrP mRNA in retinoic acid–induced differentiation of HL-60 cells. HL-60 cells were cultured in the presence of 1 mol/L all-trans retinoic and obtained at days 0, 1, and 3. Total RNA was isolated and reverse-transcribed to cDNA, which was then used for both prion and β-actin amplification by PCR. The PCR products were then Southern blotted with radioactively labeled PrP and β-actin probes. Blots were analyzed by PhosphorImager scanning.
DISCUSSION
The normal cellular isoform of the prion protein, PrPC, is highly expressed in brain as well as many peripheral tissues, including peripheral blood mononuclear cells. We have found that PrPCis expressed by CD34+ multipotential stem cells in human BM. PrPC is downregulated in stem cell differentiation to the granulocyte lineage, but is maintained in lymphoid and monocyte lineages. The downregulation of PrPC in myeloid differentiation, at both the protein and mRNA levels, was modeled in vitro by the induction of HL-60 cells to granulocytoid lineage by retinoic acid. Our findings differ from Diomede et al,21who reported PrPC expression in neutrophils, as well as lymphocytes and monocytes. However, these studies were based on cell populations of 95% purity, in which contamination by mononuclear cells may have contributed to the PrP mRNA and protein detected by PCR and immunoprecipitation, respectively. It is also at least formally possible that PrPC is a cell-surface molecule in mononuclear cells detectable by flow cytometry, and an internally sequestered protein in neutrophils.
The regulation of expression of PrPC is incompletely understood. Despite the tissue-regulated and developmentally regulated expression of PrPC, the immediate 5′ region of the transcriptional start site does not contain a TATA box, but displays a GC-rich sequence similar to that observed in many housekeeping genes.22 Enhancer elements have been mapped upstream of the start site, and are also apparently present in the long first intron (∼10 kb) detected in all vertebrate species whose genomic PrPC organization is known.23,24 Our study suggests that retinoic acid–responsive elements participate in the cell-specific regulation of PrPC, and provides a biologically relevant in vitro paradigm to investigate the molecular basis of this regulation. Alternately, retinoic acid may be inducing the expression of trans-acting proteins that act at a silencing element controlling PrP expression. The regulation of prion protein expression in granulocytes and erythrocytes may underlie a general regulatory mechanism that could be exploited to reduce the amount of PrPC available for conversion in the prion diseases, because it has been shown that endogenous expression of PrP is necessary for continued propagation of the scrapie agent.8
Prion infectivity apparently copurifies with PrPSc, the protease resistant isoform of PrPC.25,26Cell-free conversion of PrPC to PrPSc has now been accomplished in vitro.27 Replication of the prion agent appears to be strictly dependent on availability of PrPC, because mice homozygous for the targeted disruption of the PrP gene are completely resistant to scrapie, and heterozygous mice display a longer incubation and slower disease progression than wild-type mice.8 Thus, the distribution of PrPCin hematogenous cells may predict the capability of that cell to propagate and/or transmit prion infectivity in hosts transfused or transplanted with those cells. On the basis of our current report, BM cells, including CD34+ stem cells, might be expected to harbor the prion agent, despite current guidelines which do not recognize BM as high-risk tissue.28,29 In the periphery, monocytes and lymphocytes, which express PrPC, would be expected to support prion replication, whereas PrPCnegative erythrocytes and granulocytes would not. This contention is supported by a limited number of transmission experiments30,31 and by recent data from Blattler et al,32 who show that prion agent infectivity detected in spleen by bioassay is dependent on the expression of PrPCby spleen cells and does not accumulate by nonspecific “carry-over” from the original innoculum.
An additional implication of our study is that PrPC must be differentially glycosylated in the nervous system as opposed to the periphery. PrPSc, the abnormal disease-associated isoform of PrPC, has been found to possess a high proportion of N-linked glycan chains terminating in the glycosyl moiety stage-specific embryonic antigen 1 (SSEA-1), also known as CD15.33 This carbohydrate moiety has been found to participate in cell-cell adhesion and tissue differentiation early in development, and probably plays an important role in immunocyte adhesion in inflammatory processes.34,35 In the peripheral blood, granulocytes, which express no PrPC, display prominent immunoreactivity for CD15. Lymphocytes, which display no surface CD15, do express PrPC. Only peripheral monocytes possess surface immunoreactivity for both PrPC and CD15, allowing the possibility that monocyte PrPC may be modified with the SSEA-1 moiety. Because glycosylation may participate in the brain-region–specific replication of the scrapie agent,36it is possible that certain PrPC on different peripheral cells might be more likely to participate in scrapie infection. Of interest, some data indicate that the monocyte-macrophage cell lineage is critical to the propagation of scrapie infectivity in the periphery. Scrapie infection in severe combined immunodeficient mice is apparently dependent on dendritic cells,37 which are tissue macrophages specialized for immune presentation. Moreover, peripheral blood cells containing scrapie infectivity are relatively radiation insensitive,38 which is consistent with the end-mitotic nature of peripheral monocytes. Finally, monocytes may be unique among peripheral blood cells for their capability of processing PrPC to PrPSc.39 Because other peripheral blood cells also express PrPC, the capability of monocytoid cells to support scrapie agent replication may relate to a posttranslational modification, such as SSEA-1 modification of the terminal PrPC glycans.
ACKNOWLEDGMENT
The authors gratefully acknowledge Dr Josephine Nalbantoglu for helpful advice and discussion, and Dr Michelle Krakowski for critical reading and inspiration.
Supported by the Medical Research Council (MRC) of Canada Grant No. MT12214, National Institutes of Health Grant No. AG09694, and Advanced Bioconcept. V.C.D. was supported by PhD studentship grants from MRC and Fonds de la recherche en santé du Québec.
Address reprint requests to Neil R. Cashman, MD, Montreal Neurological Institute and Hospital, 3801 University, Montreal, Québec, Canada H3A-2B4.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.