Abstract
CD20 is a nonglycosylated 33 to 37 kD phosphoprotein involved in B-cell signaling that subserves important functions in the regulation of B-cell proliferation and differentiation. In addition, this B-cell surface antigen has been shown recently to be an effective target for immunotherapy of B-cell malignancies using chimeric (mouse/human) or radiolabeled murine monoclonal anti-CD20 antibodies. In this report we show that extensive crosslinking of CD20 with murine anti-CD20 monoclonal antibodies (MoAbs) in the presence of either goat anti-mouse IgG or Fc receptor (FcR)-expressing cells directly inhibits B-cell proliferation, induces nuclear DNA fragmentation, and leads to cell death by apoptosis. The apoptotic effects of these MoAbs can be inhibited by chelation of extracellular or intracellular Ca2+ by EGTA or Bapta AM, indicating that anti-CD20–mediated apoptosis may be related to changes in Ca2+ concentration. These findings suggest that ligation of CD20 in vivo by anti-CD20 antibodies in the presence of FcR-expressing cells may initiate signal transduction events that induce elevation of [Ca2+]i and lead to apoptosis of malignant B cells, thereby contributing to the impressive tumor regressions observed in mouse models and clinical trials using anti-CD20 MoAbs.
CD20 IS A nonglycosylated 33 to 37 kD phosphoprotein expressed on greater than 95% of normal and neoplastic B cells. It is expressed on the cell surface from the pre-B stage of development until terminal differentiation to plasma cells occurs and has been used as one of the most reliable markers of the B-cell lineage.1 Monocytes, resting and activated T cells, null cells, and nonlymphoid cells are uniformly CD20-negative.2The predicted amino acid sequence of CD20 suggests a structure containing four transmembrane-spanning regions with both amino and carboxyl termini located on the cytoplasmic side of the plasma membrane.3-5 Current studies suggest that CD20 is a B-cell surface protein with the capacity to serve as a calcium channel, initiate intracellular signals, and modulate cell growth and differentiation.6 Ligation of CD20 with anti-CD20 monoclonal antibodies (MoAbs) has been shown to activate tyrosine and serine/threonine protein kinases, which are noncovalently associated with CD20, thereby inducing tyrosine phosphorylation of phospholipase C-gamma (PLCγ). In addition, hypercross-linking CD20 with anti-CD20 MoAbs plus a secondary goat anti-mouse (GAM) antibody has been shown to mobilize calcium from intracellular stores.7,8 Anti-CD20 MoAbs have been shown to exert variable effects on the proliferation of B cells after binding to the CD20 antigen. The 1F5 anti-CD20 MoAb stimulates B-cell cycle transition from G0 to G1,9 whereas another anti-CD20 MoAb (B1) inhibits B-cell progression from the G1 phase of the cell cycle into the S/G2+M stages following mitogen stimulation. MoAb binding also inhibits B-cell differentiation and Epstein-Barr virus (EBV)- or pokeweed mitogen (PWM)-induced Ig secretion.10 11 These studies have shown the importance of CD20 in important regulatory signals for B cells, although the precise role of CD20 remains unclear.
The ubiquitous expression of CD20 at high surface densities on malignant human B cells, and its lack of internalization after MoAb binding,12 has suggested its utility as a tumor target for immunotherapy of B-cell lymphomas.13,14 Studies have documented the efficacy of this approach with objective tumor responses in 25% to 50% of patients treated with unmodified anti-CD20 MoAbs.13-15 Even more impressive results have been recorded using radioiodinated I-131–anti-CD20 MoAbs, which have induced objective responses in 75% to 95% of patients treated with relapsed B-cell lymphomas.16-20 Although the antitumor effects of unmodified anti-CD20 MoAbs were initially attributed solely to complement-mediated cytolysis and antibody-mediated cellular cytotoxicity (ADCC),21-23 the magnitude of the tumoricidal effects observed have exceeded those expected, particularly in settings where unmodified murine MoAbs (which generally fix human complement poorly and mediate ADCC poorly) have been used. Furthermore, objective regressions of lymphomas have been observed in 25% to 40% of patients administered murine anti-CD20 MoAbs trace-labeled with imaging doses of Iodine-131 believed inadequate to mediate radiation-induced tumor regressions18 19 (and O.W. Press and M. Corcoran, unpublished observations, July 1993).
Accumulating evidence from in vitro studies,6,9 animal tumor models,14,24 and early clinical trials21,23 suggests that a substantial portion of the tumoricidal effect of anti-CD20 MoAbs may be mediated by mechanisms independent of complement, ADCC, or radioactive emissions. Indeed, ligation of CD20 by MoAbs has been shown to disrupt normal signal transduction in B lymphoma cells, which have subsequently been shown to regress, presumably because of induction of cell cycle arrest and/or apoptosis initiated by cross-linking of CD20 by the MoAbs.7 25-28
Apoptosis is usually defined as physiological or programmed cell death and is characterized by specific morphological features, including cellular and nuclear pyknosis, cytoplasmic blebbing, and margination of condensed chromatin at the periphery of the nuclear envelope. In addition, internucleosomal DNA fragmentation typically occurs, resulting in the production of characteristic “DNA ladders” when nuclear extracts are subjected to agarose gel electrophoresis.29,30 Cross-linking of many B-cell surface antigens, including surface IgM (sIgM), CD19, CD22, and major histocompatibility complex (MHC) class II have been reported to initiate cell cycle arrest and/or apoptosis in mouse and human B cells. Soluble anti-sIgM antibodies induce apoptosis in Ramos cells,31 and anti-MHC class II MoAbs induce apoptotic death of murine splenic B cells.32 Cross-linking of CD19 and CD22 with their respective MoAbs can also induce apoptosis in B cells, but only if the cross-linking is amplified by secondary GAM antibodies.33 Because CD20 is a component of a B lymphocyte signal transduction complex involved in the regulation of B lymphocytes following activation similar to the one perturbed by anti-sIgM antibodies, we decided to test the ability of anti-CD20 MoAbs to induce apoptotic cell death in malignant human B cells.
Our results indicate that anti-CD20 MoAbs can directly inhibit the proliferation of malignant B cells independent of complement-mediated lysis or ADCC and that apoptosis can be induced in both B-cell lines and normal tonsil B cells if the CD20 cross-linking is amplified by a GAM antibody or by incubation with FcR-bearing accessory cells. The apoptotic effects of anti-CD20 MoAbs are markedly attenuated by chelation of Ca2+ with EGTA or Bapta AM, an intracellular calcium chelator. These results indicate that anti-CD20–mediated apoptosis is related to changes in intracellular Ca2+concentration and that in vivo ligation of malignant B cells by anti-CD20 MoAbs followed by FcR-mediated cross-linking by macrophages or other accessory cells may be responsible for a substantial proportion of the cytotoxic effects of anti-CD20 MoAbs observed in clinical trials.
MATERIALS AND METHODS
Cells.
The CD20-expressing human Burkitt's lymphoma cell lines, Ramos, Daudi, and Raji, were obtained from the American Type Culture Collection (ATCC; Bethesda, MD) and maintained in log-phase growth in RPMI-1640 supplemented with 12% FBS, 2 mmol/L glutamine, 1 mmol/L sodium pyruvate, 100 U/mL penicillin, and 100 μg/mL streptomycin. Normal tonsil B cells were isolated from human tonsils and cultured as previously described.34 The mouse fibroblast Ltk− cell line transfected with human FcγRII receptor (CDw32) was obtained from ATCC with the kind permission of Dr Jacques Banchereau35 and maintained in complete RPMI-1640 medium supplemented with 1 × HAT (Hypoxanthine + aminopterin + thymidine) medium.
Antibodies and reagents.
The anti-CD20 MoAb 1F5 (IgG2a) and the control anti-CD3 MoAb 64.1 (IgG2a) were produced and purified as previously described.9,21 36 The anti-CD20 MoAb B1(IgG2a) was purchased from Coulter Corporation (Miami, FL). F(ab′)2fragments of goat anti-human cell surface IgM antibody (anti-sIgM) and F(ab′)2 fragments of goat anti-mouse IgG F(ab′)2 antibodies were purchased from Jackson ImmunoResearch Laboratories, Inc (West Grove, PA). F(ab′)2fragments of a GAM antibody were obtained from Pierce Chemical Co (Rockford, IL). Herbimycin A and genistein were obtained from Calbiochem (San Diego, CA). Bapta AM was obtained from Molecular Probes (Eugene, OR).
In vitro cell proliferation assay.
The effects of anti-CD20 MoAbs on malignant B-cell growth in vitro were determined by assessing [3H]-thymidine incorporation in Ramos, Daudi, and Raji cells with and without preincubation with anti-CD20 MoAbs 1F5 or B1.24 Briefly, 5 × 103to 5 × 104 cells were resuspended in 100 μL culture medium and plated in 96-well flat-bottom microtiter plates. After incubating cells at 37°C for 24 hours with anti-CD20 MoAbs, 0.5 μCi of [3H]-thymidine/well was added, and cells were cultured for an additional 18 hours. Cells were then harvested onto glass fiber filters with an automated harvesting system from Skatron Inc (Sterling, VA), and [3H]-thymidine uptake was assayed with a 4000 series liquid scintillation counter (Downers Grove, IL). In some experiments, cells coated with anti-CD20 MoAbs were further cross-linked using an F(ab′)2 GAM reagent. In these experiments, 5 × 104 cells/100 μL were incubated with 10 μg/mL anti-CD20 MoAbs at 4°C for 30 minutes, washed, incubated with 50 μg/mL GAM for 24 hours at 37°C, pulsed with 0.5 μCi [3H]-thymidine/well for 18 hours, harvested, and counted.
Flow cytometric analysis of apoptosis and cell cycle arrest using propidium iodide staining.
Flow cytometric analysis of cellular DNA was performed following propidium iodide staining according to the method of Fried et al.37 Briefly, 106 Ramos cells were incubated with anti-CD20 MoAbs or control antibodies in the presence or the absence of GAM, washed in phosphate-buffered saline (PBS), and then gently resuspended in 0.5 mL of a hypotonic fluorochrome solution (50 μg/mL propidium iodide in 0.1% sodium citrate plus 0.1% Triton X-100; Sigma Chemical Co, St Louis, MO). Samples were stored in the dark at 4°C until flow cytometric analysis of individual nuclei using a FACScan flow cytometer (FACScan; Becton Dickinson, San Jose, CA) could be performed. The percentage of cells that were apoptotic was measured as described by Nicoletti et al.38 Briefly, cellular debris was excluded from analysis by raising the forward scatter threshold, and the DNA content of intact nuclei was recorded on a logarithmic scale. Apoptotic cell nuclei containing hypodiploid DNA were enumerated as a percentage of the total population. After measurement of apoptotic cells, the distribution of cells in each phase of the cell cycle was determined using the same samples by the cytometric method of Fried et al.37 39
In some experiments, Ramos cells were first incubated overnight at 37°C with herbimycin A (0.125 to 0.5 μg/mL) or genistein (2.5 to 10 μg/mL) before washing and incubating with anti-CD20 plus GAM. In other experiments, the calcium chelators EGTA (3 mmol/L) and Bapta AM (5 μmol/L) were added to cell cultures 30 minutes before anti-CD20 MoAbs to determine the effects of perturbations in intracellular Ca2+ concentrations on anti-CD20–induced apoptosis.
Light scatter analysis of apoptotic cells by flow cytometry.
Apoptotic cells were detected by flow cytometry using the distinct forward and 90° light scatter characteristics of apoptotic and viable cells according to Knox et al.40 By flow cytometry, apoptotic cells cause lower forward light scatter (caused by cell shrinkage) and higher side scatter (caused by increased granularity of the cell, presumably as a result of chromatin condensation and fragmentation) than their viable counterparts. Thus, apoptotic and viable cell populations can be clearly identified and large numbers of cells reproducibly and rapidly counted. The results were expressed as “percentage apoptosis” by dividing the number of apoptotic cells enumerated by the total number of cells counted in each culture.
DNA analysis by agarose gel electrophoresis.
Fragmented DNA was isolated and analyzed from Ramos cells after incubation with and without antibodies as described by Gottschalk et al.41 Briefly, 2 to 5 × 106 Ramos cells were incubated with or without 10 μg/mL 1F5 or B1 in the presence or absence of 50 μg/mL GAM for 24 hours, then lysed in 0.5 mL of lysis buffer (0.6% sodium dodecyl sulfate (SDS) + 10 mmol/L EDTA, pH 7.0). NaCl was added to a concentration of 1 mol/L and mixed by inversion. The mixture was left at 4°C for ≥ 12 hours and then centrifuged at 12,000 g for 15 minutes at 4°C. Fifty micrograms per milliliter RNAase A (Sigma) was added to the supernatant and incubated at 37°C for 30 minutes. The supernatant was then extracted with a 1:1 mixture of phenol and chloroform, precipitated with 75% ethanol, and resuspended in TE buffer (Tris buffer + EDTA). The sample was then subjected to electrophoresis on a 2% agarose gel containing 0.5 μg/mL ethidium bromide.
RESULTS
Effects of anti-CD20 MoAbs on B-cell proliferation.
The effects of anti-CD20 MoAbs on B-cell proliferation were studied by incubating malignant human B-cell lines with 3H-thymidine in the presence or absence of the anti-CD20 MoAb 1F5. The proliferation of Ramos cells was progressively inhibited by increasing concentrations of 1F5, with maximal inhibition at concentrations ≥ 1 μg/mL (Fig1A). To determine whether this inhibition was a result of the fixation of complement derived from fetal bovine serum (FBS) in the culture medium, we repeated this experiment using FBS that had been heat-inactivated at 56°C for 30 minutes (Fig1B). These experiments suggested that both complement-mediated and complement-independent mechanisms contributed to the observed inhibition of B-cell proliferation. In the absence of detectable complement activity, Ramos cell proliferation was inhibited by up to 53% at high 1F5 concentrations (P < .001; Fig1B). All subsequent experiments were performed using heat-inactivated FBS to further characterize the complement-independent mechanisms of B-cell killing induced by anti-CD20 MoAbs. Interestingly, we found that the antiproliferative effect of 1F5 was related to cell density and to the degree of saturation of this antibody on the target cell surface. When the cell density was higher than 2 × 105 cells/mL and 1F5 was used at subsaturating concentrations, the inhibitory effect of 1F5 decreased to as low as 4 ± 3.5% (P > .05). However, the inhibitory effect of anti-CD20 MoAbs could be elicited even under these suboptimal conditions if 1F5 was cross-linked on the cell surface using a GAM antibody (81 ± 2.2% inhibition,P < .001, data not shown). These results indicate that under optimal conditions, anti-CD20 MoAbs alone are sufficient to inhibit Ramos cell growth by ligation of the CD20 antigen, but under suboptimal conditions augmentation of this effect by hypercross-linking CD20 with GAM is required to inhibit cell growth. Another anti-CD20 MoAb, B1, had similar inhibitory effects on Ramos cells, and both MoAbs also inhibited the proliferation of other B lymphoma cell lines (Raji and Daudi, data not shown) in a similar fashion.
Inhibitory effect of anti-CD20 MoAb 1F5 on Ramos cells measured by tritiated thymidine incorporation. 104 cells in 100 μL per well were incubated continuously with 0.0001, 0.001, 0.01, 0.1, 1, 10, 100 μg/mL 1F5 in medium containing FBS (A) or medium containing inactivated FBS (B) at 37°C for 24 hours, followed by addition of 0.5 μCi [3H]-thymidine per well and incubating for another 18 hours. Cells incubated in medium without 1F5 were used as control. Data are representative of three concordant experiments. Similar results were obtained with the B1 anti-CD20 antibody (not shown).
Inhibitory effect of anti-CD20 MoAb 1F5 on Ramos cells measured by tritiated thymidine incorporation. 104 cells in 100 μL per well were incubated continuously with 0.0001, 0.001, 0.01, 0.1, 1, 10, 100 μg/mL 1F5 in medium containing FBS (A) or medium containing inactivated FBS (B) at 37°C for 24 hours, followed by addition of 0.5 μCi [3H]-thymidine per well and incubating for another 18 hours. Cells incubated in medium without 1F5 were used as control. Data are representative of three concordant experiments. Similar results were obtained with the B1 anti-CD20 antibody (not shown).
Induction of apoptosis in Ramos cells by binding of anti-CD20 MoAbs.
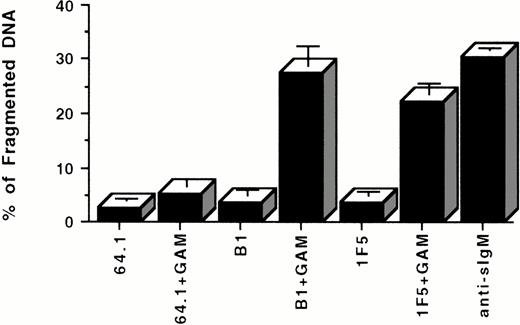
To test whether anti-CD20 MoAbs are capable of inducing B-cell death by apoptosis after inhibiting cell growth, three corroborative assays were used; propidium iodide (PI) staining of cell DNA after incubation of cells with anti-CD20 or control MoAbs, DNA fragmentation as assessed by agarose gel electrophoresis, and flow cytometric light scatter analysis. In all these assays, incubation of Ramos B cells with F(ab′)2 fragments of an anti-sIgM antibody was used as a positive control because multiple studies have documented the reliable induction of Ramos cell apoptosis with this reagent.31 33In the PI staining assay, the percentage of apoptotic nuclei detected by PI staining and flow cytometric analysis after 24 hours in culture increased from 2.7 ± 0.3% in the negative control (MoAb 64.1) to 30.6 ± 0.2% (P < .001) in the presence of anti-sIgM antibodies (Fig 2A). Soluble anti-CD20 antibodies (1F5 and B1), did not induce significant apoptosis by themselves under these conditions. However, each of these MoAbs clearly and reproducibly induced cell death when cross-linked by a GAM antibody; apoptosis occurred in 22.2 ± 1.9% of cells by 1F5 + GAM and in 27.7 ± 3.5% of cells with B1 + GAM (compared to 5.25 ± 1.3% apoptosis in control cultures incubated with MoAb 64.1 + GAM, P < .001; Fig 2A). A kinetic analysis of the induction of apoptosis by CD20 cross-linking indicated that DNA fragmentation could be detected as early as 1 hour after incubation with anti-CD20 MoAbs + GAM, with near maximal levels of apoptosis achieved after 12 to 24 hours of incubation (Fig 2B).
(A) Apoptotic effect of anti-CD20 MoAbs on Ramos cells as shown by propidium iodide staining. 106 cells/mL were incubated with 10 μg/mL of the B1 or 1F5 anti-CD20 antibodies or with the control 64.1 anti-CD3 antibody for 24 hours in the presence or the absence of GAM cross-linker (50 μg/mL). Cell nuclei were stained with propidium iodide and analyzed by flow cytometry. Hypodiploid DNA peaks corresponding to apoptotic nuclei were quantified. Data are representative of five concordant experiments. (B) Kinetics of apoptosis induced by CD20 cross-linking. 106 cells/mL were incubated with or without 10 μg/mL anti-CD20 (B1) MoAb + 50 μg/mL GAM for 0 to 48 hours. Cell nuclei were stained with propidium iodide and analyzed by flow cytometry as described above. Anti-sIgM was used as a positive control. Data are representative of three similar experiments.
(A) Apoptotic effect of anti-CD20 MoAbs on Ramos cells as shown by propidium iodide staining. 106 cells/mL were incubated with 10 μg/mL of the B1 or 1F5 anti-CD20 antibodies or with the control 64.1 anti-CD3 antibody for 24 hours in the presence or the absence of GAM cross-linker (50 μg/mL). Cell nuclei were stained with propidium iodide and analyzed by flow cytometry. Hypodiploid DNA peaks corresponding to apoptotic nuclei were quantified. Data are representative of five concordant experiments. (B) Kinetics of apoptosis induced by CD20 cross-linking. 106 cells/mL were incubated with or without 10 μg/mL anti-CD20 (B1) MoAb + 50 μg/mL GAM for 0 to 48 hours. Cell nuclei were stained with propidium iodide and analyzed by flow cytometry as described above. Anti-sIgM was used as a positive control. Data are representative of three similar experiments.
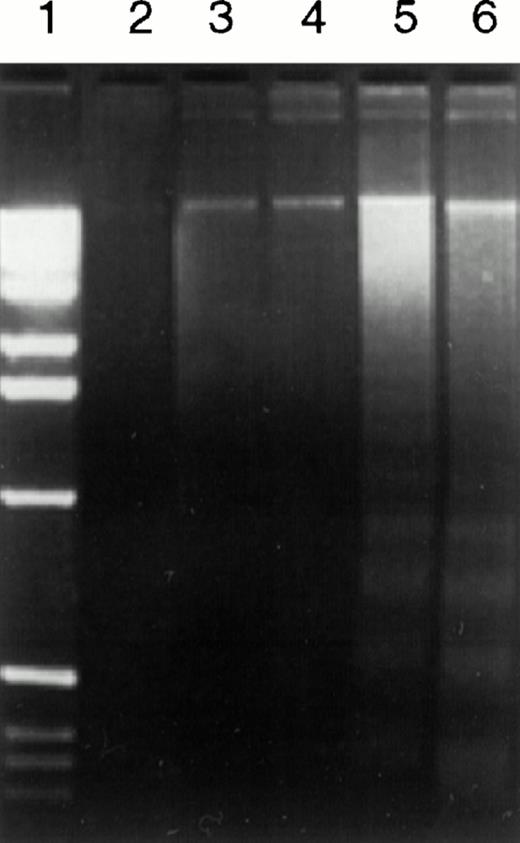
Two different assays were used to confirm this observation. Classic nucleosomal DNA ladder patterns were observed in DNA samples from cells treated by anti-CD20 MoAb (B1) after cross-linking with GAM, as shown by agarose gel electrophoresis in Fig 3(lane 5). Another anti-CD20 MoAb, 1F5, exhibited similar apoptotic effects (data not shown). Cells treated by anti-CD20 MoAbs alone did not consistently display DNA ladder formation (Fig 3, lane 4). Similar results were observed in flow cytometric light scatter analyses in five consecutive experiments, one of which is shown in Fig4.
DNA degradation in Ramos cells caused by anti-CD20 MoAb (B1) plus GAM shown by agarose gel electrophoresis. Ramos cells (2 × 106) were treated with 10 μg/mL control anti-CD3 MoAb (64.1) or anti-CD20 MoAb (B1) in the presence or the absence of GAM cross-linker (50 μg/mL) at 37°C for 24 hours. Fragmented DNA was isolated and analyzed on a 2% agarose gel. Lane 1, molecular weight markers; lane 2, 64.1; lane 3, 64.1 + GAM; lane 4, B1; lane 5, B1 + GAM; lane 6, anti-sIgM.
DNA degradation in Ramos cells caused by anti-CD20 MoAb (B1) plus GAM shown by agarose gel electrophoresis. Ramos cells (2 × 106) were treated with 10 μg/mL control anti-CD3 MoAb (64.1) or anti-CD20 MoAb (B1) in the presence or the absence of GAM cross-linker (50 μg/mL) at 37°C for 24 hours. Fragmented DNA was isolated and analyzed on a 2% agarose gel. Lane 1, molecular weight markers; lane 2, 64.1; lane 3, 64.1 + GAM; lane 4, B1; lane 5, B1 + GAM; lane 6, anti-sIgM.
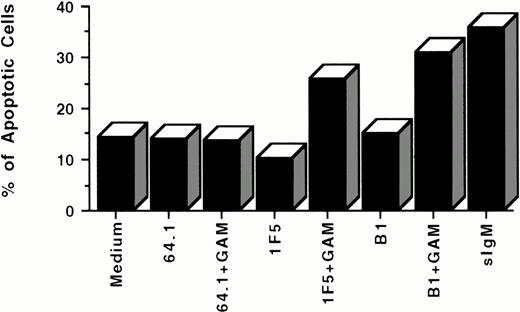
Apoptosis of Ramos cells induced by anti-CD20 MoAbs as shown by flow cytometry with light scatter analysis. 106cells/mL were incubated with 10 μg/mL antibodies for 24 hours in the presence or the absence of GAM cross-linker at 50 μg/mL. Pelleted cells were resuspended in fixative (5% formaldehyde, 1% FBS in pH 7.2 PBS), and analyzed by flow cytometry. Apoptotic cells were identified and quantified by flow cytometry according to their smaller size and higher density. The data are representative of five experiments.
Apoptosis of Ramos cells induced by anti-CD20 MoAbs as shown by flow cytometry with light scatter analysis. 106cells/mL were incubated with 10 μg/mL antibodies for 24 hours in the presence or the absence of GAM cross-linker at 50 μg/mL. Pelleted cells were resuspended in fixative (5% formaldehyde, 1% FBS in pH 7.2 PBS), and analyzed by flow cytometry. Apoptotic cells were identified and quantified by flow cytometry according to their smaller size and higher density. The data are representative of five experiments.
We next tested whether normal human B lymphocytes were also susceptible to apoptosis after ligation of CD20 by MoAbs. Tonsil B cells were cultured for 2 days with anti-CD20 MoAbs or a control anti-CD3 MoAb 64.1 in the presence or absence of GAM, followed by detection of apoptotic cells by PI staining. High rates of spontaneous apoptosis were observed in tonsil B cells in control cultures incubated alone or with control MoAb 64.1 (31 ± 0.14%). Incubation with anti-CD20 MoAbs alone slightly reduced the level of spontaneous apoptosis (28.9 ± 0.042%, P < .05), as previously described for normal human B cells.42 However, hypercrosslinking of CD20 on tonsil B cells with anti-CD20 + GAM significantly increased the percentage of tonsil B cells undergoing apoptosis (47.25 ± 0.77%, compared with 35.25 ± 0.92 in control cultures incubated with 64.1 + GAM, P < .001), similar to the effects of hypercrosslinking CD20 on the B lymphoma cell lines.
Because Fcγ receptor (FcγR) engagement is known to inhibit anti-Ig–induced B-cell responses,43 we investigated whether the cytotoxic effect of intact anti-CD20 MoAbs could be caused by the interaction of the Fc region of the MoAbs with FcγRs on Ramos cells. To test this hypothesis we compared the effects of intact anti-CD20 MoAbs with those induced by their F(ab′)2fragments. The results indicated that the inhibitory effect of F(ab′)2 fragments of the B1 anti-CD20 MoAb (34.00 ± 11.25% of counts per minute [cpm] obtained in control cultures using MoAb 64.1) were comparable with those of the intact B1 MoAb (44.78 ± 10.39% of control cpm, P > .05). Furthermore, hypercross-linking CD20 with anti-CD20 MoAbs plus GAM [goat anti-mouse Ig F(ab′)2 for anti-CD20 F(ab′)2] augmented the apoptotic effects of both anti-CD20 F(ab′)2 and intact anti-CD20 MoAb similarly (data not shown), indicating that neither binding of the Fc portion of anti-CD20 MoAbs to FcR nor FcR cross-linking was essential for the observed effects.
Induction of cell cycle arrest.
To determine whether cross-linking CD20 induces cell cycle arrest as well as apoptosis, we analyzed the distribution of Ramos cells in various phases of the cell cycle by a flow cytometric method using the same experimental samples used to measure apoptosis. The results of four separate experiments are shown in Table1. As can be seen, in all experiments small increases in the percentage of cells in the G1 phase of the cell cycle were observed after incubation with anti-CD20 antibodies, suggesting that cell cycle arrest was induced in a small fraction of the cells by CD20 ligation. The magnitude of this effect could be amplified by crosslinking with anti-CD20 MoAbs + GAM, increasing the percentage of cells in G1 phase from 30% to 43% and correspondingly decreasing the percentage of cells in S + G2/M phases from 70% to 57% (Table 1).
FcγR-expressing cells amplify apoptosis induced by anti-CD20 MoAbs.
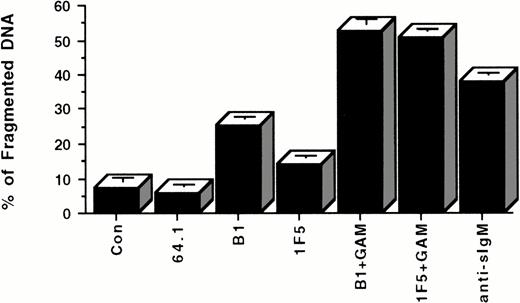
As shown in the above experiments, apoptosis of Ramos cells can only be reliably initiated by anti-CD20 MoAbs if they are cross-linked with GAM. To determine whether similar cross-linking might conceivably occur in vivo under clinical conditions in which anti-CD20 MoAbs are administered to patients bearing B-cell malignancies, we investigated whether functional cross-linking of Ramos cells coated with anti-CD20 MoAbs might be achieved by binding of the Fc domain of these antibodies to FcγR of adjacent cells. To test this possibility, we incubated Ramos cells with anti-CD20 MoAbs in the presence or absence of mouse fibroblasts transfected with the human Fc receptor (FcγRII/CDw32).35 As indicated in Fig5, these cells were capable of providing functional cross-linking and inducing apoptosis of Ramos cells bearing surface anti-CD20 MoAbs. The percentage of apoptotic nuclei increased from 6.05 to 7.35 ± 0.81% for two negative controls to 25.30 ± 0.60% (P < .001) and 14.10 ± 0.39% (P < .001) for anti-CD20 MoAbs B1 and 1F5, respectively.
Apoptosis of Ramos cells incubated with anti-CD20 MoAbs in the presence of FcγR-expressing accessory cells. Each well of a 24-well plate was coated with 5 × 105 FcγR-expressing accessory cells at 37°C overnight. 106 Ramos cells were added to each coated well with PBS, control anti-CD3 antibody (64.1), or anti-CD20 MoAbs. After 24 hours incubation at 37°C, cells were stained with PI and analyzed by flow cytometry. Anti-CD20 MoAbs (B1 and 1F5) plus GAM and anti-sIgM antibody were used as positive control in the absence of FcγR-expressing accessory cells. Data are representative of four concordant experiments.
Apoptosis of Ramos cells incubated with anti-CD20 MoAbs in the presence of FcγR-expressing accessory cells. Each well of a 24-well plate was coated with 5 × 105 FcγR-expressing accessory cells at 37°C overnight. 106 Ramos cells were added to each coated well with PBS, control anti-CD3 antibody (64.1), or anti-CD20 MoAbs. After 24 hours incubation at 37°C, cells were stained with PI and analyzed by flow cytometry. Anti-CD20 MoAbs (B1 and 1F5) plus GAM and anti-sIgM antibody were used as positive control in the absence of FcγR-expressing accessory cells. Data are representative of four concordant experiments.
Effect of inhibitors of protein kinases on apoptosis.
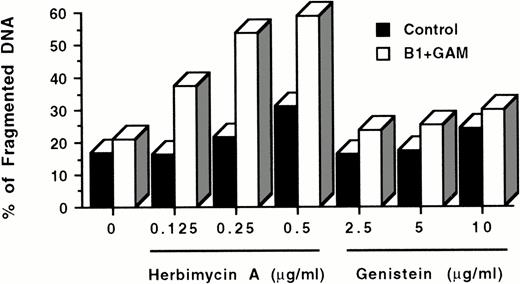
Experiments by other investigators7 as well as our own unpublished results have shown that the CD20 molecule is noncovalently associated with both tyrosine and serine kinases. To test whether these protein kinases are involved in the cascade of events induced by CD20 cross-linking and leading to apoptosis, we exposed Ramos cells to the tyrosine kinase inhibitor herbimycin A and the nonspecific protein kinase inhibitor genistein before CD20 hypercross-linking with anti-CD20 MoAbs + GAM. Unexpectedly, both inhibitors appeared to augment the induction of apoptosis by anti-CD20 MoAbs + GAM in a dose-dependent manner, rather than inhibiting it as anticipated (Fig6). In three concordant experiments, herbimycin increased the percentage of fragmented DNA by 57.5 ± 5.8% compared with cultures incubated with B1 + GAM without herbimycin. Also, a slight increase in apoptosis of Ramos cells was observed with herbimycin A and genistein by themselves, as has previously been reported for Jurkat cells treated with genistein.44
Effect of protein kinase inhibitors on apoptosis induced by CD20 cross-linking. 106 Ramos cells/mL were incubated overnight with 0.125 to 0.5 μg/mL herbimycin A or 2.5 to 10 μg/mL genistein at 37°C. After washing twice with medium, cells were cultured with or without 10 μg/mL B1 anti-CD20 MoAb + 50 μg/mL GAM for 24 hours. Cell nuclei were stained with PI and analyzed by flow cytometry as described above. Cells treated with inhibitors only were used as controls. All wells contained comparable concentrations of dimethyl sulfoxide, which was used as a diluent to dissolve herbimycin A and genistein. The data are representative of three experiments.
Effect of protein kinase inhibitors on apoptosis induced by CD20 cross-linking. 106 Ramos cells/mL were incubated overnight with 0.125 to 0.5 μg/mL herbimycin A or 2.5 to 10 μg/mL genistein at 37°C. After washing twice with medium, cells were cultured with or without 10 μg/mL B1 anti-CD20 MoAb + 50 μg/mL GAM for 24 hours. Cell nuclei were stained with PI and analyzed by flow cytometry as described above. Cells treated with inhibitors only were used as controls. All wells contained comparable concentrations of dimethyl sulfoxide, which was used as a diluent to dissolve herbimycin A and genistein. The data are representative of three experiments.
Correlation of anti-CD20–mediated apoptosis with increases in intracellular Ca2+ concentrations.
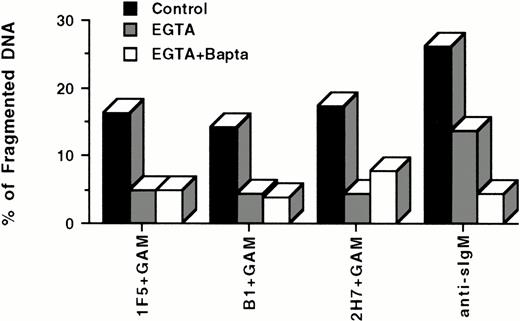
Because anti-sIgM–induced apoptosis of lymphoma cells has been reported to be dependent on increases in [Ca2+]i and to be inhibited by calcium chelators,40 we chose to investigate the role of intracellular calcium fluxes on anti-CD20–mediated apoptosis. Our unpublished results as well as reports by others7 indicate that ligation of CD20 with soluble anti-CD20 MoAbs alone is insufficient to mediate detectable increases in [Ca2+]i. However, hypercross-linking CD20 with anti-CD20 MoAbs and a GAM antibody7 does induce reproducible elevations of [Ca2+]i. These results suggested that anti-CD20–mediated apoptosis, like anti-sIgM–mediated apoptosis, may be influenced by fluctuations in [Ca2+]i. To test this hypothesis, we incubated Ramos cells in the presence and absence of the extracellular Ca2+ chelator EGTA and the intracellular Ca2+chelator Bapta AM in the presence of anti-sIgM or anti-CD20 MoAbs + GAM (Fig 7). Calcium chelation was found to inhibit apoptosis of Ramos cells induced by either anti-sIgM or anti-CD20 MoAbs + GAM, though inhibition of apoptosis was not complete in either case, suggesting that other signaling events may also be involved.
Ca2+ chelators inhibit apoptosis induced by hypercross-linking CD20. 106 Ramos cells/mL were incubated with 3 mmol/L EGTA or 3 mmol/L EGTA plus 5 μmol/L Bapta AM for 30 minutes at 37°C before incubation with 10 μg/mL antibodies for 24 hours in the presence or absence of GAM cross-linker (50 μg/mL). Cell nuclei were stained with PI and analyzed by flow cytometry. Hypodiploid DNA peaks corresponding to apoptotic nuclei were quantified, and results presented as antibody-mediated apoptosis minus spontaneous apoptosis. Cells that were not pretreated with either EGTA or Bapta were used as controls. Data are representative of four experiments.
Ca2+ chelators inhibit apoptosis induced by hypercross-linking CD20. 106 Ramos cells/mL were incubated with 3 mmol/L EGTA or 3 mmol/L EGTA plus 5 μmol/L Bapta AM for 30 minutes at 37°C before incubation with 10 μg/mL antibodies for 24 hours in the presence or absence of GAM cross-linker (50 μg/mL). Cell nuclei were stained with PI and analyzed by flow cytometry. Hypodiploid DNA peaks corresponding to apoptotic nuclei were quantified, and results presented as antibody-mediated apoptosis minus spontaneous apoptosis. Cells that were not pretreated with either EGTA or Bapta were used as controls. Data are representative of four experiments.
DISCUSSION
CD20 was the first human B-cell differentiation antigen to be identified by an MoAb after the identification of surface immunoglobulin. Accumulating evidence indicates that this molecule is an initiating component of signal transduction pathways that regulate B-cell proliferation and differentiation and that ligation of CD20 by MoAbs can inhibit B-cell proliferation and differentiation.6 Our results confirm and extend these findings by showing clearly and reproducibly that hypercross-linking of CD20 with anti-CD20 MoAbs plus a secondary GAM antibody not only inhibits B lymphoma cell growth, but also induces cell death by apoptosis. More importantly, our data show that similar hypercross-linking of CD20 can be achieved by incubation of anti-CD20 MoAb-coated lymphoma cells with FcR-expressing accessory cells and that under these conditions, apoptosis occurs in the absence of GAM. These findings suggest that anti-CD20–mediated apoptosis may occur in vivo in lymphoma patients treated with anti-CD20 antibodies and that this mechanism may be partially responsible for the clinical remissions observed in these clinical trials.13,15,16,18 Two preliminary abstracts published by other groups suggests that our findings regarding apoptosis are reproducible in other laboratories using other anti-CD20 antibodies.25 27
The intracellular signaling events that culminate in apoptosis are not fully understood and appear to vary for different stimuli and for different cell lines.45 Our experiments and those of others7 have shown that cross-linking of CD20 with anti-CD20 MoAbs induces phosphorylation of tyrosine and serine/threonine kinases and their substrates including PLCγ1, but it is insufficient to mobilize intracellular calcium stores unless hypercross-linking of anti-CD20 with GAM antibody is performed. Because our studies also showed that apoptosis can be induced in Ramos cells incubated with anti-CD20 MoAbs + GAM but not by soluble anti-CD20 MoAbs alone, we hypothesized that calcium fluxes were involved in the intracellular signaling pathways leading to apoptosis. Our results confirm this supposition and indicate that anti-CD20–mediated apoptosis can be effectively inhibited by the Ca2+chelators EGTA and Bapta AM, which prevent the increases in [Ca2+]i normally induced by hypercross-linking CD20 with anti-CD20 MoAbs + GAM. These observations are also consistent with recent reports that anti-sIgM–mediated apoptosis in group I MUTU cells40 and CD3/T-cell receptor complex–mediated apoptosis in immature thymocytes are mediated by increases in intracellular calcium concentrations.46 47
There are at least two potential mechanisms for the induction of changes in cytosolic calcium that may mediate the apoptotic effects observed using anti-CD20 MoAbs. The first explanation derives from studies by Tedder et al suggesting that the CD20 molecule, with its four membrane spanning regions, functions as a plasma membrane calcium channel when multimeric complexes of CD20 are formed by cross-linking.6,48 It has been convincingly shown that some anti-CD20 MoAbs (eg, B1) inhibit B-cell progression from the G1 phase of the cell cycle into the S/G2+M stages following mitogen stimulation,10 11 and it has been proposed that this inhibition of cell cycle progression derives from the ability of anti-CD20 MoAbs to sustain conductive calcium channel activity, because cell cycle progression is dependent on low levels of cytosolic calcium. The current studies suggest that sustained calcium channel conductance induced by CD20 hypercross-linking may also lead to apoptosis, at least under the conditions used in these experiments.
Increased levels of cytosolic calcium may also be derived from the release of calcium stores from the endoplasmic reticulum through a protein tyrosine kinase (PTK) dependent signaling pathway initiated by anti-CD20 MoAb binding, a phenomenon that is accentuated when CD20 is hypercross-linked with GAM.7 Previous reports (as well as our unpublished data) have shown that binding of anti-CD20 MoAbs increases the phosphorylation of CD20-associated serine and tyrosine kinases and their substrates, including PLCγ. Differential effects of phosphorylation of PLCγ have been reported, which appear to be dependent on the extent of CD20 cross-linking.7 When CD20 was bound by anti-CD20 MoAbs alone, phosphorylation of PLCγ occurred, but increases in [Ca2+]i required further cross-linking of CD20 with a secondary GAM antibody. These observations imply that the signals derived from binding of anti-CD20 MoAbs alone may differ either qualitatively or quantitatively from signals emanating from CD20 hyper-crosslinking with anti-CD20 MoAbs + GAM. This differential signaling may explain apparently contradictory observations with the 1F5 anti-CD20 MoAb, which stimulates cell cycle progression of normal, resting B cells9 but suppresses growth of malignant B-cell lymphomas in vitro and in vivo (J.A. Ledbetter and C. Siegall, unpublished observations, September 1997) and causes apoptosis when further cross-linked by a secondary antibody.
We used the protein kinases inhibitors, herbimycin A and genistein, in preliminary experiments to test whether protein tyrosine kinases are involved in the signaling pathways leading to apoptosis induced by CD20 hypercross-linking. These inhibitors consistently failed to inhibit the apoptotic effects of anti-CD20 + GAM, and in fact augmented the process. PTK inhibitors (Herbimycin A and tyrphostin AG135) have also been reported by others to have no effect on apoptosis of Burkitt's lymphoma cell lines induced by other stimuli.40 At least two explanations can be proposed to explain these findings. First, it is possible that PTKs are not actively involved in the signaling pathways involved in CD20-mediated apoptosis, explaining the failure of kinase inhibitors to abrogate the phenomenon. As mentioned above, it is possible that the CD20 molecule functions as a calcium channel, thereby autonomously generating the calcium flux that terminates in apoptosis after anti-CD20 hypercross-linking. Alternatively, it is conceivable that CD20 hypercross-linking activates several different signaling pathways, some of which may be mediated by protein kinases with proapoptotic downstream effects and some of which generate antiapoptotic signals. The net effect of CD20 ligation may depend on a complex summation or interplay of a multitude of positive and negative signals.49 Because the inhibitors genistein and herbimycin A are nonspecific and inhibit different protein kinases to variable degrees,50 it is plausible that these inhibitors abrogated the messages transduced by the putative antiapoptotic kinases to a greater degree than those from the postulated proapoptotic kinases. The net result of such preferential inhibition would be an augmentation of anti-CD20–mediated apoptosis by the nonspecific kinase inhibitors, as observed in our experiments (Fig 6). A similar hypothesis has been proposed by others for anti-sIgM–mediated apoptosis in B cells49 and to describe the effects of protein kinase inhibitors on positive and negative signaling pathways associated with other surface molecules (eg, CD19, CD2233). Further experiments with more selective protein kinase inhibitors or antisense oligonucleotides will be necessary to clarify the roles of protein kinases in CD20-mediated apoptosis.
Regardless of the mechanism involved, the elevated intracellular calcium levels induced by CD20 hypercross-linking appear to inhibit cell cycle progression and trigger apoptosis. Soluble anti-CD20 MoAbs appear to inhibit proliferation without induction of apoptosis, because the minuscule cytosolic calcium fluxes induced by soluble anti-CD20 MoAbs alone are insufficient to trigger the latter phenomenon.7 In contrast, extensive cross-linking of CD20 with anti-CD20 MoAbs plus GAM (or FcγR-bearing accessory cells) appears to surpass the cytosolic calcium threshold required for induction of apoptosis in a fashion analogous to that shown for anti-sIgM–mediated apoptosis.40
Several groups have investigated the differential effects of simple ligation of cell surface molecules by MoAbs (and other ligands) and contrasted these effects with those observed after extensive cross-linking of the same surface molecules.33,51-54Studies with surface Ig have suggested that soluble antibodies promote B-cell tolerance through clonal anergy, whereas membrane bound antigens, which confer extensive cross-linking of ligand-receptor pairs, readily induce B-cell death by apoptosis.51 Fas-mediated induction of apoptosis is also dependent on efficient cross-linking of the Fas molecule on the cell surface.52 Dhein et al reported that F(ab′)2fragments of anti-Fas MoAbs and some isotypes of anti-Fas MoAbs (eg, IgG2b) were unable to induce apoptosis of target cells, but that the apoptotic capabilities of these inactive or less active antibody preparations could be fully reconstituted by hypercross-linking with protein A, anti-mouse Ig, or anti-mouse Ig F(ab′)2.52 Complementary findings from another group53 indicate that (1) two different polyclonal anti-sIgM antibodies administered to severe combined immunodeficiency (SCID) mice with BCL1 tumors induce dormancy more effectively than two MoAbs directed against different sIgM epitopes, (2) a mixture of three anti-sIgM MoAbs mimic the effect of polyclonal antibodies and (3) hypercross-linking of sIgM with a single MoAb followed by a secondary anti-Ig antibody increases apoptosis markedly. By analogy with these studies of sIgM cross-linking, we hypothesize that extensive cross-linking of CD20 with anti-CD20 MoAbs plus GAM (or FcγR-expressing accessory cells) generates stronger and/or more sustained signals than simple ligation of CD20, and that the more extensive cross-linking of CD20 results in increased cytosolic calcium levels and rapid induction of irreversible cell death by apoptosis.
ACKNOWLEDGMENT
We gratefully acknowledge the secretarial assistance of Patricia Kury Adam.
Supported by Grant No. R01 CA55596 from the National Institutes of Health, Bethesda, MD.
Address reprint requests to Daming Shan, MD, Department of Biological Structure, University of Washington, PO Box 357420, Seattle, WA 98195.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 1. Inhibitory effect of anti-CD20 MoAb 1F5 on Ramos cells measured by tritiated thymidine incorporation. 104 cells in 100 μL per well were incubated continuously with 0.0001, 0.001, 0.01, 0.1, 1, 10, 100 μg/mL 1F5 in medium containing FBS (A) or medium containing inactivated FBS (B) at 37°C for 24 hours, followed by addition of 0.5 μCi [3H]-thymidine per well and incubating for another 18 hours. Cells incubated in medium without 1F5 were used as control. Data are representative of three concordant experiments. Similar results were obtained with the B1 anti-CD20 antibody (not shown).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/5/10.1182_blood.v91.5.1644/3/m_blod4052101a.jpeg?Expires=1766216610&Signature=cPDXRAd~Z88fYJurAomK5Y8ldJWEV2Y7~Gka68c6KGrcGNbhvFv-3oQ6r62-HhrwHWnlnwge3ho5a6gbHPFFOISFCUy~dofLK2XkCfxCaUZDu1o8d6fZZk6lf4c-WqN4bXObW1PinWBeb3198DPnwbHrIVKfGu2WzMrdDM9MPpIEySzeF0Qv0pFqMxFO-sNbxwNGpq33vRNRryWL1xksIQJujPqlM8oQiOFLhl~jdzO1L3yy2ZHMtEC-sBHlOOq0GYAFZsRRxMdwBofercLqH4c650D~5DKlKYhwSQo5IFJKrbR9ZJYLtLtMbDLI2pjFzz3asBwES64qqQ-gG6hJYA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Inhibitory effect of anti-CD20 MoAb 1F5 on Ramos cells measured by tritiated thymidine incorporation. 104 cells in 100 μL per well were incubated continuously with 0.0001, 0.001, 0.01, 0.1, 1, 10, 100 μg/mL 1F5 in medium containing FBS (A) or medium containing inactivated FBS (B) at 37°C for 24 hours, followed by addition of 0.5 μCi [3H]-thymidine per well and incubating for another 18 hours. Cells incubated in medium without 1F5 were used as control. Data are representative of three concordant experiments. Similar results were obtained with the B1 anti-CD20 antibody (not shown).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/5/10.1182_blood.v91.5.1644/3/m_blod4052101b.jpeg?Expires=1766216610&Signature=E~xu5mVxNePSK0rIEGQ9mha1i2~V6XBgQLvg1ZUP-BQgB1DQcKNWapw~skw6jA~B2gKJ2u4MTf5aUzbi64AyCnxQZN1pcWY8CaMX24l8y6vy7Hi9WMOOwce4Y-dvgL3hC09L0t76mdNQ~z3m6nzE44wdyk9cMHj1~3e5-fJjJ4gQwTqhOHpoHWFGsLwi6XqvVHIUd4h0I2nsupd-pTi3DKOFV-leoMwg5r7P9XLkXIjZ1rD7Sxba~qNLVsRe6lo9abjkcCwrLfkpjsIu0DJlPJgnZwPFmEfs81joA0qPHzh3I~YfJ55tGOj7mCmVNPbkDpMpYCsh8e22Q2XhVYpFlA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)