Abstract
Using a polyclonal antiserum against canine CD34, we previously found that CD34 is expressed on canine bone marrow progenitor cells in a manner analogous to that found in humans. To further characterize CD34+ cells and to facilitate preclinical canine stem cell transplant studies, monoclonal antibodies (MoAbs) were raised to CD34. A panel of 10 MoAbs was generated that reacted with recombinant CD34 and with CD34+ cell lines and failed to react with CD34− cell lines. Binding properties of five purified MoAbs were determined by BIAcore analysis and flow cytometric staining, and several MoAbs showed high affinity for CD34. Two antibodies, 1H6 and 2E9, were further characterized, and in flow cytometry studies typically 1% to 3% of stained bone marrow cells were CD34+. Purified CD34+ bone marrow cells were 1.8- to 55-fold enriched for colony-forming unit–granulocyte-macrophage and for long-term culture initiating cells as compared with bone marrow mononuclear cells, whereas CD34− cells were depleted of progenitors. Three autologous transplants were performed with CD34+ cell fractions enriched by immunomagnetic separation. After marrow ablative total body irradiation (920 cGy), prompt hematopoietic recovery was seen with transplanted cell doses of ≤1.1 × 107 /kg that were 29% to 70% CD34+. Engraftment kinetics were similar to those of dogs previously transplanted with approximately 10- to 100-fold more unmodified autologous marrow cells. This suggests that CD34+ is a marker not only of canine bone marrow progenitors but also for cells with radioprotective or marrow repopulating function in vivo. MoAbs to CD34 will be valuable for future studies of canine hematopoiesis and preclinical studies concerning stem cell transplantation, gene therapy, and ex vivo progenitor cell expansion.
THE EXPRESSION OF CD34 on hematopoietic cells is developmentally regulated in hematopoiesis such that its expression is lost beyond the committed progenitor stage.1-3 This pattern of expression allowed for the use of monoclonal antibodies (MoAbs) to human CD34 to selectively isolate for the purpose of transplantation small subpopulations from bone marrow (BM) that are highly enriched for progenitors.4,5 More recently, peripheral blood progenitor cells collected after mobilization with growth factors and/or chemotherapy have been enriched from leukapheresis products by CD34 selection techniques for use in transplantation.6-8 CD34 selection facilitates tumor cell removal from autografts7,9 and T-cell depletion of allografts to prevent graft-versus-host disease (GVHD).8,10Separations of CD34+ cells for transplantation have been accomplished using immunoadsorption columns,5,9immunomagnetic beads,11 and high-speed cell sorting.12
Canine models of autologous and allogeneic stem cell transplantation have been important for studies concerning GVHD, recombinant hematopoietic growth factors, and gene therapy. These models have proved predictive of clinical findings in humans.13,14Therefore, a marker for identifying and isolating canine hematopoietic progenitors that would facilitate graft manipulation may allow for further insights to be gained through canine studies. Recently, we cloned both the cDNA and gene for the canine homologue of CD34.15 A recombinant CD34 murine Ig fusion molecule (CD34-Ig) was used as an immunogen to generate an affinity-purified polyclonal antiserum (RPαCD34) that reacted with approximately 1% of BM cells, a cell population that was highly enriched for colony-forming unit–granulocyte-macrophage (CFU-GM). However, use of a polyclonal antiserum was suboptimal for precise fluorescence-activated cell sorting (FACS) studies and for large-scale cell separations.
This report describes the production of MoAbs that react with canine CD34, which was necessary to better characterize CD34+cells by in vitro and in vivo functional studies and to develop technology for transplantation of enriched progenitor cell populations. Ten hybridomas were cloned that produced MoAb against CD34. Several high-affinity MoAbs specific for CD34 were identified, and two, 1H6 and 2E9, were characterized in studies to isolate cell populations enriched for canine marrow progenitors. Results of initial autologous transplant studies, performed using cell populations enriched for CD34+ cells with MoAb 1H6, showed that these cell populations provided radioprotection after a myeloablative dose of total body irradiation (TBI). These studies have confirmed and extended previous observations regarding the use of CD34 as a marker for canine hematopoietic progenitors.
MATERIALS AND METHODS
Cell lines and cell culture.
Canine myelomonocytic leukemia cell lines ML1,16 ML2, ML3, 1390 (CD8+ leukemia), and CLGL (large granular lymphocyte) were cultured as previously described.15 Jugular vein endothelial cells from normal dogs were purchased from Endotech (Indianapolis, IN) and cultured according to the manufacturer's instructions.
Production of recombinant CD34.
A CD34-murine Ig fusion protein, consisting of the extracellular domain of CD34 fused to the hinge, CH2, and CH3 of a murine IgG-2a Ig molecule (CD34-Ig), was produced by transient expression in COS cells and purified as previously described.17
Anti-CD34 enzyme-linked immunosorbent assay (ELISA).
The anti-CD34 ELISA was based on a previously described protocol18 with the following modifications. Immunlon 2 (Dynex Technologies, Chantilly, VA) flat-bottom plates were coated with 3 μg/mL CD34-Ig diluted in 0.05 mol/L bicarbonate binding buffer, pH 9.6. Bound antibody was detected with a 1:8,000 dilution of horseradish peroxidase (HRP)-conjugated antimouse IgG-1 antibody (Southern Biotechnology, Birmingham, AL). Bound HRP was detected with ATBS substrate (Kirkgaard and Perry, Gaithersburg, MD)
Immunization of mice with CD34-Ig and cell lines and MoAb production.
Six-month-old Balb/c mice (obtained from Taconic, Germantown, NY), received three injections of either CD34-Ig or cells in varying combinations and sequences. ML3 or 1390 cells (100 μL at 108/mL) were injected either subcutaneously or intraperitoneally in phosphate-buffered saline (PBS) or intraperitoneally in adjuvant. When using adjuvant, protein (300 μL at 500 mg/mL in PBS) was mixed with 150 μL Montanide ISA50 and 150 mL RIBI adjuvant (Ribi Immunochem Research Inc, Hamilton, MT). CD34-Ig–specific antibody titers of mouse sera were determined by ELISA. Mice were selected for fusion based on antibody titers to CD34-Ig and a prefusion boost was administered with CD34-Ig.
MoAbs to CD34 were produced as previously described.19Briefly, spleen cells from the immunized Balb/c mice were fused with NS-1/FOX-NY myeloma cells. Viable heterokaryons were selected in RPMI 1640 medium supplemented with adenine/aminopterin/thymidine (AAT). Cultures secreting antibody specific for CD34 were identified by ELISA using an HRP-conjugated goat antibody (Zymed, San Francisco, CA) specific for mouse antibodies with an IgG1 isotype and by reactivity with ML3 and 1390 cell lines using a goat antimouse IgG fluorescein isothiocyanate (FITC) second-stage antibody. Monoclonal hybridoma cell lines were produced via two rounds of cloning via limiting dilution. Large-scale MoAb production was undertaken by ascites production using stable hybridomas. MoAb was purified from ascites using protein G columns (Pierce, Rockford, IL) according to the manufacturer's instructions. Biotinylation of purified MoAb was performed using D-biotinyl-e-aminocaproic acid N-hydroxysuccinimide ester (Boehringer Mannheim, Indianapolis, IN) according to the manufacturer's instructions. Isotypes of MoAbs were determined by using Isostrip Mouse Monoclonal Antibody Isotyping Kit (Boehringer Mannheim) as per the manufacturer's instructions.
Western blotting.
Recombinant proteins (300 ng) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using an 8% tris-glycine gel (Novex, San Diego, CA) under reducing conditions and transferred to polyvinylidene fluoride (PVDF) membranes. Membranes were blocked with 15 mg/mL nonfat dry milk in PBS, blotted with 1 mg/mL MoAb-biotin conjugate, washed in 0.5× PBS/0.5% Tween-70, and then blotted with 1:5,000 streptavidin-peroxidase in the blocking solution.
Leukemia cell lines ML1, ML3, and 1390 (2.5 × 105cells) were harvested and washed twice in PBS, and proteins were prepared by lysis in 1% NP40 in PBS followed by removal of nuclei by centrifugation at 10,000g. Proteins (25 μg) were separated by SDS-PAGE in a 10% polyacrylamide gel under reducing conditions and proteins were transferred to nitrocellulose membranes. Membranes were blocked with 5% nonfat milk in PBS and then incubated with either RPαCD34 at 500 ng/mL or MoAb (2E9 or 1H6) at 2 μg/mL. After washing, blots were incubated in blocking solution containing alkaline phosphatase-conjugated second stage goat antirabbit or goat antimouse antibodies at 1:500 dilution (Amersham, Arlington Heights, IL). Western blots were developed with ECL detection reagents (Amersham).
MoAb affinity determination.
The binding of anti-CD34 MoAbs to the CD34-Ig fusion protein was determined by BIAcore analysis. All experiments were run on BIAcore 1000 or 2000 instruments20 (Pharmacia Biosensor, Uppsala, Sweden) at 25°C, using PBS, pH 7.4 (GIBCO BRL Products, Life Technologies, Gaithersburg, MD) containing 0.005% surfactant P20 (Pharmacia Biosensor) as the running buffer. The carboxymethylated dextran matrix of research grade sensor chip CM5 (Pharmacia Biosensor) was modified using the Amine Coupling Kit (Pharmacia Biosensor) as follows.21 Equal volumes of 0.10 mol/L N-hydroxysuccinimide and 0.40 mol/L N-ethyl-N′-(3-dimethylaminopropyl) carbodimide were mixed and injected to activate the surface for 7 minutes. The ligand, a solution of 10 μg/mL of CD34-Ig in 10 mmol/L sodium formate, pH 4.0, was injected for 3 minutes. Remaining active sites were reacted by the injection of 1 mol/L ethanolamine for 5 minutes. Immobilization of approximately 900 RU of CD34-Ig was achieved. Each analyte was diluted in the running buffer to give a series of concentrations between 10 and 250 nmol/L. Appropriate volumes of these solutions were injected with a flow rate of 10 to 50 μL/min. After the injection, flow of running buffer alone was established to allow observation of the dissociation of bound protein. Association and dissociation rates of each analyte were determined by curve fitting using BIAevaluation 2.1 (Pharmacia Biosensor).22 The affinity was calculated by dividing the dissociation rate by the association rate.
Flow cytometry.
Cells were incubated with purified MoAb at 5 to 10 μg/mL or with supernatants from overgrown hybridoma cultures. MoAbs 31A (murine IgG-1, antimurine Thy-1)23 and S5 (anticanine CD44)24 were used as negative and positive controls, respectively. Second-stage reagents were FITC-conjugated or phycoerythrin (PE)-conjugated goat-antimouse polyclonal antibody, streptavidin-FITC (all Caltag, San Francisco, CA), and streptavidin PE (Southern Biotechnology). In two-color studies, RPαCD3415was used at 5 mg/mL with a PE-conjugated goat antirabbit antibody (Southern Biotechnology) as a second-stage reagent. Cell lines and cultured endothelial cells were incubated with MoAb for 20 minutes at 4°C, washed twice with PBS, incubated with FITC-conjugated second-stage antibodies for 20 minutes at 4°C, and then washed twice with PBS. Ficoll-Hypaque–separated BM mononuclear cells (BMMC), unfractionated hemolysed BM, or peripheral blood mononuclear cells at 2 to 5 × 106/mL were stained as previously described.15 In blocking experiments to demonstrate specificity of MoAbs for CD34, cell lines were incubated with MoAb (5 to 10 μg/mL), CD34-Ig (100 μg/mL), or a combination of the two. Cells were then washed with PBS and incubated with FITC-conjugated goat antimouse IgG antibody. Cells were washed and resuspended in PBS/2% fetal calf serum (FCS) for analysis. To determine whether a non–cross-blocking pair of MoAbs could be identified, ML3 cells were incubated with saturating concentrations of MoAb at 4°C, washed in PBS/FCS, and then stained with biotinylated 1H6 at 10 μg/mL. After further washing, cells were incubated with avidin-PE or avidin-FITC. Cells were washed and resuspended in PBS/FCS for FACS analysis. Flow cytometry was performed on a FACScan (Becton Dickinson, San Jose, CA) or FACStar (Becton Dickinson), and the list mode data were analyzed using Winlist (Verity Software House Inc, Topsham, ME) or Cellquest software (Becton Dickinson).
CFU-GM assays.
BMMC (5 × 107/mL) were stained with MoAb 2E9 or 1H6 at 5 to 10 μg/mL as described above, resuspended in PBS/2% horse serum, and sorted using a FACStar. Sorted CD34+ and CD34− cell fractions and control BMMC were centrifuged, resuspended in culture media containing recombinant canine (rc)–granulocyte colony-stimulating factor (G-CSF), rc–stem cell factor (rc-SCF), and rc–granulocyte-macrophage colony-stimulating factor (rc–GM-CSF) each at 100 ng/mL, and various numbers of cells per plate were assayed for granulocyte-macrophage progenitor cells (CFU-GM), as previously described.25
Long-term culture initiating cell (LTC-IC) assays.
Canine LTC-IC assays were performed as modifications of previously described procedures for human studies.26-28 Briefly, stromal layers were established in T-25 flasks and fed with Iscove's medium supplemented with 20% horse serum, 2% L-glutamine, and 10−7 mol/L hydrocortisone sodium succinate. Stromal cells were cultured at 37°C in 5% CO2 until reaching confluence. The adherent layers were trypsinized, washed, irradiated (1,800 cGy using a 137Cs source), and transferred to 24-well plates (3 × 105/plate). Two days later, the wells were seeded in triplicate with sorted CD34+ cells, CD34− cells, and BMMC at 1 × 104, 2 × 104, or 1 × 105 cells per well. Cells were cultured at 33°C in 5% CO2 and fed weekly renewing half of the media. After 5 weeks, the adherent layers were trypsinized. Pooled cells from the triplicates were washed and 5 × 104 cells plated into duplicate CFU-GM assays performed as described above.
Immunomagnetic separation and autologous transplantation of canine BM.
Beagle dogs were raised at the FHCRC and were observed for disease for at least 60 days before entering the study. Research was performed according to the principles outlined in the guide for Laboratory Animal Facilities and Care prepared by the National Academy of Sciences, National Research Council. The research protocol was approved by the Institutional Review Animal Care and Use Committee of the Fred Hutchinson Cancer Research Center. The kennels were certified by the American Association for Accreditation of Laboratory Animal Care.
BM was aspirated from the humeri and femora of anesthetized animals. Buffy coat was isolated by centrifugation at 600g for 15 minutes, washed in PBS/2% horse serum, and treated with hemolytic buffer to remove red blood cells. Cells were then incubated at 1 × 108 cells/mL with biotinylated MoAb 1H6 at 40 μg/mL for 30 minutes at 4°C. Cells were washed in PBS/2% horse serum followed by incubation with immunomagnetic streptavidin-coated microbeads (Miltenyi Biotech, Auburn, CA), and separation was performed using a miniMACS or VarioMACS (Miltenyi Biotech) separation device.
Dogs received a single dose of 920 cGy TBI delivered at 7 cGy per minute from two opposing 60Co sources.29Separated cells were resuspended in 5 to 10 mL of PBS and infused intravenously over 1 to 2 minutes within 4 hours of irradiation. The day of marrow infusion was designated as day 0. Supportive care included parenteral fluids, electrolytes, and irradiated blood transfusions from day −5 until recovery of the white blood cell count (WBC) to greater than 1,000/μL, and prophylactic broad spectrum systemic antibiotics from day 0 until the WBC recovered to greater than 1,000/μL.30
RESULTS
Production and evaluation of MoAbs to CD34.
Initial attempts to generate anti-CD34 MoAbs using CD34-Ig alone as an immunogen failed, and there was a strong and unexpected production of antibodies to the murine Ig portion of the fusion protein. Because the ML2 leukemia cell line (from which the CD34 cDNA was cloned) was found to express variable, and at times low level, surface CD34, and because we were concerned that CD34 epitopes on CD34+ endothelial cells may differ from those on hematopoietic cells,31additional canine leukemia cell lines were screened for CD34 expression with RPαCD34. Two cell lines, ML3 and 1390, were identified that consistently expressed high levels of cell surface CD34. Six mice were immunized using ML3, 1390, and CD34-Ig in various combinations, and sera were screened for antibody titers to CD34-Ig by ELISA. To test whether antibodies were specific to the extracellular domain of CD34, serum was also tested against a control fusion protein that has an identical Ig amino acid sequence to CD34-Ig. This showed that the immunization schedule was important for generating high titer responses against the extracellular domain of CD34. Antibody titers specific to CD34 were highest when CD34-Ig was used in the first or second immunization of a schedule that included immunization with ML3 or 1390 (data not shown). Immunizations with ML3 and 1390 cells alone did not produce measurable responses against CD34-Ig.
The spleen from one of several mice with high serum titers of CD34-specific antibody was chosen for fusion. This animal received a primary immunization with ML3 cells, two subsequent boosts with CD34-Ig and then with ML3, and a final immunization with CD34-Ig immediately before fusion. CD34-Ig was used in ELISA for initial screening of hybridoma culture supernatants. Because CD34-Ig contains a murine IgG-2a sequence, this was accomplished by using an IgG-1–specific antibody to detect MoAb bound to CD34-Ig. ELISA screening detected 32 hybridomas producing MoAb reactive with CD34-Ig. Twenty-one of these had MoAb reactive with at least one of the screening cell lines ML3 and 1390 cells when analyzed by FACS. Further screening for MoAbs of other isotypes, performed using both ML3 and 1390 cells using a general FITC antimouse detection step, did not detect additional MoAbs reactive with these cell lines. The positive hybridomas were cloned by limiting dilution, and the isotypes of these MoAbs were independently confirmed to be IgG-1.
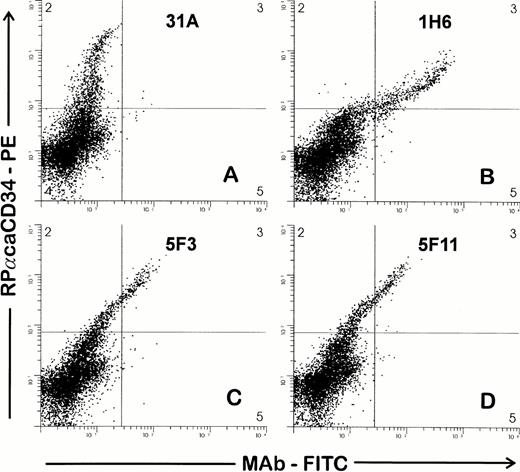
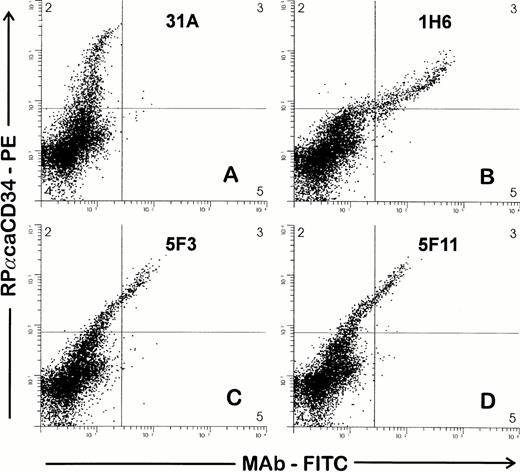
Supernatants from hybridomas were then screened by FACS against BMMC by both single- and two-color analysis in combination with RPαCD34. Nine of 21 supernatants were reactive with the same cell population (∼3% of BMMC) that was recognized by RPαCD34. Representative examples of staining from three hybridomas are shown in Fig 1 and show a high concordance between the cell populations recognized by the polyclonal antiserum and the three MoAbs. Further studies indicated that 5F11 contained two different anti-CD34 MoAbs and that these (5F11-3 and 5F11-6) were separated by additional limiting dilution cloning. Therefore, 10 hybridomas were cloned and MoAbs further tested in FACS studies against CD34+ and CD34− cell targets. Each of these MoAbs reacted with CD34+ cell targets (1390, ML3, ML2, and cultured endothelial cells) and did not react with CD34− cell lines.
Two-color staining to screen hybridomas for MoAb to CD34. Ficolle-separated BMMC were stained as described in the Materials and Methods. Cells were first incubated with hybridoma supernatants, washed, and then incubated with RPαCD34. Second-stage goat antirabbit PE and goat antimouse FITC antibodies were added simultaneously. Gates were drawn to exclude debris and to include cells with light scattering properties of lymphocytes and blasts. RPαCD34-PE staining is shown on the Y axis and staining of the MoAb-FITC on the X axis. MoAbs in (B), (C), and (D) give positive staining of the population of cells recognized by RPαCD34 as compared with 31A (control), which does not costain the CD34+ cells.
Two-color staining to screen hybridomas for MoAb to CD34. Ficolle-separated BMMC were stained as described in the Materials and Methods. Cells were first incubated with hybridoma supernatants, washed, and then incubated with RPαCD34. Second-stage goat antirabbit PE and goat antimouse FITC antibodies were added simultaneously. Gates were drawn to exclude debris and to include cells with light scattering properties of lymphocytes and blasts. RPαCD34-PE staining is shown on the Y axis and staining of the MoAb-FITC on the X axis. MoAbs in (B), (C), and (D) give positive staining of the population of cells recognized by RPαCD34 as compared with 31A (control), which does not costain the CD34+ cells.
Specificity of MoAbs for CD34.
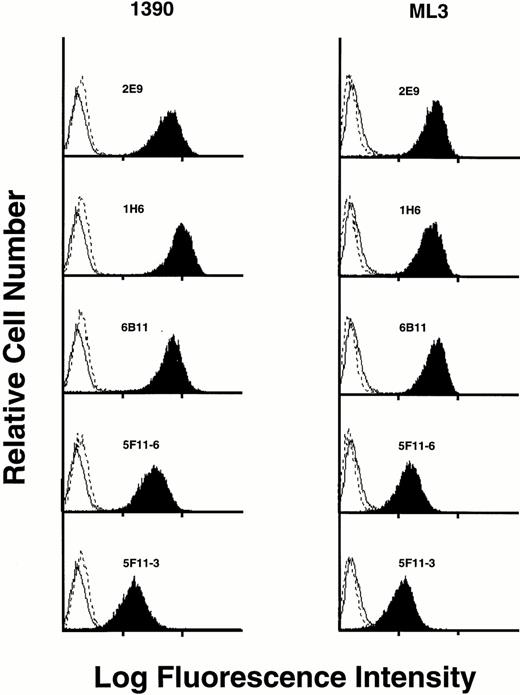
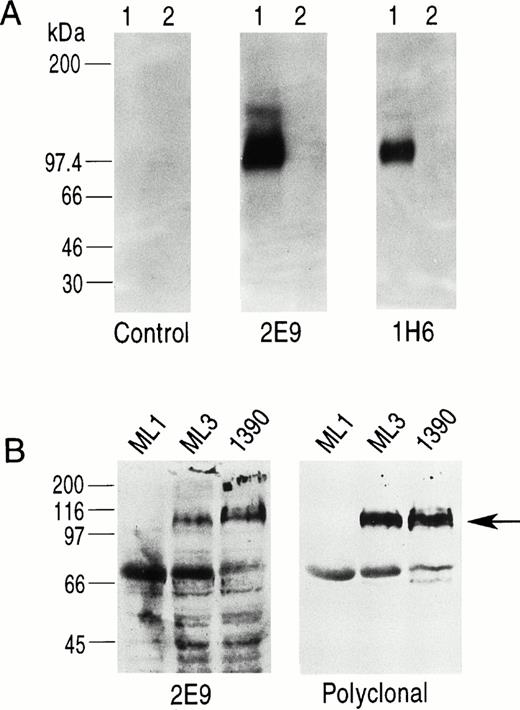
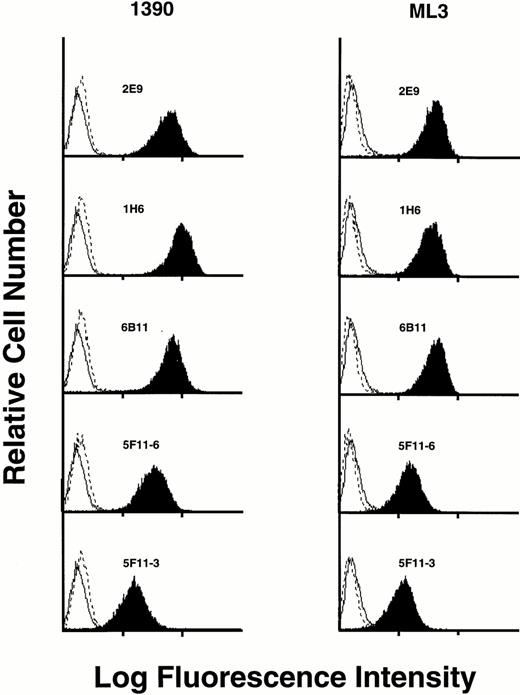
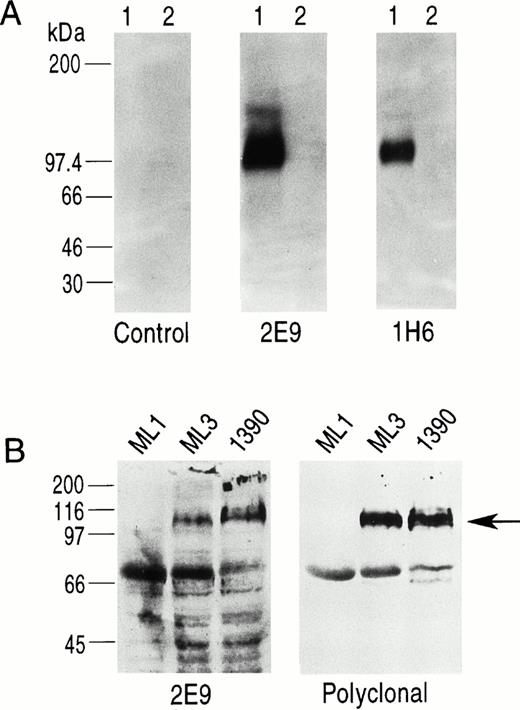
Five MoAbs (1H6, 2E9, 6B11, 5F11-3, and 5F11-6) were produced as ascites, purified, and further characterized. To show specificity for CD34, several studies were performed. First, the ML3 and 1390 leukemia cell lines were incubated with five MoAbs and the binding assessed by FACS analysis. All five antibodies stained a homogeneous population of cells from both cell lines when compared with control. Although 1390 cells were slightly brighter compared with ML3 cells, no other differences were found. Preincubation of the antibodies with excess CD34-Ig reduced binding to background, demonstrating specificity (Fig 2). This was true for both cell lines and all five MoAbs tested. Second, Western blotting studies against CD34-Ig confirmed reactivity of biotinylated MoAbs 1H6 and 2E9 to the extracellular domain of CD34. As shown in Fig 3A, both MoAbs identified a band of the expected molecular weight for CD34-Ig (∼90 kD). This band was absent in the lane containing control fusion protein. Third, in Western blotting studies of the ML1, ML3, and 1390 cell lines, MoAb 2E9 recognized a band of approximately 110 kD in the ML3 and 1390 cell lines (Fig 3B) that was absent in the CD34− cell line ML1. The results were consistent with the findings with RPαCD34 that detected a band of identical size in ML3 and 1390 cells but not ML1.
Staining of CD34+ cell lines 1390 and ML3 with various MoAbs (dark plots). In each case, staining is blocked by preincubation of MoAb with CD34-Ig (dashed line). Staining of an isotype-matched negative control is shown on each histogram as a solid line.
Staining of CD34+ cell lines 1390 and ML3 with various MoAbs (dark plots). In each case, staining is blocked by preincubation of MoAb with CD34-Ig (dashed line). Staining of an isotype-matched negative control is shown on each histogram as a solid line.
(A) Western blotting study using biotinylated MoAbs 2E9 and 1H6. Lane 1 in each panel is CD34-Ig and lane 2 in each panel is a control fusion protein (CTLA4-Ig) that has the same murine IgG2A sequence as CD34-Ig. 1H6 and 2E9 recognize CD34-Ig but not CTLA4-Ig, indicating specific binding of 2E9 and 1H6 to the extracellular domain of CD34 as expressed in the fusion construct. (B) Western blots of cell lines ML1 (CD34−), ML3, and 1390 (both CD34+) using 2E9 and RPαCD34. The band for CD34 (∼110 kD) is indicated by the arrow. The other bands present on these blots were also detected in control experiments using only the second-stage reagents.
(A) Western blotting study using biotinylated MoAbs 2E9 and 1H6. Lane 1 in each panel is CD34-Ig and lane 2 in each panel is a control fusion protein (CTLA4-Ig) that has the same murine IgG2A sequence as CD34-Ig. 1H6 and 2E9 recognize CD34-Ig but not CTLA4-Ig, indicating specific binding of 2E9 and 1H6 to the extracellular domain of CD34 as expressed in the fusion construct. (B) Western blots of cell lines ML1 (CD34−), ML3, and 1390 (both CD34+) using 2E9 and RPαCD34. The band for CD34 (∼110 kD) is indicated by the arrow. The other bands present on these blots were also detected in control experiments using only the second-stage reagents.
Binding properties of the MoAbs.
To assess properties of the MoAbs that could affect staining and separations of progenitors cells, functional affinities were determined. In FACS studies, ML3 and 1390 cell lines were both used to also assess any potential differences. Cells were incubated with increasing concentrations of antibodies and the binding determined. Using ML3 cells, 1H6 and 2E9 had similar high-affinity binding with 50% maximal binding at 1 mg/mL. Binding showed a single affinity and was saturated at approximately 3 μg/mL. Fifty percent maximal binding was at moderate concentrations for 6B11 (3 μg/mL) and 5F11-6 (7 μg/mL) and lowest for 5F11-3 (13 μg/mL). The relative binding properties of the five MoAbs were similar against 1390 but with slightly higher saturating concentrations. No significant differences were found in the maximal binding for each antibody at saturating concentration to suggest the antibodies were seeing any subset of CD34 molecules. The affinities (Kd) of the five antibodies for CD34-Ig were determined by BIAcore analysis. In these studies, 2E9 (<0.01 nmol/L) and 6B11 (<0.03 nmol/L) had the highest affinities (Table 1) with very low dissociation rates after binding antigen (<5 × 10−6 L/s), whereas 1H6 (0.42 nmol/L) had lower affinity due to a faster dissociation rate (7.6 × 10−4 L/s). Consistent with cell binding assays, 5F11-6 (0.8 nmol/L) and 5F11-3 (1 nmol/L) had the lowest affinities. In subsequent blocking studies (data not shown), all 10 MoAbs were able to block binding of biotinylated 1H6 to the ML3 cell line, suggesting that the MoAbs all recognized related epitopes. In conclusion, the antibodies bound specifically to related epitopes on CD34, had a range of affinities, and were unable to identify any epitope differences between the two cell lines tested.
Characterization of CD34+ cells.
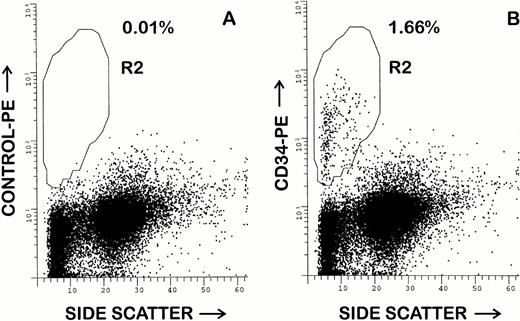
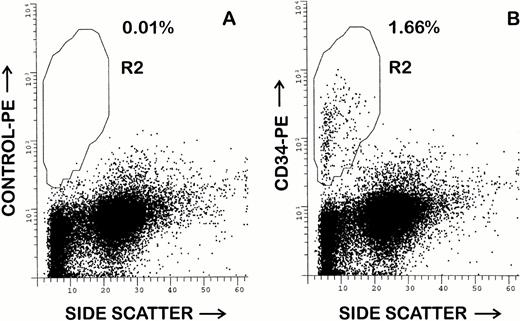
Although all five of the purified MoAbs were suitable for FACS studies, 1H6 and 2E9 were selected for additional FACS studies of canine BM and peripheral blood. Both antibodies appeared to recognize an identical cell population in canine BM. In 10 samples of unfractionated BM, a mean of 2.1% ± 1.0% (range, 0.7% to 3.7%) CD34+cells were detected amongst the leukocyte population. An example of this analysis is shown in Fig 4. The CD34+ cells were small to large in size with low to low-intermediate side scatter. Larger CD34+ cells gave consistently moderately bright staining, whereas smaller CD34+ cells showed more heterogeneous CD34 expression, including a tail of cells with dim positive staining. As compared with previous studies using RPαCD34, it was possible to more accurately delineate the CD34+ and CD34− cell populations due to less nonspecific staining of monocytes and granulocytes. In repeated (n > 5) studies of steady-state unfractionated peripheral blood leukocytes, less than 0.1% CD34+ cells were detected.
Flow cytometry analysis of canine BM using MoAb 1H6. Hemolyzed whole BM was stained as described in the Materials and Methods. Cells were incubated with propidium iodide before analysis and cells displayed have been electronically isolated from a gate that excludes debri and cells that retained propidium iodide. Staining of control antibody 31A is shown in (A). In (B), staining with the MoAb 2E9 indicates that 1.66% of BM cells are CD34+. Analysis using other projections indicated that CD34+ cells are small to large in size and have low to low-intermediate side scattering properties.
Flow cytometry analysis of canine BM using MoAb 1H6. Hemolyzed whole BM was stained as described in the Materials and Methods. Cells were incubated with propidium iodide before analysis and cells displayed have been electronically isolated from a gate that excludes debri and cells that retained propidium iodide. Staining of control antibody 31A is shown in (A). In (B), staining with the MoAb 2E9 indicates that 1.66% of BM cells are CD34+. Analysis using other projections indicated that CD34+ cells are small to large in size and have low to low-intermediate side scattering properties.
Progenitor assays.
To define in vitro functional characteristics of cells stained with either 2E9 or 1H6, CD34+ cells were isolated from BMMC by cell sorting and cultured in CFU-GM assays or LTC-IC assays. Reanalysis of sorted CD34+ and CD34− fractions showed that cell purities were routinely greater than 95% and usually 98% to 99%. In all experiments, the CD34+ population was enriched for CFU-GM as compared with control BMMC, whereas the CD34− cell fraction was depleted of CFU-GM. Results of four representative experiments are shown in Table 2. There was considerable variation in both the degree of CFU-GM enrichment (1.8- to 55-fold), progenitor depletion (2.3- to 150-fold), and progenitor yields with a constant input number of CD34+ cells (2,000 cells/plate) and BMMC or CD34− cells (both 25,000 cells/plate). In LTC-IC assays a similar pattern of progenitor enrichment within the CD34+ cell fraction was observed and CD34− cells were depleted of LTC-IC as compared with CD34+ cells and BMMC. Two representative examples of these experiments are shown in Table 3.
Autologous transplantation studies.
To determine in vivo functional characteristics of CD34+cells, transplantation studies were performed using enriched progenitor cell populations isolated by immunomagnetic positive selection of canine BM. Marrow cell doses and engraftment kinetics were compared with those of 16 historical control dogs32 that received unmodified fresh marrow autografts after conditioning with the same dose of TBI. Based on results of preliminary FACS studies and small-scale immunomagnetic separations using biotinylated 1H6, 2E9, and 6B11, we chose to use MoAb 1H6 for these separation studies. Separation conditions were determined after several small scale separations of canine BM in which various concentrations of beads (50 to 200 μL/1 × 108 cells) and 1H6 (5 to 60 μg/1 × 108 cells) were tested. CD34+ enriched cell fractions for autologous transplantation were isolated by immunomagnetic adsorption from canine BM after incubation with biotinylated 1H6 at 40 μg/mL with cells at 1 × 108cells/mL, followed by incubation with streptavidin-coated magnetic microbeads at 100 μL/ 1 × 108 cells with cells at 1 × 108 cells/mL. CFU-GM growth from CD34 selected cells did not appear to be inhibited by presence of the antibody-bead complex on the cells (data not shown) and therefore no attempt was made to remove the MoAb or beads from cells before transplant.
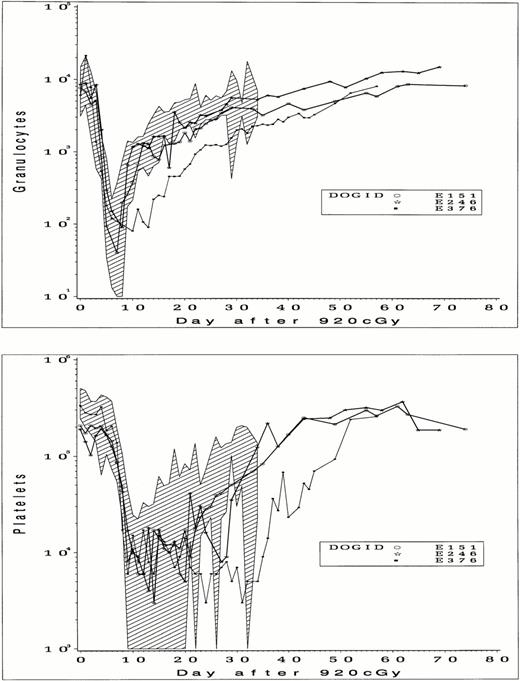
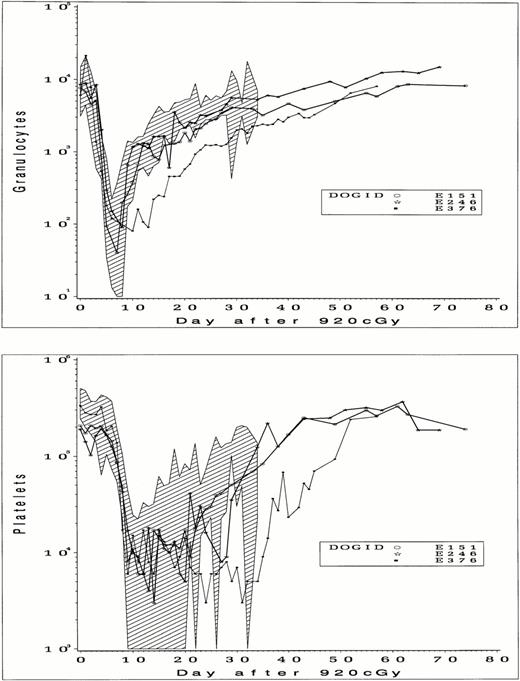
Details of the cell doses, cell separations, and transplant are shown in Table 4. For two dogs (E151 and E246), cell doses aspirated for processing were comparable to those of 16 control dogs (3.2 ± 2.0 × 108 total nucleated cells [TNC]/kg), whereas the cell dose from E376 (7.4 × 107 TNC/kg) was lower. Final cell doses used for transplantation after CD34 selection were 3.0 × 106/kg, 1.1 × 107/kg, and 1.7 × 106/kg, in each case less than 3% of the total of BM cells aspirated. Purities of CD34+ cell fractions obtained with a single pass through a magnetic column were 60%, 29%, and 70%, and doses of CD34+ cells transplanted were 1.62 × 106/kg, 3.4 × 106/kg, and 1.21 × 106/kg, respectively. In 2 dogs (E151 and E246), granulocyte recovery to an absolute neutrophil count of greater than 500/mL occurred on days 9 and 11 after TBI (Fig 5) and on day 20 in the third dog (E376). Platelet recovery to within normal levels was slower than granulocyte recovery but was complete in each case. The slowest granulocyte and platelet recovery was seen in E376, the dog that received the lowest per kilogram dose of total cells and CD34+ cells. When compared with control dogs, granulocyte and platelet recovery for E151 and E246 were within or close to the range previously observed, but recovery was slower than controls for E376, suggesting a possible effect of cell dose on recovery. Dogs were observed from 2 to 4 months after transplant, and blood counts remained stable without any evidence of secondary graft failure.
Peripheral blood granulocyte counts (upper panel) and platelet counts (lower panel) in dogs that received 920 cGy TBI followed by infusion of CD34-selected BM cells. The range of counts from 16 control dogs that received unmodified BM after 920 cGy are indicated by shading.
Peripheral blood granulocyte counts (upper panel) and platelet counts (lower panel) in dogs that received 920 cGy TBI followed by infusion of CD34-selected BM cells. The range of counts from 16 control dogs that received unmodified BM after 920 cGy are indicated by shading.
DISCUSSION
CD34 has previously been defined as a marker of hematopoietic progenitors. It is becoming increasingly important as technologies for large-scale separation of CD34+ cells have been applied to studies of clinical stem cell transplantation. Despite this, good animal models in which to refine studies of CD34+ cell fractions are limited. MoAbs to human CD34, some of which cross-react with CD34+ cells from nonhuman primate species,4 have been available since 1984.1 An MoAb to murine CD34 has only recently become available for such studies.33 However, this MoAb detected 4% to 17% CD34+ cells in murine BM,33 somewhat higher than the 1% to 5% CD34+ cells detected in human BM.1,3 Dogs have proved a valuable preclinical model for human stem cell transplantation.13 14 However, a limitation of the canine model for studies of hematopoiesis or stem cell transplantation has been the lack of a useful marker to identify and enrich hematopoietic progenitors. In this report, we have described the production of MoAbs to canine CD34 that can be used to enrich canine progenitor cell populations that appear functionally and phenotypically similar to human CD34+ cells. We have consistently detected approximately 1% to 3% CD34+ cells in canine BM, similar to the percentage of CD34+ cells present in human BM. These MoAbs should help in refining studies in several areas and facilitate the phenotypic and functional characterization of progenitors present in canine BM, peripheral blood, and cord blood. Application of these MoAbs to studies of stem cell transplantation, progenitor cell expansion, gene therapy, and culture of canine dendritic cells is anticipated.
Two reagents were developed that proved critical in making these MoAbs. The first was a fusion protein, CD34-Ig, made for immunization and screening strategies. This allowed production of the second, an affinity-purified polyclonal antiserum RPαCD34, which was used to identify cell lines expressing CD34, to screen antibody from hybridomas, and to carry out preliminary characterization of canine CD34.15 After failure initially to generate MoAbs to CD34, we found that to generate high levels of anti-CD34 specific response, it was important to use immunization schedules that included both CD34-Ig and CD34+ leukemia cell lines. CD34-specific responses were not detected after immunization of mice with CD34+ cell lines alone. Although 10 MoAbs were made, each MoAb was able to block the binding of 1H6 to CD34+ cell lines, indicating that they all recognized identical or overlapping epitopes. Therefore, more detailed characterization was limited to five MoAbs initially, and then later to two high-affinity MoAbs, 2E9 and 1H6, for evaluation of canine progenitors. Differences in marrow cell populations recognized by these two MoAbs were not identified by FACS analysis. However, in Western blotting studies, 2E9 was superior to 1H6, most likely because of its higher affinity for CD34. The reactivity of these MoAbs to CD34-Ig and native CD34 in Western blotting studies indicated that they were not dependent on the tertiary structure of CD34 for their binding.
Reactivity of these MoAbs to CD34 was confirmed by ELISA studies, in studies that used CD34-Ig to block binding to CD34+ cell lines, and by Western blotting of CD34-Ig and leukemia cell lines. The enrichment and depletion of progenitor cells in BM fractions isolated by cell sorting was consistent with binding of MoAb to CD34 on BM cells. Specificity of the MoAbs for CD34 was suggested by the lack of binding of MoAbs to CD34− cell lines as previously defined by the polyclonal antiserum and molecular studies,15 by blocking studies with CD34-Ig, and by restricted binding to a small population of BM cells that were not detected in peripheral blood. Overall, the properties suggested that we had produced several high-affinity MoAbs that should be suitable for specifically isolating highly enriched progenitor cell populations for further characterization and transplant studies. In addition, preliminary immunohistochemistry studies of frozen or paraffin sections from canine tissues showed that 1H6 and 2E9 stained vascular endothelial cells (data not shown), suggesting a possible use of these MoAbs for studying canine endothelial cells. The reactivity of the MoAb 1H6 and 2E9 in paraffin sections, frozen sections, and in Western blotting studies was a pattern described for class II epitopes but not class I or class III epitopes of human CD34.34
An important consideration for future studies to characterize canine progenitors is whether these MoAbs recognize all subsets of BM CD34+ cells. The presence of CD34 isoforms, most likely mediated through posttranslational protein modifications, has been shown in mice in which L-selectin could bind CD34 on vascular endothelium but not CD34 on murine hematopoietic cells.31Also, we have observed that cultured canine endothelial cells express a lower molecular weight form of CD34 than leukemic cell lines.15 Functionally significant different isoforms of CD34 that can be defined by flow cytometry within normal human BM have not been reported, although their presence is a possibility, as suggested by variable staining of CD34+ human leukemia samples with different MoAbs to human CD34.34 Therefore, the use of MoAbs that recognize all CD34+ BM cells may be an important prerequisite for accurately defining phenotypic and functional characteristics of CD34+ and CD34− progenitor cell populations. Two-color flow cytometry studies (Fig 1) showed that there was concordance between the cell population recognized by the polyclonal antiserum (recognizing multiple epitopes of CD34) and the MoAbs. Therefore, these MoAbs appeared to recognize an epitope(s) expressed on all BM CD34+ cells and should be suitable for defining CD34+ BM subsets.
The findings that purified CD34+ BM cells were enriched for hematopoietic progenitors and that CD34− cells were depleted of those progenitors suggested that a high proportion of canine progenitors express CD34. We found there was some variability in the degree of progenitor enrichment and growth found in the sorted CD34+ cell populations. Because conditions for culture of purified canine progenitors have not yet been well defined and may differ from those of unfractionated BMMC and because of the use of a limited number of canine specific cytokines in CFU-GM assays, further modification of the culture conditions and additional canine-specific cytokines may be needed to more accurately define the degree of progenitor enrichment in CD34+ cells as compared with BMMC.
Results of these initial transplant studies showed that it was unnecessary to remove the MoAb-bead complex from the CD34 selected cells before infusion to achieve engraftment. Complete and relatively prompt hematopoietic recovery was observed after treatment with a TBI dose greater than 2.5 times the LD99 dose for dogs29 despite infusion of cell doses comprising less than 3% of the starting marrow inoculum. Effects of marrow cell dose on engraftment were observed in a previous canine autologous transplant dose finding study35 that used cryopreserved unmodified BM. At cell doses between 1.0 × 107/kg and 1.0 × 108/kg, there was a correlation of cell dose with speed of granulocyte engraftment and death with engraftment failure. At total nucleated cell doses of less than 2.5 × 107/kg, engraftment failure was usually observed, and at cell doses between 2.5 and 5.0 × 107/kg, engraftment failure or delayed granulocyte recoveries were seen. In a second study36 at BMMC doses of greater than 1.0 × 107/kg and less than 1.5 × 107/kg, engraftment failure occurred in 7 of 7 dogs. As cell fractions transplanted in this study were substantially enriched for CD34+ cells, these results strongly suggest that the radioprotective functions of these grafts were provided by CD34+ cells rather than other cell populations. Results of these in vitro and in vivo studies support an hypothesis that CD34 is a highly conserved marker of hematopoietic progenitors. By analogy to human and mouse data, it is expected that canine CD34+cells will have long-term repopulating ability even though, as suggested by murine studies,37 there may be CD34− cells that can also provide this function. We plan to test this by allogeneic transplantation of highly purified CD34+ cell populations isolated by cell sorting and posttransplant studies to evaluate the presence of long-term multilineage donor hematopoiesis.
ACKNOWLEDGMENT
The authors thank Bonnie Larson and Harriet Childs for assistance in preparation of the manuscript and Reggie Castro, Mario Lioubin, and Gary Schoch for technical assistance. We are grateful to Doug Jones, to the technicians of the canine laboratory, and to other members of the canine transplant team for their technical assistance. We are grateful to Peter Moore (University of California, Davis, CA) for providing canine leukemia cell lines for these studies. Canine recombinant cytokines were generously supplied by Amgen Corp (Thousand Oaks, CA).
Supported in part by Grants No. HL03701, DK42716, HL36444, HL54881, CA47748, CA31787, AI37747, and AI85003 from the National Institutes of Health, Department of Health and Human Services (Bethesda, MD). Support was also received from a prize awarded by the Josef Steiner Krebsstiftung, Bern, Switzerland. L.K.-B. was a postdoctoral fellow from the Department of Experimental Biology and Medicine, Rudjer Boskovic Institute, Zagreb, Croatia.
Address reprint requests to Peter A. McSweeney, MD, Fred Hutchinson Cancer Research Center, 1124 Columbia St, M318, Seattle, WA 98104.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.