Abstract
In vivo expansion and multilineage outgrowth of human immature hematopoietic cell subsets from umbilical cord blood (UCB) were studied by transplantation into hereditary immunodeficient (SCID) mice. The mice were preconditioned with Cl2MDP-liposomes to deplete macrophages and 3.5 Gy total body irradiation (TBI). As measured by immunophenotyping, this procedure resulted in high levels of human CD45+ cells in SCID mouse bone marrow (BM) 5 weeks after transplantation, similar to the levels of human cells observed in NOD/SCID mice preconditioned with TBI. Grafts containing approximately 107 unfractionated cells, approximately 105purified CD34+ cells, or 5 × 103 purified CD34+CD38− cells yielded equivalent numbers of human CD45+ cells in the SCID mouse BM, which contained human CD34+ cells, monocytes, granulocytes, erythroid cells, and B lymphocytes at different stages of maturation. Low numbers of human GpA+ erythroid cells and CD41+ platelets were observed in the peripheral blood of engrafted mice. CD34+CD38+ cells (5 × 104/mouse) failed to engraft, whereas CD34− cells (107/mouse) displayed only low levels of chimerism, mainly due to mature T lymphocytes. Transplantation of graded numbers of UCB cells resulted in a proportional increase of the percentages of CD45+ and CD34+ cells produced in SCID mouse BM. In contrast, the number of immature, CD34+CD38− cells produced in vivo showed a second-order relation to CD34+graft size, and mice engrafted with purified CD34+CD38− grafts produced 10-fold fewer CD34+ cells without detectable CD34+CD38− cells than mice transplanted with equivalent numbers of unfractionated or purified CD34+ cells. These results indicate that SCID repopulating CD34+CD38− cells require CD34+CD38+ accessory cell support for survival and expansion of immature cells, but not for production of mature multilineage progeny in SCID mouse BM. These accessory cells are present in the purified, nonrepopulating CD34+CD38+ subset as was directly proven by the ability of this fraction to restore the maintenance and expansion of immature CD34+CD38− cells in vivo when cotransplanted with purified CD34+CD38−grafts. The possibility to distinguish between maintenance and outgrowth of immature repopulating cells in SCID mice will facilitate further studies on the regulatory functions of accessory cells, growth factors, and other stimuli. Such information will be essential to design efficient stem cell expansion procedures for clinical use.
TRADITIONAL SOURCES OF hematopoietic stem and progenitor cells for transplantation include autologous and allogeneic bone marrow (BM) and mobilized peripheral blood (PB). Recently, human umbilical cord blood (UCB) has been shown to be a realistic alternative source of stem cells.1,2 UCB contains cells of all of the hematopoietic lineages, including cells that can produce granulocyte-macrophage colony-forming unit (GM-CFU) after extended long-term stromal cell-supported culture. Most of these long-term culture-initiating cells (LTC-IC) are found in the small subset of CD34+CD38− cells.3The ability to cryopreserve, select, and expand progenitors without loss of proliferative capacity4 makes UCB an appropriate model to identify immature hematopoietic cell subsets involved in hematopoiesis in vivo, select appropriate growth factor (GF) combinations and culture conditions to maintain and expand stem cells in vitro, and design optimal gene transfer conditions aimed at efficient and stable transduction of transplantable stem cells.5
Hereditary immunodeficient SCID and NOD/SCID mice are useful recipients to assess human stem cell capacities in a transplantation assay and appear particularly suitable to assess the outgrowth of purified UCB cell subsets and the effects of ex vivo manipulation on hematopoietic capacities after transplantation. Several approaches for engrafting immunodeficient mice with normal or leukemic human hematopoietic cells have been described. The most frequently used systems involve injection of mobilized human PB, BM,6 or UCB cells in sublethally irradiated mice,7,8 electively followed by human GF treatment9-12 and/or cotransplantation with nonrepopulating CD34− accessory cells,13human BM long-term culture-derived stromal cells, or rodent cell lines that produce human GFs.14 Transgenic SCID mice expressing the genes for human interleukin-3 (IL-3), granulocyte-macrophage colony-stimulating factor (GM-CSF), and stem cell factor (SCF) have also been used to promote human cell engraftment,15 whereas human fetal liver, thymus,16,17 and/or bone fragment18 implantation has been used to create a human microenvironment in the mouse.
Injection with human cytokines or other additional treatment is not required to establish high-level human cell engraftment after transplantation of human UCB cells in immunodeficient mice, which suggested that neonatal cells either respond differently to the murine microenvironment or provide their own cytokines in a paracrine fashion.7,8 However, analysis of the hematopoietic potential of UCB cells in SCID is limited by the large number of cells required to achieve significant engraftment levels, possibly because of low seeding efficiencies of stem cells or elimination of transplanted cells by natural killer (NK) cells or the mononuclear phagocytic system, which are intact in SCID mice. More reproducible and higher levels of engraftment with smaller graft sizes have been achieved with NOD/SCID mice, which has been attributed to the lack of functional macrophages, NK cells, and complement activity in this mouse strain.19 Specific elimination of phagocytic cells in spleen and liver of SCID mice can be achieved within 24 hours after a single intravenous (IV) injection of liposome-encapsulated dichloromethylene diphosphonate (Cl2MDP).20-22As shown recently for human acute myeloid leukemia (AML) and UCB cells, macrophage-depleted SCID mice supported the production of similar levels of human cells from 10-fold fewer transplanted cells as compared with SCID mice conditioned with total body irradiation (TBI) alone. For AML cells, preconditioning of SCID mice with liposomes led to similar levels of engraftment as observed in NOD/SCID mice, which suggested that macrophages have a prominent role in eliminating injected human cells in SCID mice.22
The present study was undertaken to quantitatively analyze the maintenance and outgrowth of distinct UCB immature cell subsets in macrophage-depleted SCID mice and to assess the hematopoietic cell lineages produced.
MATERIALS AND METHODS
Human UCB cells.
UCB samples were obtained after informed consent in conformity with legal regulations in The Netherlands from placentas of full-term normal pregnancies. Mononucleated cells were isolated by Ficoll density gradient centrifugation (1.077 g/mL; Nycomed Pharma AS, Oslo, Norway) and were cryopreserved in 10% dimethylsulphoxide, 20% heat-inactivated fetal calf serum (FCS), and 70% Hank's Balanced Salt Solution (HBSS; GIBCO, Breda, The Netherlands) at −196°C as described.23 After thawing by stepwise dilution in HBSS containing 2% FCS, the cells were washed with HBSS containing 1% FCS and used for flow cytometric analysis, transplantation into SCID mice (unfractionated grafts), or subset purification.
Subset purification.
Purification of CD34+ cells was performed by positive selection using Variomacs Immunomagnetic Separation System as described24 (CLB, Amsterdam, The Netherlands). The percentage CD34+ cells in the unseparated population (unfractionated UCB) and in the purified CD34+ and CD34− fractions was determined by fluorescence-activated cell sorting (FACS) analysis. For isolation of CD34+CD38+, CD34+CD38+/−, and CD34+CD38− subsets, purified CD34+ cells were stained with fluorescein isothiocyanate (FITC)- and R-phycoerythrin (PE)-conjugated antibodies against human CD34 and CD38 (CD34-FITC, CD38-PE; Becton Dickinson, San Jose, CA) for 30 minutes on ice in HFN (HBBS, 2% [wt/vol] FCS, 0.05% [wt/vol] sodium-azide) containing 2% (vol/vol) normal human serum (NHS). After incubation, the cells were washed twice, resuspended in HBSS, and sorted using a FACS Vantage flow cytometer (Becton Dickinson).
Transplantation of UCB cells in immunodeficient mice.
Female, specified pathogen-free (SPF) CB-17-scid/scid (SCID) mice, 6 to 9 weeks of age, were obtained from Harlan (CPB, Austerlitz, The Netherlands). NOD/LtSz-scid/scid mice (NOD/SCID) were obtained from The Jackson Laboratory (Bar Harbor, ME). The mice were housed under SPF conditions in a laminar air flow unit and supplied with sterile food and acidified drinking water containing 100 mg/L ciprofloxacine (Bayer AG, Leverkusen, Germany) ad libitum. The plasma Ig levels of the mice were determined with an enzyme-linked immunosorbent assay using a sheep antimouse antibody reacting with mouse IgG (Boehringer Mannheim Biochemica, Penzberg, Germany), and animals with plasma Ig levels greater than 40 μg/mL were excluded.25 To deplete macrophages, the SCID-mice were injected IV into a lateral tail vein with 200 μL liposome stock solution containing di-chloromethylene di-phosphonate (Cl2MDP; a gift of Boehringer Mannheim GmbH, Mannheim, Germany) 1 day before transplantation of hematopoietic cells.26 In previous studies22with human acute leukemia and UCB cells, this approach required 10-fold fewer cells for uniform engraftment than in SCID mice conditioned with TBI alone. All mice received TBI at 3.5 Gy, delivered by a137Cs source adapted for the irradiation of mice (Gammacell; Atomic Energy of Canada, Ottawa, Ontario, Canada) 2 to 4 hours before transplantation. The transplants were suspended in 200 μL HBSS containing 0.1% bovine serum albumin (BSA; Sigma, St Louis, MO) and injected IV into a lateral tail vein. Transplanted cell numbers were 107 (unfractionated and CD34− cells), 105 (CD34+cells), 5 × 104 (CD34+CD38+cells), and 5 × 103(CD34+CD38− cells), unless stated otherwise in the results.
In vitro colony assay.
Unfractionated and purified CD34+ and CD34− grafts as well as chimeric mouse BM samples were assayed for the presence of GM-CFU and erythroid burst-forming units (BFU-E) by in vitro colony formation in viscous methylcellulose culture medium as previously described.27-29 Briefly, unfractionated and CD34− cells were plated at a concentration of 25,000 per 35-mm Petri dish (Becton Dickinson), CD34+ purified grafts at 1,000 per dish, and chimeric mouse BM at 50,000 per dish. Culture medium consisted of 1 mL Dulbecco's medium (GIBCO, Gaithersburg, MD), containing 0.8% (wt/vol) methylcellulose, 5% (vol/vol) FCS, and further supplemented with 1.5% (wt/vol) BSA, 10 mg/mL insulin, 0.3 mg/mL human transferrin, 15 mmol/L β-mercaptoethanol, 0.1 mmol/L sodium selenite, 1 mg/mL nucleosides, 15 μmol/L linoleic acid, 15 μmol/L cholesterol, 100 U/mL penicillin, and 50 mg/mL streptomycin. For BFU-E, cultures were supplemented with 0.2 mmol/L bovine hemin (Sigma), 200 ng/mL human SCF, and 4 U/mL (25 μg/mL) human recombinant Epo (Behringwerke AG, Marburg, Germany). For CFU-GM, cultures were supplemented with 5 ng/mL human recombinant GM-CSF (Behringwerke AG), 200 ng/mL SCF, and 30 ng/mL human recombinant IL-3. The cultures were maintained in a humidified atmosphere of 10% CO2 at 37°C for 14 days, after which the colonies were counted. Data of duplicate dishes were expressed as average number of colonies per 105 cells plated.
Tissue collections and analysis.
Mice were examined at a single time point, 35 days after transplantation, to enable meaningful comparisons between experiments, because individual hematopoietic subsets show differences in engraftment kinetics in immunodeficient mice.12 Mice were killed by CO2 inhalation followed by cervical dislocation in accordance with institutional animal research regulations. From each mouse, both femurs were collected and BM cell suspensions were prepared by flushing. After counting, the cells were cultured in colony assays and analyzed by flow cytometry to determine the percentage of human cells in the mouse BM. Cells were suspended in HBSS containing 2% (vol/vol) FCS, 0.05% (wt/vol) sodium azide, 2% (vol/vol) human serum, and 2% (vol/vol) mouse serum and stained for 30 minutes at 40°C with the pan-leukocyte surface marker CD45-FITC antibody and with CD33-PE antibody. Positive samples were further analyzed by incubation with FITC- and PE-labeled mouse monoclonal antibodies to human CD34, CD19, CD16, CD15, CD38, CD33, CD56, CD4, and CD8 (Becton Dickinson Immunocytometry Systems, San Jose, CA) and glycophorin A (GpA), CD3, and CD71 (Dako A/S, Copenhagen, Denmark). Parallel samples were incubated with isotype-matched control antibodies. Cell samples of nontransplanted mice were stained as negative controls. Fluorescence was measured using a FACScan flow cytometer and Lysis II software (Becton Dickinson). Dead cells were excluded by adding 1 μg/mL propidium iodide (PI) and gating for PI− cells in the FL3 (PI) channel. For all samples, 10,000 events were collected in a gate for PI− cells. To quantitate CD34+subsets in selected samples, 1,000 to 10,000 events were also collected in a gate that included all viable human CD34+ cells. CD34+ and CD34+CD38−expansion were calculated on the assumption that one femur contains 8.5% of all BM cells.30
In a number of experiments, PB was collected weekly from the tail vein and analyzed for the presence of human GpA+ erythrocytes and CD41+ platelets by flow cytometry. Blood samples were collected in EDTA-coated tubes and stained with CD41-FITC (PharMingen, San Diego, CA) and GpA-FITC, respectively (Dako A/S) in HBSS with 2% (vol/vol) FCS, 0.05% (wt/vol) sodium azide, 2% (vol/vol) human serum, 2% (vol/vol) mouse serum, and 2 g/L EDTA for 30 minutes at 40°C. Cell samples of nontransplanted mice and human blood cells were stained as controls.
Statistical and regression analysis.
Results are expressed as individual data or as the arithmetic mean ± standard deviation. The regression analysis of the percentage of human CD45+, CD34+, and CD34+CD38− cells in the chimeric BM as a function of the number of CD34+ cells transplanted was performed by plotting the data on a double logarithmic scale and calculating the regression using the general formula y = axb. By this method, an exponent b = 1 proves first order (single-hit) kinetics, ie, direct proportionality (linearity) of chimeric cell numbers and cells transplanted, whereas an exponent b = 2 demonstrates second order (two-hit) kinetics. The frequency of repopulating cells in the SCID mice was approximated using Poisson statistics.
RESULTS
Chimeric BM analysis.
Chimerism in SCID mouse BM was assessed by flow cytometric analysis 35 days after UCB transplantation. Typical results of chimeric BM stained with CD45-FITC versus CD33-PE and CD45-FITC versus CD34-PE are shown in Fig 1A and B, respectively. The percentage of CD45+ cells was used as a measure for engraftment levels of human cells in the mouse BM. Only mice with percentages larger than 1% CD45+ cells were considered to be engrafted. Positive staining for any of these markers was not found in nontransplanted mice (Fig 1C and data not shown), demonstrating the specificity of the antibodies for human cells. As shown in Fig 1A and B, the CD45+ cells were heterogeneous with respect to CD33 and CD34 expression.
Flow cytometric analysis of chimeric mouse BM stained with CD45-FITC versus CD33-PE (A) and CD45-FITC versus CD34-PE (B). BM of nontransplanted mice showed no staining with the CD45-FITC or CD33-PE antibody (C).
Flow cytometric analysis of chimeric mouse BM stained with CD45-FITC versus CD33-PE (A) and CD45-FITC versus CD34-PE (B). BM of nontransplanted mice showed no staining with the CD45-FITC or CD33-PE antibody (C).
Parallel groups of mice were injected with unfractionated mononucleated UCB cells or with purified CD34+ or CD34−cells (Fig 2) in SCID mice conditioned with either TBI or TBI and macrophage depletion or in TBI-conditioned NOD/SCID mice. Transplantation of unfractionated mononucleated UCB cells into macrophage-depleted SCID mice resulted in more prominent engraftment levels compared with SCID mice conditioned with TBI alone. After transplantation with 107 unfractionated or 105purified CD34+ cells, the macrophage-depleted SCID mice showed similar levels of chimerism as NOD/SCID mice preconditioned with TBI. CD34− cells (107 cells transplanted) did not result in high levels of chimerism in either mouse strain (Fig 2).
Engraftment levels of 4 different UCB samples in SCID and SCID/NOD mice. The percentages CD45+ cells are shown for individual SCID mice preconditioned with TBI and Cl2MDP (○), SCID mice preconditioned by TBI alone (•), and NOD/SCID mice preconditioned by TBI alone (□).
Engraftment levels of 4 different UCB samples in SCID and SCID/NOD mice. The percentages CD45+ cells are shown for individual SCID mice preconditioned with TBI and Cl2MDP (○), SCID mice preconditioned by TBI alone (•), and NOD/SCID mice preconditioned by TBI alone (□).
As shown in Table 1, transplantation of 107 unfractionated cells from 5 different UCB samples resulted in high levels of chimerism in all mice (n = 22) transplanted. Transplantation of 105 purified CD34+ cells also resulted in high levels of human cells in 35 of 38 mice, whereas mice transplanted with 107 CD34− cells showed only low levels of engraftment in 5 of 18 mice transplanted. These results show that relatively low numbers of purified CD34+ UCB cells are capable of proliferation in the macrophage-depleted SCID mouse microenvironment without the support of accessory cells or addition of hematopoietic growth factors. The CD34+ cells were further separated into a CD38+subset (∼50% of CD34+ cells containing >90% of clonogenic progenitors) and a CD38− subset (∼5% of the CD34+ population, enriched for immature, multipotent progenitors31) and transplanted into preconditioned SCID mice; cell numbers were 5 × 104 and 5 × 103, respectively. The CD34+CD38− subset showed high levels of engraftment in 4 of 6 mice with chimerism levels similar to those obtained with 20-fold larger numbers of CD34+ cells and 200-fold larger numbers of unfractionated UCB (Table 1). Despite the 10-fold larger cell numbers, only 1 of 4 mice engrafted with sorted CD34+CD38+ cells at the low level of 1.7%. These results show that the ability to repopulate SCID mice resides exclusively in the CD34+CD38− immature population.
Engraftment of UCB Cells in Preconditioned SCID Mice
| Graft . | Graft Size* . | Chimeric-151 Mice/ Injected . | % Chimerism (CD45+ cells) . | Characteristics of Chimerism . | |||
|---|---|---|---|---|---|---|---|
| CD34+ Cells . | CD34+CD38− Cells . | ||||||
| Cells/ Mouse BM (×106) . | Expansion Factor . | Cells/ Mouse BM (×104) . | Expansion Factor . | ||||
| Unfractionated | 107 | 22/22 | 34.5 ± 19.3-152 | 1.1 ± 1.4-153 | 12.9 ± 19.2 | 1.84 ± 2.7 | 2.19 ± 4.92 |
| CD34− | 107 | 5/18 | 7.8 ± 8.6 | 0.1 ± 0.1 | 4.0 ± 3.7 | 0 | 0 |
| CD34+ | 105 | 35/38 | 20.4 ± 16.3 | 1.1 ± 0.9 | 14.1 ± 18.6 | 6.9 ± 13.2 | 10.8 ± 16.6¶ |
| CD34+CD38+ | 5 × 104 | 1/4 | 1.7 | 0 | 0 | 0 | 0 |
| CD34+CD38− | 5 × 103 | 4/6 | 18.4 ± 8.7 | 0.1 ± 0.1 | 18.6 ± 5.2 | 0 | 0¶ |
| Graft . | Graft Size* . | Chimeric-151 Mice/ Injected . | % Chimerism (CD45+ cells) . | Characteristics of Chimerism . | |||
|---|---|---|---|---|---|---|---|
| CD34+ Cells . | CD34+CD38− Cells . | ||||||
| Cells/ Mouse BM (×106) . | Expansion Factor . | Cells/ Mouse BM (×104) . | Expansion Factor . | ||||
| Unfractionated | 107 | 22/22 | 34.5 ± 19.3-152 | 1.1 ± 1.4-153 | 12.9 ± 19.2 | 1.84 ± 2.7 | 2.19 ± 4.92 |
| CD34− | 107 | 5/18 | 7.8 ± 8.6 | 0.1 ± 0.1 | 4.0 ± 3.7 | 0 | 0 |
| CD34+ | 105 | 35/38 | 20.4 ± 16.3 | 1.1 ± 0.9 | 14.1 ± 18.6 | 6.9 ± 13.2 | 10.8 ± 16.6¶ |
| CD34+CD38+ | 5 × 104 | 1/4 | 1.7 | 0 | 0 | 0 | 0 |
| CD34+CD38− | 5 × 103 | 4/6 | 18.4 ± 8.7 | 0.1 ± 0.1 | 18.6 ± 5.2 | 0 | 0¶ |
*Percentage CD34+ cells in the graft: unfractionated UCB, 0.7% to 3.1%; CD34+, 77% to 83%; and CD34−, 0.1% to 0.6%.
Mice are considered chimeric at greater than 1% CD45+cells.
Mean ± SD of chimeric mice, results of 5 UCB.
CD34+ BM cells, calculated on the assumption that 1 femur represents 8.5% of all BM cells.30
¶Significantly different from unfractionated graft (P = .03, Fisher's exact test).
Multilineage outgrowth of UCB cells.
BM cells of chimeric mice were cultured in standard methylcellulose culture under conditions of stimulation with recombinant human GF that selectively favor the outgrowth of human monomyeloid and erythroid progenitors and failed to stimulate mouse progenitors. Comparison of clonogenic cell numbers in 15 chimeric mice with the numbers of colony-forming cells in the grafts showed a median expansion of 2.7-fold (range, 0 to 11) and 1.7-fold (range, 0 to 13) for CFU-GM and BFU-E numbers, respectively, as measured 35 days after transplantation. Because these progenitor cell populations have a high turnover rate, this observation demonstrates that monomyelocytic and erythroid progenitors are produced from more immature progenitors in the mouse hematopoietic environment.
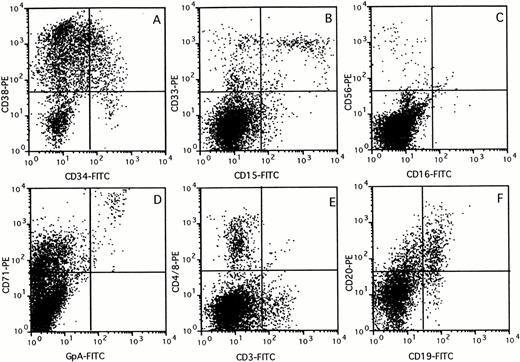
The composition of the human cell population in the BM of chimeric mice was assessed by flow cytometry using a panel of lineage-specific markers (Fig 3). The percentage of cells in each subset identified was expressed relative to the percentage cells stained with the panleukocyte marker CD45 (Fig 4). Mice transplanted with 107 unfractionated UCB cells showed multilineage outgrowth (Fig 4A). The most prominent population (25% to 50% of the human CD45+ cells) consisted of B-lymphoid cells, which contained immature CD19+CD20− as well as mature CD19+CD20+ cells (Figs 3F and 4). CD15+CD33+ monocytes, CD15+CD33+/− granulocytes, and CD15−CD33+ immature myelomonocytic cells were present at percentages ranging between 6% and 16% of the human cells (Figs 3B and 4). GpA+CD71++ erythroblasts and, occasionally, GpA+CD71− mature red blood cells (not visible in Fig 3D) were present in low numbers. In keeping with the presence of CD71 on activated nonerythroid cells, the large population of CD71+GpA− cells (Fig3D) contained cells of multiple lineages.32 The composition of the BM of mice transplanted with CD34+ (Fig 4B) or CD34+CD38− cells (Fig 4C) was similar to that of mice transplanted with unfractionated UCB. The few mice that showed detectable chimerism after transplantation of CD34− cells also had outgrowth of low numbers of myeloid, erythroid, and B-lymphoid cells, which were possibly derived from the low numbers of CD34+ cells (0.1% to 0.6%) still present in the fraction. However, greater than 50% of the cells growing in these mice consisted of mature CD3+ T lymphocytes, which also expressed CD4 or CD8. CD3+ cells were also identified in mice transplanted with unfractionated, CD34+, or CD34+CD38− cell subsets, but these CD3+ cells expressed neither CD4 nor CD8 (Figs 3 and 4A through C). These cells may represent a subset of NK cells, because CD3 is expressed on some CD56+cells33 and CD56+ cells were also identified in low numbers in chimeric mice, including those transplanted with purified CD34+CD38− cells (Figs 3C and4C). The large population of CD3− cells that expressed CD4 or CD8 (Fig 3E) most likely consisted of CD4+monocytes.
Immunophenotyping of chimeric mouse BM. BM (>10% CD45+) was stained with a panel of antibodies specific against different human blood cell lineages. FACS profiles of a representative mouse are shown for CD34 versus CD38 (A), CD15 versus CD33 (B), CD16 versus CD56 (C), GpA versus CD71 (D), CD3 versus CD4 and CD8 (E), and CD19 versus CD20 (F) expression, respectively.
Immunophenotyping of chimeric mouse BM. BM (>10% CD45+) was stained with a panel of antibodies specific against different human blood cell lineages. FACS profiles of a representative mouse are shown for CD34 versus CD38 (A), CD15 versus CD33 (B), CD16 versus CD56 (C), GpA versus CD71 (D), CD3 versus CD4 and CD8 (E), and CD19 versus CD20 (F) expression, respectively.
Composition of the human CD45+ cell population in chimeric SCID mice stained for the human markers shown in Fig 3. Results (average ± SD) are those of 23 mice in total, transplanted with unfractionated UCB (A), purified CD34+(B), CD34+CD38− (C), and CD34− (D) grafts derived from 5 UCB samples. The percentage of cells in each subset was expressed relative to the percentage of CD45+ cells present in the BM of each mouse. The percentage of chimerism ranged between 10% and 40% for the data shown in (A) through (C) and between 1% and 15% for the data shown in (D).
Composition of the human CD45+ cell population in chimeric SCID mice stained for the human markers shown in Fig 3. Results (average ± SD) are those of 23 mice in total, transplanted with unfractionated UCB (A), purified CD34+(B), CD34+CD38− (C), and CD34− (D) grafts derived from 5 UCB samples. The percentage of cells in each subset was expressed relative to the percentage of CD45+ cells present in the BM of each mouse. The percentage of chimerism ranged between 10% and 40% for the data shown in (A) through (C) and between 1% and 15% for the data shown in (D).
Despite large numbers of human cells in the BM of SCID mice, very few human cells were detected in the leukocyte fraction of PB, spleen, and thymus (data not shown). Whole tail vein blood samples of CD34+ transplanted mice collected at various time points after transplantation contained human GpA+ erythrocytes at very low levels (∼0.1%) that could only be detected if very large cell numbers (>105) were analyzed (Fig 5C). The largest quantities (0.1% to 0.2%) were found 2 weeks after transplantation. From week 3 on, the level decreased and became undetectable in week 5. Human CD41+ platelets could also be detected in the mouse PB and followed a similar time course as the erythroid cells, with peak levels of 0.5% in week 2 (Fig 5B).
Circulating CD41+ platelets and GpA+ erythrocytes in the PB of CD34+transplanted SCID mice. Blood was collected in the presence of 2 g/L EDTA and stained immediately with CD41-FITC (B) and GpA-FITC (C). (A) shows the blood of a nontransplanted mouse stained with CD41-FITC.
Circulating CD41+ platelets and GpA+ erythrocytes in the PB of CD34+transplanted SCID mice. Blood was collected in the presence of 2 g/L EDTA and stained immediately with CD41-FITC (B) and GpA-FITC (C). (A) shows the blood of a nontransplanted mouse stained with CD41-FITC.
Evidence for accessory cell requirement for immature cell expansion but not for outgrowth of human UCB cells in SCID mice.
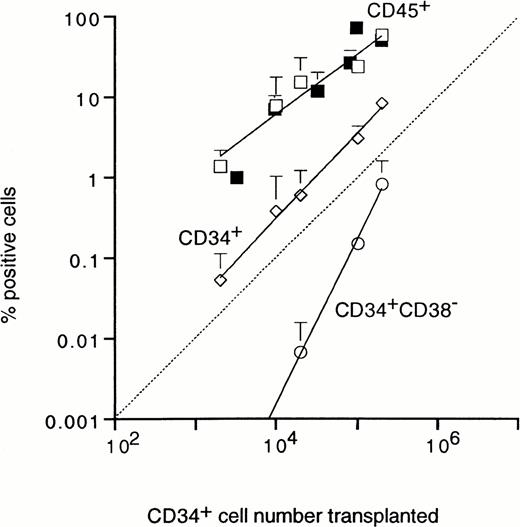
The UCB cell number required for engraftment was analyzed by injection of graded numbers of unfractionated or CD34+ cells. Transplantation of 2 × 103 CD34+ cells resulted in a low, but measurable level of chimerism of 1.4% CD45+ cells (Fig 6). The level of chimerism increased proportionally with cell dose, reaching approximately 60% human CD45+ cells after injection of 2 × 105 purified CD34+ cells. Engraftment after transplantation of unfractionated mononuclear UCB cells and purified CD34+ cells followed similar proportional patterns, with exponents of 0.8 and 1, respectively (Fig 6). Also the percentage of human CD34+ cells detected in SCID mouse BM after 35 days showed a linear relation with graft size (Fig 6). These results demonstrate that the outgrowth of human UCB cells in the SCID mouse BM does not require the support from accessory cells present in either the CD34+ or CD34− UCB fractions. Figure 3A shows that the CD34+ cells produced in SCID mouse BM were heterogeneous with respect to CD38 expression and included cells with low CD38 expression, which suggested that very immature cells were maintained and/or expanded in the mouse microenvironment. As shown in Fig 6, the production of cells with an immature CD34+CD38− phenotype showed a much steeper dependence on the number of CD34+transplanted, with a exponent of 2, demonstrating second order (two-hit) kinetics. CD34+CD38− cells were not detected in BM of mice transplanted with purified CD34+CD38− cells (Table 1 and Fig 7B), whereas, in addition, the numbers of CD34+ cells in these mice were 10-fold lower than in mice transplanted with unfractionated or CD34+grafts, despite similar levels of CD45+ cells (Table 1). Taken together, the nonlinear relation between graft size and the percentage of CD34+CD38− cells after 35 days, the lower number of CD34+ cells, and the absence of CD34+CD38− cells in mice transplanted with purified CD34+CD38− grafts show that immature CD34+CD38− cells can be maintained in the mouse microenvironment, but only with the support of accessory cells.
Relationship between the number of CD34+cells transplanted and percentage of human CD45+ (□), CD34+ (◊), and immature CD34+CD38− (○) cells detected in SCID mouse BM after 5 weeks. Results show the mean ± SD for 3 mice per data point. For comparison, the numbers of CD45+ cells detected in BM of mice transplanted with graded numbers of unfractionated cells are also shown (▪).
Relationship between the number of CD34+cells transplanted and percentage of human CD45+ (□), CD34+ (◊), and immature CD34+CD38− (○) cells detected in SCID mouse BM after 5 weeks. Results show the mean ± SD for 3 mice per data point. For comparison, the numbers of CD45+ cells detected in BM of mice transplanted with graded numbers of unfractionated cells are also shown (▪).
Distribution of human CD34 and CD38 in chimeric mouse BM after transplantation of CD34+ subsets, sorted as defined by the windows in (A). (B) through (E) provide the results 35 days after transplantation of 5 × 103CD34+CD38− cells (B), 5 × 103CD34+CD38− + 25 × 103 CD34+CD38+ cells (C), 105 CD34+ cells (D), or 25 × 103 CD34+CD38+/− (E). One thousand to 10,000 events were collected in a window containing CD34+ cells only. Quadrants were set to indicate CD34+CD38+ and CD34+CD38− cells. The percentages indicate the frequency of human CD34+ CD38− cells in mouse BM. CD34+CD38+ cells did not engraft (data not shown). The dissociation of outgrowth of CD45+cells and maintenance or expansion of CD34+CD38− cells is also in this experiment indicated by the CD45 percentages, ie, 25.1% for (B) without CD34+CD38− cells and 5.5% for (C), 46.7% for (D), and 2.9% for (E) with similar frequencies of CD34+CD38− cells.
Distribution of human CD34 and CD38 in chimeric mouse BM after transplantation of CD34+ subsets, sorted as defined by the windows in (A). (B) through (E) provide the results 35 days after transplantation of 5 × 103CD34+CD38− cells (B), 5 × 103CD34+CD38− + 25 × 103 CD34+CD38+ cells (C), 105 CD34+ cells (D), or 25 × 103 CD34+CD38+/− (E). One thousand to 10,000 events were collected in a window containing CD34+ cells only. Quadrants were set to indicate CD34+CD38+ and CD34+CD38− cells. The percentages indicate the frequency of human CD34+ CD38− cells in mouse BM. CD34+CD38+ cells did not engraft (data not shown). The dissociation of outgrowth of CD45+cells and maintenance or expansion of CD34+CD38− cells is also in this experiment indicated by the CD45 percentages, ie, 25.1% for (B) without CD34+CD38− cells and 5.5% for (C), 46.7% for (D), and 2.9% for (E) with similar frequencies of CD34+CD38− cells.
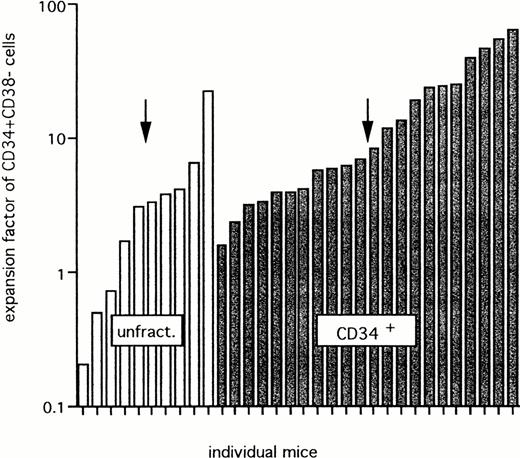
Figure 8 shows the actual expansion of CD34+CD38− cells in BM of the 32 (from 69) chimeric mice in which such cells were detectable. The expansion ranged between 0.2 and 22.1, with a median expansion of threefold for unfractionated mononucleated UCB cells, and between 1.6 and 63.1, with a median value of sevenfold after transplantation of CD34+grafts. This difference is statistically not significant.
Expansion of CD34+CD38− cells after transplantation of unfractionated or CD34+ cells. Data for 32 mice that showed detectable CD34+CD38− cells from a group of 69 chimeric mice (>1% CD45+ cells). Twenty-eight mice were transplanted with unfractionated (unfract.) cells and 41 mice were transplanted with CD34+ grafts from 5 different UCB samples. The arrow shows the median expansion factor of CD34+CD38− cells in each group.
Expansion of CD34+CD38− cells after transplantation of unfractionated or CD34+ cells. Data for 32 mice that showed detectable CD34+CD38− cells from a group of 69 chimeric mice (>1% CD45+ cells). Twenty-eight mice were transplanted with unfractionated (unfract.) cells and 41 mice were transplanted with CD34+ grafts from 5 different UCB samples. The arrow shows the median expansion factor of CD34+CD38− cells in each group.
Direct proof of an accessory role of CD34+CD38+cells in the maintenance of the transplanted CD34+CD38− population in vivo was obtained by cotransplantation of CD34+CD38− cells and CD34+CD38+ cells. Dot plots of CD38 versus CD34 expression, collected in a gate for CD34+ cells, show that transplantation of 5 × 103CD34+CD38− cells results in production of CD34+ cells, which are all CD38+ (Fig 7B). After transplantation of 5 × 103CD34+CD38− cells to which 25 × 103 CD34+CD38+ cells were added, CD34+CD38− cells were clearly produced in the mouse BM (Fig 7C) at frequencies similar to those observed in mice transplanted with 105 CD34+ cells (Fig 7D). Also in this experiment, transplantation of 5 × 104CD34+CD38+ cells alone did not result in human cell engraftment (similar to the data presented in Table 1). Sorted CD34+CD38+/− cells (corresponding to 20% of the CD34+ cells) also repopulated transplanted SCID mice with propagation of immature CD34+CD38−cells, which can be explained by the presence of repopulating and accessory cells in this subset (Fig 7E).
Repopulating cell frequency.
The maintenance or expansion of CD34+CD38− cells in SCID mice might be considered as a more significant characteristic of the capacity of repopulating stem cells than the ability to produce mature progeny. Taking into account that the seeding efficiency of repopulating cells in transplanted SCID mice is unknown and that the support provided by accessory cells may be suboptimal, a lower limit for the frequency of cells with the ability to maintain or expand the numbers of CD34+CD38− cells was estimated using the pooled data of 69 mice engrafted with graded doses of unfractionated or purified CD34+ cells from 5 different UCB samples (Table 2). By using Poisson statistics, a value of 1 repopulating cell per 70,000 CD34+ cells was estimated (95% confidence limits, 54,000 to 102,000). This would correspond to 1 repopulating cell per approximately 7 × 106 unfractionated UCB cells and 1 per 3,500 CD34+CD38− cells.
Frequency Analysis of Repopulating Cells
| Graft* . | CD34+ Cell No. Transplanted† . | Mice Positive for CD34+CD38− (n) . | Mice Negative for CD34+CD38− (n) . | % Negative . |
|---|---|---|---|---|
| CD34+ | 1,660 | 0 | 2 | 100 |
| 8,300 | 0 | 2 | 100 | |
| 16,600 | 1 | 1 | 50 | |
| 80,000 | 10 | 12 | 55 | |
| 104,097 | 8 | 2 | 20 | |
| 166,000 | 3 | 0 | 0 | |
| Total | 3,200 | 0 | 2 | 100 |
| 9,600 | 0 | 3 | 100 | |
| 32,000 | 0 | 4 | 100 | |
| 60,000 | 2 | 3 | 60 | |
| 70,000 | 3 | 3 | 50 | |
| 111,000 | 2 | 1 | 33 | |
| 198,900 | 3 | 2 | 40 |
| Graft* . | CD34+ Cell No. Transplanted† . | Mice Positive for CD34+CD38− (n) . | Mice Negative for CD34+CD38− (n) . | % Negative . |
|---|---|---|---|---|
| CD34+ | 1,660 | 0 | 2 | 100 |
| 8,300 | 0 | 2 | 100 | |
| 16,600 | 1 | 1 | 50 | |
| 80,000 | 10 | 12 | 55 | |
| 104,097 | 8 | 2 | 20 | |
| 166,000 | 3 | 0 | 0 | |
| Total | 3,200 | 0 | 2 | 100 |
| 9,600 | 0 | 3 | 100 | |
| 32,000 | 0 | 4 | 100 | |
| 60,000 | 2 | 3 | 60 | |
| 70,000 | 3 | 3 | 50 | |
| 111,000 | 2 | 1 | 33 | |
| 198,900 | 3 | 2 | 40 |
*Only chimeric SCID mice (>1% CD45+ cells by flow cytometry) were included in the analysis.
n = 5 UCB, 69 mice transplanted.
DISCUSSION
Engraftment of UCB in SCID mice preconditioned by 3.5 Gy TBI and injection of CL2MDP liposomes was more prominent than in SCID mice conditioned with TBI alone and similar to that observed in NOD/SCID mice. The macrophage-depleted SCID mice supported the multilineage outgrowth of unfractionated UCB, purified CD34+ cells and the immature subset of CD34+CD38− UCB cells, with production of B lymphocytes, monocytes, granulocytes, erythroid cells, NK cells, and platelets as well as production of CD34+ cells, including phenotypically immature CD34+CD38− cells. Small numbers of purified CD34+CD38−cells also engrafted efficiently with chimerism levels similar to those observed in accessory cell and/or GF-supported NOD/SCID mice,13 whereas CD34+CD38−cells did not engraft, indicating that the SCID repopulating potential resides exclusively in the CD34+CD38−subset.
The detection of CD34+CD38− cells in SCID mouse BM is consistent with the finding that CD34+CD38− cells recovered from the BM of engrafted SCID and NOD/SCID mice have retained the capacity to produce clonogenic progeny in long-term culture and to differentiate into myeloid and lymphoid cells in single-cell/well cultures.34,35 Results showing that purified human cells from NOD/SCID mouse BM may engraft secondary recipients also suggest that repopulating stem cells are maintained in the BM of immunodeficient mice.36 Taken together, these data show that immature CD34+CD38− UCB cells can survive and expand in transplanted immunodeficient mice.
The level of expansion of the immature CD34+CD38− subset in chimeric mouse BM, but not the multilineage production of more differentiated progeny, appeared to be dependent on accessory cells. This is most clearly demonstrated by the second order (two-hit) kinetics of the relation between graft size and the numbers of immature CD34+CD38− cells produced in the SCID mouse BM in contrast to the directly proportional relation between graft size and the numbers of mature CD45+ cells and the CD34+ population as a whole (Fig 6). Additional data show that engraftment levels and types of human cells produced in the BM of mice transplanted with 5 × 103CD34+CD38− cell were similar to those obtained with 20-fold more CD34+ cells or 2,000-fold larger numbers of unfractionated mononucleated UCB cells, which also showed that accessory cells or exogenous GFs are not needed for multilineage outgrowth of immature human cells in immunodeficient mice. In contrast, the observation that SCID mice transplanted with CD34+CD38− grafts produced 10-fold fewer CD34+ cells and no detectable CD34+CD38− cells, despite equal numbers of CD45+ cells, than mice transplanted with unfractionated or CD34+ grafts with equivalent numbers of CD34+CD38− cells (Table 1), provides additional evidence for an involvement of accessory cells in the maintenance and expansion of immature UCB cells in the SCID mouse microenvironment. Because mice transplanted with unfractionated mononucleated UCB cells did not show larger numbers of CD34+ cells (Table 1) or more extensive expansion of CD34+CD38− cells (Fig 8) than mice transplanted with purified CD34+ cells, we postulated that the accessory cells needed for the support of immature UCB cells are present in the CD34+ population. Formal proof was obtained by cotransplantation of CD34+CD38− cells and CD34+CD38+ cells (Fig 7). Whereas transplantation of CD34+CD38− cells alone did not result in the maintenance of these cells, the addition of 50% CD34+CD38+, a fraction that by itself did not result in substantial chimerism, restored the propagation of CD34+CD38− cells in engrafted mice (Fig7).
One possible function of the accessory cells UCB cells might be to prevent elimination of the small numbers of CD34+CD38− cells by residual immune-reactivity in the SCID mouse by providing an excess of human cells. However, because small numbers of CD34+CD38− cells produced equal numbers of mature progeny in the macrophage-depleted SCID mice than much larger unfractionated or CD34+ grafts (Table 1), it is unlikely that such a mechanism plays a prominent role in promoting CD34+CD38− cell engraftment. It is more likely that accessory cells provide essential GFs or other stimuli needed for the self-renewal of immature human cells that are not provided by the mouse microenvironment. CD34+ UCB cells and their immediate progeny have been shown to produce various GF, including IL-3, G-CSF, and GM-CSF, which stimulate in vitro colony formation by UCB in an autocrine or paracrine fashion.37 A role of accessory cell-derived GF in the maintenance of immature cells is also suggested by the supportive role of a cocktail of erythropoietin, Steel factor, IL-3, and GM-CSF for expansion of human cells in NOD/SCID mice transplanted with high numbers of unfractionated human BM cells, which was only observed late after transplantation, when the number of human cells were reduced.12 Further studies are required to examine to what extent optimal combinations of these or other GFs can replace accessory cells in maintaining and expanding CD34+CD38− cells in immunodeficient mice.
Estimation, by Poisson statistics, of the frequency of original UCB cells that can maintain or expand CD34+CD38− cell numbers during the 5 weeks of engraftment period yielded a value of 1 in 70,000 CD34+ cells (corresponding to 1 in 3,500 CD34+CD38− cells). This value is lower, but in the same order of magnitude, than the 1 in 600 SCID repopulating CD34+CD38− cells that has been calculated on the basis of the frequency of transplanted NOD/SCID mice with detectable numbers of human cells in the BM as assessed by Southern blots.38 The difference is most likely due to the criteria chosen in that the ability to expand CD34+CD38− cells is a more stringent parameter for engraftment of immature cells than the production of mature progeny at a level of as low as 0.05% human cells detected by DNA blotting analysis.38 Such low engraftment levels can in principle be derived from contaminating mature cells, as shown in our study by the low, but detectable (>0.5% of mouse BM) engraftment with mature T cells in some mice transplanted with purified CD34− cells. The ability to maintain or expand CD34+CD38− cell numbers in SCID mouse BM is probably more characteristic for repopulating stem cells than production of mature progeny per se, because it may reflect an essential hematopoietic stem cells feature, ie, the ability to maintain its own numbers in vivo.
The differences in repopulating cell frequencies might also be due to the cotransplantation of accessory cells and/or administration of growth factors in the NOD/SCID mouse model that may have promoted human cell engraftment.38 Although it is clear that CD34+CD38− cells still represent a heterogeneous cell population with only a minority of cells capable of hematopoietic reconstitution, all frequency estimates of the SCID mouse repopulating human cells likely underestimate the frequency of human repopulating cells and should be treated with caution. In particular, the seeding efficiency of these cells has not been assessed yet, whereas the efficacy of the growth stimuli provided by the xenogeneic environment, accessory cells in the transplant or exogenous growth factor administration might very well be suboptimal. Studies into the kinetics of human BM cell engraftment in immunodeficient mice have shown that the number of immature, CD34+Thy1+cells that can be detected in the mouse BM 2 days after transplantation is at least 2 logs lower than input numbers, suggesting that only a very small fraction of the immature human cells develop in these mice.12
The present study provides evidence for differential regulation of the expansion as opposed to multilineage outgrowth of immature human hematopoietic stem cells in transplanted SCID mice. The possibility to distinguish experimentally between these essential functions in the SCID mouse transplantation assay now opens an experimental approach to examine the effects of various GFs, cell subsets, and other agonists on the self-renewal of human immature stem cell subsets. This information will be essential to design and test conditions for ex vivo activation and expansion of immature hematopoietic cells and for various experimental purposes, such as required for the development of efficient gene transfer protocols into hematopoietic cells with retention of repopulating ability.
ACKNOWLEDGMENT
The authors thank Dr F.K. Lotgering and the staff of the Obstetrics Department at the Sophia Children's Hospital (Rotterdam, The Netherlands) and Dr A.Th. Alberda and the staff of the St Fransiscus Hospital (Rotterdam, The Netherlands) for the collection of cord blood samples used in this study. We acknowledge Dr W.A.M. Loenen for the initial cord blood work in our lab and Els van Bodegom for taking care of the SCID mice.
Supported in part by grants of the Netherlands Organization for Scientific Research NWO, the Netherlands Cancer Foundation Koningin Wilhelmina Fonds, the Royal Netherlands Academy of Arts and Sciences, and contracts of the Commission of the European Communities.
Address reprint requests to Gerard Wagemaker, PhD, Institute of Hematology, Room H Ee1314, Erasmus University Rotterdam, Dr. Molewaterplein 50, PO Box 1738, 3000 DR Rotterdam, The Netherlands.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal