Abstract
Different genes in the β-like globin locus are expressed at specific times during development. This is controlled, in part, by competition between the genes for activation by the locus control region. In mice, gene inactivation of the erythroid Krüppel-like factor (EKLF) transcription factor results in a lethal anemia due to a specific and substantial decrease in expression of the fetal/adult-stage–specific β-globin gene. In transgenic mice carrying the complete human β-globin locus, EKLF ablation not only impairs human β-globin–gene expression but also results in increased expression of the human γ-globin genes during the fetal/adult stages. Hence, it may appear that EKLF is a determining factor for the developmental switch from γ-globin to β-globin transcription. However, we show here that the function of EKLF for β-globin–gene expression is necessary even in absence of gene competition. Moreover, EKLF is not developmental specific and is present and functional before the switch from γ-globin to β-globin–gene expression occurs. Thus, EKLF is not the primary factor that controls the switch. We suggest that autonomous repression of γ-globin transcription that occurs during late fetal development is likely to be the initiating event that induces the switch.
IN MAMMALS, EXPRESSION of globin genes is erythroid specific and is developmentally regulated.1 The human β-globin locus contains five active genes: ε, which is transcribed in the embryonic yolk sac; Gγ and Aγ, which are expressed in the fetal liver; and δ (a minor contributor) and β, which are transcribed in the bone marrow throughout adult life. These five genes are arranged in their temporal order of expression (5′-ε-Gγ-Aγ-δ-β-3′; Fig 1). There is only one well-defined switch in globin gene expression from the mouse β-like globin locus. Mice express εy, βh0, and βh1 globins during embryonic life and in early fetal development. Thereafter, expression switches to the β-minor and β-major globins genes, and these two genes continue to be transcribed into adult life.
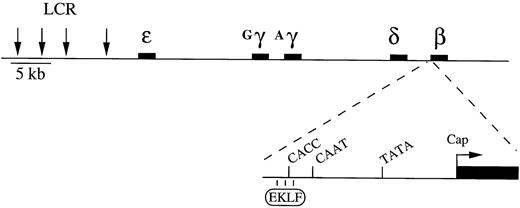
Structure of the human β-like globin locus and the β-globin gene minimal promoter. The figure also shows EKLF that interacts with the CACC motif element of the promoter.
Structure of the human β-like globin locus and the β-globin gene minimal promoter. The figure also shows EKLF that interacts with the CACC motif element of the promoter.
For high-level expression, the β-like globin genes are dependent on a locus control region (LCR). The LCR in the β-globin locus is signified by four DNase I hypersensitive site regions situated upstream of the genes (Fig 1). This DNA element has very strong erythroid-specific enhancer activity and is characterized as an LCR by its ability to confer copy-number–dependent, integration-site–independent, endogenous levels of expression onto globin transgenes.2-4
In mice, transcription of a human γ-globin transgene present alone incis with the LCR is downregulated, but only partially, during fetal development.5-9 The human β-globin gene is expressed at equivalent levels at all developmental stages in transgenic mice when it is present alone with the LCR.6,7On the other hand, if γ-globin and β-globin genes are linked together with the LCR (LCR-γ-β), both genes are appropriately regulated during development; the β-globin gene is not expressed in the embryo and then is switched on near 11.5 days in fetal life, whereas the γ-globin gene is expressed in the embryo and then is markedly downregulated during fetal life when the β-globin gene is upregulated.6,7,10 These observations, in conjunction with several other experiments,11-15 have suggested a competitive model to describe the developmental regulation of globin genes. It has been postulated that a globin gene must interact directly with the LCR through a looping mechanism for it to be activated and that the LCR can only interact with one gene at a time. Thus, the globin genes compete with each other to contact the LCR.6,7,10 At each developmental stage, the array of proteins that interact with the LCR and/or the globin genes favors interaction with a particular gene. As switching takes place, modifications in the transcription factor environment alter the relative affinity between the LCR and the genes such that the interaction switches from one gene to the next. Therefore, competition between the globin genes partly dictates their developmental pattern of expression.6,7 9 Identifying the transcription factors involved in LCR-gene interactions and/or the switching process would be a major step toward elucidating the mechanism regulating globin gene developmental specificity and the LCR mode of action.
The erythroid Krüppel-like factor (EKLF) plays an important role in transcription of the mouse fetal/adult- and human adult-stage–specific β-globin genes. Upon binding to the CACC box element in the promoters of these globin genes (Fig 1), EKLF can act as a transcriptional activator.16-20 Mice in which the EKLF gene is knocked out suffer from a severe anemia and die at approximately 16 days postcoitus.21,22 The anemia is caused by a major (approximately 20-fold) downregulation in expression of the mouse β-major gene. Other erythroid-specific genes, including other globin genes, are expressed at normal levels, and erythropoiesis is apparently otherwise unaffected. Therefore, the requirement for EKLF function appears to be very specific to the fetal/adult-specific β-major globin gene in mice. Similarly, in transgenic mice carrying the complete human β-like locus, expression of the human β-globin gene is severely hampered in an EKLF−/−background.15 23 However, in the absence of EKLF the human γ-globin transgenes are actually expressed at higher-than-normal levels during fetal life, up to the time of embryonic lethality. This result indicates that the switch in LCR interaction from the γ-globin to the β-globin gene that is expected to occur in early fetal development in mice may not take place in the absence of EKLF.
A model to account for the above results would suggest that EKLF is directly implicated in the interaction of the β-globin gene with the LCR and that EKLF activity is developmental specific. EKLF does interact with the erythroid-specific protein GATA-1,24,25for which there exist many DNA-binding sites in the LCR.26-29 Thus, there is a potential for EKLF to be involved in LCR-gene interactions. Whether or not the function of EKLF is developmentally regulated has not been tested directly, although it is known that the mRNA is present at early stages.20 30 On the other hand, the fact that the β-globin gene is expressed at all stages of development when it is alone with the LCR (see above) questions the requirement for a developmental-specific modifier acting on the β-globin gene and involved in the switching process. EKLF function might, therefore, only be necessary for competition against other globin genes. To address these questions, we have looked at EKLF function in the absence of gene competition and during development.
MATERIALS AND METHODS
Transgenic mice.
DNA analyses.
Fetal DNA was digested with HindIII, separated on agarose gels, and blotted onto nylon membranes. The membranes were hybridized to anNco I-Sma I 550-bp fragment from the 5′ region of EKLF cDNA that distinguishes between the wild-type and the targeted EKLF allele. The membranes were rehybridized with a 750-bp HindIII fragment from HSS2 of the LCR that detects this same 750-bp fragment from the μD transgene.
RNA analyses.
RNA was isolated using Trizol reagent (GIBCO-BRL, Gaithersburg, MD) as described by the manufacturer. RNase protection assays were conducted as described32 with antisense probes that span the 5′ region of the mRNA.
RESULTS
The β-globin gene requires EKLF for expression even when it is juxtaposed to the LCR and no gene competition is present.
In the complete β-like loci, the LCR is about 60 kb away from the β-globin gene (Fig 1). There are several genes that compete with the β-globin gene for interaction with the LCR, and this competition plays a role in the developmental regulation of these genes.6,7 10 Here, we aimed to test the function of EKLF in β-globin–gene expression in the absence of gene competition.
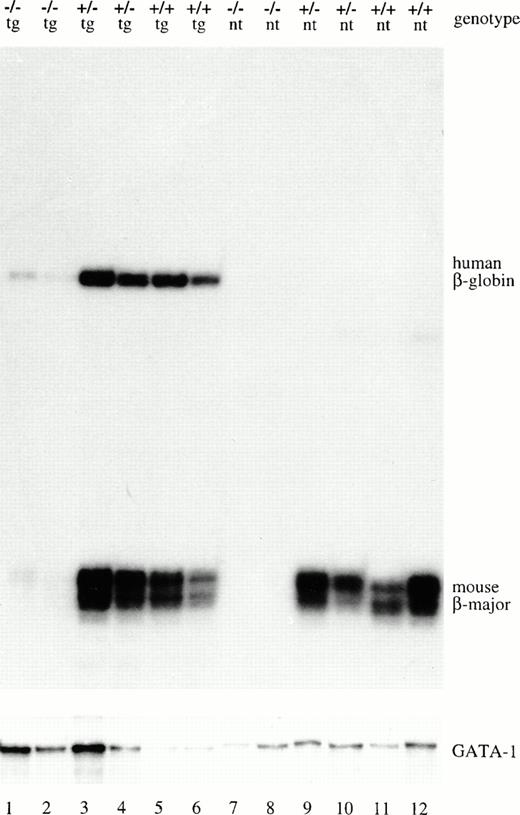
We studied a transgenic mouse line carrying the human microlocus construct33,34 in which the four hypersensitive site regions of the LCR are directly linked in cis to the complete human β-globin gene. In this construct, the LCR is within 4 kb of the TATA box of the β-globin–gene promoter, as measured from the middle of DNase hypersensitive sites 2 and 3 of the LCR. The expression of this transgene is erythroid specific and position independent.33 We used the μD14 transgenic line, which carries a single copy of the transgene and expresses human β-globin at 43% the level of the endogenous mouse β-major gene.31μD14 transgenic mice were bred to mice heterozygous for the EKLF inactivated allele. F1 animals that were transgenic and EKLF+/− were bred again to EKLF heterozygous mice to obtain μD14 fetuses null for the EKLF gene. The genotype of the animals was determined by Southern blot using a probe that distinguishes the EKLF wild-type allele from the knockout allele and a probe that is specific for the human LCR region of the transgene (Fig2). Human β-globin–transgene expression was measured relative to mouse β-major mRNA via RNase protection (Fig3). Comparison of human β-globin mRNA levels in fetal livers from EKLF knockout, heterozygous, and wild-type animals showed that expression of the transgene is as dependent on EKLF as the endogenous mouse β-major gene. Both genes gave 15- to 20-fold lower expression in the absence of EKLF. In comparison, GATA-1 expression was not affected by EKLF inactivation (Fig 3). Thus, although the β-globin promoter is juxtaposed to the LCR and no gene competition exists in the μD transgene, β-globin expression is still highly dependent on EKLF.
Genotype determination. Fetus DNA digested withHindIII was analyzed by Southern blot with an EKLF probe that distinguishes between the knockout allele (10-kb fragment detected) and the wild-type allele (6-kb fragment detected). The same blot was reprobed with human LCR sequences that hybridize to a 0.75-kbHindIII fragment from the transgene. The EKLF genotype and the presence (tg) or absence (nt) of the transgene is indicated at the top of each lane.
Genotype determination. Fetus DNA digested withHindIII was analyzed by Southern blot with an EKLF probe that distinguishes between the knockout allele (10-kb fragment detected) and the wild-type allele (6-kb fragment detected). The same blot was reprobed with human LCR sequences that hybridize to a 0.75-kbHindIII fragment from the transgene. The EKLF genotype and the presence (tg) or absence (nt) of the transgene is indicated at the top of each lane.
EKLF is required for β-globin–transgene expression in the fetal-adult stage. Fourteen-and-a-half–day fetal liver RNA was isolated from μD14 transgenic (tg) animals or nontransgenic (nt) litter mates with the EKLF genotype indicated at the top of each lane. The RNA was analyzed by RNase protection using a human β-globin and a mouse β-major probe. The RNA was also simultaneously analyzed with a GATA-1 probe as a loading control. The position of the protected fragments are indicated to the right of the figure.
EKLF is required for β-globin–transgene expression in the fetal-adult stage. Fourteen-and-a-half–day fetal liver RNA was isolated from μD14 transgenic (tg) animals or nontransgenic (nt) litter mates with the EKLF genotype indicated at the top of each lane. The RNA was analyzed by RNase protection using a human β-globin and a mouse β-major probe. The RNA was also simultaneously analyzed with a GATA-1 probe as a loading control. The position of the protected fragments are indicated to the right of the figure.
EKLF function is not developmental specific.
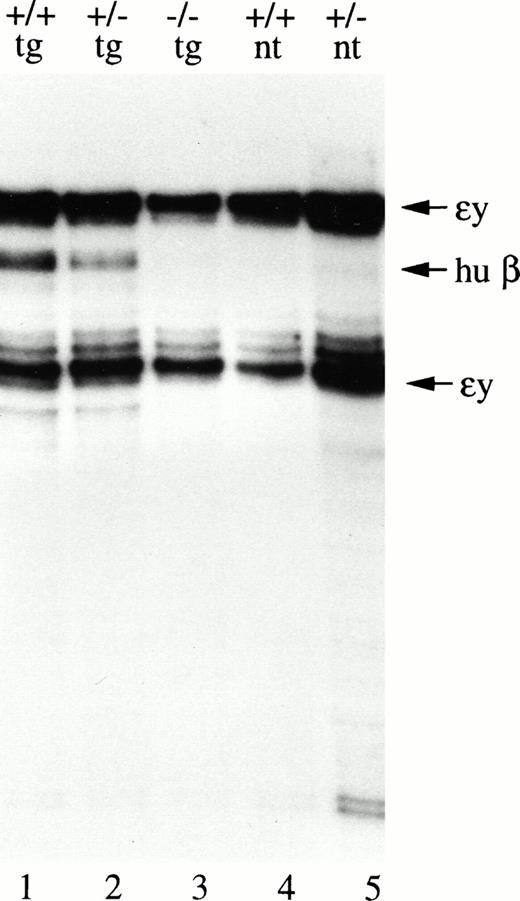
When the human β-globin gene is alone in cis with the LCR, it is expressed at all developmental stages.7,10 35 Thus, we could determine whether the EKLF protein is active at early embryonic stages by measuring the expression of the μD transgene in the presence and absence of EKLF. RNA from yolk sacs (the site of primitive erythropoiesis) was isolated from EKLF wild-type, heterozygous, and null 10.5-day embryos. Human β-globin–transgene expression was again measured by RNase protection, but the embryonic stage-specific mouse εy-globin mRNA served as the control in this case. Expression of the transgene was strongly dependent on EKLF. Very little human β-globin expression could be detected in EKLF−/− embryos, but the transgene was expressed at approximately 30% the level of the mouse εy gene in wild-type and heterozygous embryos (Fig 4,compare lane 3 with lanes 1 and 2). Results identical to those shown in Fig 4 were obtained for a second set of embryos in which two additional EKLF−/− animals carried the transgene. Thus, the dependence of β-globin gene expression on EKLF, and hence EKLF function, is not developmental specific.
EKLF is also required for β-globin–transgene expression at the embryonic stage. RNA isolated from 10.5-day yolk sac of embryos with the various EKLF genotypes and transgenicity for the μD14 construct indicated at the top of the lanes was analyzed by RNase protection with a human β-globin–transgene probe and a mouse y-globin probe. The position of the protected fragments detected by each probe is indicated by the arrows to the right of the figure.
EKLF is also required for β-globin–transgene expression at the embryonic stage. RNA isolated from 10.5-day yolk sac of embryos with the various EKLF genotypes and transgenicity for the μD14 construct indicated at the top of the lanes was analyzed by RNase protection with a human β-globin–transgene probe and a mouse y-globin probe. The position of the protected fragments detected by each probe is indicated by the arrows to the right of the figure.
DISCUSSION
It had been suggested previously that EKLF function may be developmental specific, partly because it is absent from K562 cells that express human embryonic and fetal globin genes, but not the adult β-globin gene.20 If this were the case, EKLF might serve as the predominant switch signal in the regulation of globin genes. Previously, it had been shown that EKLF mRNA and DNA-binding activity are indeed present in embryonic erythroid tissues.20,22 30However, it had not been excluded that EKLF activity is controlled in a developmental-specific manner. For example, EKLF function might depend on protein modifications and/or protein partners that are developmentally regulated. However, as reported here, we have found that the β-globin transgene in the μD construct is as dependent on EKLF for expression in embryonic erythroid tissues as it is in fetal liver (Fig 4). Thus, the function of EKLF is not developmental specific. We conclude that EKLF cannot be responsible for the initiation of globin gene switching, because it is present and fully active before this process occurs. We cannot rule out that a protein other than EKLF is involved in strengthening LCR—β-globin–gene interactions in a developmental-regulated fashion. However, expression of the β-globin gene at all developmental stages when present alone with the LCR6,7 suggests this may not be the case.
Studies with several different transgene constructs have shown that the γ-globin gene is downregulated in later development, at least partly, irrespective of the presence of the β-globin gene.6-9 We predict, therefore, that it may be this autonomous downregulation of the γ-globin genes that initiates the switch from γ-globin– to β-globin–gene expression. We suggest that the β-globin gene is fully primed to interact with the LCR well before it is actually expressed. However, the interaction between the β-globin gene and LCR cannot occur until the γ-globin genes begin to be downregulated and their interaction with the LCR is weakened. Once this occurs, the LCR can switch from the γ-globin gene to the β-globin gene. By competing for LCR interactions in this manner, the β-globin gene may in turn help to downregulate the γ-globin genes more rapidly. In this sense γ-globin downregulation appears to occur more slowly in both heterozygous and homozygous EKLF knockout mice than in wild-type animals, which results in increased γ-globin expression in fetal life.15 This suggests that EKLF is involved in helping the LCR change from the γ-globin to the β-globin gene, presumably by stabilizing LCR—β-globin–gene interactions.15 However, our results show that active EKLF in itself is not sufficient to induce the process. We therefore believe it is a combination of EKLF and the initiation of the shutdown of the γ-globin genes that is needed for the switch from γ-globin– to β-globin–gene expression to occur at the proper time and rate during development.
ACKNOWLEDGMENT
We are grateful to R. Kothary for critical reading of the manuscript. We thank Dr Tim Townes and Dr Jim Bieker for providing an EKLF clone and Dr James Ellis for providing the μD14 transgenic mice.
Supported by a grant from the Medical Research Council of Canada to L.W., through a donation from the Fondation de l'Hôpital Notre-Dame, and by salary support from Défi corporatif Canderel to Q.M. L.W. is a Scholar of the Fonds de la Recherche en Santédu Québec.
Address reprint requests to Lee Wall, PhD, Centre de recherche du Centre hospitalier de l'Université de Montréal, Room 4605, Pavillon Notre-Dame, 1560 Sherbrooke East, Montreal, Quebec, Canada H2L 4M1.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.