Abstract
Intracellular H2O2 generation, as a measure of the respiratory burst, was determined after stimulation of neutrophils by immune complex (IC)-bearing human umbilical vein endothelial cells. Under static conditions, neutrophils basically responded to the immune deposits on resting endothelial cells. The rotating shear forces of ≈0.7 dynes/cm2, corresponding to the physiological flow in postcapillary venules, completely abolished this basal H2O2 generation. After activation of the IC-bearing endothelial layers with interleukin-1 (IL-1) or tumor necrosis factor (TNF), or both, for 4 hours, rolling adhesion of the neutrophils was induced, accompanied by considerable H2O2 production. The neutrophil respiratory burst was prominently inhibited by anti-FcγRIII MoAb 3G8 (72.4%), and partially by MoAb 2E1 against FcγRII (38.5%). Both MoAbs together inhibited the Fc-mediated H2O2generation by 93.4%. The respiratory burst and rolling adhesion were markedly blocked by MoAb LAM1-3 against L-selectin (91.3%), whereas the nonfunctional anti-L-selectin MoAb LAM1-14 was ineffective. F(ab)2′ fragments of MoAb 7A9 against E-selectin inhibited neutrophil rolling by 98.6%, but not the respiratory burst. Moreover, rolling adhesion of neutrophils and the related oxidative burst were CD11b/CD18- independent. In summary, L-selectin has a unique auxiliary function in triggering the FcγR-mediated respiratory burst of rolling neutrophils to IC-bearing endothelial cells, thereby substituting CD11b/CD18 under conditions of flow.
IMMUNE COMPLEX deposition and leukocyte-mediated inflammatory reactions are common to vasculitis constituting, or accompanying, the different forms of connective tissue diseases, including systemic lupus erythematosus, rheumatoid arthritis, progressive systemic sclerosis, allograft rejection, and thrombotic thrombocytopenic purpura.1-5 Antibodies may bind by their F(ab) portion directly to unidentified structures of the endothelial cell lining.6 In addition, circulating immune complexes (ICs) may be localized in the vascular wall.7 Such IC-bearing endothelial cells are susceptible to being attacked by circulating granulocytes and monocytes, expressing Fcγ receptors. Activated complement, and many chemotactic mediators, increase the destroying potential of leukocytes.8
Neutrophils express two different low-affinity Fcγ receptors (R), FcγRII, a 40-kD protein, and FcγRIII, a 50- to 70-kD protein, whereas FcγRI, the high-affinity receptor for IgG, is not present on resting neutrophils.9 FcγRII is constitutively present and is not induced in response to neutrophil activation. By contrast, FcγRIII is expressed at low levels on resting neutrophils and is mobilized from intracellular storage pools during activation.10 Using static conditions, we have previously shown that FcγRII and FcγRIII cooperate in the generation a respiratory burst in response to IC-bearing resting endothelial cells.11 Activation of the endothelial monolayers with proinflammatory cytokines increases the number of interacting neutrophils and should consequently facilitate the ligation of neutrophil FcγRs. The extravasation of leukocytes, under physiological shear stress, is a sequential process involving multiple specific molecular interactions.12,13 L-selectin mediates the initial tethering of neutrophils to the activated endothelium and starts a transient and reversible interaction, described as “rolling” adhesion. Leukocyte rolling is mediated by lectin–carbohydrate interactions, mainly involving the selectin family of adhesion molecules.14,15 The neutrophil activation, which occurs during rolling, induces L-selectin shedding and upregulation of CD11b/CD18 (Mac-1) expression.16 17 The increasing formation of Mac-1–dependent ligations strengthens the binding forces, decelerates, and finally terminates rolling adhesion. Consequently, leukocytes firmly adhere to the activated endothelium and immediately start directed transendothelial migration. It is unknown how circulating neutrophils may recognize endothelial immune deposits. Therefore, we have studied the conditions leading to initiation of the respiratory burst to endothelial immune deposits with regard to neutrophil tethering and rolling. Purified IgG antibody against human fibronectin (FN) were bound to the extracellular fibronectin of HUVEC monolayers. The oxidative response of neutrophils to these IC-bearing endothelial cells was studied with respect to the endothelial activation with proinflammatory cytokines, using rotating shear forces in the range of 0.7 dynes/cm2.
MATERIALS AND METHODS
Cultures of human umbilical vein endothelial cells (HUVEC).
Endothelial cells from human umbilical cord veins were harvested by collagenase digestion and seeded on fibronectin-coated culture flasks. The cultures were grown in Medium 199 enriched with sodium heparin (90 μg/mL; Novo Industries, Copenhagen, Denmark), endothelial cell growth supplement (15 μg/mL; Collaborative Research, Waltham, MA), and 20% human serum as described.18 Final monolayers, in their second to fourth passage, were grown on 30-mm petri dishes (Falcon, Becton Dickinson, Oxnard, CA) after a concentric area of 20 mm in diameter was bordered with a cotton bud, wetted with nontoxic dimethylpolysiloxane (Sigma, St Louis, MO), and precoated with human fibronectin. Exhibition of cytoplasmic factor VIII activity was tested by indirect immunofluorescence with rabbit anti-human factor VIII von Willebrand antibody.18
Preparation of neutrophil suspension.
Venous blood from healthy human donors was drawn into 20-mL syringes containing lithium heparin. A total of 20 mL of blood was layered onto 15-mL Ficoll-Paque (Pharmacia, Uppsala, Sweden), and the erythrocytes were allowed to sediment spontaneously at an ambient temperature. Subsequent enrichment by buoyant density centrifugation over Ficoll-Paque, and short-time hypotonic lysis to remove residual erythrocytes was performed as described.18 The neutrophils were washed twice with Gey's buffer (GIBCO, Glasgow, Scotland) and were resuspended in Hank's buffered salt solution (HBSS), supplemented with 0.1 mg/mL of human serum albumin (HBSS-A) (OHRA 20/21, Behringwerke AG, Marburg, Germany). The cell suspension contained more than 95% CD13+ neutrophils, as assessed by flow cytofluorometry.
Pretreatment of HUVEC monolayers.
First, the culture medium was discarded, and the endothelial monolayers were washed three times with Medium 199 to completely remove fibronectin-containing serum. Thereafter, the monolayers were preincubated with the purified Ig fraction of a polyclonal goat antiserum against human fibronectin (Cappel, Organon Teknika, West Chester, PA), at a protein concentration of 20 μg/cm2 in fresh Medium 199 for 1 hour at 4°C. No endothelial detachment was observed under these conditions. To remove unbound fibronectin antibody, the monolayers were washed twice with HBSS. Subsequently, the HUVEC monolayers were preincubated with culture medium containing 30 ng/mL of human recombinant interleukin-1 (IL-1) (Roche, Nutley, NJ) or 10 ng/mL TNF (Cetus, Emeryville, CA) for 4 hours at 37°C. Before co-incubation with neutrophils, the monolayers were washed twice with 1 mL of HBSS-A.
Quantification of neutrophil adherence under rotation.
Rolling adhesion was assayed using a modified Stamper-Woodruff assay. Pretreated HUVEC monolayers were washed twice with HBSS, overlayered with 2 × 106 neutrophils in 100 μL HBSS-A, and immediately placed onto the 37°C prewarmed platform of a horizontal shaker-incubator (Lab-Shaker, A. Kühner AG, Birsfelden, Switzerland) for 10 minutes at 64 rpm. The experiments were stopped by aspiration of the leukocyte suspension. The remaining sticky leukocytes were carefully overlayered with 250 μL 2% paraformaldehyde in PBS and fixed for 15 minutes. The monolayers were washed once and protected by a glass coverslip. Adherent leukocytes were assessed in four randomly chosen fields of 1 mm2 at a magnification of 400×, using phase contrast microscopy. The fields were located at a half-radius distance from the center of the concentric HUVEC monolayer. The maximal wall shear stress at the bottom with the medium used was 0.7 dynes/cm2, corresponding to the lower range of shear in post capillary venules in vivo. The value was computed according to the formula given by Ley et al19 for a similar shaking incubator.
Flow cytometric determination of H2O2production under static and shear conditions.
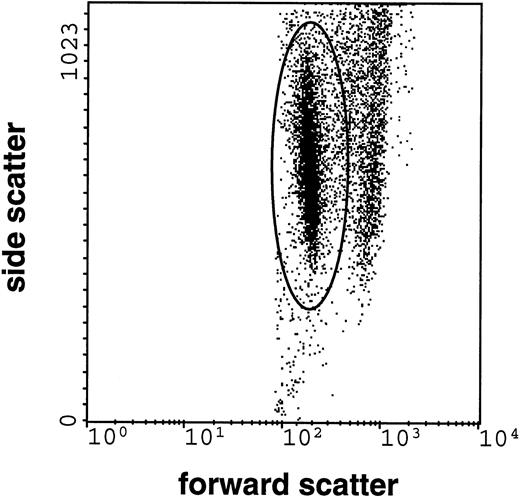
Intracellular H2O2, generated following stimulation on IC-bearing HUVEC, was assessed by quantifying the intracellular oxidation of the indicator dye dihydrorhodamine 123, a nonfluorescent and membrane-permeable fluorogenic substrate (DHR) (Molecular Probes, Eugene, OR), which is a sensitive probe for detection of the respiratory burst activity in neutrophils. DHR is oxidized mainly by H2O2 to the intracellularly accumulating fluorescent rhodamine-123.20 21 The dishes were prepared as described for the assessment of adherence. 5 × 105 nontreated neutrophils, suspended in 100 μL HBSS-A containing 1 μmol/L dihydrorhodamine-123, were allowed to interact at 37°C with IC-bearing nonactivated HUVEC or IC-bearing cytokine-activated HUVEC, respectively. Shear was applied as described above. The assay was stopped after 60 minutes on ice. The cells were scraped off the dishes and suspended in ice-cold HBSS. Dead cells were counterstained with 30 μmol/L propidium iodide (Serva, Heidelberg, Germany). A FACScan cytofluorometer (Becton Dickinson, San Jose, CA) with argon ion laser excitation at 488 nm was used to measure 10,000 cells of each stained sample. Data were acquired and processed using LYSIS-II software. Neutrophils were identified by their typical side scatter (SSC) and forward scatter (FSC) light patterns, allowing the formation of a gate for analysis of the neutrophil DHR fluorescence (Fig 1). The neutrophils positive for propidium iodide were excluded from analysis.
Flow cytometric record of the respiratory burst of neutrophils (10,000 cells counted) interacting with IC-bearing endothelial cells. Forward scatter (FSC), as a measure of the cell size, is presented on the x-axis, whereas side scatter (SSC), as a measure of granularity, is presented on the y-axis. The elliptic gate depicts neutrophils. The difference between cell populations in FSC was used to establish gates for the analysis of the dihydrorhodamine-123 oxidation in neutrophils.
Flow cytometric record of the respiratory burst of neutrophils (10,000 cells counted) interacting with IC-bearing endothelial cells. Forward scatter (FSC), as a measure of the cell size, is presented on the x-axis, whereas side scatter (SSC), as a measure of granularity, is presented on the y-axis. The elliptic gate depicts neutrophils. The difference between cell populations in FSC was used to establish gates for the analysis of the dihydrorhodamine-123 oxidation in neutrophils.
Calculation of the inhibition of neutrophil H2O2 production.
Inhibition of the neutrophil H2O2 production was calculated according to the estimation 100 − [(a − c)/(b − c)] × 100 where a is neutrophil H2O2 production in the presence of MoAb on cytokine-activated HUVEC-bearing IC, b is neutrophil H2O2 production without MoAb on cytokine-activated HUVEC bearing IC, and c is neutrophil H2O2 production on nonactivated IC-bearing HUVEC. The same type of equation was used to calculate the inhibition of neutrophil adhesion.
Antibodies.
The MoAbs LAM1-3 and LAM1-14, directed against human L-selectin, are of the IgG1 isotype.22 The MoAb LAM1-3 recognizes a functional epitope of L-selectin interfering with neutrophil rolling on cytokine-activated endothelial cells, whereas the anti-L-selectin MoAb LAM1-14 does not functionally block L-selectin ligations.23F(ab)2′ fragments of MoAb 7A924 against E-selectin and the MoAb W6/32 (IgG2a, HLA class I α chain) were generously supplied by Dr F. W. Luscinskas (Department of Pathology, Brigham and Women's Hospital, Boston, MA). MoAb IB-4 is of the IgG2a class and was a gift of Dr M. Patarroyo (Karolinska Institute, Stockholm, Sweden). MoAb 3G8 (anti FcγRIII, Immunotech, Marseille, France) and 2E1 (anti FcγRII, Immunotech) were used for functional inhibition of the IC-mediated respiratory burst of the neutrophils. All these antibodies were used at a saturating concentration of 10 μg/mL.
Statistical analysis.
Statistical validation was performed using Student's two-tailedt-test for unpaired observations.
RESULTS
The respiratory burst of neutrophils in response to IC-bearing HUVEC monolayers with and without shear.
In a previous study we showed that pretreatment of HUVEC monolayers with antibodies against extracellular matrix proteins, may serve as a model to study in vitro the response of neutrophils to deposited IC.11 Antibody to FN were found to be most suitable, because their deposition did not interfere with the integrity of the HUVEC monolayers. By direct immunofluorescence using FITC-labeled FN antibody, extracellular FN was detected on the surface of endothelial cells and the fibrous structure of the subendothelial matrix. After 1 hour of incubation, the FITC-labeled FN antibody accumulated at the intercellular regions of adjacent endothelial cells, from which it continued to diffuse into the subendothelial matrix. Regardless of whether IC deposition was performed before or after cytokine activation, there was no difference in terms of neutrophil respiratory burst induction (data not shown).
Consideration of the physiological shear stress is necessary to investigate the physiological conditions, leading circulating neutrophils to recognize immune deposits at the vascular barrier. Therefore, adhesion and respiratory burst were studied under nonstatic conditions, using a rotating adhesion assay that was initially introduced by Stamper and Woodruff25 to assess lymphocyte attachment to high endothelial venules under physiological flow. The assay was modified by Spertini et al23 to study in vitro the adhesion of leukocytes to endothelial cells in culture. In our study, the assay was adapted to assess the adherence and respiratory burst of neutrophils to IC-bearing endothelial monolayers. HUVEC monolayers were grown in the central area of culture dishes within the circular limits of a nontoxic silicon oil coat. The resulting monolayers of 20 mm in diameter were pretreated with cytokines or MoAbs, or both, washed, and overlayered with 2 × 105neutrophils suspended in 100 μL medium, before the dishes were incubated under flow or static conditions (for details see Materials and Methods).
The respiratory burst of neutrophils gaining access to the immobilized IC was quantified by assessing oxidation of the indicator dye dihydrorhodamine-123 to rhodamine-123 by flow cytofluorometry, a measure of the intracellular H2O2production.20 21 Endothelial cells and neutrophils could be clearly distinguished by the different forward- and side-scatter patterns (Fig 1). Because of the scraping dispersion procedure using cold phosphate-buffered saline (PBS), barely any HUVEC-neutrophil aggregates were detectable.
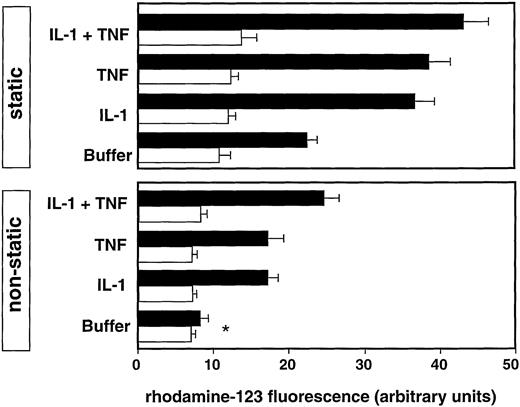
The spontaneous H2O2 generation of freshly isolated neutrophils in suspension was low (6.4 ± 0.3 U; mean ± SEM of three experiments). After activation with 100 nmol/L phorbol myristate acetate (PMA), H2O2generation markedly increased (172.3 ± 5.0 U; mean ± SEM of three experiments). Under static conditions, spontaneous settling of neutrophils on resting IC-bearing endothelial cells led to impressive respiratory burst induction, which was significantly enhanced after preincubation of the endothelial cells for 4 hours with IL-1 or tumor necrosis factor (TNF), or both (P ≤ .001; Fig 2). Control experiments with F(ab)2′ fragments of anti-FN Ab did not induce the respiratory burst (data not shown).
Respiratory burst of neutrophils on endothelial cells bearing ICs (black bars) or untreated (white bars) under static conditions (static) or rotating shear stress (nonstatic). The endothelial monolayers were preincubated for 2 hours with the indicated stimuli. Values are expressed as mean ± SEM of three experiments;P < .001 between experiments with untreated and IC-bearing HUVECs; *nonsignificant experiment. Particular experiments were performed using the same batch of neutrophils and endothelial cells.
Respiratory burst of neutrophils on endothelial cells bearing ICs (black bars) or untreated (white bars) under static conditions (static) or rotating shear stress (nonstatic). The endothelial monolayers were preincubated for 2 hours with the indicated stimuli. Values are expressed as mean ± SEM of three experiments;P < .001 between experiments with untreated and IC-bearing HUVECs; *nonsignificant experiment. Particular experiments were performed using the same batch of neutrophils and endothelial cells.
Under rotating shear, the respiratory burst of neutrophils to nonactivated HUVECs was very low. Even in the presence of endothelial immune deposits, the neutrophil response did not increase and was comparable to the spontaneous respiratory burst of freshly isolated neutrophils in suspension. Similarly, no significant respiratory burst was observed after cytokine activation of HUVECs without immune deposits. Significant H2O2 generation was only detected in the presence of immune deposits and after preactivation of the endothelial cells with TNF or IL-1. It reached about 50% of the amount obtained under static conditions (Fig2). Thus, activated endothelial cells provide adhesive conditions, which are indispensable for the Fc-mediated activation of the respiratory burst of rolling neutrophils.
Effect of MoAbs against FcγRII and FcγRIII.
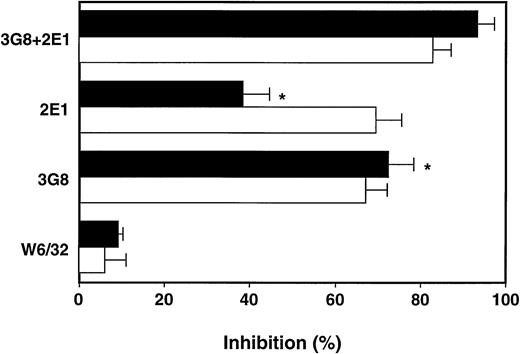
Under static conditions, both low-affinity FcγRs similarly participated in binding to endothelial immune deposits.11However, the Fc-mediated respiratory burst of rolling neutrophils was predominantly inhibited by the MoAb 3G8 against FcγRIII, whereas MoAb 2E1 against FcγRII was less effective. Both MoAbs together almost completely blocked the FcγR-mediated respiratory burst (Fig3).
Inhibition of the neutrophil respiratory burst to IC-bearing endothelial cells by the MoAbs 2E1 against anti-FcγRII and 3G8 against FcγRIII. The IC-bearing endothelial cells were activated for 4 hours with IL-1 and TNF before the respiratory burst was estimated with the indicated MoAbs at 10 μg/mL under shear (black bars) and static conditions (white bars). For calculation of inhibition refer to Materials and Methods. Values are expressed as means ± SEM of three experiments; *P < .001 for the effect of anti-FcγRII 2E1 versus anti-FcγRIII 3G8 MoAbs.
Inhibition of the neutrophil respiratory burst to IC-bearing endothelial cells by the MoAbs 2E1 against anti-FcγRII and 3G8 against FcγRIII. The IC-bearing endothelial cells were activated for 4 hours with IL-1 and TNF before the respiratory burst was estimated with the indicated MoAbs at 10 μg/mL under shear (black bars) and static conditions (white bars). For calculation of inhibition refer to Materials and Methods. Values are expressed as means ± SEM of three experiments; *P < .001 for the effect of anti-FcγRII 2E1 versus anti-FcγRIII 3G8 MoAbs.
The role of β2 integrins and selectins.
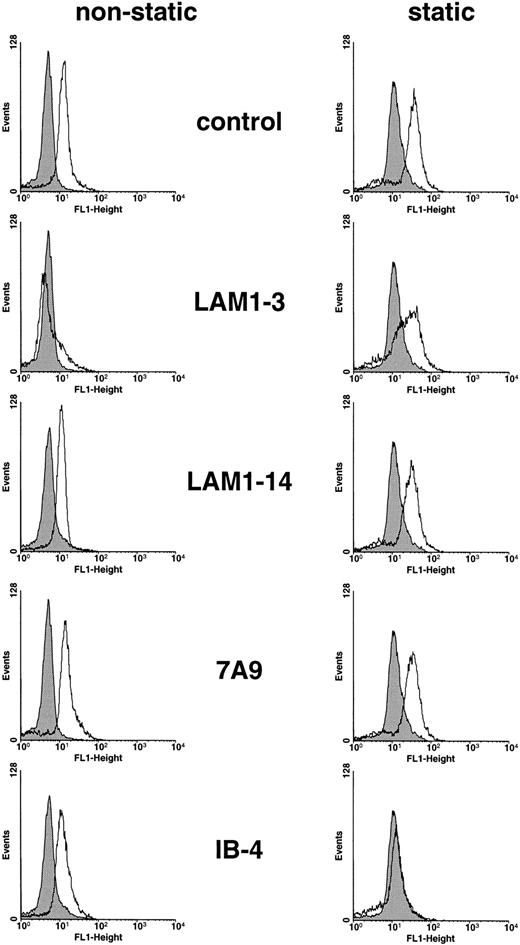
The neutrophil respiratory burst, generated under static conditions, was significantly blocked by MoAb IB-4 against CD18 (inhibition: 76.0% ± 2.3%; mean ± SEM of three experiments;P < .001; Fig 4). So far, the data correspond to the function of CD11b/CD18 in mediating adhesion under static conditions. By contrast, the anti-L-selectin MoAb LAM1-3 (inhibition, 8.4% ± 2.4%; mean ± SEM of three experiments) or F(ab)2′ fragments of MoAb 7A9 against E-selectin did not inhibit (inhibition, 1.5% ± .5%; mean ± SEM of three experiments; Fig 4).
Inhibitory effects of MoAbs against L-selectin (LAM1-3), E-selectin (7A9), and CD18 (IB-4) on the respiratory burst of neutrophils under shear stress (left histograms) and static conditions (right histograms). Control experiments were performed in the presence of buffer instead of MoAbs (control), or the MoAb LAM1-14, recognizing a nonfunctional epitope of L-selectin (LAM1-14). Endothelial cells were preactivated with IL-1 (30 ng/mL) for 4 hours. Neutrophils on resting endothelial cells in the absence of ICs, were used as control (filled histograms). The data are representative of three experiments.
Inhibitory effects of MoAbs against L-selectin (LAM1-3), E-selectin (7A9), and CD18 (IB-4) on the respiratory burst of neutrophils under shear stress (left histograms) and static conditions (right histograms). Control experiments were performed in the presence of buffer instead of MoAbs (control), or the MoAb LAM1-14, recognizing a nonfunctional epitope of L-selectin (LAM1-14). Endothelial cells were preactivated with IL-1 (30 ng/mL) for 4 hours. Neutrophils on resting endothelial cells in the absence of ICs, were used as control (filled histograms). The data are representative of three experiments.
Under rotating shear, MoAb IB-4 lost its effect (inhibition: 9.0% ± 3.2%; mean ± SEM of three experiments; Fig 4), indicating that the respiratory burst of rolling neutrophils to immobilized IC is CD18 independent. However, the functionally blocking MoAb LAM1-3 was almost completely inhibiting (91.3% ± 2.6%; mean ± SEM of three experiments; P < .001), whereas MoAb LAM1-14, recognizing a nonfunctional epitope of L-selectin, did not block the Fc-mediated H2O2 generation of neutrophils. Thus, adhesive ligation by L-selectin is crucial to the induction of the respiratory burst in our assay. By flow cytofluorometry, saturating concentrations of MoAb LAM1-3 and LAM1-14 did not compete with the binding of MoAb 2E1 and MoAb 3G8 (data not shown), thus excluding artifactual steric competition of FcγRII and FcγRIII by the anti-L-selectin MoAbs.
By contrast, F(ab)2′ fragments of the adhesion blocking MoAb 7A9 against E-selectin did not inhibit the H2O2 generation of rolling neutrophils (5.6% ± 3.7%; mean ± SEM of three experiments; Fig 4). The optimal expression of E-selectin in our experimental setup and its function in providing rolling adhesion (see below) indicates that E-selectin does not support the FcγR-mediated respiratory burst of rolling neutrophils to immobilized IC.
P-selectin, by its ligation to PSGL-1, is another candidate to support the response of rolling neutrophils to endothelial immune deposits. P-selectin is constitutively expressed on HUVECs and decreases with the number of passages. By flow cytofluorometry and laser scan microscopy, early passages of HUVEC cultures, expressed detectable levels of P-selectin (data not shown). We previously showed by transmission electron microscopy that early passage HUVECs contain Weibel-Palade bodies.26 Nevertheless, the neutrophil respiratory burst induced by nonactivated IC-bearing HUVECs was not significantly different from that induced by control HUVEC monolayers (Fig 2). Hence, an auxiliary role of the P-selectin–PSGL-1 ligand pair in this model is unlikely. In summary, the data demonstrate that functional epitopes of L-selectin are crucial to the recognition of endothelial immune deposits under fluid shear.
The rolling adherence of neutrophils to IC-bearing HUVEC monolayers under rotating shear.
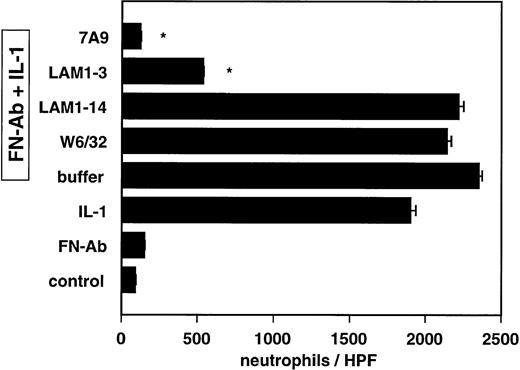
The spontaneous rolling adherence of neutrophils to resting HUVECs was relatively low, regardless of whether IC were present (Fig5). By contrast, the adhesion of neutrophils greatly increased after activation of the endothelial monolayers with IL-1 (Fig 5) or TNF (data not shown). The MoAb LAM 1-3 against L-selectin (inhibition: 80.3% ± 0.2%; mean ± SEM of three experiments; P < .001), markedly decreased neutrophil adhesion to cytokine-activated IC-bearing HUVECs, whereas MoAb LAM1-14 and W6/32 showed no significant inhibition. Of note, F(ab)2′ fragments of the anti-E-selectin MoAb 7A9 (inhibition, 98.6% ± .1%; mean ± SEM of three experiments; P < .001) were even more potent in inhibiting neutrophil rolling adhesion. This predominant role of E-selectin is consistent with its ability to maintain the lowest rolling velocity,15 which initiates stable adhesion.
Adhesion of neutrophils to IC-bearing and IL-1 activated (30 ng/mL for 4 hours) or resting endothelial cells under nonstatic conditions. The role of L-selectin and E-selectin in mediating neutrophil adhesion to IC-bearing, IL-1-activated endothelial cells was determined by preincubating neutrophils and endothelial cells for 30 minutes at 4°C with the indicated MoAbs diluted at 10 μg/mL. The adhesion assay was performed under rotation (64 rpm) for 10 minutes at 37°C, keeping the concentration of MoAbs at 10 μg/mL. Experiments with resting, IC-bearing, and IL-1-activated endothelial cells (first three bars from the bottom) were performed. Control experiments contained buffer instead of MoAbs (Buffer). Values are expressed as mean ± SEM of three experiments; *P < .0001 for MoAbs v buffer.
Adhesion of neutrophils to IC-bearing and IL-1 activated (30 ng/mL for 4 hours) or resting endothelial cells under nonstatic conditions. The role of L-selectin and E-selectin in mediating neutrophil adhesion to IC-bearing, IL-1-activated endothelial cells was determined by preincubating neutrophils and endothelial cells for 30 minutes at 4°C with the indicated MoAbs diluted at 10 μg/mL. The adhesion assay was performed under rotation (64 rpm) for 10 minutes at 37°C, keeping the concentration of MoAbs at 10 μg/mL. Experiments with resting, IC-bearing, and IL-1-activated endothelial cells (first three bars from the bottom) were performed. Control experiments contained buffer instead of MoAbs (Buffer). Values are expressed as mean ± SEM of three experiments; *P < .0001 for MoAbs v buffer.
DISCUSSION
We have previously shown that, under static conditions, neutrophils basically adhere to nonactivated, IC-bearing endothelial cells, generating a CD11b/CD18-dependent respiratory burst by ligation of FcγRII and FcγRIII.11 The data presented in this report show that, under shear conditions, neutrophils do not adhere to nonactivated, IC-bearing endothelial cells. Accordingly, no respiratory burst was induced, suggesting that the FcγRs of circulating neutrophils cannot bind to immobilized IC. Activation of the endothelial cells with proinflammatory cytokines caused neutrophil rolling adhesion, which was sufficient to induce the IC-mediated respiratory burst. Unlike spontaneously settling neutrophils, the respiratory burst of rolling neutrophils was CD11b/CD18 independent. Under static conditions, cooperation between CD11b/CD18 and FcγRIII is a prerequisite for the phosphorylation of FcγRII, initiation of downstream signaling, and oxidase assembly in neutrophils.27 The fact that ligation by β2-integrins is lost under fluid shear stress23 may explain these data and rises the question whether a similar receptor cooperation is working under fluid shear.
The process of rolling has extensively been analyzed as a coordinated action of different members of the selectin family of adhesion molecules sustaining leukocyte rolling within a broad range of fluid shear forces.15 L-Selectin is known to predominate tethering of neutrophils at higher fluid shear forces. On high endothelial venules, leukocyte adhesion through L-selectin to peripheral lymph node addressin has been shown to require a minimum level of fluid shear stress to sustain rolling interactions.28 Lawrence et al29 showed that fluid shear above a threshold of 0.5 dynes/cm2 wall shear stress significantly enhances HL-60 myelocyte rolling on P- and E-selectin. As a result, a rank order in terms of rolling velocity has been defined for neutrophils, with L-selectin > P-selectin > E-selectin15 representing an overlapping functional cascade dedicated to decelerate circulating leukocytes. The selected flow rate in our assay is well beyond the critical threshold and covers the shear requirements to study the different selectins. Accordingly, rolling adhesion on activated HUVECs was markedly inhibited by the MoAb LAM1-3 against L-selectin, and by F(ab)2′ fragments of the MoAb 7A9 against E-selectin, supporting the previous findings of Spertini et al.23 The data presented here show that rolling neutrophils specifically use L-selectin to respond to endothelial immune deposits. The observation that L-selectin is uniquely localized at microvillous sites of initial anchoring neutrophils may explain the key role of L-selectin.30-32Similarly, nonactivated neutrophils, preferentially express FcγRIII at membrane protrusions,33 whereas β2-integrins and CD44 are exclusively localized on the cell body.34,35 The respiratory burst of rolling neutrophils was mainly FcγRIII dependent, whereas in arrested adhesion FcγRII and FcγRIII equally contributed to the neutrophil H2O2 generation.11 These data further support a concept in which spatial proximity at microvillous protrusions favors ligation of FcγRs during initial capture.
Rolling adhesion of neutrophils is a self-limited process during which continuous shedding of L-selectin and gradual upregulation of functionally competent CD11b/CD18 is paralleled by a decrease in rolling velocity, ultimately leading to arrested adhesion.12,36 Several studies suggest that juxtacrine signals, exchanged by engaged adhesion molecules, induce these phenotypic changes.36-41 In particular, L-selectin ligation by anti-L-selectin MoAbs40 and sulfatides42 generates intracellular signals leading to potentiation of the neutrophil respiratory burst induced by soluble mediators.43 In this context, the rapid phosphorylation of downstream signaling proteins, including the 42-kD mitogen-activated protein kinase, corresponded to priming rather than direct induction of reactive oxidative intermediates.40 43 These data support our finding that the MoAbs LAM1-3 and LAM1-14 did not increase the Fc-mediated respiratory burst under static conditions. Thus, in our experimental setup, the outside-in signaling function of L-selectin likely primes for Fc-mediated respiratory burst induction, although the adhesive function of L-selectin was indispensable.
Its constitutive expression makes P-selectin the earliest mediator of leukocyte rolling during an inflammatory response and might therefore be another candidate contributing to ligation of FcγRs in our assay. The main ligand of P-selectin is P-selectin glycoprotein ligand (PSGL-1), which is expressed on leukocytes and platelets. Corresponding with the microvillous expression of L-selectin, PSGL-1 confers rolling on P-selectin.44 Despite the fact that early passages of resting HUVECs in our experiments expressed P-selectin, no Fc-mediated respiratory burst was detectable. This indicates that P-selectin does not support the recognition of endothelial immune deposits. An auxiliary role for PSGL-1 as a ligand of E-selectin45-48 is excluded by the lack of an active function of E-selectin. Future studies have to focus on the possibility that L-selectin can also interact with PSGL-1 to mediate neutrophil rolling on adherent neutrophils,48 49 in order to contribute to the L-selectin–supported initiation of the respiratory burst.
In summary, the study demonstrates that L-selectin has a unique auxiliary function in triggering the FcγR-mediated respiratory burst of rolling neutrophils to IC-bearing endothelial cells. Hence, L-selectin covers the function of CD11b/CD18, which is restricted to static conditions.
Supported by the Swiss National Science Foundation Grant No. 3100-40796.94 (R.M.); by the Müller-Hartmann Stiftung, Zurich, Switzerland; and by Deutsche Forschungsgemeinschaft, Grant No. FR1165/1-1 (D.F.).
Address reprint requests to René Moser, IBR GmbH, PO Box, CH-9545 Waengi, Switzerland.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.