The complete remission (CR) rate after intensive chemotherapy for acute myelogenous leukemia (AML) remains low in elderly patients, mainly because of a higher infectious mortality rate related to neutropenia and an increased incidence of adverse prognostic factors. Granulocyte-macrophage colony-stimulating factor (GM-CSF) has been shown to potentially recruit leukemic blasts into cell cycle and improve cytotoxic effects when given during chemotherapy, and to shorten the duration of neutropenia when administered after chemotherapy. Two hundred forty patients aged 55 to 75 years who had newly diagnosed AML were randomly assigned to receive placebo orEscherichia coli–derived GM-CSF (5 μg/kg/d by 6-hour intravenous infusion) starting during induction chemotherapy on day 1 and continued through and after chemotherapy until recovery of neutrophils, or evidence of regrowth of leukemia, or up to day 28. Induction chemotherapy consisted of idarubicin (8 mg/m2/d on days 1 to 5) and cytarabine (100 mg/m2/d on days 1 to 7). The study drug was not administered subsequent to the induction course. Patients who achieved a CR received continuous maintenance therapy for 1 year with four quarterly reinduction courses; in the 55- to 64-year age subgroup, patients were randomly assigned to receive or not a consolidation course before maintenance therapy. The CR rate was similar in the GM-CSF and placebo groups (63% and 60.5%, respectively; P = .79). The mortality, rate of resistant disease, and rate of regrowth of leukemia were also similar in both groups. The time to neutrophil recovery was shorter in patients who received GM-CSF (24 v 29 days; P = .0001), but the incidence and characteristics of infectious events were not different. The 2-year disease-free survival (DFS) rate was significantly improved in the GM-CSF group (48% v 21% in the placebo group;P = .003). This effect was highly significant in the cohort of patients aged 55 to 64, but only marginal in patients ≥65 years of age. There was a trend toward a longer overall survival (OS) in the GM-CSF group (P = .082). In summary, the administration of GM-CSF, concomitantly with chemotherapy and thereafter during induction course in AML, shortened the time to neutrophil recovery, but did not improve the CR rate in patients aged 55 to 75. Nonetheless, DFS and OS were significantly prolonged in patients aged 55 to 64 treated with GM-CSF. These results are promising and further evaluation of myeloid growth factors in AML is warranted.
THE INCIDENCE OF acute myelogenous leukemia (AML) increases with age and more than half of the patients are over 60 years at the time of the diagnosis.1 2Treatment results after intensive chemotherapy remain disappointing in elderly patients with AML3-5 for two main reasons: a high rate of mortality during the neutropenic period, which is mainly due to infection; and an increased incidence of adverse prognostic factors, which explains the higher failure rate.
Therefore, the use of hematopoietic growth factors (HGFs), in particular granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF), in combination with intensive chemotherapy, has appeared to be a potentially highly effective approach to improve outcome in older patients with AML. Two separate mechanisms of HGFs have been shown in AML: (1) when given before and/or during chemotherapy, HGFs can recruit leukemic blast cells into cell-cycle, thus making them more susceptible for killing by S-phase–specific agents, like cytarabine (Ara-C)6-13; and (2) when given after chemotherapy, HGFs may shorten the duration of neutropenia and therefore decrease the rate of infections, particularly fatal infections.14 15
Here, we report the results of a multicenter, randomized, placebo-controlled, double-blind clinical trial conducted by the Groupe Ouest Est Leucémies Aiguës Myéloblastiques (GOELAM) and designed to evaluate the effects of GM-CSF given both during and after intensive induction chemotherapy for de novo AML in elderly patients aged 55 to 75.
PATIENTS AND METHODS
Patients
Eligibility for enrollment in the study was limited to patients aged 55 to 75 with previously untreated de novo AML as defined morphologically by the French-American-British (FAB) system of classification.16 The bone marrow aspirate had to show at least 30% nonerythroid blast cells. Patients were excluded if they had a performance status before diagnosis of ≥2 according to the World Health Organization (WHO) grading system,17 an abnormal left ventricular ejection fraction, or severe hepatic or renal disturbances (hepatic enzymes levels > four times the normal values, serum bilirubin level >35 μmol/L, creatinine level >150 μmol/L). Patients with previous unexplained cytopenia were eligible for the study. Conversely, patients with a history of documented myelodysplastic syndrome or prior treatment with chemotherapy or radiation could not enter the study. Cytogenetic abnormalities were classified as favorable—t(8;21), t(15;17), and inv(16); unfavorable— -5, 5q-, -7, 7q-, and complex rearrangements; or intermediate—all other abnormalities.18 19 The protocol received approval from the Ethics Board of the Nantes Hospital and written informed consent was given by all eligible patients before undergoing remission therapy. The enrollment period was from May 1992 to November 1994. After May 1993, patients with acute promyelocytic leukemia were no longer included in the study because of the initiation of another study focusing on treatment with all-trans retinoic acid.
Study Design
Randomization of induction treatment with either GM-CSF or placebo was balanced within each center and stratified according to age with a cut-off at 65 years. Induction chemotherapy consisted of idarubicin (8 mg/m2/d for 5 consecutive days) combined with Ara-C (100 mg/m2/d by continuous infusion for 7 consecutive days). Human recombinant Escherichia coli–derived GM-CSF was provided by Pharmacia & Upjohn Laboratories (previously Farmitalia, Guyancourt, France) and administered at a dose of 5 μg/kg/d as a 6-hour intravenous infusion. The study medication was started on day 1, 12 hours after the initiation of chemotherapy, and continued until evidence of neutrophil recovery (absolute neutrophil count >500/μL for 3 consecutive days) or up to day 28 of the induction course. In patients with greater than 50,000/μL white blood cells before treatment, the study medication was delayed by 1 or 2 days. Medication was discontinued in patients with evidence of resistance, or regrowth of circulating leukemic cells, or in case of toxic effects attributable to the study drug. A bone marrow aspiration for evaluation of therapy was performed after neutrophil recovery or at approximately day 30 in the case of persistent neutropenia.
GM-CSF (or placebo) was not administered subsequent to the induction course, either during postremission treatment (consolidation course and/or maintenance phase) or during salvage therapy in patients resistant to the induction course. Postremission treatment was identical in the GM-CSF and placebo groups. Two strategies were used according to age to evaluate the impact of a consolidation course in the younger cohort of patients: (1) patients aged 65 to 75 received maintenance therapy for 1 year with 6-thioguanine (2 mg/kg/d given orally on days 1 through 4 every week) and Ara-C (60 mg/m2administered subcutaneously on day 5 every week) interrupted every 3 months by a reinduction course consisting of lomustine (CCNU; 40 mg given orally on day 1), mitoguazone (350 mg/m2 on day 1), and Ara-C (40 mg/m2 administered subcutaneously twice daily on days 1 through 5); (2) patients aged 55 to 64 were randomly assigned to receive one course of consolidation therapy followed by maintenance therapy or maintenance therapy alone. Consolidation therapy consisted of intermediate-dose Ara-C (1 g/m2 given on a 3-hour infusion every 12 hours on days 1 through 4) and amsacrine (100 mg/m2 administered intravenously on days 5 through 7).
Evaluation of Therapy
The efficacy of induction therapy was evaluated after one course. Complete remission (CR) was defined as a normocellular bone marrow containing less than 5% blasts and peripheral blood counts showing greater than 1,000/μL neutrophils and greater than 100,000/μL platelets. Treatment failure was defined as resistant leukemia (partial response or no response) or death (early death during the 7 days of induction treatment or death during chemoinduced bone marrow hypoplasia). Leukemic cell regrowth was defined, after a clearance of circulating blast cells consecutive to chemotherapy, as a rapid increase of these cells between days 15 and 28 to greater than 1,000/μL. Relapse was defined as the reappearance of leukemic cells in the bone marrow or peripheral blood or evidence of extramedullary leukemia. Neutropenia-related infectious complications such as septicemias, pneumonias, and other significant clinically or microbiologically documented infections were registered and compared in the two treatment groups. Severity of treatment-related toxicity was graded according to the National Cancer Institute (NCI) common toxicity criteria.20
Statistical Analysis
The main objective of the study was to assess the ability of GM-CSF to improve the antileukemic effect of induction chemotherapy by increasing the CR rate and/or duration of disease-free survival (DFS). Assuming a 50% remission rate and a 20% DFS rate at 2 years in the placebo group, a total of 240 randomized patients were required to demonstrate either a 20% CR rate improvement or a 20% increase of DFS rate (α = 5%, β = 20%, unilateral test). The secondary objectives were to evaluate the reduction of the time to neutrophil recovery, to evaluate the reduction of neutropenia-related infectious complications, and to detect an increase in overall survival (OS). All eligible patients who received the randomly assigned treatment were included in the analysis for comparison between the two treatment groups whether the planned program was completed or the study drug prematurely discontinued. Comparison between the two treatment groups were performed with Fisher's exact test for binary variables and Student's t test in case of normal distribution, for continuous variables. Survival curves and time to neutrophil recovery were estimated by the Kaplan-Meier method and comparison was made with the log-rank test. OS was calculated from the date of random assignment to the time of death. DFS was calculated from the date of first CR to the date of first relapse. The time to neutrophil recovery was calculated from the first day of chemotherapy. The duration of hospitalization was calculated from the first day of induction chemotherapy to the date of discharge from the hospital. The following factors were analyzed for their impact on CR achievement, CR duration, and survival with a logistic regression analysis (for the CR rate) and the Cox model with the likelihood ratio test (for DFS and OS): age, performance status, white blood cell count, FAB classification, and cytogenetic features.
RESULTS
Between May 1992 and November 1994, 244 patients were registered in the study by 17 institutions. Four patients (one in the GM-CSF group and three in the placebo group) were considered not eligible for entry onto the study: one because of an inadequate diagnosis, one because of poor clinical status, and two for withdrawal consent before receiving the study medication.
Characteristics of Patients
Of 240 eligible patients, 114 were randomly assigned to receive GM-CSF and 126 to receive placebo. There was no significant difference between the two treatment groups at diagnosis in terms of age, sex, performance status, FAB subtype, blood cell counts, and cytogenetic analysis (Table1). The incidence of unexplained cytopenia before diagnosis was also similar in both groups (8.5% in the GM-CSF group and 5% in the placebo group; P = .31) as was the incidence of morphologic features of myelodysplasia at the time of the diagnosis (23% and 15%, respectively; P = .09). Among 240 eligible patients included in the study, eight (four in each treatment group) were judged nonassessable for induction treatment: one because of death before the first administration of the study medication and seven because of major protocol violation (one never received the medication because of a supply problem, three did not receive the assigned medication, one received the medication only during induction treatment, one received the medication only after induction treatment, and one received chemotherapy with high-dose Ara-C).
Overall Results
Of 232 patients assessable for induction treatment, 143 (62%) achieved a CR. There were 17 early deaths (7%), 22 deaths during marrow hypoplasia (9%), and 50 (22%) patients with resistant leukemia. The CR rate was not different in the two age subgroups (55 to 64 and 65 to 75 years) (Table 2).
Of 62 patients who achieved a CR in the younger age subgroup, 48 were randomized for postremission treatment. Twenty-three were assigned to receive a consolidation course before maintenance treatment and 25 to receive only maintenance treatment. As of July 1, 1996, the median follow-up duration was 36 months. The probability of remaining alive and free of disease at 2 years among the 143 patients who achieved a CR was 33% (95% confidence interval, 25% to 41%) with no significant difference between the two age subgroups (P = .12). The median DFS duration was 15 months.
The median OS duration of the 240 eligible patients was 12 months and the 2-year actuarial OS rate was 33% (95% confidence interval, 27% to 40%) with a significant difference between the two age subgroups in favor of younger patients (P = .034).
Outcome According to Prognostic Factors
Among the factors tested for their impact on CR rate, two were of significant predictive value: the initial white blood cell count (cut-off, 30,000/μL) and cytogenetic features (normal and favorable karyotype v intermediate or unfavorable) (P = .003 and P = .0003, respectively) (Table 2).
None of the prognostic factors was related to the DFS. Among patients aged 55 to 64 years, DFS was not significantly longer after the consolidation plus maintenance regimen than after maintenance therapy alone (P = .27 by the log-rank test) (Table3).
Univariate analysis indicated that the OS was related to performance status, initial white blood cell count, and to cytogenetic features. In multivariate analysis, only two parameters remained significant: initial white blood cell count (P = .0002) and the karyotype (P = .0001). OS was marginally improved in patients assigned to receive postremission treatment that included a consolidation course with a 2-year actuarial survival rate of 76%, as compared with 46% in patients who only received the maintenance regimen (P = .052, log-rank test).
Outcome According to Treatment Group (GM-CSF v Placebo)
There was no significant difference between the two treatment groups according to patient characteristics and prognostic factors at study entry (Table 1).
Extrahematologic toxicities.
GM-CSF treatment was discontinued due to intolerance in 13.5% of patients (15 of 110), whereas the rate for placebo discontinuation was only 4% (five of 122 patients) (P = .02). The main reasons for discontinuation of the study medication were flushing, hypotension, or fever (in four and two patients, respectively, of the GM-CSF and placebo arms), fluid overload symptoms (in four and one patient, respectively), serious cardiopulmonary or renal events with or without capillary leak syndrome (in seven and two patients), and bone pain in one patient of the placebo group. Extrahematologic toxicities attributable to the induction regimen or adjuvant therapies were similar in both groups. Severe extrahematologic toxicities (grade ≥3 by NCI common toxicity criteria) included hepatic toxicity in 13% and 16% of patients, respectively, in the GM-CSF and placebo arms (P = .58), cardiac toxicity in 7% and 4% (P = .39), oral mucositis in 9% and 7% (P = .62), vomiting in 5% and 11% (P = .09), and intestinal toxicity in 7% and 12% (P = .27).
Time to neutrophil recovery, neutropenia-related infectious complications, and transfusion support.
The median time from the start of chemotherapy to the recovery of a neutrophil count greater than 500/μL was 24 days (95% confidence interval, 23 to 26 days) in the GM-CSF group compared with 29 days (95% confidence interval, 28 to 32 days) in the placebo group. Neutrophil recovery was significantly shorter in the GM-CSF group (P = .0001, log-rank test). The duration of febrile episodes higher than 38.5°C was similar among the two treatment groups, as were the incidence and characteristics of significant clinically or microbiologically documented infections (Table4). Although the duration of intravenous antibiotic treatment was significantly reduced among the patients randomly assigned to GM-CSF (P = .018), the duration of hospitalization of these patients was only marginally decreased (P = .10) (Table 4). The shortening of neutropenia did not decrease the duration of systemic antifungal therapy in the GM-CSF group (P = .90), mainly because of frequent continuation of antifungal treatment beyond the end of neutropenia for persistence of either presumed or documented fungal infection. Median platelet and red blood cell transfusion requirements were not different in the two treatment groups (P = .67 and P = .10, respectively) (Table 4).
Results of induction therapy.
Sixty-nine of 110 assessable patients (63%; 95% confidence interval, 54% to 72%) achieved a CR in the GM-CSF group, as compared with 74 of 122 assessable patients (60.5%; 95% confidence interval, 52% to 69%) in the placebo group. The CR rates were not different in the two treatment groups (P = .79). Table5 gives the breakdown of the results after induction therapy. Adjustment for prognostic factors using the logistic regression model did not modify the result (P = .76, likelihood ratio test). The reasons for not achieving CR were similar in both treatment groups. The mortality rate during induction phase was 18% in patients receiving GM-CSF and 15.5% in patients receiving placebo; the rates of resistant leukemia (partial response or no response) were 19% and 24%, respectively (P = .70). Leukemic regrowth occurred in two patients in each group.
The outcome of induction treatment was also analyzed only for patients who did not have the study drug discontinued because of extrahematologic toxicities (95 patients given GM-CSF and 117 given placebo). The results were not different from those based on intent-to-treat analysis: the GM-CSF and the placebo groups were similar regarding the CR rate (67% v 61%; P = .32) and the proportion of patients who contracted documented infectious events (69% v 73%; P = .61); the time to neutrophil recovery was shorter in patients who actually completed the study drug in the GM-CSF group (24 days v 30 days; P = .0001).
Postremission therapy.
There were no significant differences between the two treatment groups considering chemotherapy administered after remission. Among patients aged 55 to 64, 10 of 32 (31%) in the GM-CSF group were randomly assigned to receive a consolidation course before maintenance treatment, as compared with 13 of 30 (43%) in the placebo group (P = .39). The proportion of patients in whom the intensity of maintenance chemotherapy had to be reduced at least once by more than 20% (in dose and/or in duration and/or by delay of therapy) was also similar in both treatment groups (47% and 54%, respectively, in the GM-CSF and placebo groups).
DFS.
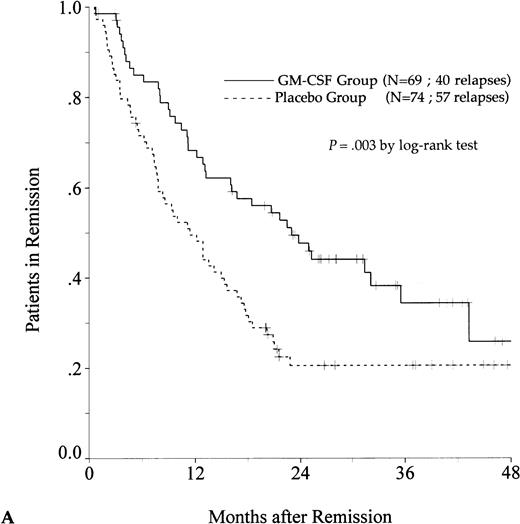
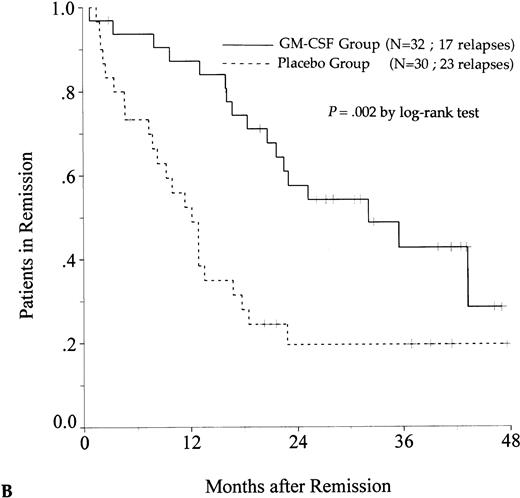
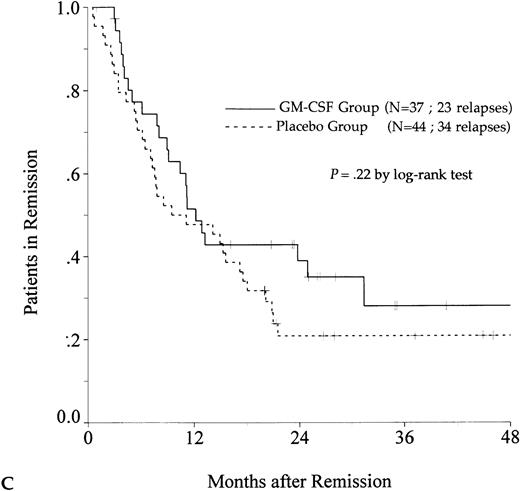
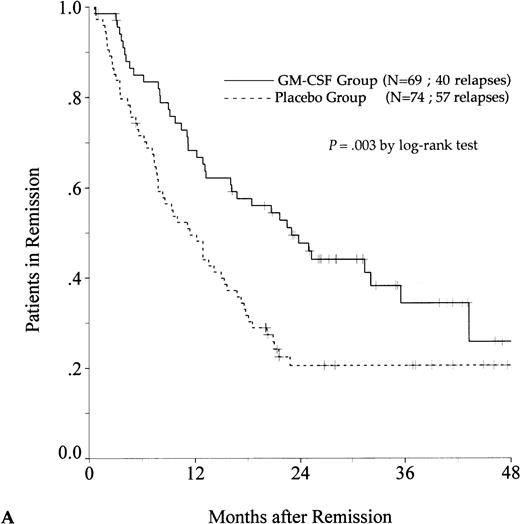
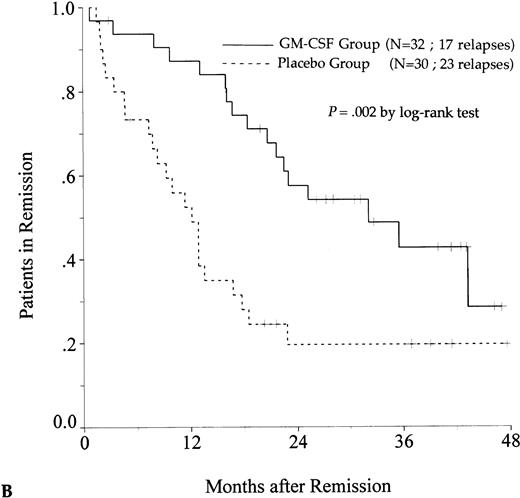
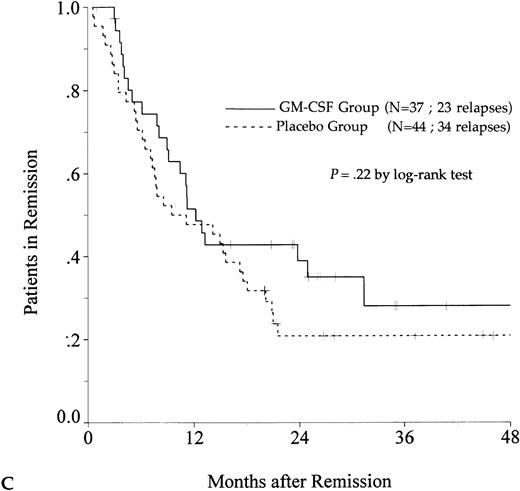
DFS was affected by whether patients had received GM-CSF or placebo concomitantly with induction course. Among 69 patients who achieved a CR in the GM-CSF group, the median DFS duration was 23 months (95% confidence interval, 16 to 30) and the probability of remaining alive and free of disease at 2 years was 48% (95% confidence interval, 35% to 60%), as compared with 11 months (95% confidence interval, 8 to 15) and 21% (95% confidence interval, 11% to 30%), respectively, for patients randomly assigned to receive placebo (P = .003, log-rank test) (Fig 1A). Adjustment for prognostic factors did not modify this result (P = .007, likelihood ratio test). The impact of GM-CSF on DFS was significantly different according to age (P = .002 and P = .22, log-rank test, between DFS in the GM-CSF and placebo groups among younger and older age subgroups, respectively) (Fig 1B and C). A more beneficial impact of GM-CSF on DFS also appeared in patients with M1, M2, and M3 FAB subtypes (P = .013); initial leukocytosis less than 30,000/μL (P = .012); and in patients receiving maintenance therapy without prior consolidation as postremission treatment in the younger age subgroup (P = .0002, log-rank test) (Table 3).
Probability of DFS for all patients who achieved CR (A), patients aged 55 to 64 years (B), and patients aged 65 to 75 years (C), according to assigned treatment group. Tick marks indicate surviving patients in continuous CR.
Probability of DFS for all patients who achieved CR (A), patients aged 55 to 64 years (B), and patients aged 65 to 75 years (C), according to assigned treatment group. Tick marks indicate surviving patients in continuous CR.
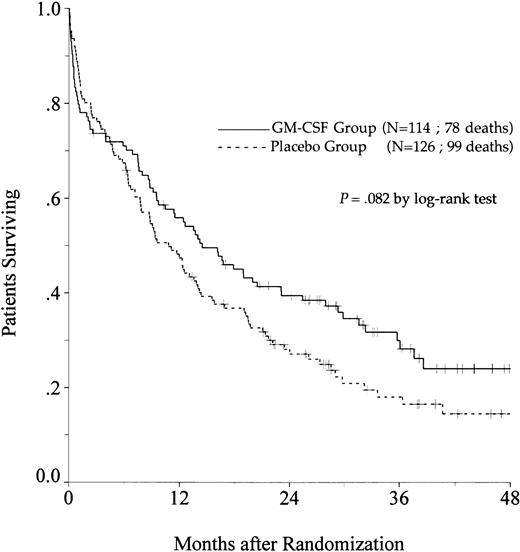
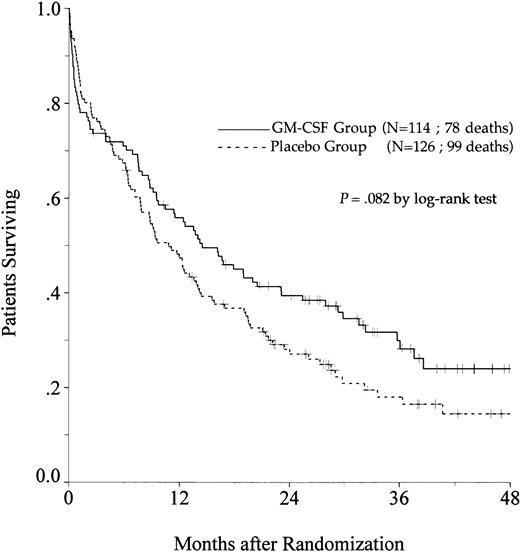
OS.
There was a trend toward an improvement in OS in the GM-CSF group (P = .082, log-rank test) (Fig2). The estimated OS rate at 2 years was 39% in the GM-CSF group (95% confidence interval, 30% to 48%) and 27% in the placebo group (95% confidence interval, 19% to 35%). Among patients greater than 65 to 75 years of age, OS was similar in the two treatment groups (P = .97). In contrast, among patients aged 55 to 64, the positive impact of GM-CSF on OS was significant (P = .014) and, in this age subgroup, the use of GM-CSF during the induction course appeared to be a predictive factor for survival independent of the other predictive factors (initial white blood cell count, karyotype, and consolidation therapy before maintenance) (P = .039, likelihood ratio test).
Probability of OS among all eligible patients, according to assigned treatment group. Tick marks indicate surviving patients.
Probability of OS among all eligible patients, according to assigned treatment group. Tick marks indicate surviving patients.
DISCUSSION
The aim of this randomized trial was to assess the ability of recombinant E coli–derived GM-CSF to improve the outcome of induction therapy and the DFS in elderly patients with AML when administered simultaneously with chemotherapy and thereafter. Our study confirms the positive result of most other controlled trials on the use of myeloid growth factors for supportive care in AML with a reduction of the median neutrophil recovery by 5 days in patients assigned to receive GM-CSF. However, this faster recovery did not result in a substantial reduction of the incidence of documented infections or mortality during hypoplasia or length of hospitalization. The shortening in duration of intravenous antibiotic requirement was the only benefit observed according to neutropenia-related morbidity.
With our treatment option, we failed to demonstrate any improvement in the CR rate among patients who were given GM-CSF, despite the significantly shorter period of neutropenia and the timing of GM-CSF for a priming effect. However, the DFS was prolonged in patients who received GM-CSF; this effect was highly significant in the cohort of patients aged 55 to 64, but only marginal in patients ≥65 years of age. This impact of GM-CSF on the length of remission suggests an increase in the antileukemic effect of chemotherapy, which could result from a reduction of the residual leukemia burden among responding patients. Finally, the use of GM-CSF did prolong OS only in the cohort of younger patients, aged 55 to 64.
Another issue raised in our trial was the comparison of two different postremission regimens evaluated only in patients aged 55 to 64: one course of consolidation followed by maintenance therapy or maintenance therapy alone. The proportion of patients assigned to consolidation course was similar in the GM-CSF group and the placebo group. DFS was not different in consolidation plus maintenance versus maintenance arms, whereas OS was marginally improved in patients who received consolidation treatment. Thus, postremission treatment did not introduce a selection bias in this study that could account for improved DFS or OS in patients who were given GM-CSF during the induction course.
Over the past decade, the myeloid growth factors, in particular GM-CSF and G-CSF, have been administered to AML patients with two major objectives: (1) shortening the duration of chemotherapy-induced neutropenia and thereby reducing the incidence of severe infections; and (2) enhancing the antileukemic effect of chemotherapy by recruitment of dormant leukemia cells into a sensitive phase of the cell cycle. A number of large, prospective, controlled trials, most of them conducted on older AML patients, have investigated these supportive or priming strategies using either GM-CSF or G-CSF.21-24 They produced positive conclusions such as safety of the administration of myeloid growth factors in the treatment of AML, considering the theoretical risk of leukemia stimulation,11 and reduction in the duration of neutropenia in the vast majority of studies. However, no clear or still debated results have been obtained in terms of improvement in CR rate, CR duration, and OS. It is difficult to compare results between these trials because of substantial differences in regard to patient population, design of the treatment program, and origin or dosage of growth factors. The major difference in the design of these trials could be the timing of myeloid growth factor in relation to initial induction chemotherapy (either before and/or during and/or after).
Twelve clinical comparative studies were designed in AML, mainly in an attempt to evaluate the supportive approach of myeloid growth factors, aimed to shorten neutropenia by administering growth factor only after chemotherapy.14,15,25-34 Most of these studies showed a significant reduction in the median time to neutrophil recovery, but no difference in infectious-related mortality rates. Even so, an additional beneficial effect was shown on the CR rate and on the OS rate in three trials. In the trial reported by the AML Cooperative Study Group,30 the CR rate was increased in patients treated with G-CSF, whereas the induction mortality and survival rate were not improved. The first clinical study testing GM-CSF14 showed a reduction in early mortality, which was more pronounced in older patients. Another study on yeast-derived GM-CSF, conducted in elderly patients,26 showed prolonged survival in the GM-CSF arm.
In another approach, the myeloid growth factor was used in 11 clinical studies for priming strategies and administered either before and during, or before and during and after induction chemotherapy.28,35-45 G-CSF was evaluated in three recent trials designed for priming43-45; they failed to show any improvement in leukemia outcome, except a trend toward improvement in the CR rate reported in one study.45 In contrast, two available studies among eight testing GM-CSF concluded there was an improvement in DFS. A German randomized trial on yeast-derived GM-CSF priming (GM-CSF starting 1 day before induction therapy) and long-term administration (over five consecutive chemotherapy courses) showed an improvement in DFS37,38; this effect was significant only in patients younger than 60 years. In the South German placebo-controlled study, E coli–derived GM-CSF was administered 2 days before chemotherapy and continued until recovery of neutrophils in the second induction course and in two subsequent consolidations39; an increase in DFS, only observed among patients younger than 50, was the major difference in the outcome between the two groups. Conversely, a pilot study in which GM-CSF was started up to 8 days before chemotherapy, showed a negative impact on the CR rate and survival rate when compared with historical control groups.36 The trials conducted by the European Organization for Research and Treatment of Cancer (EORTC)-GIMEMA Leukemia Cooperative Group28 and the EORTC-HOVON Groups40 did not show evidence of beneficial effect of the priming approach when E coli–derived GM-CSF was started 1 day before chemotherapy and continued until completion of chemotherapy or neutrophil recovery. In our study, GM-CSF was started on the first day of chemotherapy, 12 hours after the initiation of chemotherapy. Thus, the discrepancy about remission duration between these trials with similar design is difficult to explain and might suggest some detrimental effects of one or more days of blast cell stimulation without simultaneous cytotoxic therapy.
In our study, the addition of GM-CSF instead of placebo to initial induction therapy was the unique significant predictive factor for prolonged DFS. Subgroup analysis based on prognosis factors demonstrated that in patients who received GM-CSF, the improvement in DFS time was greater in patients aged 55 to 64, in patients with initial leukocytosis less than 30,000/μL, and in those with FAB cytology subtype M1, M2, or M3, when compared with patients without these characteristics. In both German studies that tested GM-CSF for priming strategies,37-39 the positive effect on DFS time was also only observed among younger patients.
In conclusion, in elderly patients with AML, the administration ofE coli–derived GM-CSF simultaneously with chemotherapy and thereafter during the induction course is safe, but does not increase the CR rate. However, DFS is prolonged in patients who receive GM-CSF; this effect is highly significant in the cohort of patients aged 55 to 64 years and translates in this subgroup to an improved OS. These results are promising and justify further evaluation of myeloid growth factors in AML by in vitro studies, as well as clinical randomized trials to identify the optimal conditions for their use.
Presented in part at the 36th and 37th annual meetings of the American Society of Hematology, Nashville, TN December 1994, and Seattle, WA December 1995.
Address reprint requests to Francis Witz, MD, Service de Médecine A, Hôpital de Brabois, CHU de Nancy, Rue du Morvan, 54511 Vandoeuvre Cedex, France.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.