The CC chemokine macrophage inflammatory protein 1β (MIP-1β), has been shown to be a chemoattractant preferentially activating CD4+ CD45RA+ T lymphocytes. Further analysis of chemokine action on lymphocytic cells has shown the potent migration-promoting capacity of MIP-1β on human thymocytes. The responding cells were the CD4+ and CD8+single-positive (SP), as well as the CD4+CD8+ double-positive (DP) populations, with little if any migratory activity on the double-negative (DN) population. The activation of thymocytes by MIP-1β appeared to be a direct, receptor-mediated event as evidenced by the rapid mobilization of intracellular calcium, increase in proteins phosphorylated on tyrosine, and activation of the mitogen-activated protein kinase (MAPK) pathway. Radioligand binding analyses showed specific and displaceable binding of MIP-1β to thymocytes with a Kd of approximately 1 nmol/L, a profile that was comparable with MIP-1β binding to CCR-5–transfected NIH 3T3 cells. In addition, CCR-5 mRNA was detected in total thymocyte populations indicating that activation of thymocytes by MIP-1β may occur through binding to CCR-5. Further dissection of the subpopulations showed that only the DP and CD8+ SP populations expressed CCR-5 and expression data on these two populations was confirmed using anti–CCR-5 monoclonal antibody. These data may be suggestive of a role for MIP-1β in human thymocyte activation, and show a potential route for HIV infectivity in the developing immune system.
THE CHEMOKINES ARE a superfamily of small molecular mass proteins (8 to 16 kD) considered to play an important role in the induction and maintenance of leukocytic infiltrates in inflammation.1,2 Presently, the superfamily can be subdivided into four subfamilies based on the conservation of an N-terminal cysteine motif. Thus, the CXC subfamily, characterized by molecules such as IL-8, MGSA/Groα, and NAP-2, are primarily activators of neutrophils, whereas the CC family, characterized by among others RANTES, MIP-1α, MIP-1β, and MCP-1 through 4, are active on leukocyte populations other than neutrophils.1-4The CX3C prototype Fractalkine has been shown to preferentially activate monocytes and T lymphocytes, whereas the C-family–member lymphotactin thus far preferentially attracts lymphocytes and natural killer (NK) cells.5-8
We originally hypothesized that chemokines can have leukocyte-specific chemoattractant effects, after demonstrations of CD4+CD45RO+ lymphocyte migration induced by RANTES, and CD8+- and CD4+-specific migration of lymphocytes induced by MIP-1α and MIP-1β, respectively.9-11 However, in heterogenous cell populations, little is known about the receptors that bind these chemokines. CC chemokine receptor 1 (CCR-1) shows high affinity binding for MIP-1α and RANTES.12 Although subsequent cloning efforts have increased the repertoire of CC chemokine receptors, these receptors all appear to show a broad specificity of action, binding two or more of the CC-chemokines.13 Moreover, whereas MIP-1α binds CCR-1 and CCR-5, evidence for specific, competable binding of MIP-1β leading to signal transduction has thus far only been shown with transfected CCR-5.14 15
More recently, MIP-1α, MIP-1β, and RANTES have been shown to act as soluble inhibitors of infectivity by M-tropic strains of HIV-1.16 Subsequently, it was shown that CCR-5 acts as an HIV-1 coreceptor mediating infection by M-tropic strains,17-21 explaining the inhibitory role of these chemokines. Thus, characterization of cell types expressing functional CCR-5, or closely related receptors, will be an important exercise in determining the course of HIV transmission and infectivity.
In our further analyses of the action(s) of MIP-1β on lymphoid cells, we have found that this chemokine can potently stimulate migration of human thymocytes. In this report we show that this effect is mediated by direct receptor ligation, eliciting rapid and transient calcium flux and tyrosine phosphorylation of multiple protein species. Cell fractionation has shown that CD4+ CD8+double-positive (DP) and CD4+ and CD8+single-positive (SP) thymocytes migrate in response to MIP-β. In addition, thymocytes respond to MIP-1β by activation of mitogen-activated protein kinase (MAPK). Reverse transcriptase PCR analyses have also shown the presence of message for CCR-5 in the DP and CD8+ SP thymocyte subpopulations, and surface expression was confirmed using anti–CCR-5 monoclonal antibody. Ligand binding analyses have shown high-affinity binding of this chemokine to thymocytes, comparable with that detected in CCR-5–transfected NIH 3T3 cells.
MATERIALS AND METHODS
Thymocyte preparation.
Human thymus, removed as a consequence of cardiac surgery (average age, 3 months; range, 1 week to 2 years; n = 18), was gently teased apart in cold Hank's balanced salt solution (HBSS), under sterile conditions. Total thymocytes were separated after Ficoll density gradient centrifugation and multiple HBSS washes at 4°C. Minor contaminating populations of monocytes/macrophages were removed after Dynal bead separation of anti–CD14-labelled cells according to standard protocols. Contaminating erythrocytes were rigorously removed by multiple centrifugation on Ficoll. Populations of whole thymocytes were then stored on ice before use or stained for fluorescence activated cell sorting (FACS) of individual subpopulations.
FACS and CCR-5 staining.
For cell sorting, thymocytes were labelled with anti–CD4-fluorescein isothiocyanate (FITC) and anti–CD8-PE (Becton Dickinson, Mountain View, CA). After gating on appropriate populations, SP (CD4+ or CD8+) cells, CD4+CD8+ DP and CD4− CD8 double-negative (DN) cells were sorted using a FACStar Plus (Becton Dickinson) cell sorter by standard methods. Sorted populations were used immediately for bioassay, or frozen at −80°C before RNA preparation.
Surface expression of CCR-5 was assessesed using a monoclonal anti–CCR-5-FITC antibody according to the manufacturer's instructions (R&D Systems, Minneapolis, MN; IgG2b). Briefly, human thymocytes were prepared and stained with anti–CD4-PE, anti–CD8-PE-cychrome, and CCR-5-FITC antibodies as described above. Three color-standard FACS analyses were performed, with data being collected on 10,000 events, and CCR-5 expression was assessed on populations gated for CD4+, CD8+, CD4−/CD8−, and CD4+/CD8+expression.
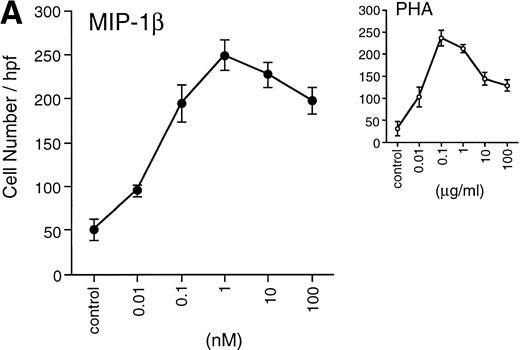
(A) Migration of unfractionated thymocytes in response to MIP-1β (0.01 to 100 nmol/L), or PHA (0.01 to 100 μg/mL). Each point represents the mean ± SEM for migration from n = 4 experiments performed in duplicate.
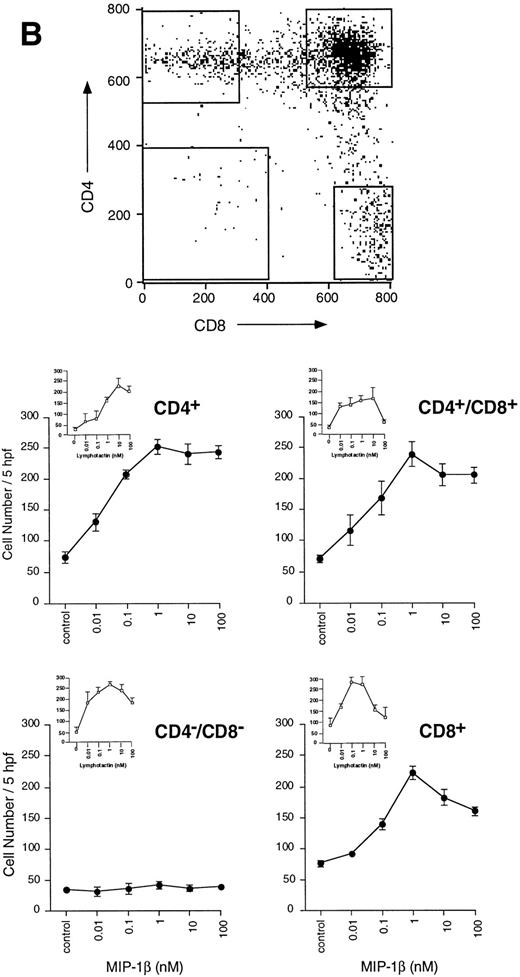
(B) Migration of thymocytes from each purified subpopulation in response to MIP-1β. Antibody-labelled cells were sorted on the FACStar Plus cell sorter, as detailed in the Materials and Methods section. Sorted cells were collected from the populations delineated by the gates in the dot-plot. Cells were washed once in PBS, then used immediately for assay of migration. Each point represents the mean ± SEM migration from n = 3 experiments performed in duplicate.
(A) Migration of unfractionated thymocytes in response to MIP-1β (0.01 to 100 nmol/L), or PHA (0.01 to 100 μg/mL). Each point represents the mean ± SEM for migration from n = 4 experiments performed in duplicate.
(B) Migration of thymocytes from each purified subpopulation in response to MIP-1β. Antibody-labelled cells were sorted on the FACStar Plus cell sorter, as detailed in the Materials and Methods section. Sorted cells were collected from the populations delineated by the gates in the dot-plot. Cells were washed once in PBS, then used immediately for assay of migration. Each point represents the mean ± SEM migration from n = 3 experiments performed in duplicate.
Chemotaxis assay.
Chemotaxis of thymocytes was performed by modification of the previously described method22 by using the 48-well modified Boyden chamber from NeuroProbe (Cabin John, MD). Cells that had migrated onto the underside of an 8 or 5 μm PVPF polycarbonate membrane, after 1-hour incubation at 37°C, were quantified under high power (400x) and results expressed as mean ± SEM for five high-power fields in comparison with lymphotactin-induced migration used as a positive control.
Calcium flux analyses.
Whole thymocyte populations were labelled with indo-1AM (3 μm final concentration; Molecular Probes, Eugene, OR) for 45 minutes at room temperature. After labelling, cells were resuspended in 1 mL HBSS containing 1% bovine serum albumin (BSA). Measurement of calcium flux was performed according to previously published methods23 by using a PTI fluorimeter (South Brunswick, NJ).
Phosphorylation analyses.
Unfractionated thymocytes were used for phosphorylation analyses as a determination of additional signal transduction mechanisms stimulated by MIP-1β. Whole thymocytes were used immediately after separation from the thymus. Cell viability at the time of assay was consistently greater than 95%. After agonist stimulation, cell pellets were lysed in buffer (1% NP-40, 50 mmol/L Tris-HCl, pH 8.0, 150 mmol/L NaCl, 0.25% deoxycholate, 5 mmol/L EDTA) containing protease and phosphatase inhibitors (1 mmol/L PMSF, 10 μg/mL each of aprotinin and leupeptin, 1 mmol/L sodium orthovanadate, 1 mmol/L EGTA, 100 μmol/L β-glycerophosphate, 10 mmol/L sodium fluoride, 1 mmol/L tetrasodium pyrophosphate). Lysates were mixed with 2x SDS running buffer and loaded onto 12% tris-glycine gels (Novex; San Diego, CA). Equivalent loading of samples was determined by BCA assay (Pierce Chemical Co, Rockford, IL). Separated proteins were transferred to PVDF membranes (Millipore Corp, Bedford, MA), nonspecific sites blocked with block-buffer (5% BSA in Tris-buffered saline [TBS], containing 0.1% [vol/vol] Tween-20 and 0.05% [vol/vol] thimersol) then stained overnight with antiphosphotyrosine antibody (4G10; UBI). After staining, phosphorylated proteins were shown with an antimouse IgG-HRP conjugate, washed three times as above, detected using ECL reagent (Amersham Corp, Arlington Heights, IL) and Biomax MR film (Eastman Kodak Co, Rochester, NY).
In parallel, whole cell lysates from thymocytes (5 × 106) stimulated for 3 minutes with MIP-1β (10−7 mol/L) or 10 μg anti-CD3 (UCHT-1; Sigma) were run on 10% tris-glycine gels, transferred as detailed above then the blots stained with anti-ACTIVE MAPK antibody (rabbit polyclonal; 1:5000 dilution; Promega Corp, Madison, WI). Activated MAPK was detected using ECL reagent, as described above. The blot was subsequently stripped and reprobed with anti-ERK2 (p42 MAPK) antibody (1:1000 dilution; Transduction Laboratories, Lexington, KY).
Reverse transcriptase-polymerase chain reaction (RT-PCR) analyses of thymocyte populations.
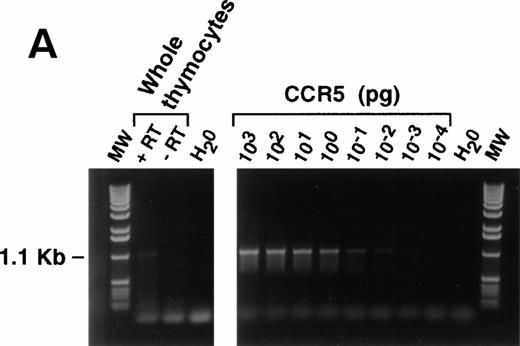
Total thymocytes, or individually-sorted populations (>98% purity; 2 × 106-107 cells) were washed once in phosphate-buffered saline (PBS) and RNA was extracted using the S.N.A.P. Total RNA Isolation Kit (Invitrogen, San Diego, CA) according to the manufacturer's protocol. First-strand cDNA synthesis for use in PCR reactions was generated using the cDNA Cycle Kit (Invitrogen). Parallel reactions were set up without AMV RT enzyme in order to control for genomic DNA contamination in subsequent PCR reactions. PCR analysis was then performed using the following primers specific for human CCR-5: sense, 5'-ATGGATTATCAAGTGTCAAGT-CCAATC; antisense, 3'-TCACAAGCCCACAGATATTTCCTGC,14 and 40 cycles of 94°C, 1 minute; 55°C, 2 minutes; 72°C, 3 minutes. Similar reactions were run with human CCR-5 cDNA (positive control), and water in place of first-strand cDNA (negative control). Amplified product (1.1 Kb) was resolved on a 1% agarose gel containing ethidium bromide.
Radioligand–binding analyses.
For displacement binding studies, purified human thymocytes were incubated with 0.2 nmol/L 125 I-labeled chemokine (2200 Ci/mmol/L; DuPont NEN, Boston, MA) for 3 hours at 4°C in the presence of increasing concentrations of unlabeled chemokine. All reactions were performed in duplicate and were repeated three to five times. Each reaction contained 107 cells in a final volume of 150 μL of binding buffer (PBS, 0.5% BSA, 1 mmol/L CaCl2, 5 mmol/L MgCl2). The reaction was terminated by layering the cell suspension over 200 μL oil (80% silicone oil [Dow Corning 550 fluid] and 20% paraffin oil), and the cells were separated from the buffer by centrifugation. The tube bottom containing the cell pellet was cut and placed into a fresh vial and the radioactivity quantified in a Packard Cobra 5010 g counter (Packard Instruments Co). The binding data was analyzed using Igor Pro software (Wavemetrics) to determine the displacement binding, Kd, and the number of binding sites per cell.
RESULTS
MIP-1β induces chemotaxis of human thymocytes.
Freshly isolated unfractionated thymocytes and sorted DN, DP, CD4+, and CD8+ SP thymocytes were assayed for chemotaxis in response to MIP-1β, using phytohemagglutinin (PHA) as a control agonist. Figure 1A shows the mean number of cells migrating in response to PHA (0.01 μg/mL to 100 μg/mL) compared with that induced by MIP-1β. Surprisingly, MIP-1β induced potent migratory effects on the whole thymocyte population over a broad range of concentrations ranging from 0.1 nmol/L to 100 nmol/L. Maximal migration of thymocytes was obtained with 10−9 mol/L MIP-1β ( Fig 1A).
Further analysis showed that induced migration was occurring within the DP, CD4+, and C8+ SP populations, but not in the DN population. The migratory potential of the DN population was confirmed by their response to lymphotactin (Fig 1B, inset). Little, if any, difference was noticed in the potency of MIP-1β in stimulating migration of the individual responder populations (Fig 1B). In all responder populations, maximal migration was obtained with 1 nmol/L MIP-1β, with significant migration above background occurring with concentrations as low as 0.01 nmol/L. In contrast, there was significantly greater migration induced by MIP-1β in the CD4+ SP and DP populations at subnanomolar concentrations, with almost double the number of cells responding at 0.1 nmol/L MIP-1β compared with the CD8+ SP population.
MIP-1β stimulates calcium flux in thymocytes.
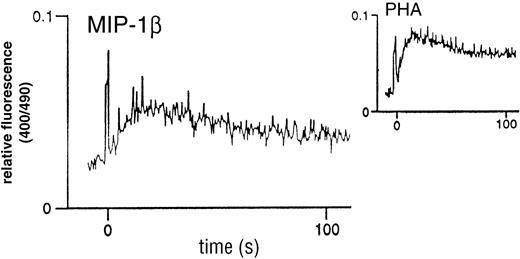
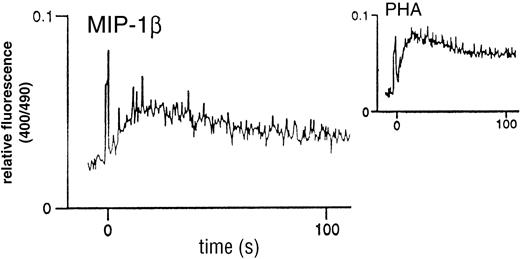
Analysis of classical chemokine function prompted an investigation of the capacity of MIP-1β to stimulate calcium flux in the thymocyte population. As shown in Fig 2, there was an elicited flux with unfractionated thymocytes, characterized by a rapid transient flux, in contrast to the more prolonged flux associated with PHA. These flux profiles are comparable with those obtained using other chemokines on unfractionated peripheral blood lymphocytes (PBL) [not shown]. In addition, the short duration of the response was suggestive of a classical chemokine-chemokine receptor-induced transient Ca2+ flux.
MIP-1β–induced Ca2+ mobilization in unfractionated human thymocytes. Freshly-isolated cells were loaded with 3 mmol/L (final) Indo-1AM and kept in a constantly-stirred cuvette, as detailed in the Materials and Methods section. Stimulation was with 100 nmol/L recombinant human MIP-1β. The inset shows the flux profile obtained in response to 2 μg/mL PHA as a positive control. Flux profiles are representative examples taken from n = 6 experiments.
MIP-1β–induced Ca2+ mobilization in unfractionated human thymocytes. Freshly-isolated cells were loaded with 3 mmol/L (final) Indo-1AM and kept in a constantly-stirred cuvette, as detailed in the Materials and Methods section. Stimulation was with 100 nmol/L recombinant human MIP-1β. The inset shows the flux profile obtained in response to 2 μg/mL PHA as a positive control. Flux profiles are representative examples taken from n = 6 experiments.
Phosphorylation analyses.
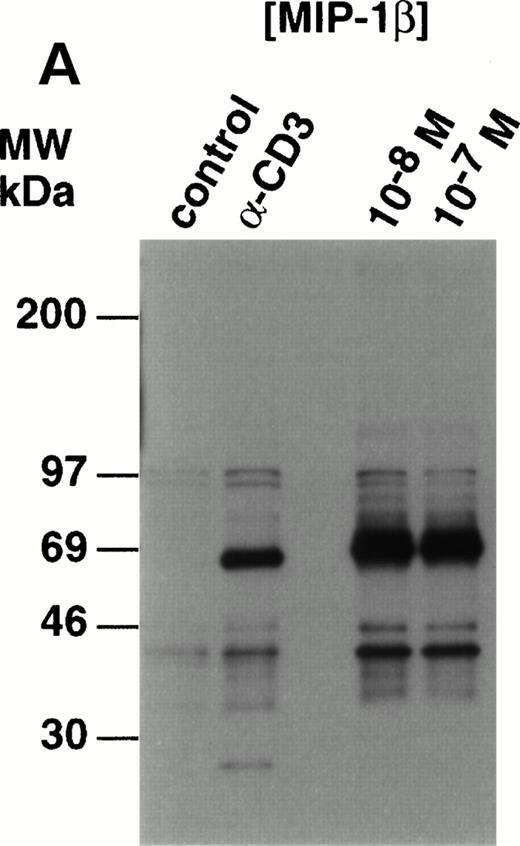
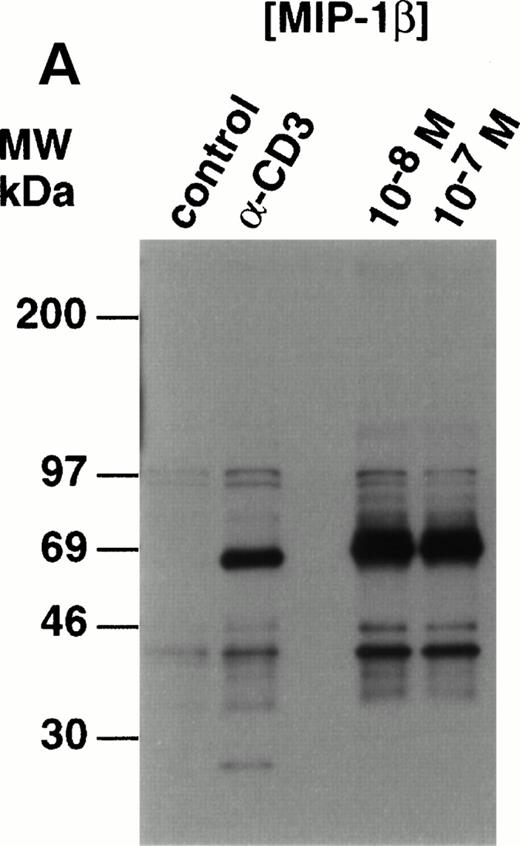
Analysis of whole cell lysates from freshly-prepared thymocytes stimulated with MIP-1β, showed potent upregulation of tyrosine phosphorylation (Fig 3A). The species phosphorylated appeared similar to those observed in the anti–CD3-stimulated thymocytes with one exception; a species of 21 to 24 kD was clearly phosphorylated in the thymocytes in response to anti-CD3 but not MIP-1β. This was shown to be TCRζ (p24) by Western analysis (data not shown). Compared with control, unstimulated lysates, proteins of 120 to 130 kD, 65 to 90 kD, and 40 to 46 kD were phosphorylated in MIP-1β–stimulated cells. Preliminary studies using monoclonal antibodies in Western analysis of antiphosphotyrosine immunoprecipitated proteins, have shown that p130Cas and p56lck constituted at least two of the phosphorylated species (K.B.B., manuscript in preparation).
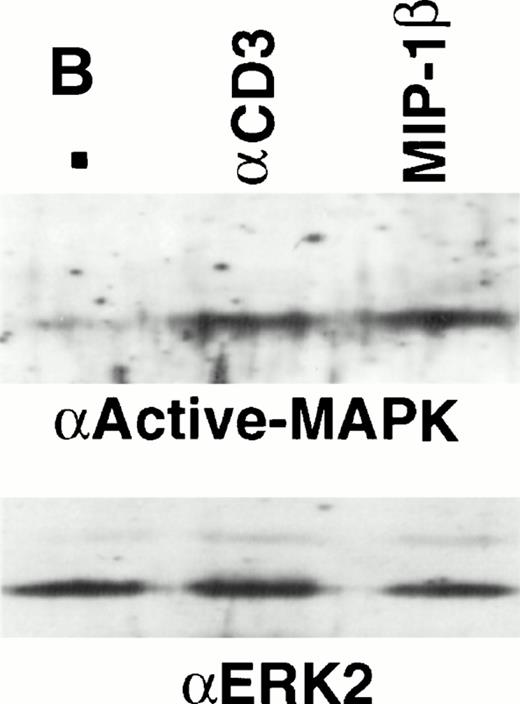
Analysis of tyrosine phosphorylated proteins in thymocytes in response to MIP-1β. (A) Freshly-isolated unfractionated thymocytes were kept on ice before stimulation wth 10 nmol/L and 100 nmol/L MIP-1β, or anti-CD3 as a control, for 3 minutes at 37°C. After stimulation, the lysates were prepared as described in the Materials and Methods section. Electrophoresis under reducing conditions was performed using 12% tris-glycine gels and Western blots probed with antiphosphotyrosine (clone 4G10). (B) Thymocytes were purified and stimulated with anti-CD3 (10 mg) or 100 nmol/L MIP-1β as outlined in the Materials and Methods section. Electrophoresis under reducing condition was performed using 10% tris-glycine gels and Western blots probed with antiactive MAPK. Blots were stripped and reprobed with anti-ERK2 (lower panel) to show equivalent loading.
Analysis of tyrosine phosphorylated proteins in thymocytes in response to MIP-1β. (A) Freshly-isolated unfractionated thymocytes were kept on ice before stimulation wth 10 nmol/L and 100 nmol/L MIP-1β, or anti-CD3 as a control, for 3 minutes at 37°C. After stimulation, the lysates were prepared as described in the Materials and Methods section. Electrophoresis under reducing conditions was performed using 12% tris-glycine gels and Western blots probed with antiphosphotyrosine (clone 4G10). (B) Thymocytes were purified and stimulated with anti-CD3 (10 mg) or 100 nmol/L MIP-1β as outlined in the Materials and Methods section. Electrophoresis under reducing condition was performed using 10% tris-glycine gels and Western blots probed with antiactive MAPK. Blots were stripped and reprobed with anti-ERK2 (lower panel) to show equivalent loading.
The phosphorylation of species of 40 to 46 kD suggested the potential activation of MAPK. Analyses using antiphosphoMAPK polyclonal antibodies clearly showed activated MAPK from cells treated with either anti-CD3 or MIP-1β (Fig 3B). No phosphoMAPK was observed with anti–ERK-1 antibodies (not shown).
Competition-binding analyses.
Binding studies with the unfractionated thymocyte population indicated displaceable MIP-1β binding, albeit with a unique dissociation profile. Two phases were observed, one consisting of an initial partial displacement, followed by a second more complete displacement (Fig 4A). Strong initial binding of125I-MIP-1β was rapidly competed by addition of unlabelled MIP-1β. Addition of this competitor to 1 nmol/L resulted in a significant reduction in binding, indicating a high-affinity interaction (Kd of 0.1 nmol/L). For the initial phase, maximal displacement occured with addition of 1 nmol/L to 5 nmol/L unlabelled MIP-1β. Interestingly, however, addition of MIP-1β to 10 nmol/L resulted in potentiation of total125I-MIP-1β binding (n = 5) , marking the second phase of the displacement profile. Addition of unlabelled MIP-1β completely dissociated the bound 125I-MIP-1β. The Kdvalue for this latter displacement profile was 35 nmol/L; a value also derived if all the points are included in the calculations, as shown in Fig 4A.
(A) Biphasic thymocyte binding of125I-MIP-1β. Initial binding of 125I-MIP-1β to thymocytes is competed by increasing concentrations of unlabelled MIP-1β. Scatchard analysis of all points indicate a total Kd value of 35 nmol/L with an estimated 4,500 sites per thymocyte over the entire population (not shown). If each phase of the reaction is analyzed separately, a Kd value of 0.1 nmol/L (first phase; dotted line) and 35 nmol/L (second phase) are determined. This reaction carried out at 4°C contains 70,000 cpm of input 125I-MIP-1β per reaction, and is representative of n = 5 experiments each using thymocytes from different donors. (B and C) Heterologous competiton of125I-MIP-1β bound to thymocytes by MIP-1α and RANTES, respectively. This result is taken from the same experiment as depicted in (A). (D) Ligand binding study using 125I-MIP-1β and CCR-5/NIH 3T3 transfectants. 125I-MIP-1β was added at 120,000 cpm per reaction. (E) and (F) Heterologous competition of125I-MIP-1β bound to CCR-5/NIH 3T3 cells by MIP-1α and RANTES, respectively.
(A) Biphasic thymocyte binding of125I-MIP-1β. Initial binding of 125I-MIP-1β to thymocytes is competed by increasing concentrations of unlabelled MIP-1β. Scatchard analysis of all points indicate a total Kd value of 35 nmol/L with an estimated 4,500 sites per thymocyte over the entire population (not shown). If each phase of the reaction is analyzed separately, a Kd value of 0.1 nmol/L (first phase; dotted line) and 35 nmol/L (second phase) are determined. This reaction carried out at 4°C contains 70,000 cpm of input 125I-MIP-1β per reaction, and is representative of n = 5 experiments each using thymocytes from different donors. (B and C) Heterologous competiton of125I-MIP-1β bound to thymocytes by MIP-1α and RANTES, respectively. This result is taken from the same experiment as depicted in (A). (D) Ligand binding study using 125I-MIP-1β and CCR-5/NIH 3T3 transfectants. 125I-MIP-1β was added at 120,000 cpm per reaction. (E) and (F) Heterologous competition of125I-MIP-1β bound to CCR-5/NIH 3T3 cells by MIP-1α and RANTES, respectively.
The CC chemokines, MIP-1α, and RANTES, which together with MIP-1β are ligands for CCR-5, were able to compete 125I-MIP-1β binding as shown in Fig 4B and 4C, respectively. RANTES and MIP-1α were able to compete the first phase of MIP-1β binding. However, as also observed with 10 nmol/L MIP-1β, after addition of 20 nmol/L MIP-1α or 500 nmol/L RANTES, the total binding of 125I-MIP-1β increased significantly. We tested a CCR-5/NIH 3T3 transfectant for comparison with the thymocyte–MIP-1β binding profile. As shown in Fig 4D, the binding profiles observed with the thymocytes could be reproduced qualitatively in the transfected cells, albeit at smaller amplitudes. 125I-MIP-1β binding to the CCR-5 transfectant could be competed with unlabelled MIP-1β, giving rise to an extended displacement profile. MIP-1α competed125I-MIP-1β binding with a Ki of 1.0 nmol/L, whereas RANTES competed with a Ki of 0.3 nmol/L (Fig 4E and F, respectively). At concentrations of 30 nmol/L MIP-1α or 100 nmol/L RANTES, there was also a potentiation of 125I-MIP-1β total binding.
Surface expression of CCR-5.
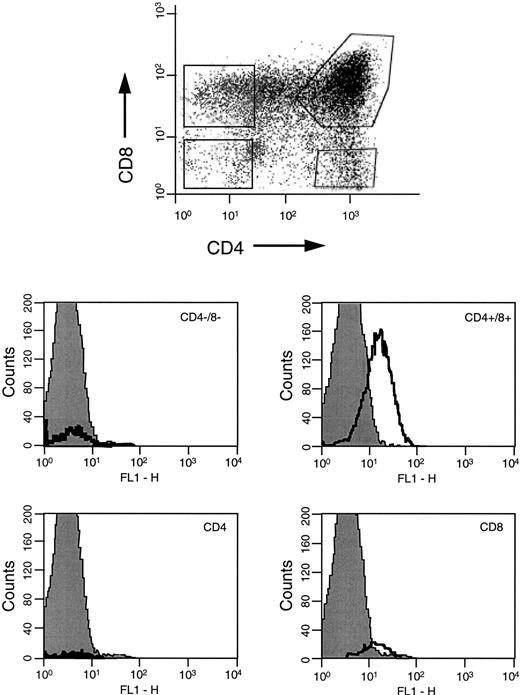
Using a specific antihuman CCR-5 FITC-coupled monoclonal antibody, the thymocyte population was analyzed for surface expression of CCR-5, the receptor for which MIP-1β has high affinity. Specific anti–CCR-5 staining was detected on the whole thymocyte population (not shown) so individual populations were analyzed for surface expression of CCR-5. Gating on the individual populations, Fig 5shows that greatest CCR-5 expression was detected on DP populations. There was slight positivity on the CD8+ SP population, however the DN and CD4 populations were negative.
FACS analyses of CCR-5 surface expression on human thymocytes. Thymocytes labelled with CD4-PE-cychrome, CD8-PE and CCR-5-FITC were gated on DN, CD4+, DP, and CD8+ subpopulations for analyses of CCR-5 expression. The FACS profiles are representative of experiments using n = 4 different human thymus donors.
FACS analyses of CCR-5 surface expression on human thymocytes. Thymocytes labelled with CD4-PE-cychrome, CD8-PE and CCR-5-FITC were gated on DN, CD4+, DP, and CD8+ subpopulations for analyses of CCR-5 expression. The FACS profiles are representative of experiments using n = 4 different human thymus donors.
RT-PCR analyses.
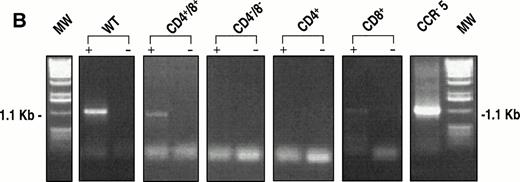
Messenger RNA for CCR-5 was detected using RT-PCR. Figure 6A shows a representative example of the expression pattern of CCR-5 mRNA in the unfractionated thymocyte population, and shows the specificity of the assay, as the message was absent from the samples lacking reverse transcriptase enzyme. A total of 2 × 106 thymocytes were used for this experiment, and the level of specific message corresponds to approximately 10−2 pg when compared with the CCR-5 cDNA control. Experiments on sorted populations (Fig 6B) also showed the presence of CCR-5 in DP and CD8+ SP thymocyte subpopulations, correlating with the surface expression noted from FACS analyses.
(A) RT-PCR analysis of CCR-5 message in thymocytes. Messenger RNA for CCR-5 was detected as described in the Materials and Methods section. An unfractionated population of thymocytes (2 × 106 total cells) was used for the reaction. Freshly isolated thymocytes were analyzed for migration capacity in response to MIP-1β (not shown) then aliquots frozen for molecular analyses. Human CCR-5 cDNA, cloned into the pBluescript Sk + vector (Stratagene), was used as a positive control, for the specificity of the primers outlined in the Methods. (B) Identical experiments were performed with thymocyte subpopulations (8 × 106) with or without the addition of reverse transcriptase enzyme.
(A) RT-PCR analysis of CCR-5 message in thymocytes. Messenger RNA for CCR-5 was detected as described in the Materials and Methods section. An unfractionated population of thymocytes (2 × 106 total cells) was used for the reaction. Freshly isolated thymocytes were analyzed for migration capacity in response to MIP-1β (not shown) then aliquots frozen for molecular analyses. Human CCR-5 cDNA, cloned into the pBluescript Sk + vector (Stratagene), was used as a positive control, for the specificity of the primers outlined in the Methods. (B) Identical experiments were performed with thymocyte subpopulations (8 × 106) with or without the addition of reverse transcriptase enzyme.
CONCLUSION
Until recently, the biology of MIP-1β has been restricted to limited reports on the migration- and adhesion-promoting capacity of this CC chemokine.12,13,24 More recent studies have shown the ability of MIP-1β to act as a soluble inhibitory factor for HIV entry into macrophages and T cells.16 Significantly, the receptor that binds and signals in response to MIP-1β, CCR-5, is a specific coreceptor for macrophage-tropic strains of HIV.17-21Irrespective of this revelation, little evidence existed for a signal transducing role of MIP-1β in lymphoid cells, with data only on activation of T-cell and NK-cell migration. We now show a novel bioactivity for MIP-1β; stimulation of human thymocyte migration in response to this chemokine. In addition, MIP-1β stimulated intracellular calcium mobilization and induced tyrosine phosphorylation of multiple protein species, including MAPK in thymocytes. Using PCR primers specific to the CCR-5 gene,14 and a specific monoclonal antibody to CCR-5 we were also able to detect specific message and protein expression for this receptor in freshly isolated DP and CD8+ SP thymocytes. This data provides some of the first evidence to support an activation role for this chemokine in lymphoid populations. The lack of expression of CCR-5 on CD4+ SP populations does not correlate with the MIP-1β–induced migration seen in vitro. Whether or not MIP-1β is activating cell migration through CCR-1, or there is a novel MIP-1β–binding receptor on thymocytes, is currently under investigation and such studies will be enhanced by use of specific neutralizing antibodies in relevant bioassays.
In equilibrium-binding experiments, MIP-1β showed a unique biphasic pattern in addition to an increase in 125I-MIP-1β binding. The increase in 125I-MIP-1β binding observed after addition of 10 nmol/L MIP-1β (or significantly higher concentrations of MIP-1α and RANTES) is potentially indicative of an increase in binding sites for this chemokine on thymocytes. This phenomenon appeared to be unique to 125I-MIP-1β because neither 125I-MIP-1α nor RANTES gave similar patterns of binding on thymocytes or the CCR-5 transfectants. The biphasic nature of 125I-MIP-1β displacement may reflect MIP-1β binding to two different, but closely related receptors. Because the ligand binding reactions were performed at 4°C, membrane trafficking events leading to increased receptor expression can be excluded. Alternatively, it is possible that CCR-5 or similar receptors undergo conformational changes in the presence of higher concentrations of MIP-1β leading to enhanced binding of this specific chemokine with the receptor. The full molecular characterization of this binding entity awaits further studies.
Interestingly, as observed in other systems where chemokines stimulated intracellular calcium mobilization,23,25,26MIP-1β–induced protein tyrosine phosphorylation in lymphoid cells. The precise role this signaling plays in stimulating thymocytes is unclear. It is also interesting in so far as PTK activity is only observed when cells are stimulated immediately after preparation, having been kept at 4°C. When cells are left at room temperature or at 37°C, there is a complete loss of apparent PTK signal transduction capacity. Such a loss of signaling may be the result of negative regulation of individual signaling components including kinases known to interact in other chemokine-mediated signal transduction cascades.27 Further studies are required to examine such possibilities, as well as the identities of phosphorylated species of 120 to 130 kD and 69 to 90 kD.
The revelation that p42 MAPK constituted one of the phosphorylated species of 40 to 46 kD leads us to speculate on the role of this chemokine in thymocyte activation. This is the first indication of the induction of MAPK activation in thymocytes using a stimulus other than anti-CD3. We have not observed proliferative activity in whole thymocyte populations using MIP-1β as a stimulus in standard assays, however there may be other subtle effects on specific subpopulations that are masked by the whole population. It is also interesting that no obvious activation of p44 MAPK was observed. Potential activation of p38 MAPK or of alternative JNK/SAPK pathways remains to be assessed to obtain a more complete picture of MIP-1β–induced signal transduction systems occuring in thymocytes. Interestingly, p38 MAPK phosphorylation has been detected in IL-8–stimulated human PMN27 and p44 MAPK phosphorylation in MCP-1–activated monocytes,28 both functions being related to cell migration, thus an investigation of the relation of this kinase activity to thymocyte migration is a logical next step.
The biological significance of thymocyte stimulation by MIP-1β is as yet unclear. Whether this chemokine plays a significant role during T-cell ontogeny and development, is difficult to determine. The absolute requirement for IL-7 in T cell commitment29suggests that MIP-1β may have synergistic and/or additive signaling roles with other cytokines and chemokines at different stages of development. The chemokine action may be localized to the microenvironment of the thymus, recruiting progenitor cells to specific areas of the thymic superstructure, or retaining them in proximity to thymic stromal cells. Because the expression pattern of MIP-1β in pro-T cells and thymocytes shows high frequency,30 this chemokine may fulfill as yet undescribed functions vital to thymocyte homeostasis. Interestingly, the DP thymocyte population showed considerable migratory activity and both the DN and DP subpopulations exhibit calcium flux responses when stimulated by MIP-1β ( not shown ). Whether or not MIP-1β stimulates the differentiation and proliferation of these cells into single-positive thymocytes is under investigation.
One result of major significance is the expression of CCR-5 on thymocytes. Because CCR-5 is an HIV coreceptor, the presence of this receptor in the thymic environment potentially provides easy access for viral infection of the host's developing immune system. Further studies are now required to extensively catalog the expression pattern of CCR-5 at different stages of development in neonates. In doing so, vital information will be obtained concerning the temporal infectivity pattern of HIV from maternal sources, which may in turn determine therapeutic regimens with which to combat such infection.
The first two authors made equal contributions to this study.
DNAX is supported by Schering Plough Corp.
Address correspondence to Kevin B. Bacon, PhD, Department of Immunology, Neurocrine Biosciences Inc, 3050 Science Park Rd, San Diego, CA 92121.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.