Interleukin-7 (IL-7) has been shown to be a critical factor in B and T lymphopoiesis, and to influence the differentiation of myeloid cell lineages. In the present study we extend these results demonstrating that IL-7 also plays an important role in the development of thymic dendritic cells (DC). The addition of IL-7 to rat fetal thymus organ cultures (FTOC) resulted in a drastic increase in the number of CD3−CD4−CD8− cells, which mostly expressed typical DC markers, including major histocompatibility complex class II, OX-62, CD11b, CD68, and CD54. These cells exhibited morphological and ultrastructural features of DC, and were potent stimulators of the allogeneic mixed leukocyte reaction. Although increased numbers of DC were continuously generated throughout the culture period in the presence of IL-7, they were not actively dividing, indicating that DC in IL-7–treated cultures did not arise by expansion of pre-existing cells. Reduced DC numbers obtained after the addition of neutralizing anti–IL-7 antibodies to mouse FTOC confirmed the relevance of endogenously produced IL-7 on thymic DC development. Furthermore, the addition of IL-7 to FTOC derived from severe combined immunodeficient mice also generated large numbers of DC in the absence of thymocyte maturation.
DENDRITIC CELLS (DC) are a heterogeneous population comprising irregularly shaped, migratory cells of sparse but wide distribution in both lymphoid and nonlymphoid tissues. In functional terms, DC are potent stimulators of T-cell–mediated immunity and, as such, have extraordinary antigen-presenting capacity.1-3 The origin of DC and the growth factor requirements for their development are not fully characterized. Several reports have shown that substantial numbers of functional DC can be generated from blood or bone marrow (BM) precursor cells under the influence of granulocyte-macrophage colony-stimulating factor (GM-CSF) alone or in combination with tumor necrosis factor-α (TNF-α) and stem cell factor (SCF).4-10 Peripheral blood monocytes can also differentiate into DC upon culture with GM-CSF and interleukin-4 (IL-4).11-13 However, although GM-CSF appears to be necessary in vitro, recent in vivo studies have shown that increased levels of GM-CSF or the lack of GM-CSF activity do not modify the numbers of DC in the lymphoid tissues,14,15 suggesting that other growth factors are important for DC generation. Additionally, DC can arise from intrathymic progenitor cells also capable of forming T lymphocytes16-18 and, interestingly, these DC develop in vitro in the absence of GM-CSF.19 According to these findings and those from previous reports suggesting that IL-7 can enhance DC formation from thymic precursors,18,19 we examined the effects of IL-7 on the development of thymic DC. IL-7 is a unique and nonredundant cytokine known to play a crucial role in thymocyte maturation,20-24 and has also been involved in the differentiation of B cells20,21,25,26 and other hematopoietic cell lineages, including megakaryocytes and myeloid cells.27-31 We now report that the addition of IL-7 to fetal thymus organ cultures (FTOC) results in the continuous generation of large numbers of functional DC, whereas deprivation of endogenously produced IL-7 drastically reduces the numbers of DC, implying a new role of IL-7 in thymic DC maturation.
MATERIALS AND METHODS
Animals.
Wistar rats and Swiss mice were maintained in our animal facilities. CB-17 severe combined immunodeficient (SCID) mice were obtained from the specific pathogen-free breeding facilities at the Severo Ochoa Center for Molecular Biology (Madrid, Spain). Rat fetuses at day 16 of gestation and mouse fetuses at day 14 of gestation were obtained from timed pregnancies. The day of finding a vaginal plug was designated day 0 of gestation.
Fetal thymus organ cultures.
Thymic lobes were aseptically removed from 16-day-old rat embryos or 14-day-old mouse fetuses using a stereoscopic microscope, trimmed of surrounding mesenchyme, and organ-cultured as follows. Four to six individual thymic lobes were placed on the surface of 0.8-μm polycarbonate filters (Millipore Ibérica, Madrid, Spain), which rested on stainless steel screen pieces attached to the central well of organ tissue culture dishes (Becton Dickinson, San Diego, CA). Lobes were cultured in 1 mL of RPMI 1640 medium (2 mmol/L L-glutamine) supplemented with sodium pyruvate (1 mmol/L), streptomycin (100 mg/mL), penicillin (100 U/mL) (all reagents: GIBCO-BRL, Eragny, France), and 10% fetal calf serum (FCS) (Biosys, Compiègne, France). The peripheral well of the culture dishes was filled with 5 mL of sterile distilled water. Cultures were incubated at 37°C in a humidified incubator containing 10% CO2 in air. Medium was replaced daily. The IL-7–treated organ cultures were performed at a concentration of 2,000 U/mL of recombinant human IL-7 (ampoule code 90/530; National Institute for Biological Standards and Control, Hertfordshire, UK).
For neutralizing studies, mouse anti-human/mouse IL-7 (mouse IgG2b) was used at a concentration of 75 μg/mL. In the presence of 5 ng/mL IL-7, 5 μg/mL antibody achieved 90% neutralization of bioactivity in the 2B clone proliferation assay (Genzyme, Cambridge, MA). In this case, control cultures were supplemented with purified mouse IgG2b monoclonal antibody (MoAb) from the MOPC 141 tumor line (ICN Biomedicals, Costa Mesa, CA).
Antibodies.
Mouse anti-rat MoAbs of the following specificities were used in our study: CD8 (OX-8 fluorescein isothiocyanate [FITC] or phycoerythrin [PE]), CD4 (OX-38 FITC or PE), CD3 (G4.18 biotin), major histocompatibility complex (MHC) class II (OX-6 FITC), CD11b (WT.5 FITC), CD54 (1A29 FITC), CD44 (OX-49 FITC), and CD25 (OX-39 FITC) were obtained from Pharmingen (San Diego, CA). Antibodies recognizing an integrin-like antigen specific for rat DC (OX-62) and CD68 (ED1 FITC) were purchased from Serotec (Oxford, UK). The following rat anti-mouse MoAbs were from Pharmingen: CD8 (53-6.7 FITC) and CD4 (H129.19 PE). The biotinylated antibodies were revealed with second-step streptavidin-CyChrome (Pharmingen), and unlabeled antibodies with FITC-conjugated F(ab′)2 fragment of rabbit anti-mouse IgG (Serotec).
Immunofluorescence staining and flow cytometry analysis.
Single-cell suspensions were obtained by passing disrupted thymic lobes through a 25-gauge hypodermic needle, and maintained on ice in phosphate-buffered saline (PBS) containing 1% FCS and 0.1% NaN3 before use. A total of 1 to 2 × 105cells were incubated with saturating amounts of FITC- and PE-labeled MoAbs for 30 minutes at 4°C. After staining, cells were washed twice and resuspended for analysis. Stainings with unlabeled antibodies were followed, after washing, by incubation with FITC-conjugated F(ab′)2 fragment of rabbit anti-mouse IgG. For three-color immunofluorescent labeling, cells were stained with anti–CD3-biotin, followed by anti–CD8-FITC, anti–CD4-PE, and streptavidin-CyChrome. Stained cells were analyzed in a FACScan flow cytometer (Becton Dickinson) from the Research Center, Faculty of Biology, Complutense University of Madrid. Debris and dead cells were excluded from the analysis by forward and side scatter and propidium iodide gatings. The data were analyzed using PC-Lysis research software (Becton Dickinson).
Cell-cycle analysis.
To determine the proportion of proliferating cells, 1 to 2 × 105 cells were stained with anti–CD8-FITC and anti–CD4-PE for 30 minutes at 4°C. Cells were washed twice with PBS and fixed in 30% ethanol for a minimum of 30 minutes, but usually overnight at 4°C. The cells were then washed, resuspended in a solution of 25 μg/mL 7-AAD (Sigma España, Madrid, Spain) in PBS with 0.025% Nonidet P-40 (Sigma), and incubated in the dark at 4°C for 2 hours. Analysis was performed in a FACScan, using Cell Fit and PC-Lysis softwares (Becton Dickinson).
Mixed leukocyte reaction (MLR) assay.
Cells recovered from 12-day control and IL-7–treated FTOC were incubated with a cocktail of anti-CD4, anti-CD8, and anti-CD3 MoAbs followed by two rounds of immunomagnetic bead depletion (Dynabeads; Dynal, Oslo, Norway). The purity of the recovered CD3−CD4−CD8− cells was 90% to 95%. Thymic DC were isolated from adult rat thymus as described.32 Subsequently, both CD3−CD4−CD8− cells and DC were treated with 25 μg/mL mitomycin C (Sigma) for 30 minutes at 37°C, extensively washed, and used at different numbers (1 cell to 2.5 to 5 × 104 cells) as stimulators for allogeneic T cells (2 × 105) isolated from rat lymph nodes. The cultures were performed in 96-well round-bottom culture plates, using 0.1 mL RPMI 1640/10% FCS. After 5 days at 37°C in 10% CO2-in-air incubator, the cultures were pulsed for 4 hours with 10 μmol/L 5-bromo-2′-deoxy-uridine (BrdU). A specific kit from Boehringer Mannheim (BrdU Labeling and Detection Kit III; Mannheim, Germany) was used to measure BrdU incorporation into newly synthesized DNA. Briefly, the labeling medium was removed and cells were dried (2 hours at 60°C), fixed in 70% ethanol in HCl (0.5 mol/L) for 30 minutes at −20°C, treated with nucleases (30 minutes at 37°C), and then incubated with peroxidase-conjugated Fab fragments of mouse anti-BrdU (30 minutes at 37°C). The peroxidase reaction was developed with ABTS-substrate (Boehringer Mannheim), and the sample absorbance was measured using an enzyme-linked immunosorbent assay (ELISA) reader at 405 nm with a reference wavelength at 492 nm.
Electron microscopy.
Organ-cultured thymic lobes were fixed by immersion in 4% glutaraldehyde, buffered to pH 7.3 with Millonig's fluid, postfixed in 1% osmium tetroxide in the same buffer, and dehydrated in acetone for embedding in Araldite (Fluka, Buchs, Switzerland). Sections were obtained with a Reichert OM-U3 ultramicrotome (Reichert-Jung, Wien, Austria). Semithin sections stained with an alkaline solution of toluidine blue were used in the histological analysis, and ultrathin sections were double-stained with uranyl acetate and lead citrate, and examined in a JEOL 10.10 electron microscope (Jeol, Tokyo, Japan).
Immunocytochemistry.
Air-dried cytospin preparations were fixed for 5 minutes in acetone at −20°C. Slides were incubated with anti-rat (or mouse) MHC class II antibodies for 60 minutes. Endogenous peroxidase activity was inhibited with 0.33% H2O2 in methanol for 5 minutes, and preparations were then incubated for 45 minutes with a 1/100 solution of peroxidase-conjugated rabbit anti-mouse (or rat) Igs in PBS (Dako, Glostrup, Denmark). The peroxidase reaction was developed with 0.05% 3, 3′-diaminobenzidine (Sigma) in PBS with 0.1% H2O2 for 10 minutes. The reaction was stopped and slides were counterstained with methylene blue and mounted.
RESULTS
Treatment of rat FTOC with IL-7.
Rat FTOC were performed using day 16 fetal thymus lobes, containing 90% to 95% of triple-negative (TN) CD3−CD4−CD8− cells,33 and were cultured for 12 days in the continuous presence of recombinant human IL-7 (2,000 U/mL based on previous titration in FTOC, data not shown). Under these conditions, IL-7 decreased the total cell yield of these lobes compared with control cultures (Table 1). The recovered cells were triple-labeled for flow cytometric analysis and the expression of CD3, CD4, and CD8 was examined. Two major effects were observed: there was a reduction in the proportion of CD4+CD8+ thymocytes, and an accumulation of mature CD4−CD8+ thymocytes and TN cells (Fig1). Despite the reduced cellularity, the absolute numbers of mature CD8+ thymocytes and TN cells were significantly higher after IL-7 treatment, showing a fourfold and a threefold increase, respectively, when compared with the control cultures (Table 1).
Phenotypic analysis of cells recovered from FTOC treated with IL-7. Cells were recovered from fetal thymic lobes after 12 days of culture, and triple-labeled for flow cytometry analysis as described in Materials and Methods. CD4 versus CD8 expression is represented in the dot plots with percentages of cells included in the corner of each quadrant. The cells within each quadrant were also examined for expression of CD3. The percentages in the corner of each histogram represent the proportion of cells expressing high levels of CD3. The profiles shown are representative of six independent experiments.
Phenotypic analysis of cells recovered from FTOC treated with IL-7. Cells were recovered from fetal thymic lobes after 12 days of culture, and triple-labeled for flow cytometry analysis as described in Materials and Methods. CD4 versus CD8 expression is represented in the dot plots with percentages of cells included in the corner of each quadrant. The cells within each quadrant were also examined for expression of CD3. The percentages in the corner of each histogram represent the proportion of cells expressing high levels of CD3. The profiles shown are representative of six independent experiments.
The specificity of the IL-7 effect could be shown by the absence of these effects, when FTOC were grown in the continuous presence of IL-7 with neutralizing anti-human IL-7 MoAb for 12 days of culture (data not shown).
IL-7 treatment generates large numbers of dendritic cells.
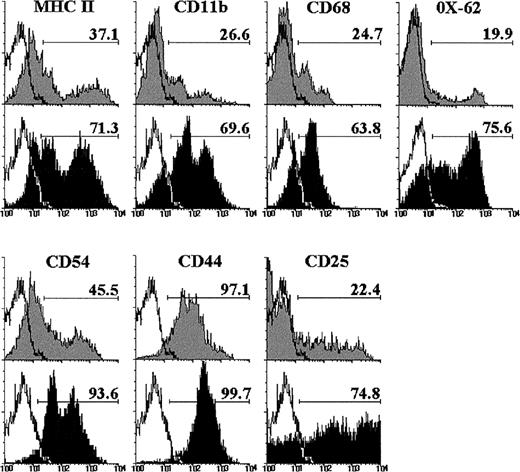
The expression of different cell markers was analyzed on double-negative (DN) CD4−CD8− cells raised after 12 days of culture with IL-7, to better characterize this thymic cell subset. As shown in Fig 2, the proportion of DN cells expressing MHC class II, CD11b, and CD68 increased after culture with IL-7. Similarly, the MoAb OX-62, which recognizes rat dendritic cells,34 reacted with the majority of the DN cell subset from IL-7–treated FTOC, but only with a small percentage of control DN cells (Fig 2). The frequency of CD54+ DN cells also increased in the presence of IL-7. In addition, most DN cells expressed CD44 in both control and IL-7–treated lobes, although with a higher intensity after IL-7 administration, and the proportion of CD25+ DN cells was three to four times higher in IL-7–treated FTOC than in control cultures (Fig 2).
Phenotype of CD4−CD8− cells raised after 12 days of culture in the absence (gray histograms) or presence of IL-7 (black histograms). These CD4−CD8− cells were determined by gating on negative fluorescence of thymic cells stained with PE-conjugated anti-CD4 and anti-CD8. A minimum of 5,000 cells was analyzed for the expression of MHC class II, CD11b, CD68, OX-62, CD54, CD44, and CD25. Black lines represent the background fluorescence using isotype-matched irrelevant FITC-conjugated MoAb, or omitting the particular MoAb under study. The results are representative of a series of three independent experiments.
Phenotype of CD4−CD8− cells raised after 12 days of culture in the absence (gray histograms) or presence of IL-7 (black histograms). These CD4−CD8− cells were determined by gating on negative fluorescence of thymic cells stained with PE-conjugated anti-CD4 and anti-CD8. A minimum of 5,000 cells was analyzed for the expression of MHC class II, CD11b, CD68, OX-62, CD54, CD44, and CD25. Black lines represent the background fluorescence using isotype-matched irrelevant FITC-conjugated MoAb, or omitting the particular MoAb under study. The results are representative of a series of three independent experiments.
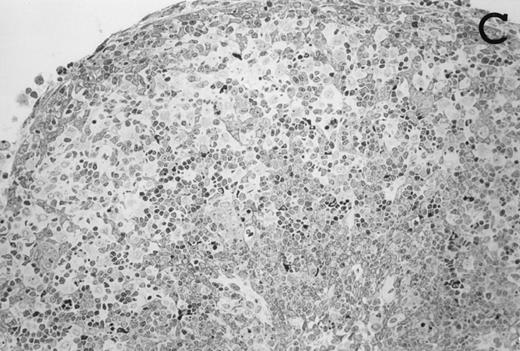
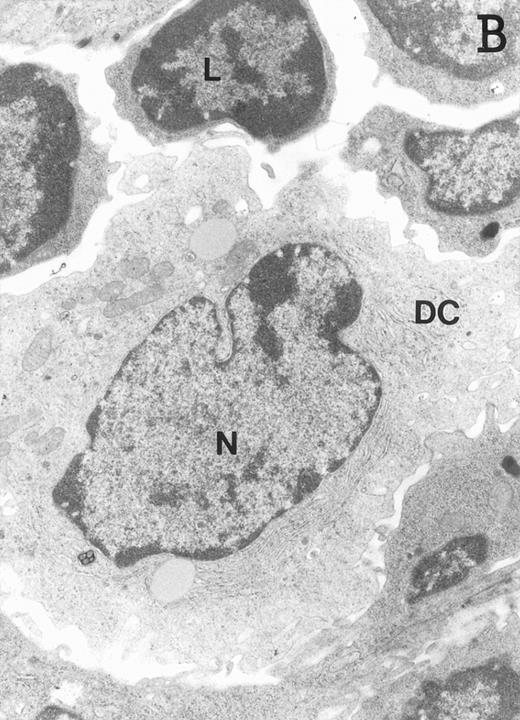
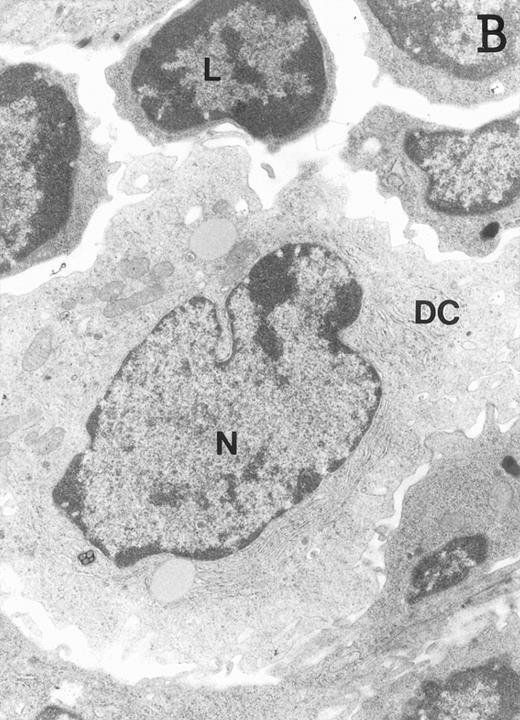
Accordingly, the DN cell subset seemed to include a large number of nonlymphoid cells after 12 days of treatment with IL-7. A histological and ultrastructural study of IL-7–treated and control FTOC was then performed to confirm the nature of these cells. A peripheral band of pale cells, morphologically identifiable as DC, was clearly present in IL-7–treated lobes (Fig 3). In contrast, thymocytes largely predominated in control FTOC (Fig 3). The ultrastructural analysis confirmed the existence of a large number of mature DC in IL-7–treated cultures. They were large, irregular, electron-lucent elements, with lobulated nuclei and cytoplasmic organelles arranged close to the nucleus (Fig4A). Moreover, immature DC, which had shorter cell processes, a less irregular nucleus, and few membranous organelles scattered throughout the cytoplasm, were also observed in these lobes (Fig 4B).
Large numbers of DC develop in the presence of IL-7. Thymic lobes were organ-cultured under control conditions (original magnifications: A, 130×; B, 650×) or in the presence of IL-7 (original magnifications: C, 130×; D, 650×) for 12 days. Note the peripheral band of pale cells in IL-7–treated lobes (C). These cells mostly correspond to DC (asterisks; D). In contrast, thymocytes (arrows) predominate in control lobes (B).
Large numbers of DC develop in the presence of IL-7. Thymic lobes were organ-cultured under control conditions (original magnifications: A, 130×; B, 650×) or in the presence of IL-7 (original magnifications: C, 130×; D, 650×) for 12 days. Note the peripheral band of pale cells in IL-7–treated lobes (C). These cells mostly correspond to DC (asterisks; D). In contrast, thymocytes (arrows) predominate in control lobes (B).
Ultrastructure of DC. DC appear as large, irregular elements showing a lobulated nucleus (N), and an electron-lucent cytoplasm with few organelles (stars) arranged close to the nucleus. Note the interdigitating cell processes in close contact (arrows) with the neighboring lymphocytes (L) (A; original magnification ×3,400). Immature DC exhibit shorter cell processes, a less irregular but usually indented nucleus, and membranous organelles scattered throughout the cytoplasm (B; original magnification ×7,900).
Ultrastructure of DC. DC appear as large, irregular elements showing a lobulated nucleus (N), and an electron-lucent cytoplasm with few organelles (stars) arranged close to the nucleus. Note the interdigitating cell processes in close contact (arrows) with the neighboring lymphocytes (L) (A; original magnification ×3,400). Immature DC exhibit shorter cell processes, a less irregular but usually indented nucleus, and membranous organelles scattered throughout the cytoplasm (B; original magnification ×7,900).
T-cell stimulatory activity of DC generated in IL-7–treated FTOC.
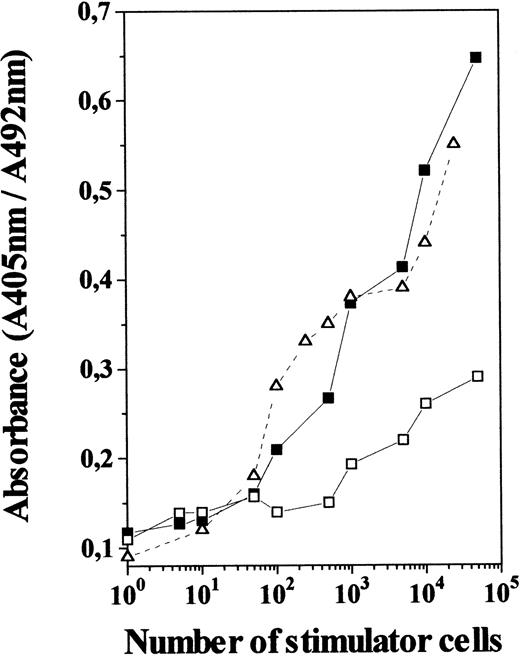
To determine whether IL-7 produced functional DC, we evaluated the allostimulatory capacity of TN cells generated in 12-day IL-7–treated FTOC, using purified T cells as responder cells. As shown in Fig5, these TN cells were found to be as efficient as freshly isolated thymic DC at stimulating the proliferation of allogeneic T cells. In contrast, TN cells from control cultures gave a poor proliferative response, even when used at high numbers, which agrees with the low incidence of DC among control TN cells. This indicates that TN cell subset raised in IL-7–treated cultures is not only composed of cells that appear morphologically like DC and express cell-surface markers characteristic of DC, but are also efficient antigen-presenting cells.
MLR-stimulatory capacity of TN cells generated in the presence of IL-7. TN cells derived from 12-day IL-7–treated FTOC (including 75% to 85% of OX-62+ cells; ▪) and control FTOC (including 15% to 25% of OX-62+ cells; □) were used at different numbers as stimulators for allogeneic T cells (2 × 105) isolated from lymph nodes. Those were compared with normal thymic DC isolated from rat thymus (▵). After 5 days the cultures were pulsed for 4 hours with BrdU. A specific kit was used to measure BrdU incorporation into newly synthesized DNA. Full details are given in Materials and Methods. Results are the means of the pooled data from two to three independent experiments, each with five cultures per point. Standard deviations represented less than 10% of the mean values.
MLR-stimulatory capacity of TN cells generated in the presence of IL-7. TN cells derived from 12-day IL-7–treated FTOC (including 75% to 85% of OX-62+ cells; ▪) and control FTOC (including 15% to 25% of OX-62+ cells; □) were used at different numbers as stimulators for allogeneic T cells (2 × 105) isolated from lymph nodes. Those were compared with normal thymic DC isolated from rat thymus (▵). After 5 days the cultures were pulsed for 4 hours with BrdU. A specific kit was used to measure BrdU incorporation into newly synthesized DNA. Full details are given in Materials and Methods. Results are the means of the pooled data from two to three independent experiments, each with five cultures per point. Standard deviations represented less than 10% of the mean values.
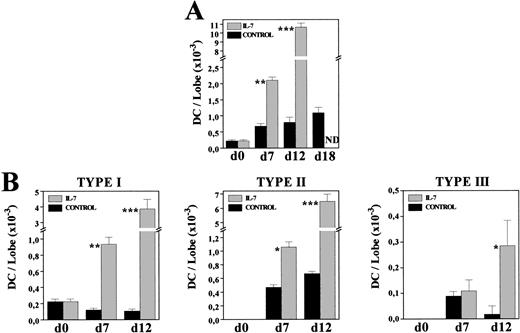
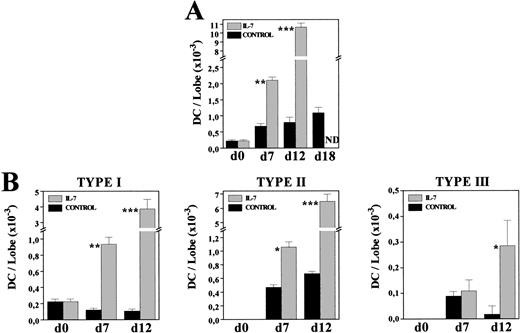
Kinetics of DC production in IL-7–treated FTOC.
To know whether the increased DC numbers were due to a gradual process occurring throughout the culture period, we comparatively analyzed the frequency and morphology of MHC class II+ cells present in both control and IL-7–treated FTOC after 7 and 12 days of culture. The proportion of MHC class II–expressing cells had almost doubled after 1 week of culture in the presence of IL-7 (C: 2.7%; IL-7: 4.7%), whereas a 10-fold increase was obtained at day 12 of culture (C: 1.4%; IL-7: 14.7%). Furthermore, on both days 7 and 12, most MHC class II+ cells (60% to 75%) exhibited a typical DC morphology, while under control conditions around 70% of these MHC class II+ cells corresponded to macrophages (data not shown). The gradual production of DC throughout the culture period was clearly evidenced by comparing the absolute numbers of DC in both culture conditions (Fig 6A). After 7 days in culture, DC numbers increased three times under control conditions, whereas a ninefold increment occurred in the presence of IL-7. In the following days, DC production practically stopped in control FTOC, increasing 1.1 and 1.4 times between days 7-12 and 12-18, respectively, but new DC were generated with IL-7, which experimented a fivefold increase between days 7-12 (Fig 6A).
Kinetics of DC generation in the presence of IL-7. Cells recovered from control and IL-7–treated FTOC were cytospun onto slides, and stained with anti–MHC class II MoAbs. Percentages of MHC class II+ cells exhibiting DC morphology were determined at days 0, 7, 12, and 18. Five thousand cells were scored in 5 to 10 cytospin preparations. At each time point, the absolute numbers of total DC (A) and their distinct subpopulations (B) were obtained by multiplying the total number of cells per lobe by the corresponding percent for each population. Values represent the mean ± SEM of two to three independent experiments. Asterisks refer to the statistical significance differences between control and IL-7–treated FTOC: *P ≤ .05, **P ≤ .01, ***P ≤ .001. ND, not determined.
Kinetics of DC generation in the presence of IL-7. Cells recovered from control and IL-7–treated FTOC were cytospun onto slides, and stained with anti–MHC class II MoAbs. Percentages of MHC class II+ cells exhibiting DC morphology were determined at days 0, 7, 12, and 18. Five thousand cells were scored in 5 to 10 cytospin preparations. At each time point, the absolute numbers of total DC (A) and their distinct subpopulations (B) were obtained by multiplying the total number of cells per lobe by the corresponding percent for each population. Values represent the mean ± SEM of two to three independent experiments. Asterisks refer to the statistical significance differences between control and IL-7–treated FTOC: *P ≤ .05, **P ≤ .01, ***P ≤ .001. ND, not determined.
Also supporting the continuous production of DC in IL-7–treated FTOC were the changes undergone by the proportion of distinct DC subsets, previously defined in rat thymus according to their morphology and MHC class II expression.35 Briefly, type I DC were considered as immature DC because they constituted the first DC subpopulation appearing during thymus development, and because they showed short, thin or bulbous-like cell processes, and a weak expression of MHC class II antigens. Type II DC corresponded to elements exhibiting the features of typically mature DC, including irregular shape and a strong reactivity for MHC class II molecules, whereas among the type III DC, which transiently appeared during thymus ontogeny, there were some very irregular ones with long, thin cell processes, and a stronger expression of MHC class II molecules than that found in mature type II DC. The three DC subsets appeared in both control and IL-7–treated lobes, but their relative proportions were substantially modified by the presence of IL-7. As described in vivo,35 in control cultures mature type II DC predominated (70% to 80%), although there was an important proportion of type III DC (13%) in 7-day FTOC, which decreased after 12 days of culture (2%). In contrast, in IL-7–treated FTOC the frequency of immature, type I DC was always significantly higher (C: 14% to 18%; IL-7: 35% to 45%). Accordingly, while the absolute numbers of type I DC gradually decreased throughout the culture period in control FTOC, a continuous increase was observed after culture with IL-7 (Fig 6B). The cell numbers of types II and III DC also increased in IL-7–treated cultures, whereas in control lobes the numbers of type II DC remained unchanged and those of type III DC even decreased (Fig 6B).
Cell-cycle analysis.
A cell-cycle analysis was performed using 7-AAD in combination with anti-CD4 and anti-CD8 MoAbs to examine the proliferative status of CD4−CD8− cell subset. Comparison of the percentage of cycling cells within DN cell subpopulation did not show significant differences between control and IL-7–treated FTOC after 7 and 12 days of culture (Table 2). The frequency of proliferating cells in the DN MHC class II+cell subset did not change either (data not shown). Therefore, these results suggest that DC in IL-7–treated cultures did not arise by expansion of pre-existing cells.
IL-7 deprivation with neutralizing anti–IL-7 antibodies results in a drastic reduction in the numbers of DC.
To determine whether in the absence of added IL-7 DC development involves endogenous IL-7, we analyze the generation of DC after the addition of a neutralizing anti-human/mouse IL-7 MoAb to mouse FTOC, because this antibody did not neutralize rat IL-7. A maximal effect was obtained with an MoAb concentration of 75 μg/mL (data not shown). After a 12-day culture period in the presence of the antibody the total cell recovery from thymic lobes was 66% reduced as compared with the control cultures (Table 3), whereas a higher reduction (78%) in DC numbers was observed (Table 3). Therefore, these results indicate that DC development is highly dependent of endogenously produced IL-7.
IL-7–induced DC generation in the absence of maturing thymocytes.
As previously shown in Fig 1, IL-7 treatment increased the numbers of mature CD8+ thymocytes, as a consequence of the ability of IL-7 to promote the differentiation of TcRαβ cells, selectively to the CD8 cell lineage.36 It has also been demonstrated that the treatment of mature T cells with IL-7 upregulates the expression and secretion of GM-CSF and IL-4,37,38 two cytokines that have been involved in the maturation pathway of DC.4-13 To avoid the possibility that accumulating mature thymocytes in IL-7–treated FTOC could be influencing the development of DC, fetal thymus lobes derived from SCID mice were organ-cultured in the presence of IL-7 for 12 days. Treatment with IL-7 resulted in a threefold increase in the total number of cells (Table 4), but did not promote T-cell maturation, development being arrested at the CD4−CD8− stage in both control and IL-7–treated FTOC (data not shown). In contrast, IL-7 administration increased the proportion of DC, the number of which increased ninefold (Table 4).
DISCUSSION
Several reports have shown that the in vitro differentiation of the so-called myeloid-derived DC,1-3 generated from peripheral blood or BM CD34+ hematopoietic progenitors, or from blood monocytes, is induced principally by GM-CSF, usually in combination with other cytokines including TNF-α, IL-4, and SCF.4-13On the contrary, the named lymphoid-related DC, coming from the earliest intrathymic precursor cell subsets CD44+CD25− and CD44+CD25+,16,17 develop in the absence of GM-CSF.19 In addition, it has been recently shown that there are no important variations in the number of DC in spleen and thymus from GM-CSF overproducing transgenic mice,15 or after in vivo administration of GM-CSF or GM-CSF plus IL-4.14 Likewise, mice lacking expression of GM-CSF receptor and GM-CSF-null mice do not have decreased numbers of DC.15 These results indicate that GM-CSF does not appear to be a major growth factor for the differentiation of the known DC subsets and, therefore, other cytokines would be involved in the process. In this regard, it has been said that transforming growth factor β1 is essential for the development of epidermal Langerhans cells,39,40 and that the injection of Flt3 ligand into mice results in increased numbers of DC in several organs.14
We show here that IL-7 plays an important role in the development of thymic dendritic cells. Addition of IL-7 to FTOC results in a drastic numerical increase in cells expressing characteristic DC markers and exhibiting typical morphological and ultrastructural features of DC, as well as the capacity to stimulate the proliferation of allo-reactive T cells.
The phenotype of DC developing in the presence of IL-7 is similar to that of freshly isolated rat thymic DC, including the lack of CD4 and CD8 surface expression.32,35,41 In contrast, DC from the human thymus express CD4, whereas mouse thymic DC predominantly express CD8.42 The relevance of CD4 and CD8 expression for the biology of DC is still unknown, although the distinct expression in different species argues against an important and specific role for these T-cell markers in DC function. In agreement, a recent study with CD8-null mice has shown that neither the development nor the functionality of DC is affected by the lack of CD8 molecule.43
On the other hand, increased numbers of both immature and mature DC are continuously generated in the presence of IL-7, whereas in control cultures the numbers of immature DC progressively decrease, and those of mature DC remain practically unchanged. Because no differences are found in the proportion of proliferating cells in this thymic cell subset, these results indicate that the higher DC production is the result of a continuous and enhanced DC differentiation throughout the culture period, rather than the product of the IL-7–induced proliferation of the pre-existing DC population. Furthermore, endogenous IL-7 deprivation with a neutralizing anti–IL-7 MoAb results in a drastic inhibition of DC formation. In agreement with our findings, Márquez et al18 reported that human intrathymic CD34+ precursors cultured in the presence of IL-7 were able to develop simultaneously into both T and non-T (DC and monocytes)-lineage cells, while Saunders et al19 described that murine CD4low thymic precursors proliferated and differentiated to DC, but not to T-lineage cells, after a 4-day culture period with a mixture of five to seven cytokines including IL-7, the omission of which had a marked effect on DC generation. Likewise, it has been described that the differentiation of DC from a human BM cell precursor population in liquid cultures supplemented with a cocktail of nine cytokines, including IL-7.44 The addition of IL-7 to mouse FTOC has also been reported to result in the accumulation of TN cells.45 However, these cells were identified as early T-cell precursors on the basis of the expression of CD44, CD25, and Sca-1, cell markers that have been repeatedly reported on DC.18,32,46,47 Although no morphological study was performed, it is highly likely that these cells were DC, as is borne out by our results. Increased numbers of DN/TN thymic cells are also seen in IL-7–transgenic mice48 and after hematopoietic reconstitution with BM infected with IL-7–producing retrovirus,49 but the real nature of those cells was not investigated.
The development of large numbers of DC in IL-7–treated FTOC established from SCID mice shows that IL-7–induced DC maturation does not depend on growth factors produced by maturing thymocytes in response to IL-7. An indirect effect through other thymic cell components can be also discarded because thymic epithelial cells do not express IL-7 receptors,50 and because IL-7 concentrations required to stimulate the secretion of cytokines (including IL-1α, IL-1β, IL-6, and TNF-α) from monocytes are higher than those used in our study.51 To our knowledge there is no information available related to a possible IL-7–induced cytokine secretion from thymic progenitor cells. Therefore, we favor a direct role for IL-7 in DC development, which is also supported by the evidence that the highest levels of IL-7 receptor expression are found in the CD44+CD25− and CD44+CD25+ murine progenitor populations,52 which constitute the intrathymic precursor cell subsets able to give rise to both thymic DC and to the thymic T-lineages.17 Moreover, the expression of IL-7 receptor on DC has been recently shown.53 Other factors, such as IL-1 and TNF-α, have been pointed out to be essential for the in vitro generation of DC from intrathymic precursor cells.19However, the highest proportion of thymic DC is found very early in ontogeny (R.S., unpublished results, May 1995), when the expression of IL-7 is maximal,54 and that of IL-1 or TNF-α undetectable in the thymus.55-57
Therefore, we can conclude that IL-7 is another cytokine to incorporate in the ever-increasing list of growth factors that affect the development of DC.
Supported in part by CAYCIT Grants No. PB91-0374 and PB94-0332 from the Spanish Ministry of Education and Culture. R.S. is a fellow of the Spanish Ministry of Education and Culture.
Address reprint requests to Agustı́n G. Zapata, PhD, Department of Cell Biology, Faculty of Biology, Complutense University, 28040 Madrid, Spain.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.