Abstract
Multiple sclerosis, systemic lupus erythematosus, and rheumatoid arthritis are immune-mediated diseases that are responsive to suppression or modulation of the immune system. For patients with severe disease, immunosuppression may be intensified to the point of myelosuppression or hematopoietic ablation. Hematopoiesis and immunity may then be rapidly reconstituted by reinfusion of CD34+progenitor cells. In 10 patients with these autoimmune diseases, autologous hematopoietic stem cells were collected from bone marrow or mobilized from peripheral blood with either granulocyte colony-stimulating factor (G-CSF) or cyclophosphamide and G-CSF. Stem cells were enriched ex vivo using CD34+ selection and reinfused after either myelosuppressive conditioning with cyclophosphamide (200 mg/kg), methylprednisolone (4 g) and antithymocyte globulin (ATG; 90 mg/kg) or myeloablative conditioning with total body irradiation (1,200 cGy), methylprednisolone (4 g), and cyclophosphamide (120 mg/kg). Six patients with multiple sclerosis, 2 with systemic lupus erythematosus, and 2 with rheumatoid arthritis have undergone hematopoietic stem cell transplantation. Mean time to engraftment of an absolute neutrophil count greater than 500/μL (0.5 × 109/L) and a nontransfused platelet count greater than 20,000/μL (20 × 109/L) occurred on day 10 and 14, respectively. Regimen-related nonhematopoietic toxicity was minimal. All patients improved and/or had stabilization of disease with a follow-up of 5 to 17 months (median, 11 months). We conclude that intense immunosuppressive conditioning and autologous T-cell–depleted hematopoietic transplantation was safely used to treat these 10 patients with severe autoimmune disease. Although durability of response is as yet unknown, all patients have demonstrated stabilization or improvement.
INTENSIVE immunosuppression and hematopoietic stem cell transplantation has been proposed or initiated as a therapy for patients with severe autoimmune diseases (SADS) who have poor prognostic features.1-11 The rationale is to maximally suppress or ablate the immune system and then rescue the patient from prolonged cytopenias or hematopoietic failure by infusing either autologous or allogeneic hematopoietic progenitor cells (CD34+ cells). This approach is supported by hematopoietic stem cell transplantation in animal autoimmune disorders and in patients undergoing transplantation for a hematologic disease who also had a coincidental autoimmune disease. Animal autoimmune diseases may occur spontaneously or be induced either by immunization with self-peptides or adoptive transfer of disease-initiating lymphocytes. A spontaneous onset lupus-like illness occurs in Murthy Roth Lab lymphoproliferative (MRL/lpr) mice and New Zealand Black/New Zealand White (B/W) mice.12,13 In MRL/lpr and B/W mice, an allogeneic transplant from a nonsusceptible strain is required to cure disease.12,13 Alternatively, disease can be transferred from susceptible to nonsusceptible mice after bone marrow transplantation. In MRL/lpr mice, a single gene defect in Fas expression, a protein that signals for apoptosis, results in a lymphoproliferative response with lupus-like features.14 15Therefore, it appears in these animal models that spontaneous-onset autoimmune disease may arise from a hematopoietic stem cell defect predisposing to immune dysregulation.
Induced autoimmune diseases require manipulation of a normal immune system (ie, immunization) to break self-tolerance. Why a potentially self-reactive repertoire exists is unknown, but environmental influences are necessary to break tolerance. Experimental autoimmune encephalomyelitis (EAE) is an induced animal autoimmune disease that mimics multiple sclerosis (MS). EAE may be cured by allogeneic, syngeneic, or autologous bone marrow transplantation,16-20although the relapse rate is higher after an autologous or syngeneic transplant. In animal autoimmune disorders, genetically preordained diseases require an allogeneic transplant from a nonsusceptible strain for cure, whereas environmentally induced disease may be cured with either allogeneic or autologous transplantation.
Patients with aplastic anemia, leukemia, or lymphoma and a coincidental autoimmune disease such as rheumatoid arthritis (RA), scleroderma, Crohn’s disease, or MS have been treated by hematopoietic stem cell transplantation for their hematologic disorder.21-29 In most reported cases this has also resulted in subsequent remission of their autoimmune disease. Although the number of anecdotal case reports is small, duration of remission appears better with an allogeneic graft compared with an unmanipulated autologous graft. Virtually no information is available on lymphocyte-depleted autologous transplantation in patients with autoimmune diseases. Because of the higher expected morbidity of allogeneic transplantation, consensus conferences have recommended initiating this approach with autologous stem cells.3
MATERIALS AND METHODS
Patient Selection
All three protocols (MS, systemic lupus erythematosus [SLE], and RA) are approved by the US FDA under IDE numbers BB-IDE 6440, BB-IDE 6559, and BB-IDE 6778, respectively. For all protocols, patients must be less than 60 years of age at the time of pretransplant evaluation and must meet the following criteria.
MS.
Patients must have clinically definite MS using Poser criteria supported by characteristic magnetic resonance imaging (MRI) changes and absence of serologic or clinical signs of other autoimmune diseases.30 In addition, the patients must fulfill both of the following criteria: (1) failure to stabilize active clinical progression with intravenous methylprednisolone administered for a minimum of 3 days at 1 g per day; and (2) a Kurtzke extended disability status scale (EDSS)31 of 5.0 to 8.0, with an increase in the EDSS by 1.5 points within the preceding 12 months in patients with an EDSS of 5.5 or less at the start of the evaluation period or an increase of 1 point in patients with an EDSS of 6 or greater at the start of the evaluation period. The increased EDSS must be sustained for at least 3 months before enrollment. Final eligibility is determined by a selection and safety monitoring committee consisting of Drs Jerry Wolinsky (University of Texas, Houston, TX) and Henry McFarland (National Institutes of Health, Bethesda, MD).
SLE.
Patients may be enrolled if they fulfill any one of the following criteria: (1) biopsy-proven World Health Organization (WHO) class III or IV glomerulonephritis that has failed to respond to NIH short course cyclophosphamide therapy32 (500 to 1,000 mg/m2monthly for at least 6 months), with treatment failure defined as a failure of serum creatinine to return to normal or pre-exacerbation level; (2) vasculitis and/or immune complex deposition causing end organ signs or symptoms, eg, cerebritis, transverse myelitis, pulmonary hemorrhage, or cardiac failure, not controlled with corticosteroids and cyclophosphamide; (3) transfusion-dependent cytopenias that are immune-mediated and not controlled with danazol, prednisone, and an alkylating agent (cyclophosphamide or vincristine); or (4) catastrophic antiphospholipid syndrome, which is defined as an antiphospholipid titer greater than 5 standard deviations above the mean and two or more antiphospholipid related manifestations, including either cytopenias or vascular thrombosis that failed to respond to anticoagulant therapy.
RA.
Patients must fulfill all of the following criteria: (1) an established clinical diagnosis of RA by the American College of Rheumatology criteria33; (2) a positive rheumatoid factor; and (3) failure of at least two disease-modifying agents (methotrexate, gold, penicillamine, and hydroxychloroquine), where failure is defined as at least six swollen joints and either 30 or more involved (swelling, tenderness, deformity, pain on motion, and decreased motion) joints or answering less than 75% of the Activities of Daily Living (ADL) Health Assessments Questionnaire “without any difficulty.”34
Hematopoietic Stem Cell (HSC) Procurement
Hematopoietic stem cells were collected from bone marrow in the first 3 patients, but due to low CD34+ cell yield, the graft was supplemented with peripheral blood stem cells (PBSCs). All subsequent hematopoietic stem cells were collected using only peripheral blood. PBSCs were mobilized with either granulocyte colony-stimulating factor (G-CSF; Amgen, Thousand Oaks, CA) at 10 μg/kg subcutaneous daily with leukapheresis beginning on day 5 or cyclophosphamide (2.0 g/m2) and G-CSF (10 μg/kg/d) with leukapheresis initiated when the white blood cell count reached 1,000/μL (1.0 × 109/L). Apheresis was continued daily until the number of harvested progenitor cells reached a minimum of 2.0 × 106 CD34+ cells/kg body weight after CD34 enrichment. The mobilized peripheral blood stem cells were lymphocyte-depleted via positive selection for CD34+ cells using the CEPRATE SC Stem Cell Concentrator (CellPro, Bothell, WA).
Conditioning Regimen
For MS, cyclophosphamide (120 mg/kg) in divided doses of 60 mg/kg/d was administered intravenously over 2 hours on days −6 and −5 and total body irradiation (TBI) was administered as 1,200 cGy divided 150 cGy twice a day on days −4, −3, −2, and −1 in the AP/PA position with 50% lung and 30% kidney and right lobe of the liver transmission blocks. One gram of methylprednisolone was administered intravenously on days −4, −3, −2, and −1.
For lupus and RA, cyclophosphamide (200 mg/kg) was administered in divided doses of 50 mg/kg/d intravenously over 1 to 2 hours on days −7, −6, −5, and −4. Antithymocyte globulin (ATG; 90 mg/kg) was administered in doses of 30 mg/kg/d on days −6, −5, and −4 and infused over 10 to 12 hours beginning 8 to 10 hours after the infusion of cyclophosphamide. Methylprednisolone (1 g) was administered intravenously 30 minutes before each dose of ATG.
Definition of Disease Status
Outcome was based on assessments before transplant and at 1, 2, 3, and 6 months and yearly after transplantation.
MS.
Improvement was defined as a decrease in the Kurtzke EDSS31by at least 1 point or increase in the Scripps NRS35 by at least 10 points. Deterioration was defined as an increase in the Kurtzke EDSS by at least 1 point or decrease in the Scripps NRS by at least 10 points. Stabilization of active disease was defined as absence of any new or progressive neurologic deficits and no significant change in the EDSS or NRS scores. MRI was performed at approximately the same intervals to monitor occurrence of new lesions and activity of lesions as determined by gadolinium enhancement.
SLE.
Outcome was based on serology (C3, C4, anti-Ds-DNA, ANA, Sm, anti-SSA, anti-SS-B, and lupus anticoagulant), lupus disease activity index (SLEDAI),36 and response of pretransplant abnormalities in involved organ systems (eg, serum creatinine; 24-hour urine protein and creatinine clearance in nephritis; left ventricular ejection fraction in myocarditis; and chest radiograph and pulmonary function tests in pneumonitis). Improvement was defined as a 50% improvement in any baseline parameter with no deterioration in any objective parameter.
RA.
Assessment parameters were tender joint count, swollen joint count, patient’s assessment of pain, patient’s global assessment of disease, physician’s global assessment, Health Assessment Questionnaire Activities of Daily Living (ADL), and acute-phase reactant value. Definition of improvement was greater than 20% improvement in both tender and swollen joint count and 20% improvement in at least three of the other five assessment parameters.37 Criteria for complete remission requires that five or more of the following be fulfilled for at least 2 consecutive months: (1) duration of morning stiffness not exceeding 15 minutes, (2) no fatigue, (3) no joint pain (by history), (4) no joint tenderness or pain on motion, (5) no soft tissue swelling in joints or tendon sheaths, and (6) erthyrocyte sedimentation rate less than 30 mm/h for a female or 20 mm/h for a male.38
Immunologic Assays
Pretransplant and posttransplant, two- and three-color immunophenotyping was performed on EDTA anticoagulated whole blood. The infused stem cell products were assessed by three-color immunophenotyping. The panel of fluorescein isothiocyanate (FITC), phycoerythrin (PE), PE-cyanin 5 (PE-Cy5), or PerCp fluorochromes included antibodies to CD45 and CD34 (Becton Dickinson, Mountain View, CA), and CD3, CD4, CD8, CD29, CD45RA, CD19, CD16, and CD56 (Coulter Cytometry; Coulter, Hialeah, FL).
Supportive Care
Patients were treated on a hepa-filtered hematology/oncology floor. A low microbial diet, fluconazole (400 mg/d oral or intravenous), and valacyclovir (500 mg TID oral or intravenous) were started upon admission and discontinued when the absolute neutrophil count (ANC) rebounded to 500/μL. Oral ciprofloxacin (750 mg orally BID) was started upon admission and switched to intravenous piperacillin/tazobactam or cefipime when the ANC fell below 500/μL. Subcutaneous G-CSF (5 μg/kg) was started the day of hematopoietic stem cell infusion and continued until the ANC was greater than 1,000/μL for 3 consecutive days. For the first 6 months after transplantation, patients were treated with either daily oral fluconazole or itraconazole and either Bactrim DS once orally three times a week or, for patients with lupus, aerosolized pentamidine (300 mg) monthly.
RESULTS
Hematopoietic Stem Cell Collection
In general, one to five daily 10 to 20 L apheresis were required to obtain greater than 2.0 × 106 CD34+cells/kg after lymphocyte depletion. In 3 patients with MS, apheresis was used to supplement the bone marrow harvests (Table 1). Positive selection for CD34+ stem cells resulted in a median 2.3 log depletion of T (CD3+) and B (CD19+) cells.
Toxicity
Nonhematologic toxicity was limited to grade 0-1 for the gastrointestinal system (nausea, vomiting, and diarrhea) according to NCI common toxicity criteria (Table2). Median time to an absolute neutrophil count greater than 500/μL (0.5 × 109/L) and platelet count greater than 20,000/μL (20 × 109/L) occurred on day 10 and 14, respectively. The median time to hospital discharge was day 14 after transplant. Five patients had positive cultures from either stool (candida and clostridium), blood (staphylococcus), or percutaneous intravenous central catheter (streptococcus) during the period of neutropenia. After engraftment, no posttransplant opportunistic infections have occurred, with the exception of a single case of dermatomal varicella zoster occurring 6 months after transplantation.
MS.
Pretransplant G-CSF was well tolerated without exacerbation of neurologic symptoms. Transient elevation of hepatic transaminases (5× normal) occurred in 2 patients while receiving G-CSF for stem cell mobilization. These values normalized within 7 days without intervention. Patients with MS tolerated chemotherapy, TBI, and G-CSF without neurologic deterioration or exacerbation.
SLE.
The first patient with lupus started transplantation while in acute renal failure with a creatinine level of 5.0 mg/dL. In this patient, dialysis was initiated before starting the conditioning regimen. High-dose chemotherapy was associated with a cell-lysis effect, acid base and electrolyte disorders, and volume disturbances. The second patient underwent transplant for recurrent alveolar hemorrhage refractory to corticosteroids and cyclophosphamide. Before starting transplantation, she required supplemental face mask oxygen. With the exception of volume and electrolyte disturbances, organ function generally stabilized or began to improve during conditioning or shortly after onset of neutropenia.
RA.
In both patients, conditioning was well tolerated without any pulmonary, renal, cardiac, or hepatic dysfunction. However, 1 patient had an immediate wheel and flare response to the subcutaneous test dose of ATG. After desensitization, full-dose ATG was administered by continuous intravenous infusion for 24 hours and then discontinued without administration over the last 48 hours due to urticaria.
Clinical Outcome
MS.
All 6 patients had rapidly progressive disease despite maximal immunosuppressive therapy during the year before transplant. Since transplantation, disease has not progressed despite stopping all immunosuppressive and immune modulating medications. Posttransplant follow-up ranges from 5 to 17 months, with a median of 11 months, and 3 patients are greater than 1 year from transplant. All patients have experienced subjective and objective neurologic improvements (Table 3), but the Kurtzke EDSS remains unchanged due to its dependence in the upper range on lower extremity motor function for scoring. In contrast, the Scripps NRS, which includes more emphasis on upper extremity function, incontinence, and cognitive ability, has demonstrated a greater than 10 point improvement in 3 patients. Improvement in the NRS did not begin until several months after transplantation. The 3 patients whose NRS improved by more than 10 points had gadolinium enhancement on their pretransplant MRI and had lower EDSS scores (6.0 to 7.5). In contrast, 2 of the 3 patients who had stabilization of disease but no significant improvement did not have pretransplant gadolinium enhancement and higher pretransplant EDSS scores (8.0 to 8.5). In all 6 patients, MRI showed no new or enhancing lesions after transplantation.
SLE.
Both patients have had no evidence of active disease since transplantation (Table 4). The first patient, a 24-year-old white woman, was diagnosed with SLE at 11 years of age. Her C3, C4, ANA, and anti-ds DNA have never been normal, even during clinical remissions. She was treated at various times with plasmapheresis, corticosteroids, pulse cyclophosphamide, hydroxychloroquine, methotrexate, and azathioprine, none of which allowed steroid tapering to less than 20 mg/d. At transplantation, she exhibited active lupus manifested by a malar rash, arthralgias, hematuria, diffuse abdominal pain, ascites, and a large pericardial effusion. Renal function was rapidly decreasing, with a serum creatinine level of 5.0 mg/dL (398 μmol/L), red blood cell (RBC) casts in the urine, a 24-hour urine protein level of 3.8 g, and biopsy-proven WHO class IV glomerulonephritis. Pancytopenia was present and serology and complement were abnormal (Table 4). Over the 12 months since transplant, the malar rash, arthralgias, pleural and pericardial effusions, and cytopenias have resolved. Renal function has stabilized at a creatinine level of 1.9 mg/dL and 24-hour urine protein level of 0.2 g. For the first time since disease onset 13 years ago, complement components (C3, C4) and antinuclear antibodies are normal with the patient off all immunosuppressive medications, including corticosteroids.
The second patient presented at 15 years of age with flu-like symptoms followed by respiratory arrest. She was transferred to a tertiary care facility in which pulmonary biopsy showed alveolar hemorrhage and vasculitis and renal biopsy showed WHO class IV glomerulonephritis. The ANA was 1:1,280. Subsequently, her disease manifested predominately as alveolar hemorrhage requiring intubation on a second occasion despite high-dose corticosteroids, plasmapheresis, and pulse cyclophosphamide. Within 3 months of disease onset she was referred for transplantation. At the time of transplant, the patient had active pneumonitis manifested as hemoptysis, pulmonary infiltrates, and hypoxia. Since transplantation, hemoptysis and pulmonary infiltrates have resolved. Pulmonary diffusion capacity has remained unchanged at 50% of normal. Corticosteroids are being gradually tapered and have been decreased from 80 to 25 mg/d.
RA.
The first patient has met the 50% response criteria for improvement (Table 5). She was a 46-year-old woman diagnosed with RA 7 years before transplantatiion. She was treated with nonsteroidal anti-inflammatory drugs, hydroxychloroquine, cyclosporine, gold, methotrexate, dapsone, sulfasalazine, minocycline, intra-articular corticosteroids, and oral prednisone at 10 to 15 mg/d for 5 years. At the time of transplantation, she had 41 tender joints and 27 swollen joints and was unable to answer any of 20 health assessment questionnaire parameters “without any difficulty.” Since transplantation, the swollen and tender joint counts have been, respectively, 0 and 3 at 1 month, 4 and 7 at 3 months, 3 and 3 at 6 months, and 2 and 4 at 12 months. By 6 months, the patient’s medications have been decreased to 3 mg/d of prednisone and 200 mg of hydroxychloroquine twice a day. By 8 months, corticosteroids were discontinued. Health assessment questionnaire parameters have improved with the patient answering “without any difficulty” to 14 of 20 questions at 1 month, 16 of 20 at 3 months, 17 of 20 at 6 months, and 16 of 20 at 12 months. Patient and physician assessment of disease has improved 70% and 40%, respectively. Rheumatoid factor became negative at 1 month but returned to low level positive at 3 months. Sedimentation rate, which was elevated before transplant, has remained normal for the 12 months since transplantation.
The second patient was a 42-year-old woman diagnosed 7 years before transplantation. She was treated with nonsteroidal anti-inflammatory drugs, hydroxychloroquine, cyclosporin, gold, methotrexate, sulfasalazine, intra-articular corticosteroids, and oral prednisone at 10 mg/d for 5 years. At the time of transplantation, she had 21 tender joints and 18 swollen joints and was unable to perform any of 20 health assessment questionnaire parameters “without any difficulty.” Although she experienced greater than a 50% reduction in swollen joints after transplantation, her tender joint count did not improve. At 3 months after transplantation, she has 22 tender and 5 swollen joints and answers 6 health assessment questionnaire parameters “without any difficulty.” She is currently off corticosteroids but remains on 20 mg per week of methotrexate. Patient and physician assessment of disease has improved 25% and 32%, respectively. Her rheumatoid factor and sedimentation rate remain elevated.
Immune Reconstitution
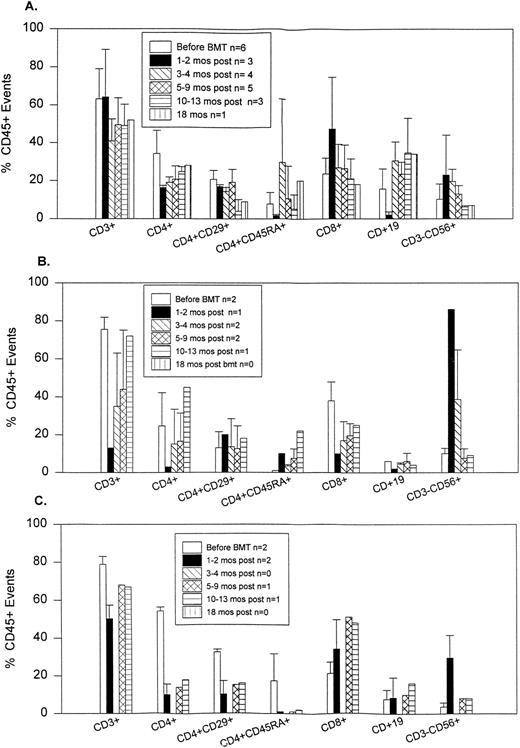
Patients had markedly reduced numbers of CD4+ cells during the first 12 months posttransplantation and all had inverted CD4/CD8 ratios (Fig 1). CD4+ cells were almost exclusively CD45RA− early posttransplantation. The number of CD45RA+ (naive) T cells gradually increased after 6 months. Early posttransplantation, CD4+ T cells persistently coexpressed CD29, a marker for the helper-inducer subset. The percentage of cells that are CD56+ (NK) increased for the first 6 posttransplant months, returning thereafter to normal. CD19+ (B) cells reached the normal range by 6 months.
Flow cytometry results of peripheral blood performed before and after transplantation. n, number of patients at that time point. (A) Patients with MS. (B) Patients with SLE. (C) Patients with RA.
Flow cytometry results of peripheral blood performed before and after transplantation. n, number of patients at that time point. (A) Patients with MS. (B) Patients with SLE. (C) Patients with RA.
DISCUSSION
These data indicate the safety and short-term benefit of hematopoietic stem cell transplantation for patients with severe manifestations of autoimmune disease. Although these results suggest a benefit from intense immunosuppressive therapy, it is unclear if a transplant or intense but nonablative immunosuppressive regimen is sufficient. Nevertheless, all patients responded to treatment with either stabilization of disease or improvement. The 6 patients with MS have remained off immunosuppressive medications since transplantation. Because the Kurtzke EDSS is heavily weighted by ambulation, some improvement may not be reflected in the score. Subtle improvements have occurred in most patients, including improved cognition, decreased incontinence, improved speech, correction of deviated eye gaze, less fatigue, and in 1 patient the newly reacquired ability to swim. MRIs also demonstrated absence of disease progression with no new lesions posttransplantation. Therefore, despite a rapid progression of neurologic impairment in the year preceding transplant and despite discontinuation of immunosuppression after transplantation, disease has improved or stabilized without new symptoms, signs, or MRI findings. In a report by Fassas et al,5 the Kurtzke EDSS improved after autologous hematopoietic stem cell transplantation using a non–radiation-containing chemotherapy regimen. Ex vivo lymphocyte purging was not performed, but ATG was administered in an attempt to obtain postinfusion in vivo purging. They also transplanted patients with less severe disease and a lower median EDSS. The pathophysiology of progressive MS is probably a spectrum with both active inflammatory and chronic degenerative components. Aiming for transplant during active disease as evidenced by gadolinium enhancement on MRI may result in greater functional improvement. Four patients had small volume gadolinium enhancement on pretransplant MRI. Three of these patients improved with more than a 10-point NRS increase. The 2 patients without pretransplant MRI enhancement had stabilization of disease for more than 1 year but no significant improvement in the NRS or EDSS.
For the first 3 patients with MS, bone marrow harvests failed to yield the CD34+ cell target. Therefore, all subsequent patients had stem cells mobilized from the peripheral blood using G-CSF without clinical flare in disease activity. Moderate to no gadolinium enhancement was present on MRI at the time of mobilization. It remains to be determined if patients with significant disease activity as manifested by large volume gadolinium enhancement will suffer exacerbations from G-CSF.
The conditioning agents were selected to maximize immunosuppression. For patients with MS, TBI was combined with cyclophosphamide and corticosteroids, because radiation could penetrate to lymphocytes sequestered within the central nervous system without regard for permeability of the blood brain barrier. However, to our knowledge, there are no data available on survival of lymphocytes within the central nervous system after a non–TBI- versus TBI-containing conditioning regimen. The dose of TBI (1,200 cGy) was based on prior experience using TBI conditioning in hematologic malignancies as well as data from hematopoietic stem cell transplantation in experimental autoimmune encephalomyelitis, an animal model of MS.16-20 A nonmyeloablative dose of total lymphoid irradiation (1,000 to 1,920 cGy) has been reported to improve functional scores in patients with progressive MS compared with untreated controls.39 Similarly, cyclophosphamide doses (200 to 2,000 mg/m2) that are approximately 3 to 10 times below the dose administered for transplant result in a 1-to 2-year stabilization of progressive MS.40 Conditioning regimen-related toxicities were minimal. Transplants for MS were uncomplicated. No acute neurologic deterioration occurred in patients with MS.
Following transplantation, both patients with lupus have no evidence of active disease for the first time since disease onset, despite gradual tapering and discontinuation of immunosuppressive drugs. The rate of corticosteroid taper remains controversial. At time of referral, prednisone dosing was 80 and 100 mg per day, respectively. Hematologists favored a rapid taper due to risk of opportunistic infections. Rheumatologists favored a slow withdrawal, because pretransplant attempts at steroid tapering resulted in disease exacerbation. Since transplant, the patients have had no evidence of disease and cushingoid habitus has disappeared. Renal biopsy on the first patient with a creatinine level of 5.0 mg/dL showed both acute inflammation and chronic scarring. It was anticipated that the creatinine level would stabilize with minimal further improvement after the first few months. However, 1 year after transplantation, renal function continues to improve. Both patients were on multiple antihypertensive medications with difficulty controlling hypertension. Since transplantation, hypertension has been easily controlled and most antihypertensive medications have been discontinued.
For RA, complete remissions were not obtained. However, both patients have improved activities of daily living and a decreased number of swollen joints on less immunosuppressive medications. The improvement in both patients was immediate and has persisted in the first patient beyond 12 months. The second patient’s response has been less impressive. In a previous case report by Joske,7 a patient with RA who underwent autologous transplantation improved from wheel chair bound to ambulation with ease.
Because it was anticipated that patients with lupus and RA would have active disease at the time of transplantation, mobilization with cyclophosphamide was administered before initiation of G-CSF to avoid the possibility of disease exacerbation. The dose of cyclophosphamide (2 g/m2) is at least double the standard NIH dose (500 to 1,000 mg/m2) for lupus. This dose resulted in transient amelioration of symptoms. In the patients with lupus, active disease persisted, and in patients with RA, the number of swollen and tender joints returned to baseline within weeks of mobilization. Cyclophosphamide mobilization was limited to 2 g/m2 due to concerns about myocardial damage from the cumulative effects of cyclophosphamide used during both mobilization and conditioning.
The conditioning regimen of cyclophosphamide, corticosteroids, and ATG was chosen for patients with SLE and RA because of its effectiveness as a conditioning regimen for aplastic anemia, which may often represent an autoimmune suppression of hematopoiesis. Some patients with aplastic anemia have rejected the donor’s graft and subsequently reconstituted normal endogenous hematopoiesis. This suggests that maximal immunosuppression, even without an allogeneic source of stem cells, may improve some autoimmune diseases. Use of a nonradiation containing regimen avoids the risk of radiation-associated second malignancies. It also avoids radiation-induced pneumonitis, especially in patients with rheumatoid interstitial lung disease. However, unlike cyclophosphamide and TBI, the cyclophosphamide and ATG regimen is not myeloablative. CD34+ stem cells were reinfused to shorten the duration of severe neutropenia and thrombocytopenia to decrease the risk of serious infection and bleeding. For these safety reasons, the FDA recommended a minimum postenrichment CD34 mobilization target of 2.0 × 106/kg to proceed with conditioning and transplantation.
Subjective and objective improvement in patients with RA and lupus generally began with onset of neutropenia. Transplants for RA were uncomplicated. In contrast, patients undergoing transplant for lupus required intense supportive care due to preexisting renal and pulmonary dysfunction. Cyclophosphamide conditioning dose was administered by ideal weight regardless of creatinine level without adverse nonhematopoietic effects. The pulmonary, renal, and hepatic toxicities listed in Table 2 for lupus patients are in reality disease-related and not due to the treatment regimen. Normal end organ function is a criteria for most cancer patients to be candidates for transplantation. NCI regimen-related toxicity is graded according to the absolute laboratory value and not relative change in function. The NCI common toxicity scales are, therefore, not accurate treatment-related toxicity scores for patients with disease-related pretransplant organ dysfunction.
In several patients, CD34+ cell yields after peripheral mobilization with either G-CSF or cyclophosphamide and G-CSF were low. Multiple apheresis were required to obtain more than 2.0 × 106 CD34+ cells/kg. Possible but hypothetical explanations include prior exposure to immunosuppressive and cytotoxic agents such as cyclophosphamide and methotrexate. Collections may also be impaired by chronic corticosteroid dependence or elevated proinflammatory cytokines associated with ongoing immune activation. Whatever the cause, platelet and neutrophil recovery after transplantation was unaffected.
It is unclear if the autologous graft needs to be depleted of lymphocytes or if there is a threshold dose of T cells acceptable for reinfusion. Most anecdotal case reports suggest nonsustained remission of a coincidental autoimmune disease after unmanipulated autologous stem cell transplantation for a hematologic disease.28 For this reason, a CD34-enriched stem cell product was reinfused. Theoretically, purging the graft of lymphocytes may prevent reinfusion of potential disease-causing cells. However, aggressive lymphocyte depletion may result in late fungal and viral infections and lymphoproliferative disorders. For this reason, a partial depletion of 2.3 logs was performed via CD34+ selection.
The mechanism(s) of posttransplant disease stabilization or improvement remains obscure. Previous studies of immune function after hematopoietic transplantation demonstrated an immunosuppressive phenotype for up to 6 to 24 months.41 42 We have demonstrated that a similar prolonged immunosuppressive phenotype prevails after a lymphocyte-depleted autologous transplant for autoimmune diseases. The natural course of several autoimmune diseases is relapsing remitting, suggesting that the immune system is dynamic and normally fluctuates between tolerance and immunity. Theoretically, aggressive immunosuppression followed by autologous stem cell reinfusion may reset the balance between self-tolerance and autoimmunity by shifting the scales towards immunosuppression. We are currently assessing immune recapitulation by studying lymphocyte proliferative responses, intracellular cytokine profile, and T-cell receptor repertoire to determine relationship to clinical disease. Analysis of posttransplant immune function may help determine whether autologous T-cell–depleted transplantation results in regeneration of self-tolerance or is simply dose-intense immunosuppression.
In these 10 patients with severe autoimmune diseases, stabilization of disease progression, remissions, or improvements have occurred for a median of 11 months since transplantation. No disease progression or relapses have occurred in patients with MS or lupus, respectively. Although complete remissions were not obtained in the 2 patients with RA, an improved standard of living with easier control of pain has been achieved. It may be that some autoimmune diseases will require immune manipulation after transplantation, more aggressive immunoablative regimens, or more complete lymphocyte purging of the autograft before reinfusion. Alternatively, autoimmune disorders or some subset of patients with autoimmune disease may require an allogeneic graft from an unaffected sibling for curative therapy. Nevertheless, lymphocyte-depleted autologous transplantation in poor prognosis patients with severe disease unresponsive to current standard therapies has resulted in early improvement or stabilization of disease with minimal toxicity. Attempting hematopoietic stem cell transplantation for autoimmune disease requires careful selection of patients and, in the case of MS, use of an outside selection and safety monitoring committee has proven very effective. Further advances in identifying high-risk patients may allow for transplantation in an earlier stage of disease before development of severe end organ damage. Longer follow-up will also be important to determine if these responses are sustained. It is also important that the conclusions from this or any other phase I study be addressed in additional randomized trials before drawing conclusions on efficacy.
ACKNOWLEDGMENT
The authors acknowledge the nursing staff and support from Northwestern Memorial Hospital and the Froedtert Memorial Lutheran Hospital.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Richard K. Burt, MD, Northwestern Memorial Hospital, Wesley Pavilion, Room 1456, 250 E Superior, Chicago, IL 60611.