Abstract
The objectives of the present study were to investigate the prognostic value of the International Prognostic Index (IPI) at relapse in the 215 patients with intermediate- or high-grade non-Hodgkin’s lymphoma (NHL) included in the PARMA trial. The IPI at relapse was available in 204 (95%) of these patients. Response rates to 2 courses of DHAP were 77%, 54%, 55%, and 42% in patients with an IPI of 0, 1, 2 and 3, respectively (P < .02), whereas complete response (CR) rates were 33%, 29%, 20%, and 0% in the same groups of patients (P < .03). With a median follow-up period of 79 months, overall survival (OS) at 5 years was 46%, 25%, 25%, and 11% in these four groups (P < .001). One hundred nine patients responding to 2 courses of DHAP were randomized to receive either BEAC (carmustine, etoposide, cytarabine, cyclophosphamide and mesna) followed by autologous bone marrow transplantation (ABMT) or 4 additional courses of DHAP: IPI at relapse was found highly correlated to OS in patients treated in the DHAP arm (5-year OS: 48%, 21%, 33%, and 0% for IPI 0, 1, 2, and 3, respectively; P = .006), but not in the BEAC arm (5-year OS: 51%, 47%, 50%, and 50% for IPI 0, 1, 2, and 3, respectively; P = .90). OS was significantly superior in the BEAC arm as compared with the DHAP arm in patients with an IPI >0 (P < .05), but not in patients with an IPI of 0. In conclusion, these results show that IPI correlates to response and overall survival in patients with aggressive NHL in relapse and enables us to identify patients with a significantly different outcome among those treated with conventional chemotherapy alone.
THE PROGNOSIS OF aggressive non-Hodgkin’s lymphoma (NHL) in relapse treated with conventional chemotherapy is poor, with a 5-year survival less than 20% in most series.1-6 Since 1978, several pilot studies have suggested that high-dose chemotherapy (HDCT) followed by hematopoietic stem cell transplantation may improve the survival of patients with NHL in relapse.7-16 Recently, the international prospective randomized phase III PARMA trial demonstrated that consolidation with HDCT improves both overall survival (OS) and progression-free survival (PFS) in patients with intermediate- or high-grade NHL responding to conventional salvage chemotherapy.17 However, still 40% to 80% of these patients experience a subsequent relapse and will die of their disease after HDCT.6-17
Prognostic factors correlated to response to chemotherapy and survival after the date of relapse have been identified: serum lactate dehydrogenase (LDH) and clinical stage have been found correlated to response rate and survival in patients with aggressive lymphoma treated with conventional5 and high-dose chemotherapy.7,11,12,14 The international prognostic index (IPI) for aggressive lymphoma at initial diagnosis has been recently described on a series of 3,273 previously untreated patients with aggressive lymphoma.18,19 This index relies on five different adverse prognostic factors (age >60 years; performance status [PS] >1; LDH >normal; clinical stage >2; and extranodal sites >1) and enables us to distinguish patients with different response rates, patients who are progression free, and OS at initial diagnosis. For patients less than 60 years of age, an age-adjusted index relying solely on 3 prognostic factors (PS, LDH, and clinical stage) was also reported.18
The objective of the present study was to investigate the prognostic value of the IPI determined at the date of relapse in the 215 patients with intermediate- or high-grade NHL in relapse treated in the prospective PARMA trial.
MATERIALS AND METHODS
Patients.
A total of 215 patients, 18 to 60 years of age at the time of the first relapse (188 patients) or second relapse (27 patients), with diffuse centroblastic (n = 73, 34%), diffuse immunoblastic (n = 40,19%), diffuse mixed small and large cells (n = 43, 20%), follicular large cell (n = 12, 6%), diffuse small cleaved cell (n = 8, 4%), lymphoblastic (n = 7, 3%), small noncleaved cell NHL (n = 5, 2%), and diffuse histologies not otherwise specified (n = 27, 12%) NHL were initially registered in the study between July 1987 and June 1994 in 51 participating centers.17
To be eligible, patients were required to have been previously treated with a doxorubicin-containing regimen and to have had a first complete response during at least 4 weeks. Relapses while on and while off therapy were defined by the investigators. Central nervous system (CNS) and bone marrow (BM) involvement at the time of relapse were considered as exclusion criteria. Informed consent, according to the PARMA protocol and to the rules of each institution and country, was obtained from each patient.
Treatment.
All patients received the DHAP regimen (dexamethasone, cisplatin, and cytarabine).4,17 After 1 course of DHAP, BM was harvested under general anesthesia (except in 41 patients with clearly progressive disease) and frozen, unless the marrow had been stored previously (24 patients). BM biopsies taken at the time of harvesting were normal in all patients. No patient received growth factors before harvesting the BM and peripheral blood stem cells were collected in none of these patients. Twenty days after a second course of DHAP, patients with complete or partial responses were defined as having chemotherapy-sensitive relapses and were eligible for random assignment to one of the treatment groups: the autologous BM transplantation (ABMT) arm or the conventional arm.17 The conditioning regimen in the ABMT arm was BEAC (carmustine, etoposide, cytarabine, cyclophosphamide, and mesna) with or without involved field radiotherapy, followed by BMT.17 The conventional treatment consisted of 4 additional courses of DHAP administered at intervals of 3 to 4 weeks. Radiotherapy was indicated if the sites of bulky disease at the time of relapse were ≥5 cm in diameter or if extranodal T3 or T4 lesions (according to the European Organization for Research and Treatment of Cancer) were recorded. Details of all treatments were given in a previous report.17
After randomization, each patient was evaluated in the allocated arm, even if treatment was incomplete. In both arms, additional treatment was allowed if the patient failed to respond to the assigned treatment. Such additional treatment included high-dose chemotherapy and ABMT for patients in the DHAP conventional arm. No specific conditioning regimen was recommended at this stage.
IPI at relapse and at initial diagnosis.
The analysis was performed with the data base updated at the end of 1995, with a median follow-up period of 79 months.
Only age-adjusted IPI was used in this cohort of patients 60 years of age or less.18 19 The IPI was calculated from the available data at the date of registration, ie, serum LDH, clinical stage, and performance status at that date. Serum LDH, clinical stage, and performance status were available in, respectively, 208 (97%), 213 (99%), and 213 (99%) of the 215 patients at the date of relapse and 184 (86%), 214 (99%), and 213 (99%) at initial diagnosis, respectively. The IPI at initial diagnosis and relapse were thus available respectively in 181 (84%) and 204 (95%) of the 215 patients.
Statistical methods.
Survival curves were computed according to the method of Kaplan and Meier20 and compared using the two-sided log rank test21 at the .05 significance level using the LIFETEST procedure of the SAS statistical package (SAS Institute Inc, Cary, NC). Other comparisons were performed using the χ2 of Pearson and Fisher exact tests.22Correlation between IPI at diagnosis and at relapse was examined using the Spearman correlation coefficient. Analysis of variance was performed to compare time from initial diagnosis to relapse in the different IPI subgroups. Because of insufficient number of patients, subgroups of patients with different IPI were grouped together for comparisons of survival.
RESULTS
Description of patients.
The characteristics of patients at relapse are given in Table 1. Fifty-two patients (25%) were in the low-risk group, 82 (40%) in the intermediate- to low-risk group, 51 (25%) in the intermediate- to high-risk group, and 19 (9%) in the high-risk group. The correlation between IPI at initial diagnosis and IPI at relapse was investigated among the 177 patients with an IPI evaluable both at initial diagnosis and relapse. Only a limited correlation was observed (Spearman’s correlation coefficient = .33), because 23% of patients with an IPI 0-1 had an IPI 2-3 at relapse, whereas 29% of patients with an IPI 2-3 at initial diagnosis had an IPI 0-1 at relapse (Table 2). Time from initial diagnosis to relapse was not significantly different according to IPI at relapse: median times were 15, 14, 13, and 15 months for IPI 0, 1, 2, and 3, respectively.
Prognostic value of IPI at relapse in the whole cohort.
The overall response rate and complete response rate were compared in patients from the different risk groups (Table 3). Response rates ranged from 75% to 42% for patients with 0, 1, 2, or 3 risk factors (P < .02), whereas complete response rates in the same groups ranged from 33% to 0% (P < .03).
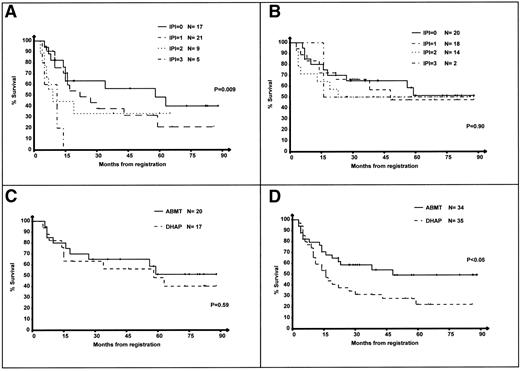
OS from the date of the first course of DHAP was compared in the patients with an IPI 0, 1, 2, and 3 at relapse. Results are presented in Fig 1. The median OS from the date of relapse of the low-, intermediate- to low-, intermediate- to high-, and high-risk groups were 58, 16, 9, and 5 months, respectively (P < .001), indicating that IPI at relapse enables us to discriminate patients with a significantly different OS after relapse.
OS of the 204 patients according to IPI at relapse. 0: IPI = 0, low-risk group; 1: IPI = 1, intermediate- to low-risk group; 2: IPI = 2, intermediate- to high-risk group; and 3: IPI = 3, high-risk group. Survival is calculated from the first day of the first course of DHAP.
OS of the 204 patients according to IPI at relapse. 0: IPI = 0, low-risk group; 1: IPI = 1, intermediate- to low-risk group; 2: IPI = 2, intermediate- to high-risk group; and 3: IPI = 3, high-risk group. Survival is calculated from the first day of the first course of DHAP.
OS according to the IPI at relapse and treatment arm.
One hundred nine patients in partial or complete response after 2 courses of DHAP were randomized and received either 4 additional courses of DHAP or a single course of HDCT with the BEAC regimen. Of these 109 patients, IPI at relapse was available in 106 (97%). The OS of patients treated in the DHAP and ABMT arms according to their IPI is shown in Fig 2A and B. The 5-year OS of patients treated in the DHAP arm were 48%, 21%, 33%, and 0% for IPI 0, 1, 2, and 3, respectively (P = .009). In contrast, in the ABMT arm, the 5-year OS were 51%, 47%, 50%, and 50% for IPI 0, 1, 2, and 3, respectively (P = .90).
OS of the 106 randomized patients according to IPI at relapse. Survival is calculated from the first day of the first course of DHAP. (A) Survival of patients in the DHAP arm according to the IPI. (B) Survival of patients in the ABMT arm according to the IPI. (C) Patients with IPI = 0, DHAP versus ABMT arm. (D) Patients with IPI = 1-3, DHAP versus ABMT arm.
OS of the 106 randomized patients according to IPI at relapse. Survival is calculated from the first day of the first course of DHAP. (A) Survival of patients in the DHAP arm according to the IPI. (B) Survival of patients in the ABMT arm according to the IPI. (C) Patients with IPI = 0, DHAP versus ABMT arm. (D) Patients with IPI = 1-3, DHAP versus ABMT arm.
Figure 2C and D shows the outcome of the 106 patients from the low-risk group (Fig 2C) or the intermediate- to low-, intermediate- to high-, and high-risk groups (Fig 2D) according to their randomization arm. The OS of patients in the low-risk group was slightly superior in the ABMT arm, but the difference was not significant (Fig 2C; P = .59). In contrast, patients with IPI >0 had a significantly superior survival in the ABMT arm (Fig 2D; P < .05).
PFS according to the IPI at relapse and treatment arm.
The PFS of randomized patients was then investigated. The 5-year PFS of patients treated in the DHAP arm were 21%, 14%, 0%, and 0%, respectively, for IPI 0, 1, 2, and 3 (P = .03). In contrast, in the ABMT arm, the 5-year PFS were 40%, 49%, 61%, and 100% for IPI 0, 1, 2, and 3, respectively (P = .51); it is noteworthy that there were only 2 patients in the IPI 3 subgroup with a 100% PFS, including 1 who died without disease at 16 months postregistration. Figure 3A and B show the PFS of patients from the low-risk group (Fig 3A) or the intermediate- to low-, intermediate- to high-, and high-risk groups (Fig 3B) according to their randomization arm. The PFS of patients in the low-risk group was slightly superior in the ABMT arm, but the difference was not significant (Fig 3A; P = .50). In contrast, patients with IPI >0 had a significantly superior survival in the ABMT arm (Fig 3B,P < .0001). There were not significant differences in patients with an IPI 0 treated in the DHAP and ABMT arms in terms of histological subtypes or tumor mass (Table4). Thirteen of the 17 IPI 0 patients randomized in the DHAP arm relapsed subsequently; 7 of these patients received ABMT and 2 of them are alive 19 and 73 months after registration in the PARMA; 6 patients were not grafted and 2 of them are reported to be alive 10 and 82 months after registration in the PARMA.
PFS of the 106 randomized patients according to IPI at relapse. PFS is calculated from the first day of the first course of DHAP. (A) Patients with IPI = 0, DHAP versus ABMT arm. (B) Patients with IPI = 1-3, DHAP versus ABMT arm.
PFS of the 106 randomized patients according to IPI at relapse. PFS is calculated from the first day of the first course of DHAP. (A) Patients with IPI = 0, DHAP versus ABMT arm. (B) Patients with IPI = 1-3, DHAP versus ABMT arm.
Prognostic value of IPI at relapse in patient refractory to DHAP.
The outcome of patients with aggressive NHL in relapse refractory to salvage chemotherapy is very poor in all series of the literature. Even with HDCT and hematopoietic stem cell transplantation, only 10% to 15% of these patients are alive disease-free at 5 years.10
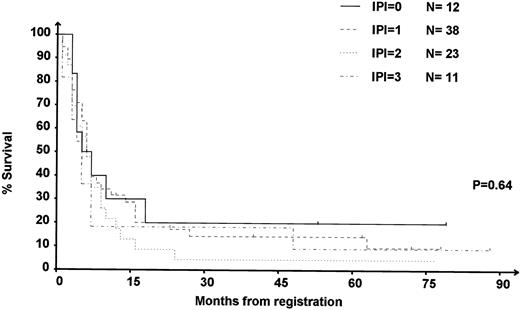
IPI at relapse was available in 84 of the 90 (91%) DHAP-refractory patients. Among these refractory patients, OS was not significantly different (P = .64) in the different IPI subgroups (Fig 4).
OS of the 84 patients refractory to DHAP according to IPI at relapse. 0: IPI = 0, low-risk group; 1: IPI = 1, intermediate- to low-risk group; 2: IPI = 2, intermediate- to high-risk group; 3: IPI = 3, high-risk group.
OS of the 84 patients refractory to DHAP according to IPI at relapse. 0: IPI = 0, low-risk group; 1: IPI = 1, intermediate- to low-risk group; 2: IPI = 2, intermediate- to high-risk group; 3: IPI = 3, high-risk group.
Information on the salvage treatment after failure of DHAP were available in 88 of these 90 patients. The OS from the date of relapse is significantly superior in the 18 patients who latter received HDCT (Fig 5A; P = .01). It must be noted that this treatment was not randomized, because patients were excluded from the PARMA study at that point. Finally, IPI at relapse was available in 82 of the 88 DHAP-refractory patients in whom information on salvage treatment after DHAP was available. Seventeen of these 82 patients received HDCT as salvage treatment within the 3 months after exclusion from PARMA, despite the absence of response to DHAP, and 65 received conventional chemotherapy (Fig 5B and C). Patients with an IPI 0-1 who were treated with HDCT were found to have a very significantly superior survival as compared with patients who had not received HDCT (P = .01; Fig 5B), whereas the OS of patients with an IPI 2-3 at relapse who were treated with HDCT was comparable to that of other patients (P = .4; Fig 5C). Because this was not randomized, these results do not demonstrate the superiority of HDCT over conventional salvage chemotherapy in patients with an IPI 0-1 at relapse not responding to DHAP, although this hypothesis deserves to be tested in a randomized trial.
OS of the 84 refractory patients according to IPI at relapse and the randomization arm. (A) Patients treated with HDCT versus others among refractory patients. (B) Patients treated with HDCT versus others among refractory patients with an IPI 0-1. (C) Patients treated with HDCT versus others among refractory patients with an IPI 2-3. Of note, the sum of patients in (B) and (C) is not equal to the total number of patients in the new (A), because IPI at relapse was not available in 6 patients for whom information on treatment after failure of DHAP was available (1 received HDCT and 5 received conventional chemotherapy after failure of DHAP).
OS of the 84 refractory patients according to IPI at relapse and the randomization arm. (A) Patients treated with HDCT versus others among refractory patients. (B) Patients treated with HDCT versus others among refractory patients with an IPI 0-1. (C) Patients treated with HDCT versus others among refractory patients with an IPI 2-3. Of note, the sum of patients in (B) and (C) is not equal to the total number of patients in the new (A), because IPI at relapse was not available in 6 patients for whom information on treatment after failure of DHAP was available (1 received HDCT and 5 received conventional chemotherapy after failure of DHAP).
DISCUSSION
This study was undertaken to investigate the prognostic value of the IPI at relapse in a prospective cohort of patients with aggressive NHL in relapse treated in a multicentric phase III protocol. The objectives were also to evaluate the benefit of HDCT in the different prognostic subgroups to identify patients who still have a poor outcome despite HDCT and who therefore could be candidates for pilot studies with new therapeutic approaches.
The results presented here show that the IPI at the date of relapse enables us to distinguish patients with a very different response rate to the DHAP regimen and a different survival. The proportion of patients in each risk group was comparable to that reported in the initial report of the IPI.18 However, it must be noted that the PARMA trial selected a population of favorable patients without BM or CNS involvement. In published series, 25% or more of relapsing patients have either BM or CNS involvement10,11; therefore, the incidence of patients in the unfavorable IPI subgroups is likely to be higher in an unselected population. Previous reports have shown that the response rate of patients with relapsing aggressive NHL is in the range of 50% and, therefore, notably inferior to that of untreated patients.1-7 Accordingly, the overall and complete response rate to chemotherapy were found to be inferior to those observed for untreated patients of the same IPI group.18 Importantly, the response rate, in particular complete response rate, decreased significantly according to the risk index. The complete response rate in particular was 0% in patients with an IPI of 3, a subgroup that should be proposed for other salvage regimens.
The IPI at relapse was also capable of distinguishing subgroups of patients with a different survival after relapse. Interestingly, patients in the low-risk group were found to have a relatively favorable outcome, with a median survival of 56 months after relapse in both randomization arms; 40% of these patients treated with 6 courses of DHAP and no HDCT were alive at 6 years and 21% remained disease-free at that date. This survival rate is greater than that usually reported for patients with aggressive NHL in relapse and indicates that conventional chemotherapy without ABMT may be curative in greater than 20% for patients with an IPI of 0. These results have important consequences for the evaluation of salvage chemotherapy regimens published in phase II trials, because the proportion of patients within the low-risk group may strongly influence the response rate and survival. The IPI at relapse is therefore an important parameter to evaluate salvage chemotherapy regimens and to avoid selection processes that would result in artificially high response rates and OS in patients with aggressive NHL in relapse.
Although the number of patients in each subgroup is small, the results presented here suggest that the magnitude of improvement achieved with high-dose chemotherapy in terms of OS is different among risk groups. HDCT apparently improved modestly the 5-year OS of patients in the low-risk group, with an observed difference of 7% in 5-year OS between the ABMT and the DHAP arms. Similarly, PFS was not significantly different in the 2 groups, although it must be noted that the PFS of IPI 0 patients treated in the DHAP arm was only 21% at 5 years (v 40% in the ABMT arm), with 13 patients experiencing relapse in the DHAP arm. The relatively favorable outcome of the subgroup of IPI 0 patients treated in the DHAP arm is in part due to the fact that 4 of these patients experienced relatively prolonged survival after relapse. In contrast, the 5-year OS and PFS of patients in the intermediate-or high-risk subgroups were 30% to 50% higher in the ABMT arm as compared with the DHAP arm. Although the number of patients in each group was actually small and differences between intermediate- and high-risk groups are only marginally significant, this result is consistent with the recently reported conclusions of the LNH87 group 2 trial in patients receiving first-line chemotherapy.23 In this study, patients with intermediate- or high-grade NHL with an IPI >1 at initial diagnosis treated with HDCT were found to have a significantly better disease-free survival and a marginally better OS as compared with patients treated with standard consolidation regimens.23 These results suggest that the survival benefits of HDCT are superior in patients within the high-risk groups as compared with those in the low-risk group. However, it must be emphasized that these results do not indicate that low-risk group patients do not benefit from HDCT, given the very low power of this analysis and the absence of stratification on this criteria.
Patients who do not respond to salvage treatment have a uniformly poor prognosis, with a 5-year survival close to 10% in all series.7-16 This indicates that response to conventional chemotherapy in itself remains the most important prognostic factor for OS in relapsing lymphoma patients. Eighteen patients not responding to 2 courses of DHAP received HDCT and hematopoietic stem cell (HSC) transplantation within the 3 months after the second course of DHAP, including 17 patients with an available IPI at relapse. These 18 patients were found to have a survival significantly superior to that of the 70 remaining patients; importantly, the difference was significant only for patients with an IPI 0-1. Despite the well-established fact that patients refractory to salvage chemotherapy have a very poor outcome with HDCT and are generally not proposed for this therapeutic approach, these results suggest that patients with an IPI 0-1 at relapse may behave differently and could possibly benefit from HDCT, even though they are resistant to salvage chemotherapy. This hypothesis could be tested in a prospective randomized trial.
In conclusion, the results presented here indicate that the IPI at relapse enables us to distinguish patients with a significantly different response rate and OS after salvage treatment. They strongly suggest that HDCT improves the survival of patients in response to DHAP and with an IPI >0 and also possibly in nonresponding patients with an IPI 0-1.
ACKNOWLEDGMENT
The authors thank M.D. Reynaud for expert secretarial assistance during the making of this manuscript.
The PARMA study was supported by grants from the Ligue Nationale de Lutte contre le Cancer (Ligues Départementales de la Haute-Savoie, Saône et Loire, Rhône, Ain, and Ardèche), the University of Nebraska Medical Center, and the “Programme Hospitalier de Recherche Clinique.”
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to J.-Y. Blay, MD, PhD, Centre Léon Bérard, 28, rue Laennec, 69008 Lyon, France; e-mail:blay@lyon.fnclcc.fr.