Abstract
Mural thrombi form on exposed arterial subendothelium by a two-step process of platelet adhesion and aggregation. At high shear stresses such as are found in stenotic arteries, both steps are mediated by von Willebrand factor (vWF). Platelets initially adhere on vWF affixed to the subendothelial matrix through the glycoprotein (GP) Ib-IX-V complex. To examine the role of the GP Ib-IX-V complex under dynamic conditions, we modeled initial platelet adhesion at shear stresses ranging from 2 to 40 dyn/cm2 using vWF-coated glass slides, mammalian cells expressing full or partial GP Ib-IX-V complexes, and a parallel plate flow chamber with phase contrast video microscopy and digital image processing. Mammalian cells expressing the full complex tethered and rolled on the vWF substrate, whereas control cells did not. The rolling was completely inhibited by the monoclonal GP Ib antibody, AK2, or the vWF antibody, 5D2, both shown previously to block vWF-dependent platelet aggregation. Other GP Ib antibodies, WM23 and SZ2, did not significantly change the number or mean velocity of rolling cells. At low levels of GP Ib surface expression, cells expressing the full complex rolled slower than cells expressing the complex without GP V, indicating that GP V strengthens the interactions with the vWF surface under these conditions. Preshearing vWF for 5 minutes at 40 dyn/cm2 immediately before introducing cells into the chamber did not significantly change the number or the mean velocity of rolling cells. Inhibiting sulfation of the tyrosine residues within the GP Ib subunit reduced the number but did not change the mean velocity of the rolling cells. Our results indicate that, under the conditions of these experiments, bonds between vWF and GP Ib constantly form and break under fluid shear stress. Additionally, our results suggest that GP Ib-IX-V complexes behave like selectin receptors in their ability to mediate smooth rolling while cells maintain continuous surface contact. Such a mechanism, in vivo, would allow platelets to slow down and eventually arrest on the blood vessel wall. The system described provides a valuable approach for investigating the structure-function relationship of individual receptors and ligands in the process of platelet adhesion and thrombosis.
PATHOLOGIC ARTERIAL thrombosis is mediated by platelets and depends on the local fluid environment in the vessel.1-4 In the presence of elevated shear stresses, such as occur in stenotic arteries, mural thrombus formation is initiated when the platelet glycoprotein (GP) Ib-IX-V complex binds to von Willebrand factor (vWF) immobilized on the exposed subendothelium of a vessel injured as the result of angioplasty or atherosclerotic plaque rupture.2,5-7 It has been demonstrated that the integrin GP IIb-IIIa complex also has a role in the adhesion of platelets to the subendothelial matrix.8 Previous studies using surfaces coated with purified vWF suggest that vWF–GP Ib complex binding mediates both the initial tethering of platelets from the bulk fluid to the surface and the transient platelet translocation that follows.9 These studies also indicate that firm adhesion of platelets is supported by activation of the GP IIb-IIIa complex, which then binds the immobilized vWF.9,10 Firm adhesion occurs rapidly, therefore, platelet translocation is most readily observed under specific conditions that inhibit platelet activation or GP IIb-IIIa complex binding.9 Under arterial shear conditions, aggregation to the initial and subsequent layers of platelets is mediated by soluble vWF binding to GP IIb-IIIa complexes on adjacent platelets.2,5,11,12 In contrast to thrombosis occurring in high shear conditions, thrombosis under low shear conditions is supported mainly by fibrinogen-platelet rather than by vWF-platelet interactions.9 13 Currently, it is unknown whether a change in vWF itself or a change in the platelet complex enhances vWF–GP Ib-IX-V mediated adhesion under high shear conditions.
High shear conditions also impact vWF–GP Ib-IX-V binding by limiting the contact time between this ligand-receptor pair. A fast on-rate is required to support binding during the relatively short contact time available under high shear conditions. Additionally, a higher bond strength is necessary to support firm adhesion in the presence of elevated shear stresses. Compared with vascular adhesion processes in the venous system, such as selectin-mediated neutrophil binding to activated endothelial cells, binding between vWF and the GP Ib-IX-V complex, must withstand shear stresses that are greater by at least an order of magnitude.
The GP Ib-IX-V complex is of significant research interest because of its role in pathologic thrombosis as well as in hemostasis. This complex contains four polypeptide subunits, GP Ibα, GP Ibβ, GP IX, and GP V, present in a stoichiometry on the platelet surface of 2:2:2:1.14 The first three polypeptides are required for expression of the complex on the cell surface15 and GP V is necessary to form a high-affinity site for thrombin on the complex.16 GP Ibα contains the regions that bind both thrombin and vWF, within a 300 amino acid domain at its N-terminus.14 This region is separated from the platelet plasma membrane by an elongated mucin-like sequence called the macroglycopeptide, whose function may be to position the ligand-binding domain above the surrounding molecules on the platelet surface.17
We have developed an experimental model to isolate the vWF–GP Ib-IX-V interactions that mediate initial platelet adhesion to exposed subendothelium. To evaluate these interactions under dynamic conditions, we used vWF-coated glass coverslips, mammalian cells expressing full or partial GP Ib-IX-V complexes, and a parallel plate flow chamber with phase contrast video microscopy and digital image processing. Our data demonstrate that purified vWF supports tethering and rolling of cells expressing the GP Ib-IX-V complex and that this interaction is dependent on vWF–GP Ibα binding. Furthermore, the data suggest an unexpected role for GP V in enhancing the avidity of interaction between GP Ib-IX and vWF independent of its effect on the degree of GP Ib-IX expression. Additionally, our data indicate that exposing immobilized vWF to high shear stresses does not enhance vWF–GP Ib-IX-V interactions. Finally, our results suggest that, under dynamic conditions, sulfation of the tyrosine residues within the GP Ibα subunit contributes to the formation of GP Ibα–vWF bonds but may not affect the off-rate of bond formation.
MATERIALS AND METHODS
Cell lines.
The transfected mammalian cell lines used in our studies are summarized in Table 1. L cell lines were grown in Dulbecco’s modified Eagle’s medium (DMEM; Life Technologies, Inc, Grand Island, NY). The Chinese hamster ovary (CHO) cell lines were grown in α-minimal essential medium (α-MEM; Life Technologies). Both media were supplemented with 10% heat-inactivated fetal bovine serum (FBS) and the selection drugs indicated in Table 1. For each assay, a portion of the cells to be used in flow chamber experiments was stained with fluorescein isothiocyanate (FITC)-conjugated GP Ibα antibody and analyzed for surface expression by flow cytometry (see below). With the exception of experiments specifically investigating differences between L and CHO cell lines, all experiments used L cells expressing full or partial GP Ib-IX-V complexes. Control cells included L and CHO cells expressing only GP Ibβ and GP IX.
Inhibition of tyrosine sulfation.
Inhibition of sulfation of the tyrosine residues within the ligand-binding region of GP Ibα was completed as described previously.18 L cells expressing full GP Ib-IX-V complexes were grown in 100-mm culture dishes to 80% confluence and then switched to sulfate-free DMEM medium with 2% of the normal concentration of methionine and cysteine and supplemented with 10% dialyzed FBS. Sodium chlorate and guaiacol were then added to the medium at final concentrations of 5 and 0.2 mmol/L, respectively. Cells were maintained under these conditions for 24 hours before further assays. Control cells were grown in complete medium.
Flow cytometry.
Surface expression of GP Ibα was determined by flow cytometry after surface labeling GP Ib-IX and GP Ib-IXV expressing cells with the FITC-conjugated monoclonal GP Ibα antibody, AN51 (DAKO, Carpinteria, CA). Before labeling, the cells were harvested with 0.54 mmol/L EDTA. The cells were then washed with phosphate-buffered saline (PBS) and incubated for 30 minutes in culture medium containing 1% bovine serum albumin (BSA; fraction V; Sigma Chemical Co, St Louis, MO) to block nonspecific antibody binding. The cells were then incubated with the FITC-AN51, at a concentration of 1.4 μg/mL for 1 hour at room temperature. After the cells were washed twice with PBS, the geometric mean fluorescence of each sample was determined using a FACScan flow cytometer (Becton Dickinson, San Jose, CA) that stimulates the fluorescent dye with an argon-ion laser at 488 nm and collects the light emitted above 530 nm. Nonspecific binding was determined by the background fluorescence from LβIX and CHOβIX cells stained with the same antibody. The data were analyzed using Cellquest software from Becton Dickinson. All experiments except those specifically investigating the effect of receptor density evaluated L and CHO cells with comparable GP Ibα surface expression.
Monoclonal antibodies (MoAbs).
The MoAbs AK2, SZ2, 5D2, and WM23 were generously provided by Dr Michael Berndt (Baker Medical Research Institute, Prahran, Victoria, Australia). AK2 and SZ2 bind to GP Ibα, AK2 within the first 275 residues and SZ2 between residues 276-282.19 WM23 is directed against the macroglycopeptide region of GP Ibα and does not interfere with vWF–GP Ibα binding.20 5D2 was raised against a 39/34-kD dispase fragment of vWF (residues 480-718 of the mature protein) that contains the GP Ib binding site.21Before introduction into the flow chamber, cells were incubated with one of the four antibodies: 6 μg/mL AK2, 25 μg/mL SZ2, 50 μg/mL 5D2, and 25 μg/mL WM23. All of these antibodies saturate at a concentration of less than 5 μg/mL, based on previous studies (M. Berndt, personal communication, June 26, 1998).19-22 After 15 to 20 minutes of incubation, the cells were resuspended in Dulbecco’s PBS (Sigma Chemical Co) to a final concentration of 500,000 cells/mL.
Preparation of vWF coverslips.
Purified vWF was obtained from normal human cryoprecipitate by glycine and NaCl precipitation23,24 and then purified on a Sepharose 4B column (2.5 × 50 cm with a bed volume of 3,000 ml; Pharmacia, Inc, Piscataway, NJ). Using an enzyme-linked immunoassay (Spectro vWF Catalog No. V-46; Ramco Laboratories, Inc, Houston, TX), the vWF concentration of the pooled peak fractions was determined relative to normal vWF plasma concentration. For each flow chamber experiment, the required amount of vWF was diluted to 30%, 250%, 500%, or 750% of normal plasma concentration in Dulbecco’s PBS (Sigma Chemical Co). For this concentration range, it has been shown that the amount of vWF absorbed on glass coverslips will increase as the concentration of vWF in solution increases.25Therefore, we used the concentration of the vWF solution coated on the coverslip as an index of vWF surface density. Glass coverslips (No. 1, 24 × 50 mm; Corning, Inc, Corning, NY) were coated with 200 μL of the desired vWF solution and incubated for a minimum of 45 minutes in a humid chamber. When 30% vWF was used, after the initial 45 minutes of incubation, the coverslips were rinsed and then coated with 400 μL of PBS containing 1% bovine serum albumin (Boehringer Mannheim, Indianapolis, IN) for a minimum of 20 minutes. To aid in handling the coverslips, a 15-mm edge was left uncoated. Immediately before using each coverslip, excess vWF or bovine serum albumin was rinsed off with 5.0 mL of 0.9% NaCl. The parallel plate flow chamber was then assembled with the coverslip forming the bottom of the chamber.26-28 All experiments were completed on surfaces prepared using a 500% vWF solution, with the exception of those experiments specifically investigating the effect of vWF surface density.
Parallel plate flow chamber and digital image processing.
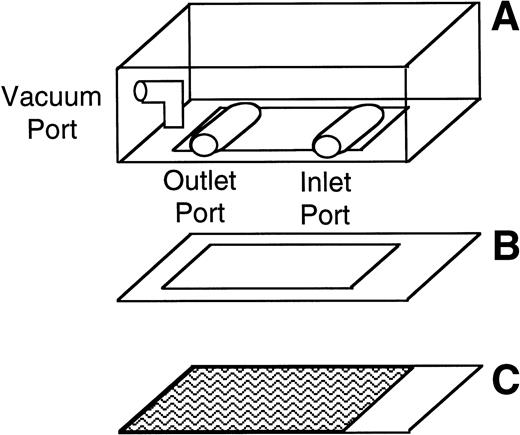
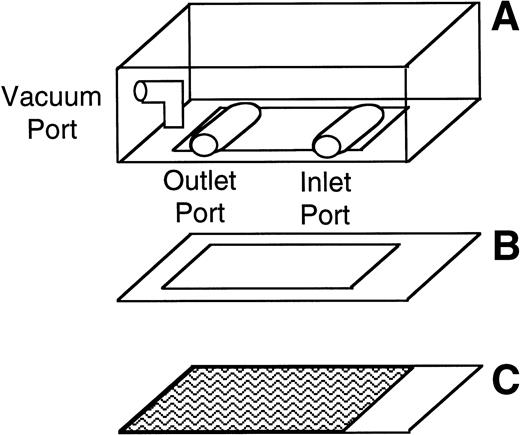
The number and velocity of rolling cells were determined using a parallel plate flow chamber and phase contrast video microscopy. The parallel plate flow chamber consists of a polycarbonate slab, a silicon gasket, and a vWF-coated glass coverslip (Fig 1). These three components are held together by vacuum. The thickness of the silicon gasket determines the height of the gap between the coverslip and the polycarbonate slab. A syringe pump connected to the outlet port draws fluid across this gap through the chamber. The wall shear stress depends on the height of the gap, the width of the chamber, the fluid viscosity, and the flow rate through the chamber.29 30
Schematic of the parallel plate flow chamber. The chamber consists of a polycarbonate slab (A), a silicon gasket (B), and a vWF-coated glass coverslip (C) held together by vacuum. During experiments, the parallel plate flow chamber is mounted on an inverted-stage microscope connected to a video camera and a video- cassette recorder.
Schematic of the parallel plate flow chamber. The chamber consists of a polycarbonate slab (A), a silicon gasket (B), and a vWF-coated glass coverslip (C) held together by vacuum. During experiments, the parallel plate flow chamber is mounted on an inverted-stage microscope connected to a video camera and a video- cassette recorder.
During experiments, the parallel plate flow chamber was mounted on an inverted-stage microscope (DIAPHOT-TMD; Nikon, Garden City, NY) equipped with a ×20 phase objective (Nikon), a ×5 projection lens (Nikon), and a silicon-intensified target video camera (Model C2400; Hammatsu, Waltman, MA) connected to a video cassette recorder. In some experiments after the chamber was assembled and mounted, vWF immobilized on the glass coverslip was sheared by perfusing Dulbecco’s PBS through the chamber for 5 minutes at a constant flow rate to produce a constant wall shear stress of 40 dyn/cm2. In most experiments, 0.6 mL of either L or CHO cells at a concentration of 500,000 cells/mL was transferred into the parallel plate flow chamber and allowed to settle on the immobilized vWF for 1 minute. Perfusion of Dulbecco’s PBS was then initiated and continued for 4 minutes at wall shear stresses ranging from 5 to 40 dyn/cm2. In other experiments, after the immobilized vWF was presheared, a cell suspension was continuously perfused through the chamber for 5 minutes without an initial settling period. In these experiments, cells were perfused through the chamber at a concentration of 100,000 cells/mL and a wall shear stress of 2 dyn/cm2. Throughout all experiments, the parallel plate flow chamber and PBS or cell solution were maintained at 37°C by a thermostatic air bath (Model 279; Laboratory Products, Boston, MA). Cell rolling in a single field of view was recorded in real time for 4 or 5 minutes on videotape. The video data were analyzed off-line using Inovision imaging software (IC-300 Modular Image Processing; Workstation Inovision Corp, Durham, NC) to quantify the number of rolling cells and the mean velocity of the cells.31 32 Mean velocity was calculated by overlapping sequential maximization images snapped at 30 frames per second and determining the distance the cells rolled during the time period of the snaps. An average of 30 to 50 cells was used to determine the mean velocity in each experimental run. For each condition investigated, three to eight experimental runs were completed, so final velocity calculations were based on measurements from approximately 100 to 350 cells. The number of rolling cells was determined by focusing on a single field of view and counting each cell that rolled on the vWF surface during the entire 4- or 5-minute flow period. Only cells that rolled maintaining continuous surface contact were included in the determination of the number and velocity of rolling cells.
All experiments were conducted after vWF had been sheared for 5 minutes at 40 dyn/cm2 before the introduction of cells, with the exception of the experiments completed to investigate the potential functional change in immobilized vWF after exposure to elevated shear conditions. With the exception of experiments to investigate the tethering ability of cells, all experiments were completed by injecting the cells into the flow chamber and allowing the cells to settle for 1 minute before the initiation of flow.
Statistics.
Results are reported as the mean ± SEM. The statistical significance of the difference between means was determined by ANOVA using the Fischer’s protected least significant difference (PLSD) test.
RESULTS
vWF–GP Ib-IX-V–mediated cell rolling.
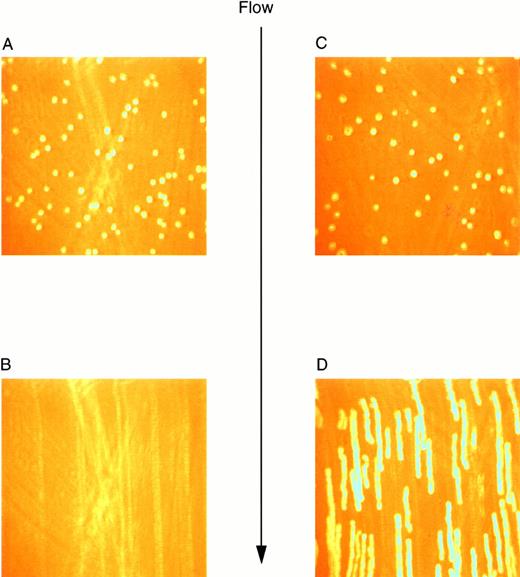
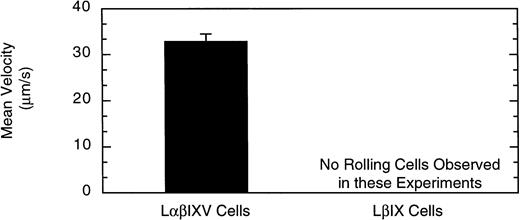
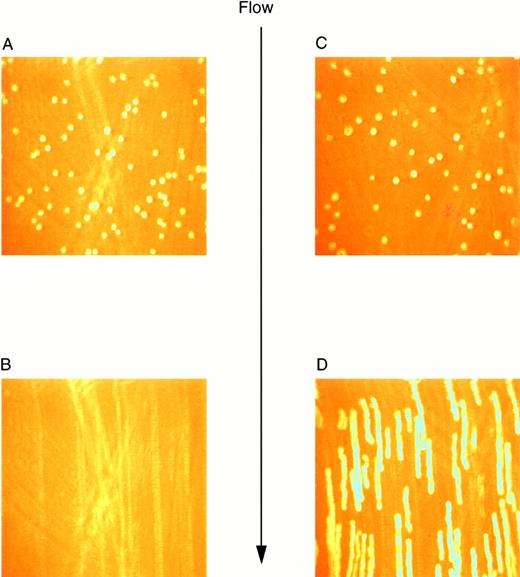
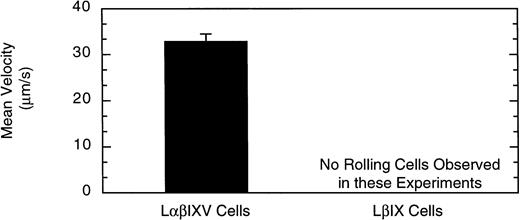
Previous studies investigating the interaction of platelets with purified vWF under dynamic conditions have demonstrated a sequential process in which the initial tethering and translocation of the platelets involves the GP Ib-IX-V complex, whereas firm adhesion requires binding of vWF by GP IIb-IIIa.9 To examine the vWF–GP Ib-IX-V interactions that initiate platelet adhesion, we first isolated these interactions using L cells expressing the GP Ib-IX-V complex and immobilized vWF in a parallel plate flow chamber. In the presence of shear, L cells expressing the full GP Ib-IX-V complex rolled on immobilized vWF (Fig 2C and D). At a shear stress of 10 dyn/cm2, greater than 700 LαβIXV cells rolled on the immobilized vWF in a single field of view during the 4-minute flow period (Fig3B). The average velocity of the rolling LαβIXV cells was 80 μm/s (Fig 3A). In contrast, not a single LβIX cell rolled on the immobilized vWF after flow was initiated (Fig 2A and B). Instead, the resulting shear force caused these cells to be lifted from the vWF surface and carried toward the center of the flowstream, suggesting that rolling was dependent on the presence of the GP Ibα subunit. Our results also suggest that cell tethering from the flowstream to immobilized vWF depends on the presence of the GP Ibα subunit. At a wall shear stress of 2 dyn/cm2, greater than 190 LαβIXV cells tethered to the vWF surface and rolled with an average velocity of 33 μm/s when a cell suspension was perfused through the chamber for 5 minutes (Fig 4). When LβIX cells were perfused through the chamber, no rolling cells were observed (Fig4).
Video images of L-cell rolling on purified vWF at a wall shear stress of 10 dyn/cm2. Images were created using a digital image processing system to snap frames of previously recorded experiments. LβIX cells (A) and LβIXV cells (C) were introduced into the chamber and allowed to incubate for 1 minute before the initiation of flow. After 30 seconds of flow, LβIX cells (B) had been swept away from the vWF surface, whereas LβIXV cells (D) rolled maintaining continuous surface contact. Images of rolling cells were created by snapping 30 frames per second and overlapping all 30 frames.
Video images of L-cell rolling on purified vWF at a wall shear stress of 10 dyn/cm2. Images were created using a digital image processing system to snap frames of previously recorded experiments. LβIX cells (A) and LβIXV cells (C) were introduced into the chamber and allowed to incubate for 1 minute before the initiation of flow. After 30 seconds of flow, LβIX cells (B) had been swept away from the vWF surface, whereas LβIXV cells (D) rolled maintaining continuous surface contact. Images of rolling cells were created by snapping 30 frames per second and overlapping all 30 frames.
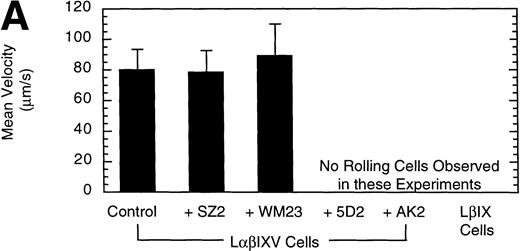
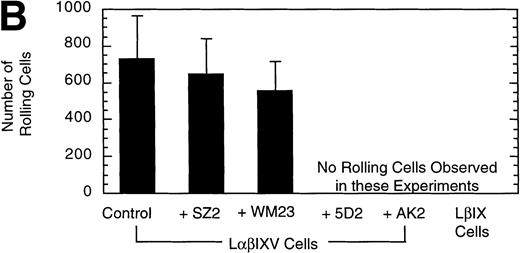
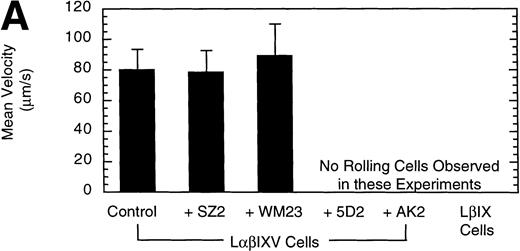
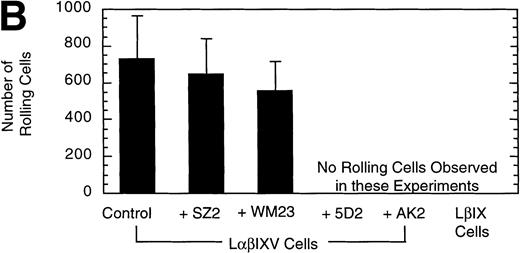
Effect of MoAbs on LβIXV cell rolling. Before introduction into the parallel plate flow chamber, LβIXV cells were incubated with one of four MoAbs: SZ2 (anti-GP Ib), WM23 (anti-GP Ib), 5D2 (anti-vWF), or AK2 (anti-GP Ib). The mean velocity (A) was calculated on a digital image processing system using 1-second maximization images. The number of rolling cells (B) was determined by counting the total number of cells rolling on the vWF surface in a single field of view over a 4-minute flow period. During experimental runs, the entire system was maintained at 37°C. Dulbecco’s PBS was pumped through the chamber at a constant flow rate, producing a constant shear stress of 10 dyn/cm2. Values are the mean ± SEM, n = 3.
Effect of MoAbs on LβIXV cell rolling. Before introduction into the parallel plate flow chamber, LβIXV cells were incubated with one of four MoAbs: SZ2 (anti-GP Ib), WM23 (anti-GP Ib), 5D2 (anti-vWF), or AK2 (anti-GP Ib). The mean velocity (A) was calculated on a digital image processing system using 1-second maximization images. The number of rolling cells (B) was determined by counting the total number of cells rolling on the vWF surface in a single field of view over a 4-minute flow period. During experimental runs, the entire system was maintained at 37°C. Dulbecco’s PBS was pumped through the chamber at a constant flow rate, producing a constant shear stress of 10 dyn/cm2. Values are the mean ± SEM, n = 3.
Shear-dependent cell tethering and rolling. The velocity of cells that tethered and rolled on the vWF surface was determined at a wall shear stress of 2 dyn/cm2. Over a 5-minute flow period, LβIXV cells tethered to the vWF surface and rolled maintaining continuous surface contact. In contrast, LβIX cells did not tether and roll on the vWF surface. Values are the mean ± SEM, n = 3 to 4.
Shear-dependent cell tethering and rolling. The velocity of cells that tethered and rolled on the vWF surface was determined at a wall shear stress of 2 dyn/cm2. Over a 5-minute flow period, LβIXV cells tethered to the vWF surface and rolled maintaining continuous surface contact. In contrast, LβIX cells did not tether and roll on the vWF surface. Values are the mean ± SEM, n = 3 to 4.
To confirm the specificity of LαβIXV cell rolling on immobilized vWF, flow chamber experiments were performed in the presence of monoclonal GP Ibα and vWF antibodies. Four MoAbs were used in our studies: AK2, 5D2, SZ2 and WM23. AK2, directed against an epitope in the N-terminal 275 residues of GP Ibα,19 and 5D2, directed against the A1 domain of vWF,21 are both strong inhibitors of ristocetin- and botrocetin-induced binding of vWF to platelets as well as asialo-vWF–dependent platelet aggregation.19-21,33 SZ2, directed against the sulfated tyrosine-containing sequence of GP Ibα (residues 276 to 282), inhibits botrocetin-induced binding of vWF to platelets and asialo-vWF–induced platelet aggregation, but is only a weak inhibitor of ristocetin-induced binding of vWF to platelets.19 WM23 is directed against the macroglycopeptide core of GP Ibα and has no effect on vWF–GP Ib-IX-V interactions.20 33 LαβIXV cell rolling on immobilized vWF was completely inhibited by both AK2 and 5D2 (Fig 3). The two other GP Ibα antibodies, WM23 and SZ2, did not significantly change the number or the mean velocity of rolling cells (Fig 3).
Comparison of cell lines.
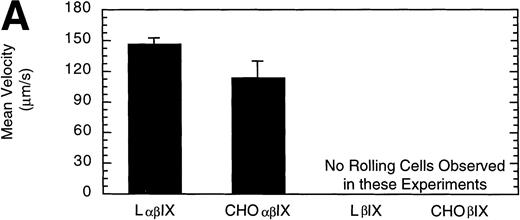
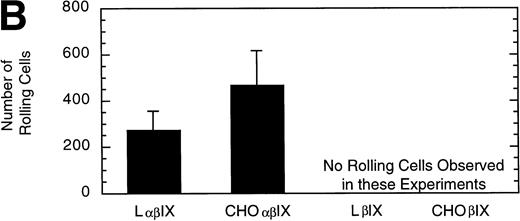
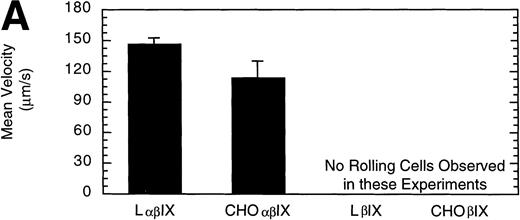
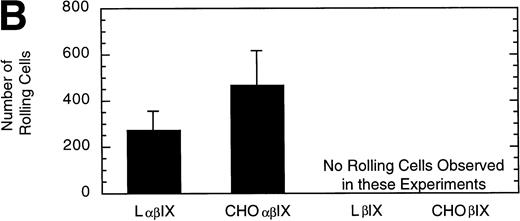
L and CHO cells expressing the GP Ib-IX complex (no GP V) or a complex of GP Ibβ and GP IX (no GP V, no GP Ibα) were used to evaluate potential differences attributable to the use of different cell lines. No significant difference between the number or velocity of rolling cells was observed between the two cell lines expressing GP Ib-IX (Fig 5). L and CHO cells lacking GP Ibα did not roll on the immobilized vWF after flow was initiated.
Comparison of L and CHO cell lines. The mean velocity (A) and the total number (B) of rolling LβIX and CHOβIX cells were determined at a wall shear stress of 10 dyn/cm2. Values are the mean ± SEM, n = 4 to 7.
Comparison of L and CHO cell lines. The mean velocity (A) and the total number (B) of rolling LβIX and CHOβIX cells were determined at a wall shear stress of 10 dyn/cm2. Values are the mean ± SEM, n = 4 to 7.
Influence of wall shear stress.
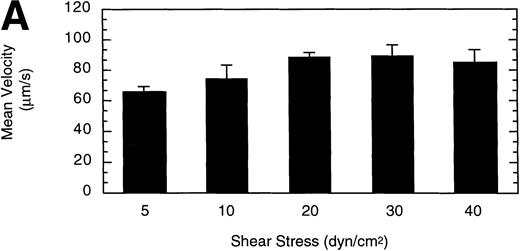
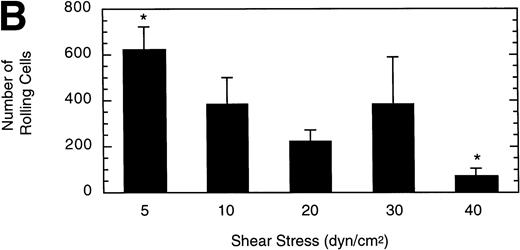
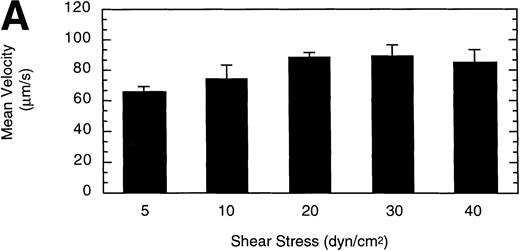
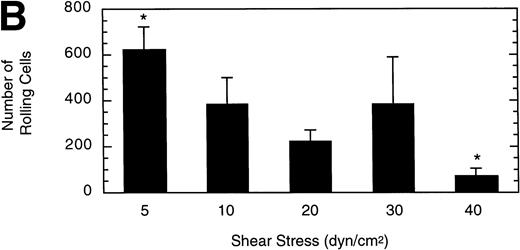
To gain insight into the strength and rate of vWF–GP Ib-IX-V binding, the influence of wall shear stress on rolling LαβIXV cells was determined. Shear stresses ranging from 5 to 40 dyn/cm2were investigated by varying the flow rate of Dulbecco’s PBS through the chamber. The mean velocity of rolling cells increased slightly as shear stress increased from 5 to 20 dyn/cm2, but did not change between 20 and 40 dyn/cm2(Fig 6A). In contrast to this modest effect on velocity, increasing the shear stress from 5 to 40 dyn/cm2 decreased the number of rolling cells by 88% (Fig6B).
Influence of shear stress on LβIXV cell rolling. The mean velocity (A) and the total number (B) of rolling cells were determined at wall shear stresses ranging from 5 to 40 dyn/cm2. Values are the mean ± SEM, n = 3 to 6. *Differences between means are statistically significant, P < .05.
Influence of shear stress on LβIXV cell rolling. The mean velocity (A) and the total number (B) of rolling cells were determined at wall shear stresses ranging from 5 to 40 dyn/cm2. Values are the mean ± SEM, n = 3 to 6. *Differences between means are statistically significant, P < .05.
Effect of vWF surface density.
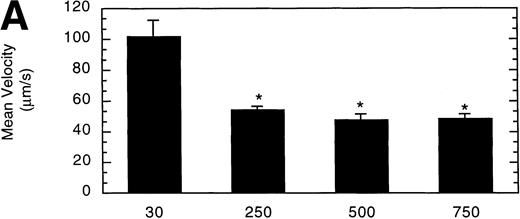
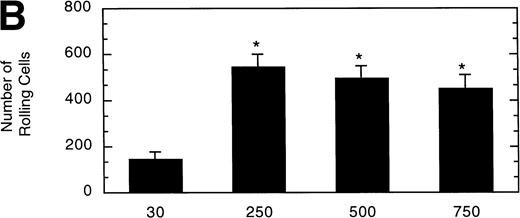
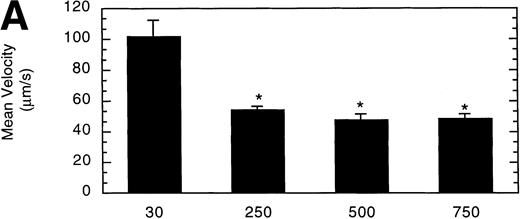
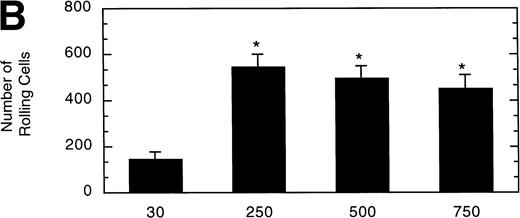
To determine the effect of vWF surface density on LαβIXV cell rolling, glass coverslips were coated with solutions of varying vWF concentration. The velocity of LαβIXV cells decreased by 50%, whereas the number of rolling cells increased by 240% when the concentration of vWF coated on the glass coverslip was increased from 30% to 250% (Fig 7). No significant differences were observed between the number or the velocity of rolling LαβIXV cells on coverslips coated with solutions containing 250%, 500%, or 750% vWF (Fig 7).
Influence of vWF surface density on LβIXV cell rolling. Mean velocity (A) and the total number (B) of rolling cells were determined on surfaces prepared using solutions with vWF concentrations ranging from 30% to 750% of normal plasma concentration. Values are the mean ± SEM, n = 4 to 8. *Statistically significant from 30% surface, P < .05.
Influence of vWF surface density on LβIXV cell rolling. Mean velocity (A) and the total number (B) of rolling cells were determined on surfaces prepared using solutions with vWF concentrations ranging from 30% to 750% of normal plasma concentration. Values are the mean ± SEM, n = 4 to 8. *Statistically significant from 30% surface, P < .05.
Effect of receptor density.
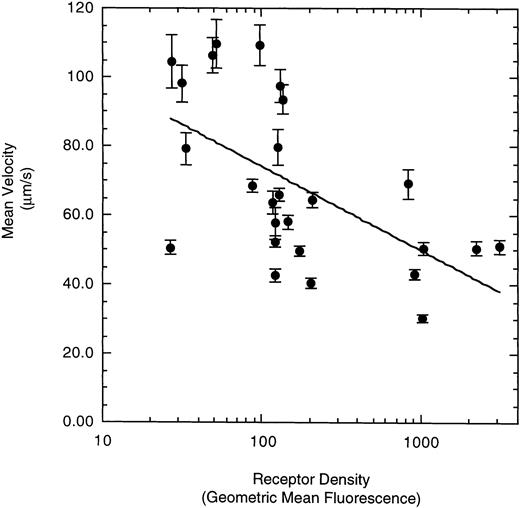
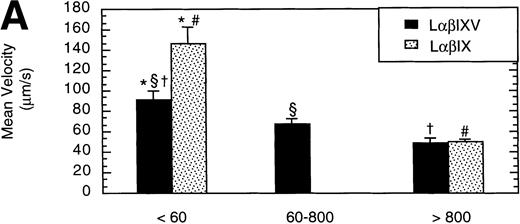
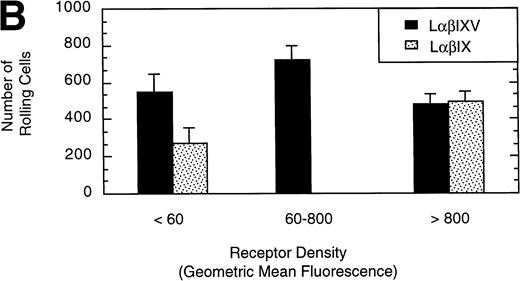
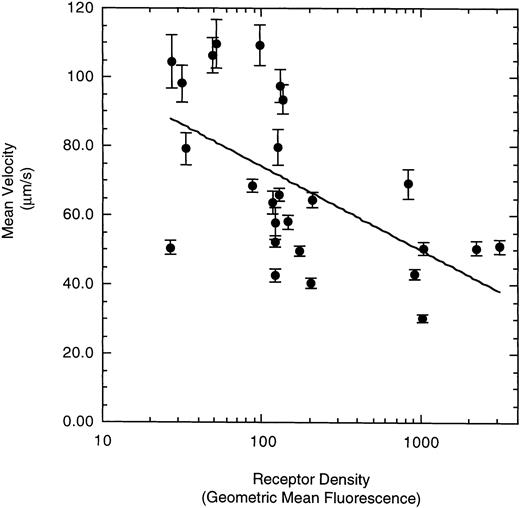
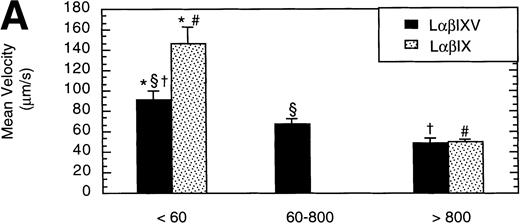
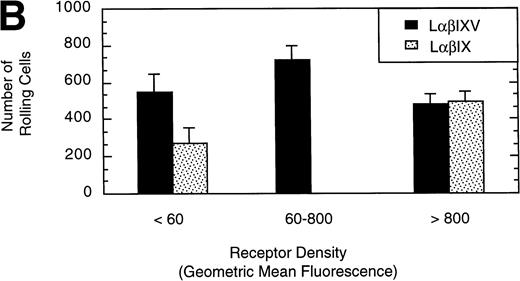
To examine the effect of receptor density on cell rolling, populations of LαβIXV cells with different levels of GP Ibα surface expression were evaluated in the parallel plate flow chamber. Before beginning flow chamber experiments, an aliquot of cells from each sample was removed and used to assess the level of GP Ibα surface expression by FITC-AN51 staining and flow cytometry. The geometric mean fluorescence of each cell sample was used as an index of receptor density. A wide range of receptor densities was present in the different cell samples as indicated by the greater than 100-fold variation in geometric mean fluorescence. The velocity of the rolling LαβIXV cells appeared to decrease as GP Ibα surface expression increased over this very broad range of receptor densities (y = 122.42 − 24.191 log (x), R = .57; Fig 8). To determine whether the apparent decrease in velocity was statistically significant, the cells were divided into three groups: low receptor density cells (10 < geometric mean fluorescence < 60 fluorescent units), medium receptor density cells (60 < geometric mean fluorescence < 800), and high receptor density cells (geometric mean fluorescence > 800). LαβIXV cells with low receptor density rolled significantly faster than those cells with medium or high receptor density (Fig 9A). No significant difference in rolling velocities was observed between LαβIXV cells with a medium receptor density and those with a high receptor density (Fig 9A). The number of rolling cells was independent of receptor density (Fig 9B).
Effect of receptor density on LβIXV cell rolling. The mean velocities were calculated based on an average of 30 to 50 cells from an experimental run. Each datapoint represents a single experimental run. Values are the mean ± SEM.
Effect of receptor density on LβIXV cell rolling. The mean velocities were calculated based on an average of 30 to 50 cells from an experimental run. Each datapoint represents a single experimental run. Values are the mean ± SEM.
Effect of receptor density and role of the GP V subunit. Mean velocity (A) and the total number (B) of rolling LβIXV and LβIX cells with different receptor densities were determined at a wall shear stress of 10 dyn/cm2. Values are the mean ± SEM, n = 6 to 14. *, §, †, #Differences between means are statistically significant, P < .05.
Effect of receptor density and role of the GP V subunit. Mean velocity (A) and the total number (B) of rolling LβIXV and LβIX cells with different receptor densities were determined at a wall shear stress of 10 dyn/cm2. Values are the mean ± SEM, n = 6 to 14. *, §, †, #Differences between means are statistically significant, P < .05.
Role of GP V.
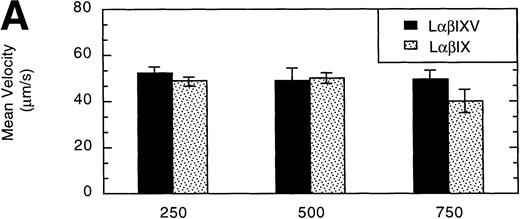
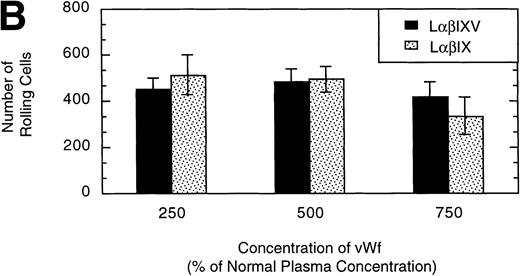
L cells expressing either the GP Ib-IX complex or the full complex containing GP V were evaluated to determine the influence of GP V on cell rolling. Cells with a similar receptor density were compared: the geometric mean fluorescence of samples from either cell line ranged from 10 to 60 fluorescent units (low receptor density group) or from 800 to 3,500 fluorescent units (high receptor density group). In the low receptor density group, the average velocity of LαβIX cells (no GP V) was 61% greater than that of LαβIXV cells (Fig 9A). Additionally, within the low receptor density group, the number of rolling LαβIX cells was 50% lower than the number of rolling LαβIXV cells (Fig 9B). When L cells with a high receptor density were evaluated, the number and velocity of rolling LαβIX cells was not significantly different from that of LαβIXV cells (Fig 9). Additionally, as observed using fully transfected cells, LαβIX cells with a low receptor density rolled significantly faster than LαβIX cells with a high receptor density (Fig 9A). LαβIX cells with a high receptor density were also evaluated on surfaces with different vWF densities. Similar to the fully transfected cells, the number and velocity of high receptor density LαβIX cells did not significantly change as the concentration of vWF coated on the glass coverslip varied from 250% to 750% (Fig10).
Influence of vWF surface density and the role of the GP V subunit. LβIXV and LβIX cells with a high receptor density (geometric mean >800 fluorescent units) were evaluated on surfaces prepared using vWF solutions with concentrations ranging from 250% to 750% of normal vWF plasma concentration. The mean velocity (A) and the total number (B) of rolling cells were determined at a wall shear stress of 10 dyn/cm2. Values are the mean ± SEM, n = 4 to 8.
Influence of vWF surface density and the role of the GP V subunit. LβIXV and LβIX cells with a high receptor density (geometric mean >800 fluorescent units) were evaluated on surfaces prepared using vWF solutions with concentrations ranging from 250% to 750% of normal vWF plasma concentration. The mean velocity (A) and the total number (B) of rolling cells were determined at a wall shear stress of 10 dyn/cm2. Values are the mean ± SEM, n = 4 to 8.
Functional effect of shear on immobilized vWF.
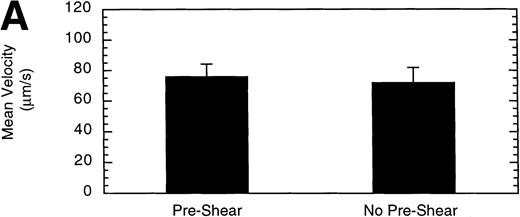
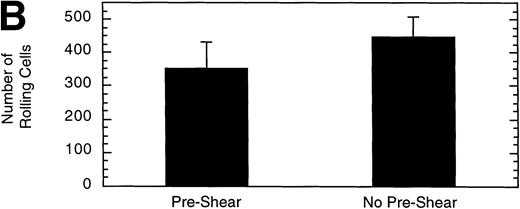
It has been demonstrated that high shear forces change the structure of immobilized vWF from a globular conformation to an open, extended conformation.34 Therefore, we investigated whether exposing vWF to high shear before perfusing LαβIXV cells might influence cell adhesion and rolling. Preshearing of vWF was accomplished by perfusing Dulbecco’s PBS through the flow chamber for 5 minutes at 40 dyn/cm2 before introducing cells. Preshearing the vWF did not significantly change the number of rolling cells or the mean velocity of the cells (Fig 11).
Effect of preshearing vWF. The mean velocity (A) and the total number (B) of rolling LβIXV cells were determined at a wall shear stress of 10 dyn/cm2. In preshear experiments, before cells were introduced into the chamber, vWF immobilized on the glass coverslip was exposed to a shear stress of 40 dyn/cm2 for 5 minutes by perfusing Dulbecco’s PBS through the chamber. Values are the mean ± SEM, n = 4 to 7.
Effect of preshearing vWF. The mean velocity (A) and the total number (B) of rolling LβIXV cells were determined at a wall shear stress of 10 dyn/cm2. In preshear experiments, before cells were introduced into the chamber, vWF immobilized on the glass coverslip was exposed to a shear stress of 40 dyn/cm2 for 5 minutes by perfusing Dulbecco’s PBS through the chamber. Values are the mean ± SEM, n = 4 to 7.
Functional effect of GP Ibα tyrosine sulfation.
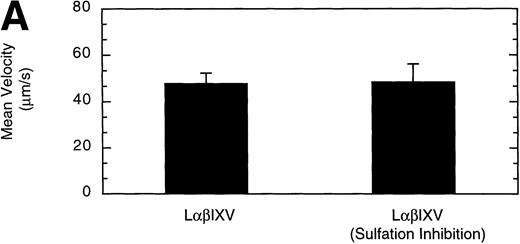
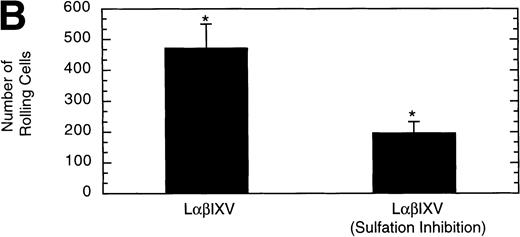
Previous studies investigating the structure of the GP Ib-IX-V complex have determined that the GP Ibα subunit is modified by the sulfation of three tyrosine residues (Y276, Y278, and Y279).18 19 To examine whether sulfate modification of the tyrosine residues impacts GP Ibα-vWF interactions under dynamic conditions, we compared rolling of sulfated LαβIXV cells with that of LαβIXV cells lacking sulfation. The velocity of LαβIXV cells lacking sulfation was not significantly different than the velocity of sulfated LαβIXV cells (Fig 12A). In contrast, the number of rolling LαβIXV cells decreased by 58% when tyrosine sulfation was inhibited (Fig 12B).
Effect of sulfation of the tyrosine residues within the GP Ib subunit. The mean velocity (A) and the total number (B) of rolling cells were determined at a wall shear stress of 10 dyn/cm2. Sulfation inhibition experiments were completed using LβIXV cells grown in complete or sulfate-depleted media. Values are the mean ± SEM, n = 4. *Differences between means are statistically significant,P < .05.
Effect of sulfation of the tyrosine residues within the GP Ib subunit. The mean velocity (A) and the total number (B) of rolling cells were determined at a wall shear stress of 10 dyn/cm2. Sulfation inhibition experiments were completed using LβIXV cells grown in complete or sulfate-depleted media. Values are the mean ± SEM, n = 4. *Differences between means are statistically significant,P < .05.
DISCUSSION
The platelet GP Ib-IX-V complex initiates pathologic arterial thrombosis by binding to vWF immobilized on the subendothelium of a denuded vessel. It has been shown previously that blocking this initial step of platelet adhesion decreases formation of thrombi and reduces long-term restenosis of narrowed vessels after injury.2 35To better understand the specific molecular mechanisms that support platelet adhesion, we have developed a dynamic experimental model that isolates vWF–GP Ib-IX-V interactions. Our system consists of vWF-coated glass coverslips, mammalian cells expressing full or partial GP Ib-IX-V complexes, and a parallel plate flow chamber with phase contrast video microscopy and digital image processing. We have demonstrated that, in the presence of shear, L and CHO cells expressing the GP Ib-IX-V complex will tether and roll on immobilized vWF, whereas cells expressing subcomplexes lacking GP Ibα do not exhibit any strong interactions. These studies indicate that the GP Ib-IX-V complex supports cell tethering and rolling on immobilized vWF independent of other platelet adhesive components.
In support of this, we have also shown that cell rolling is completely inhibited by the monoclonal GP Ibα antibody AK2 or the vWF antibody 5D2. These antibodies have been shown previously to block asialo-vWF–, ristocetin-, and botrocetin-induced platelet aggregation.19-22,33 The GP Ibα antibody SZ2 did not significantly change the number or mean velocity of rolling cells. SZ2 has been shown previously to block asialo-vWF– and botrocetin- but not ristocetin-induced platelet aggregation.19 22 These contrasting results suggest that different vWF binding sites may be involved in platelet aggregation induced by botrocetin and asialo-vWF versus aggregation induced by ristocetin and platelet adhesion under flow, even though all of these processes require a vWF–GP Ibα interaction. Thus, these findings suggest that ristocetin-dependent vWF binding to the GP Ib-IX-V complex may better reflect the binding reaction that occurs under shear in vivo.
Our results indicate that, under the conditions of these experiments, bonds between vWF and GP Ibα continually form and break under fluid shear stress. When the wall shear stress was increased from 5 to 40 dyn/cm2, the number of rolling LαβIXV cells significantly decreased, whereas no significant change in velocity was observed. We selected a wall shear stress of 10 dyn/cm2 to use in all other experiments. Although this shear stress is lower than shear stresses present in the arterial system, it is important to consider the resultant shear force acting on platelets in the blood stream relative to the shear force acting on the mammalian cells in our system. The shear force acting on cells near a vessel wall depends on both the fluid shear stress and the cell size.36,37Neglecting differences in shape, the resultant shear force used in our experiments with L and CHO cells corresponds to the shear force a platelet would experience in the presence of a wall shear stress of 110 dyn/cm2. Physiologically, shear stresses of this magnitude are only present in stenosed arteries, where platelet adhesion requires vWF–GP Ib-IX-V binding. At wall shear stresses of 10 dyn/cm2, the average velocity of the rolling cells was only 2% of the calculated velocity of a neutrally buoyant, rigid sphere of the same size flowing near a plane wall.36
In platelets, there are approximately 25,000 copies of GP Ibα, GP Ibβ, and GP IX38 and half as many copies of GP V,39 indicating that the number of complexes may be either 12,000 or 6,000, depending on the numbers of each polypeptide present in the complex. These complexes are anchored to the cytoskeleton, with this anchorage providing for an orderly spatial distribution on the plasma membrane.14 In the mammalian cells used in our study, the number of GP Ib-IX-V complexes present on the surface varied, but, as in platelets, they were anchored to the cytoskeleton and evenly distributed throughout the cell’s smooth, spherical membrane.15 In our studies, we found that cells with a low GP Ibα receptor density rolled significantly faster than cells with a high receptor density. We also observed that, beyond a certain level of GP Ibα expression, cell velocity was independent of receptor density. Similarly, significant differences in cell velocity were observed on surfaces coated with high and low vWF concentrations, but, once a threshold level of vWF surface density was reached, cell velocity remained nearly constant. Recent studies with whole blood in a similar system have shown that platelet translocation velocity is also independent of vWF surface density above a threshold level.25
Our results suggest that GP Ib-IX-V complexes behave like selectin receptors in their ability to mediate smooth rolling while cells maintain continuous surface contact. Such a mechanism, in vivo, will allow platelets to slow down and eventually arrest on the blood vessel wall. Selectin-mediated rolling has been studied in parallel plate flow chamber systems similar to the one presented here. In those studies, neutrophils were perfused through the chamber over activated endothelial cells expressing P-selectin. Previous work31has demonstrated that optimal selectin-mediated neutrophil rolling occurs at wall shear stresses of 1 to 2 dyn/cm2. At higher shear stresses, the number of rolling neutrophils rapidly decreases and is nearly zero at 10 dyn/cm2.31 In contrast, we found that LαβIXV cells roll on immobilized vWF in significant numbers at shear stresses as high as 40 dyn/cm2. Because neutrophils and the mammalian cells used in our study are similar in size, this suggests that either vWF GP Ib-IX-V bonds are stronger or that these bonds are able to form more rapidly than the bonds between P-selectin glycoprotein ligand-1 (PSGL-1) and P-selectin.
We have used this new system to evaluate the role of GP V in vWF–GP Ib-IX-V mediated cell rolling. Previous studies suggest that GP V aids in establishing the topology of the GP Ib-IX-V complex.40Additional work using transfected mammalian cells has shown that the GP V subunit is not required for ristocetin-41 or botrocetin-induced aggregation (unpublished data). In contrast to these modulator-induced interactions, we observed that the shear-dependent cell rolling in our system is influenced by the presence of GP V. When GP Ibα surface expression was low, LαβIX cells (no GP V) rolled 61% faster and the total number of rolling cells decreased by 50% relative to cells expressing the full complex. These differences were not observed when cells with a higher receptor density were compared. The results using low receptor density cells suggest that GP V may function to maintain the optimal conformation of the complex required for its interaction with surface-bound vWF under dynamic conditions. The lack of impact of GP V at high receptor densities suggests that, under these conditions, the ability of GP V to assist in optimal spacing of the full complex is diminished. Alternatively, as has been suggested for its role in thrombin binding, GP V may juxtapose adjacent GP Ibα subunits such that they can more efficiently interact with the two A1 domains in the vWF dimer. This effect would be less pronounced at high receptor densities. As with other functions of the GP Ib-IX-V complex, further studies of the role of GP V are needed.
We have also used this new system to investigate the effect of shear on immobilized vWF function. Past studies using atomic force microscopy have demonstrated that exposure to elevated shear conditions results in a structural change in immobilized vWF.34 It has been suggested that this observed structural change enhances vWF–GP Ibα binding under high shear conditions. We investigated the functional effect of shearing vWF by conducting parallel plate flow chamber experiments with and without exposing immobilized vWF to high shear immediately before use. We found that preshearing vWF did not significantly change the number of rolling cells or the mean velocity of the cells, suggesting that shear does not change the function of immobilized vWF with respect to GP Ib-IX-V complex binding. However, although the shear stress used in non-preshear experiments was well below the established critical shear stress required to alter vWF,34 it is possible that the shear conditions present in the non-preshear system did change the conformation of the immobilized vWF. It is also possible that the vWF conformation induced by preshearing could have reverted within the incubation time before reinitiating flow.
Finally, we have used this new system to evaluate the effect of sulfation of GP Ibα tyrosine residues on cell rolling. Past evidence suggests that sulfation of these residues may be required for optimal GP Ibα-vWF binding.18,19,42 The velocity of LαβIXV cells lacking sulfation was not significantly different than the velocity of sulfated LαβIXV cells. These results are consistent with those obtained in our system using the MoAb SZ2, which binds to the region of the GP Ibα subunit containing the sulfated tyrosine residues.19 Although cell velocity did not change, the number of rolling LαβIXV cells decreased by 58% when tyrosine sulfation was inhibited. This decrease in the number of rolling cells suggests that sulfation impacts the on-rate of GP Ibα–vWF bond formation, that is, sulfation impacts GP Ibα–vWF interactions by altering the ability of GP Ibα to form bonds with immobilized vWF under dynamic conditions. In contrast, the off-rate of bond formation, indicated by cell velocity, appears to be independent of sulfation.
To summarize, we have developed an experimental model of the vWF–GP Ib-IX-V interactions that mediate initial platelet adhesion to an injured vessel wall. This system provides a valuable tool for investigating the roles of individual receptors and ligands in the process of platelet adhesion and thrombosis. Our results with this new system suggest the novel finding that optimal binding between immobilized vWF and GP Ibα requires the presence of the GP V subunit within the GP Ib-IX-V complex. Additionally, our results indicate that the function of immobilized vWF with respect to GP Ib-IX-V binding does not change after exposure to high shear. Finally, our results demonstrate that, under dynamic conditions, sulfation of the GP Ibα tyrosine residues contributes to the ability of GP Ibα–vWF bonds to form but does not affect the off-rate of bond formation.
This system can now be used to further analyze mutant receptor complexes under conditions that approximate more closely the in vivo situation than do the currently used methods of inducing GP Ib-IX-V–vWF interactions with the modulators ristocetin and botrocetin. Basic mechanism studies such as these will lead to an improved understanding of the pathophysiology of arterial thrombosis and accelerate the development of novel, more potent antithrombotic agents.
ACKNOWLEDGMENT
The authors thank Nancy A. Turner and Leticia H. Nolasco for their assistance in preparing the purified vWF and Shan Gao for her technical assistance with the cells. The authors also thank Dr Michael Berndt for providing reagents and for helpful discussions about the manuscript.
Supported by National Institutes of Health (NIH) Grants No. HL-18672, NS-23327, HL-02463, and HL-46416; the Robert A. Welch Foundation Grant No. C-938; a Grant in Aid from the American Heart Association–Texas Affiliate; the NIH Medical Scientist Training Program at Baylor College of Medicine; and a Graduate Fellowship from the Whitaker Foundation.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Larry V. McIntire, PhD, Cox Laboratory for Biomedical Engineering, Rice University, PO Box 1892, Houston, TX 77251.