Abstract
Platelet IIbβ3 is a prototypic integrin and plays a critical role in platelet aggregation. Occupancy of IIbβ3 with multivalent RGD ligands, such as fibrinogen, induces both expression of ligand-induced binding sites (LIBS) and IIbβ3 clustering, which are thought to be necessary for outside-in signaling. However, the association between LIBS expression and outside-in signaling remains elusive. In this study, we used various IIbβ3-specific peptidomimetic compounds as a monovalent ligand instead of fibrinogen and examined the association between LIBS expression and outside-in signaling such as IIbβ3-mediated intracellular Ca2+ signaling. Using a set of monoclonal antibodies (MoAbs) against LIBS, we showed that antagonists can be divided into two groups. In group I, antagonists can induce LIBS on both IIb and β3 subunits. In group II, antagonists can induce LIBS on the IIb subunit, but not on the β3 subunit. Inhibition studies suggested that group I and group II antagonists interact with distinct but mutually exclusive sites on IIbβ3. Neither group I nor group II antagonist increased intracellular Ca2+concentrations ([Ca2+]i) in nonactivated platelets. All antagonists at nanomolar concentrations abolished the increase in [Ca2+]i in 0.03 U/mL thrombin-stimulated platelets, which is dependent on both fibrinogen-binding to IIbβ3 and platelet-aggregation. However, only group I antagonists at higher concentrations dose-dependently augmented the [Ca2+]i increase, which is due to aggregation-independent thromboxane A2 production. This increase in [Ca2+]i was not observed in thrombasthenic platelets, which express no detectable IIbβ3. Thus, only the group I antagonists, albeit a monovalent ligand, can initiate IIbβ3-mediated intracellular Ca2+ signaling in the presence of thrombin stimulation. Our findings strongly suggest the association between β3LIBS expression and IIbβ3-mediated intracellular Ca2+ signaling in platelets.
INTEGRINS ARE heterodimeric glycoproteins consisting of α and β subunits that are a family of cell surface molecules that mediate cellular attachment to the extracellular matrix and cell cohesion.1 Integrins are involved in many physiological functions such as development, immune response, tissue repair, and hemostasis, and they are now recognized as important signaling molecules that can mediate the bidirectional transfer of information from the outside to the inside of the cell and also from the inside to the outside of the cell.2-4
αIIbβ3 (GPIIb-IIIa), a prototypic integrin, is expressed exclusively on platelets and megakaryocytes and acts as a receptor for fibrinogen, von Willebrand factor, vitronectin, and fibronectin. The interaction of this integrin with fibrinogen or von Willebrand factor appears to be mediated, at least in part, via an Arg-Gly-Asp (RGD) sequence, and αIIbβ3 is essential for platelet aggregation that leads to hemostatic plug formation and pathological thrombus formation.5 Recent studies have demonstrated that conformations of αIIbβ3 are dynamically regulated and that the following steps are necessary for maximal platelet aggregation6: (1) agonist-induced αIIbβ3 activation via a process termed inside-out signaling, (2) ligand (fibrinogen) binding, and (3) postreceptor occupancy events via a process termed outside-in signaling that involves change in intracellular Ca2+ level and pH, tyrosine phosphorylation, and cytoskeletal reorganization.7,8 Binding of fibrinogen, a multivalent ligand, to αIIbβ3 leads to expression of neo-epitopes on αIIbβ3, termed ligand-induced binding sites (LIBS), as well as clustering of αIIbβ3. LIBS expression has been well documented on both αIIb and β3 subunits and might explain the capacity of αIIbβ3 to initiate outside-in signaling.9-11 However, the association between LIBS expression and integrin outside-in signaling remains elusive.
In this report, using six unrelated αIIbβ3-specific peptidomimetic compounds as a monovalent ligand instead of the multivalent ligand, fibrinogen, we attempted to determine whether LIBS expression on αIIbβ3 may be associated with outside-in signaling such as αIIbβ3-mediated intracellular Ca2+ changes. Using a panel of monoclonal antibodies (MoAbs) against LIBS, we showed that αIIbβ3-specific peptidomimetic antagonists can be divided into two groups. In group I, antagonists can induce LIBS on both αIIb and β3 subunits. In group II, antagonists can induce LIBS on the αIIb subunit, but not on the β3 subunit. Interestingly, only group I antagonists dose-dependently augmented the [Ca2+]i increase in thrombin-stimulated platelets in an aggregation-independent manner. Our data suggest that β3 LIBS expression is associated with αIIbβ3-mediated intracellular Ca2+ changes.
MATERIALS AND METHODS
MoAbs.
OP-G2 is an MoAb specific for αIIbβ3-complex. OP-G2 behaves like a macromolecular RGD-containing ligand and has been shown to bind at or near the ligand recognition site.12,13 AP5 (anti-β3 amino-terminus, residues 1-6) was kindly provided by Dr Thomas J. Kunicki (Scripps Research Institute, La Jolla, CA). PMI-1 (anti-αIIb heavy chain, residues 844-859), anti-LIBS1 (anti-β3), and anti-LIBS2 (anti-β3, residues 602-690) were generously donated by Dr Mark H. Ginsberg (Scripps Research Institute).14-16 AP5, anti-LIBS1, and anti-LIBS2 recognize LIBS on the β3subunit, and PMI-1 recognizes LIBS on the αIIb subunit. Monoclonal IgG was purified from ascites fluid by affinity chromatography on Protein A Sepharose CL-4B (Pharmacia, Piscataway, NJ).
αIIbβ3-specific peptidomimetic compounds.
All αIIbβ3-specific peptidomimetic compounds were synthesized in Fujisawa Pharmaceutical Co (Osaka, Japan) and the chemical structures of these compounds are shown in Fig 1. FK633, MK383, Ro44-9883 and SC54701 have been reported previously.17-20 FR169824 and FR184764 were newly designed and synthesized by Fujisawa based on the chemical structure described previously.21 The high pressure liquid chromatography (HPLC) profile of each compound showed a single sharp peak with the expected molecular weight. In addition, nuclear magnetic resonance (NMR) studies of FK633 gave only a normal NMR spectrum (data not shown). These data indicate that each compound is monomeric in solution. The purity of each compound was more than 98%.
Chemical structures of IIbβ3-specific peptidomimetic compounds used in this study.
Chemical structures of IIbβ3-specific peptidomimetic compounds used in this study.
BM13505, a thromboxane A2 (TXA2) receptor antagonist, was also synthesized in Fujisawa.22
Preparation of fluorescein isothiocyanate (FITC)-labeled fibrinogen.
Fibrinogen (Kabi, Stockholm, Sweden) was labeled basically according to the method of Faraday et al.23 Briefly, fibrinogen at 17 mg/mL was incubated with FITC (20 μg/mg fibrinogen; Sigma, St Louis, MO) for 3 hours at 22°C. Excess FITC was removed by exhaustive dialysis against modified Tyrode-HEPES buffer (137 mmol/L NaCl, 2 mmol/L KCl, 12 mmol/L NaHCO3, 0.3 mmol/L NaH2PO4, and 5 mmol/L HEPES, pH 7.4). FITC-fibrinogen was stored at 4°C and used within 1 week of preparation.
Preparation of platelets.
Platelet-rich plasma (PRP) was obtained by differential centrifugation of the acid-citrate-dextrose–anticoagulated blood as described previously.24 Prostaglandin E1(PGE1; Sigma) was added to a final concentration of 20 ng/mL. The PRP was then centrifuged at 750g for 10 minutes to sediment platelets. After three washes with Ringer’s citrate-dextrose containing PGE1, pH 6.5, the platelet pellet was resuspended in an appropriate buffer. For loading of platelets with fura-2, the diluted PRP with modified Tyrode-HEPES buffer containing 1 mmol/L MgCl2 (1:1 dilution) was incubated with 3.3 μmol/L acetoxymethyl esters (AM) of fura-2 (Dojin Chemical Co, Ltd., Kumamoto, Japan) for 15 minutes at 37°C in the dark, and then platelets were washed twice with Ringer’s citrate-dextrose containing PGE1, pH 6.5, as described above.
Flow cytometry.
Flow cytometry was performed as described previously, with slight modifications.14 Five-microliter aliquots of the washed platelets (1 × 109/mL) suspended in 20 mmol/L HEPES, 137 mmol/L NaCl, 2 mmol/L CaCl2, pH 7.4, plus 1% bovine serum albumin (BSA), and 20 ng/mL PGE1 (test buffer) were added to tubes containing serial concentrations of synthetic antagonists in 40 μL test buffer. Five microliters of each biotinylated MoAb examined was then added to the mixture to make a final concentration of 5 μg/mL and incubated for 30 minutes at room temperature. The platelet suspensions were then incubated with a 1:320 final dilution of FITC-conjugated streptavidin (Sigma) for an additional 30 minutes without an intermittent washing step. The platelets were then diluted to 0.5 mL Tris-buffered saline (TBS; pH 7.4) and analyzed in a flow cytometer (FACScan; Becton Dickinson, Mountain View, CA).
For the analysis of FITC-fibrinogen binding to platelets, 40-μL aliquots of washed platelets (1.2 × 108/mL) suspended in modified Tyrode-HEPES buffer plus 1 mmol/L CaCl2 and 1% BSA were added to tubes containing 5 μL of serial concentrations of synthetic antagonists and 5 μL of 3 mg/mL FITC-fibrinogen. After adding 20 μmol/L ADP, the mixtures were incubated for 15 minutes at 37°C without stirring. The platelet suspensions were then diluted to 0.5 mL with modified Tyrode-HEPES buffer containing 1 mmol/L CaCl2 and analyzed in the flow cytometer.
Measurement of platelet aggregation and intracellular calcium concentration.
To examine the inhibitory effects of antagonists on ADP-induced platelet aggregation, a model PAP-4 NKK platelet aggregation tracer (Nikou Bioscience Inc, Tokyo, Japan) was used as described previously.24
Change of intracellular free calcium concentrations ([Ca2+]i ) and platelet aggregation were simultaneously measured using a Calcium Ion Analyzer FS-100 (Kowa, Osaka, Japan) that detects intensities of Fura-2 fluorescence at 380 nm (F380) and 320 nm (F320). Fura-2–loaded platelets were preincubated with each synthetic antagonist for 1 minute and then stimulated with 0.03 U/mL thrombin at 37°C with a stirring rate of 1,000/min. Changes in the fluorescence and the light transmittance were recorded. The [Ca2+]i was automatically calculated from the ratio of F380 and F320 by a FS-100 computer program connected with a calcium-ion analyzer.
Thromboxane B2 (TXB2) production.
Platelets that have been analyzed for [Ca2+]iwere incubated with 10 mmol/L EGTA and 100 μmol/L indomethacin at 4°C to stop the reaction and then pelleted by centrifugation at 1,600g for 5 minutes. TXB2 in the supernatant was measured with Thromboxane B2 [125I] RIA Kit (Dupont, NEN Research Products).
RESULTS
Pharmacological properties of αIIbβ3-specific peptidomimetic compounds.
FK633, MK383, Ro44-9883, and SC54701 have been characterized as a compound that selectively inhibits αIIbβ3.17-20 FR169824 and FR184764 were newly synthesized at Fujisawa Pharmaceutical Co and inhibited fibrinogen binding to activated αIIbβ3 (Table1). None of these antagonists inhibited the adhesion of human umbilical vein endothelial cells (HUVEC) to vitronectin-coated plates or the adhesion of Chinese hamster ovary (CHO) cells stably expressing recombinant human αvβ3 to fibrinogen-coated plates even at 10 μmol/L, suggesting that these compounds do not inhibit αvβ3 or other integrins (data not shown). Fifty percent inhibitory concentrations (IC50) of these compounds for platelet aggregation induced by ADP and fibrinogen binding to ADP-stimulated platelets were summarized in Table 1. These antagonists were highly active against platelet aggregation (∼160- to 900-fold potency as compared with RGDW) and their IC50values were in a similar range (19 to 110 nmol/L). As expected, these antagonists showed larger IC50 values for platelet aggregation than for fibrinogen binding to activated αIIbβ3.
Inhibition of ADP-Induced Platelet Aggregation and Fibrinogen Binding to IIBβ3 by Antagonists
| αIIbβ3 Antagonist . | Platelet Aggregation IC50 (nmol/L) . | Fibrinogen Binding IC50 (nmol/L) . |
|---|---|---|
| FR184764 | 19.6 ± 2.5 | 9.0 ± 2.1 |
| SC54701 | 19.3 ± 6.2 | 11.5 ± 5.3 |
| FR169824 | 33.7 ± 4.6 | 19.7 ± 4.5 |
| FK633 | 110.0 ± 28.6 | 39.3 ± 7.7 |
| Ro44-9883 | 20.3 ± 3.4 | 4.4 ± 0.4 |
| MK383 | 33.0 ± 10.8 | 6.1 ± 0.9 |
| RGDW | 17,333.3 ± 6,549.0 | ND |
| αIIbβ3 Antagonist . | Platelet Aggregation IC50 (nmol/L) . | Fibrinogen Binding IC50 (nmol/L) . |
|---|---|---|
| FR184764 | 19.6 ± 2.5 | 9.0 ± 2.1 |
| SC54701 | 19.3 ± 6.2 | 11.5 ± 5.3 |
| FR169824 | 33.7 ± 4.6 | 19.7 ± 4.5 |
| FK633 | 110.0 ± 28.6 | 39.3 ± 7.7 |
| Ro44-9883 | 20.3 ± 3.4 | 4.4 ± 0.4 |
| MK383 | 33.0 ± 10.8 | 6.1 ± 0.9 |
| RGDW | 17,333.3 ± 6,549.0 | ND |
Values are given as the mean ± SD (n = 3). Platelet aggregation and fibrinogen binding were performed by using 10 and 20 μmol/L ADP, respectively.
Abbreviation: ND, not determined.
Effects of αIIbβ3-antagonists on LIBS expression.
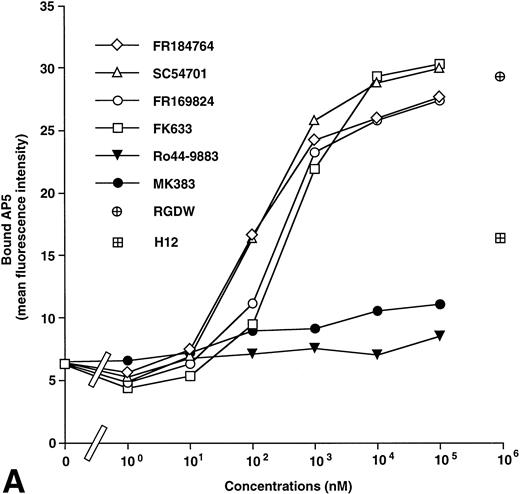
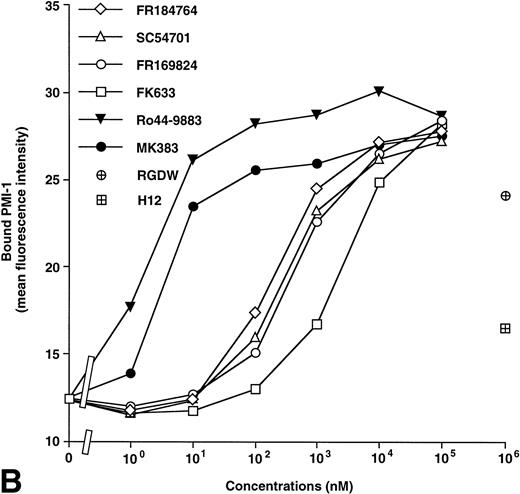
AP5 and anti-LIBS2 MoAb recognize residues 1-6 and residues 602-690 on the β3 subunit, respectively.14,16 Anti-LIBS1 MoAb recognizes different regions from those for AP5 and anti-LIBS2.14 PMI-1 MoAb recognizes residues 844-859 on the αIIb heavy chain.15 Using these MoAbs, we examined the effects of antagonists on LIBS expression on αIIbβ3. A typical set of results using AP5 and PMI-1 is shown in Fig 2. Ro44-9883 and MK383 had little effect on the induction of AP5 (Fig 2), anti-LIBS1, and anti-LIBS2 epitopes (not shown) even at 100 μmol/L, whereas FR184764, SC54701, FR169824, and FK633 markedly induced these LIBS on the β3 subunit. However, all antagonists induced PMI-1 epitope on the αIIb subunit. In this study, we designated antagonists inducing LIBS on both αIIb and β3 as group I and those not inducing LIBS on β3 as group II. RGDW and fibrinogen γ-chain peptides [HHLGGAKQAGDV (H12)] at 1 mmol/L induced both AP5 and PMI-1 epitopes, although the effects of H12 on LIBS expression were weaker than RGDW, probably due to a low affinity of H12 for αIIbβ3.12 Thus, RGDW and H12 belong to group I. We then compared the extent of LIBS expression induced by fibrinogen bound to ADP-stimulated platelets with those induced by antagonists. Maximal LIBS expression was obtained at 4 μmol/L of fibrinogen. When LIBS expression induced by FK633 was taken as 100%, fibrinogen induced only 33.7% ± 15.0% and 6.1% ± 3.7% of AP5 and PMI-1 expression, respectively (n = 3).
Effects of IIbβ3-specific peptidomimetic compounds on (A) AP5 and (B) PMI-1 epitope expression. Washed platelets (1 × 109/mL) were incubated with serial concentrations of synthetic antagonists for 30 minutes at room temperature, and then biotinylated AP5 or PMI-1 was added to the mixtures at a final concentration of 5 μg/mL. After 30 minutes of incubation at room temperature, FITC-conjugated streptavidin was added at a final dilution of 1:320, and bound antibody was analyzed by flow cytometry. Open symbols represent the antagonists that induce AP5 epitope (group I) and solid symbols represent the antagonists that do not induce AP5 epitope (group II). As controls to IIbβ3-specific peptidomimetic compounds, RGDW and HHLGGAKQAGDV (H12) were tested. These results are representative of six and three separate experiments, respectively.
Effects of IIbβ3-specific peptidomimetic compounds on (A) AP5 and (B) PMI-1 epitope expression. Washed platelets (1 × 109/mL) were incubated with serial concentrations of synthetic antagonists for 30 minutes at room temperature, and then biotinylated AP5 or PMI-1 was added to the mixtures at a final concentration of 5 μg/mL. After 30 minutes of incubation at room temperature, FITC-conjugated streptavidin was added at a final dilution of 1:320, and bound antibody was analyzed by flow cytometry. Open symbols represent the antagonists that induce AP5 epitope (group I) and solid symbols represent the antagonists that do not induce AP5 epitope (group II). As controls to IIbβ3-specific peptidomimetic compounds, RGDW and HHLGGAKQAGDV (H12) were tested. These results are representative of six and three separate experiments, respectively.
Fifty percent effective doses (ED50) for AP5, anti-LIBS1, anti-LIBS2, and PMI-1 expression are summarized in Table 2. As compared with IC50s for fibrinogen binding, ED50s of these antagonists for LIBS expression were much higher. ED50s for the induction of each LIBS on the β3 subunit were anti-LIBS1 < AP5 < anti-LIBS2, indicating that anti-LIBS1 epitope is most sensitive for ligand binding among these LIBS. The group I antagonists (Ro44-9883 and MK383) were more potent in the induction of PMI-1 epitope than group II antagonists (FR184764, SC54701, FR169824, and FK633) ([ED50 for PMI-1 expression/IC50 for fibrinogen binding] ratio; group I, 29.8 ± 19.1; group II, 1.6 ± 0.8; P < .05; Table 2). We also examined inhibitory effects of antagonists on the binding of a ligand-mimic MoAb, OP-G2, to platelets. Although OP-G2 recognizes at or near the ligand recognition site, OP-G2, like small RGD-containing peptides, binds to nonactivated platelets.12 Interestingly, group II antagonists were much more potent in the inhibition of OP-G2 binding than group I antagonists ([IC50 for OP-G2 binding/IC50 for fibrinogen binding] ratio; group I, 102.3 ± 32.2; group II, 6.7 ± 4.9;P < .01; Table 2). The apparent differences in the (ED50 for PMI-1 expression/IC50 for fibrinogen binding) ratio and the inhibitory effects on OP-G2 binding suggest that the binding sites of antagonists are distinct between the two groups.
Effects of Antagonists on AP5 and PMI-1 Expression and OP-G2 Binding
| αIIbβ3 Antagonist . | AP5 Expression ED50 (nmol/L) (n = 6) . | Anti-LIBS1 Expression ED50 (nmol/L) (n = 3) . | Anti-LIBS2 Expression ED50(nmol/L) (n = 3) . | PMI-1 Expression ED50(nmol/L) (n = 3) . | OP-G2 Binding IC50(nmol/L) (n = 3) . | PMI-1/FBG Ratio . | OP-G2/FBG Ratio . | Group . |
|---|---|---|---|---|---|---|---|---|
| FR184764 | 104.5 ± 61.5 | 48.0 ± 11.8 | 270.0 ± 104.4 | 226.7 ± 36.8 | 583.3 ± 102.7 | 25.2 | 64.8 | |
| SC54701 | 173.0 ± 74.5 | 95.0 ± 35.0 | 443.3 ± 136.5 | 246.7 ± 103.7 | 1,233.3 ± 262.5 | 21.5 | 107.2 | I |
| FR169824 | 258.3 ± 75.7 | 150.0 ± 43.6 | 560.0 ± 87.1 | 290.0 ± 96.3 | 1,866.7 ± 368.2 | 14.7 | 94.8 | |
| FK633 | 520.0 ± 195.2 | 260.0 ± 121.2 | 1,230.0 ± 311.0 | 2,266.7 ± 1,228.4 | 5,600.0 ± 496.7 | 57.7 | 142.5 | |
| Ro44-9883 | (−) | (−) | (−) | 3.3 ± 1.4 | 5.8 ± 0.8 | 0.75 | 1.3 | II |
| MK383 | (−) | (−) | (−) | 14.7 ± 7.5 | 46.7 ± 8.1 | 2.4 | 7.7 |
| αIIbβ3 Antagonist . | AP5 Expression ED50 (nmol/L) (n = 6) . | Anti-LIBS1 Expression ED50 (nmol/L) (n = 3) . | Anti-LIBS2 Expression ED50(nmol/L) (n = 3) . | PMI-1 Expression ED50(nmol/L) (n = 3) . | OP-G2 Binding IC50(nmol/L) (n = 3) . | PMI-1/FBG Ratio . | OP-G2/FBG Ratio . | Group . |
|---|---|---|---|---|---|---|---|---|
| FR184764 | 104.5 ± 61.5 | 48.0 ± 11.8 | 270.0 ± 104.4 | 226.7 ± 36.8 | 583.3 ± 102.7 | 25.2 | 64.8 | |
| SC54701 | 173.0 ± 74.5 | 95.0 ± 35.0 | 443.3 ± 136.5 | 246.7 ± 103.7 | 1,233.3 ± 262.5 | 21.5 | 107.2 | I |
| FR169824 | 258.3 ± 75.7 | 150.0 ± 43.6 | 560.0 ± 87.1 | 290.0 ± 96.3 | 1,866.7 ± 368.2 | 14.7 | 94.8 | |
| FK633 | 520.0 ± 195.2 | 260.0 ± 121.2 | 1,230.0 ± 311.0 | 2,266.7 ± 1,228.4 | 5,600.0 ± 496.7 | 57.7 | 142.5 | |
| Ro44-9883 | (−) | (−) | (−) | 3.3 ± 1.4 | 5.8 ± 0.8 | 0.75 | 1.3 | II |
| MK383 | (−) | (−) | (−) | 14.7 ± 7.5 | 46.7 ± 8.1 | 2.4 | 7.7 |
Values are given as the mean ± SD with indicated (n).
Abbreviations: FBG, fibrinogen; PMI-1/FBG ratio, ED50 for PMI-1 epitope expression/IC50 for FBG binding; OP-G2/FBG ratio, IC50 for OP-G2 binding/IC50 for FBG binding.
Inhibition between group I and group II antagonists.
The binding characteristics of group I (FR184764, SC54701, FR169824, and FK633) and group II antagonists (Ro44-9883 and MK383) were further examined in an inhibition assay. The binding of group I antagonists such as SC54701 was monitored by the binding of AP5 MoAb. As shown in Fig 3, the binding of SC54701 was markedly inhibited by all of the group II antagonists. Similarly, the binding of FR184764, FR169824, and FK633 was also markedly inhibited by all of group II antagonists (data not shown). These results indicate that the binding of a group I antagonist is inhibited by the binding of a group II antagonist.
Inhibition of SC54701 binding to IIbβ3 by group II antagonists. Washed platelets (1 × 109/mL) were incubated with 1 μmol/L SC54701 for 30 minutes at room temperature, and then varied concentrations of group II antagonists (Ro44-9883 or MK383) were added to the mixtures as a competitor and incubated for 30 minutes at room temperature. Biotinylated AP5 (5 μg/mL) was incubated with the mixtures, followed by adding FITC-conjugated streptavidin (1:320 dilution). AP5 binding to platelets was analyzed by flow cytometry. Mean fluorescence intensity (MFI) is the value obtained by subtracting AP5 binding in the absence of any antagonists. These results are the average of two separate experiments.
Inhibition of SC54701 binding to IIbβ3 by group II antagonists. Washed platelets (1 × 109/mL) were incubated with 1 μmol/L SC54701 for 30 minutes at room temperature, and then varied concentrations of group II antagonists (Ro44-9883 or MK383) were added to the mixtures as a competitor and incubated for 30 minutes at room temperature. Biotinylated AP5 (5 μg/mL) was incubated with the mixtures, followed by adding FITC-conjugated streptavidin (1:320 dilution). AP5 binding to platelets was analyzed by flow cytometry. Mean fluorescence intensity (MFI) is the value obtained by subtracting AP5 binding in the absence of any antagonists. These results are the average of two separate experiments.
Effects of αIIbβ3-antagonists on [Ca2+]i change induced by thrombin.
Group I and group II antagonists induced LIBS on αIIbβ3 differently, especially on the β3 subunit. Using these antagonists as a monovalent ligand, we examined whether the difference in LIBS expression of αIIbβ3 induced by antagonists might affect outside-in signaling via αIIbβ3. None of these antagonists affected the [Ca2+]i in nonactivated platelets, even at a high concentration of 10 μmol/L, indicating that LIBS expression alone is not sufficient to cause this outside-in signaling (data not shown). As previously demonstrated by Yamaguchi et al,25 26 a low concentration of thrombin (0.03 U/mL) induces a two-peaked [Ca2+]i increase (Fig 4). The latter peak has been shown to be dependent on both fibrinogen binding to αIIbβ3 and platelet aggregation. Each antagonist, irrespective of the group, abolished the latter [Ca2+]i peak as well as platelet aggregation. However, when the concentrations of FK633 were increased up to 10 μmol/L to induce full expression of LIBS, another second [Ca2+]i peak was induced even in the absence of platelet aggregation (Fig 4). All group I antagonists showed essentially the same effects on the [Ca2+]ichange. In addition, 1 mmol/L RGDW peptide also induced the second [Ca2+]i peak, indicating that this phenomenon is not specific for peptidomimetic antagonist (data not shown). In contrast, none of the group II antagonists showed such effects even at 10 μmol/L (Fig 4). When ADP or epinephrine was used as an agonist instead of thrombin, none of these antagonists had effects on the [Ca2+]i change (data not shown).
Different effects between group I and group II antagonists on [Ca2+]i changes induced by thrombin. Platelets were preincubated with 10 μmol/L of each antagonist and then stimulated with 0.03 U/mL thrombin. (A) None. (B) FK633 (group I). (C) FR169824 (group I). (D) Ro44-9883 (group II). (E) MK383 (group II). AG and Ca2+ indicate aggregation curve and trace of changes in [Ca2+]i, respectively. These results are representative of two separate experiments.
Different effects between group I and group II antagonists on [Ca2+]i changes induced by thrombin. Platelets were preincubated with 10 μmol/L of each antagonist and then stimulated with 0.03 U/mL thrombin. (A) None. (B) FK633 (group I). (C) FR169824 (group I). (D) Ro44-9883 (group II). (E) MK383 (group II). AG and Ca2+ indicate aggregation curve and trace of changes in [Ca2+]i, respectively. These results are representative of two separate experiments.
Using platelets derived from a patient with Glanzmann thrombasthenia who has no detectable αIIbβ3,27we further examined whether the second [Ca2+]i peak induced by group I antagonists may be specifically mediated by αIIbβ3 on platelets. Thrombin induced only first [Ca2+]i peak in thrombasthenic platelets. Neither FK633 nor FR169824 induced the additional second [Ca2+]i peak, even at 10 μmol/L (Fig 5). This patient possesses a molecular genetic defect in the αIIb gene27 and expresses normal level of αvβ3 on platelets (data not shown). Therefore, the second [Ca2+]i peak induced by group I antagonists is specifically mediated by αIIbβ3.
Effects of group I antagonists on the second [Ca2+]i peak in thrombasthenic platelets. Platelets obtained from a patient with Glanzmann thrombathenia were preincubated with 10 μmol/L of each group I antagonist and then stimulated with 0.03 U/mL thrombin. (A) None. (B) FK633. (C) FR169824.
Effects of group I antagonists on the second [Ca2+]i peak in thrombasthenic platelets. Platelets obtained from a patient with Glanzmann thrombathenia were preincubated with 10 μmol/L of each group I antagonist and then stimulated with 0.03 U/mL thrombin. (A) None. (B) FK633. (C) FR169824.
To elucidate the nature of the second peak that was induced by group I antagonists, the effects of aspirin and BM135052 (TXA2receptor antagonist) were examined. Aspirin as well as BM13505 markedly inhibited the second [Ca2+]i peak induced by FK633 (Fig 6), FR184764, SC54701, or FR169824 (data not shown). These data suggest that the second [Ca2+]i peak is caused by the production of TXA2. In addition, apyrase also abolished the second [Ca2+]i peak induced by FK633, suggesting that endogenous ADP played some role in the induction of the second peak.
Inhibition of the second [Ca2+]i peak induced by group I with aspirin, BM13505, or apyrase. The second peak of [Ca2+]i induced by 10 μmol/L FK633 and 0.03 U/mL thrombin was inhibited by 100 μmol/L aspirin (cyclooxygenase inhibitor), 10 μmol/L BM13505 (TXA2receptor antagonist), or 0.5 U/mL apyrase (ADP scavenger). (a and d) 10 μmol/L FK633; (b) 10 μmol/L FK633 plus 100 μmol/L aspirin; (c) 10 μmol/L FK633 plus 10 μmol/L BM13505; (e) 10 μmol/L FK633 plus 0.5 U/mL apyrase. These results are representative of two separate experiments.
Inhibition of the second [Ca2+]i peak induced by group I with aspirin, BM13505, or apyrase. The second peak of [Ca2+]i induced by 10 μmol/L FK633 and 0.03 U/mL thrombin was inhibited by 100 μmol/L aspirin (cyclooxygenase inhibitor), 10 μmol/L BM13505 (TXA2receptor antagonist), or 0.5 U/mL apyrase (ADP scavenger). (a and d) 10 μmol/L FK633; (b) 10 μmol/L FK633 plus 100 μmol/L aspirin; (c) 10 μmol/L FK633 plus 10 μmol/L BM13505; (e) 10 μmol/L FK633 plus 0.5 U/mL apyrase. These results are representative of two separate experiments.
Effects of αIIbβ3-antagonists on TXB2 formation.
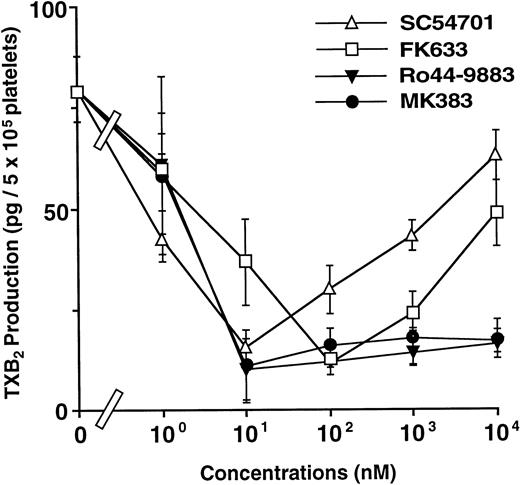
To confirm that a group I antagonist induces TXA2production with the costimulation by thrombin, TXB2, a major metabolite of TXA2, was measured. As shown in Fig 7, TXB2 was initially produced by thrombin stimulation. All antagonists at low concentrations markedly inhibited TXB2 production dose-dependently, indicating that the greater part of the TXB2 production under these conditions is dependent on both fibrinogen binding and platelet-aggregation. However, at higher concentrations, group I antagonists induced TXB2 production in a concentration-dependent manner. In contrast to group I antagonists, the group II antagonists did not induce TXB2 production even at high concentrations. In addition, the levels of TXB2production in the absence of platelet aggregation correlated with the induction of AP5 epitope (compare Figs 2A and 7). These data confirm that the TXA2 production, induced by a high concentration of group I antagonists, is responsible for the second [Ca2+]i peak.
Different effects between group I and group II antagonists on TXB2 formation (TXA2metabolites). Platelets were preincubated with various concentrations of antagonists and then stimulated with 0.03 U/mL thrombin. TXB2 was measured by RIA Kit. Values are given as the mean ± SD (n = 3). Open and solid symbols represent group I and group II antagonists, respectively.
Different effects between group I and group II antagonists on TXB2 formation (TXA2metabolites). Platelets were preincubated with various concentrations of antagonists and then stimulated with 0.03 U/mL thrombin. TXB2 was measured by RIA Kit. Values are given as the mean ± SD (n = 3). Open and solid symbols represent group I and group II antagonists, respectively.
DISCUSSION
In the present study, we demonstrated that αIIbβ3 antagonists can be divided into two groups, group I and group II, according to the effects on LIBS expression on the αIIb and the β3 subunits. We designated antagonists inducing LIBS on the β3 subunit as group I (FR184764, SC54701, FR169824, and FK633) and those not inducing as group II (Ro44 9883 and MK383). However, in contrast to the data reported by Steiner et al,28 29 using the PMI-1 MoAb as a probe, we demonstrated that all six antagonists can induce LIBS on the αIIb subunit. A group II antagonist was apparently more potent in the induction of PMI-1 epitope than a group I antagonist. Using these antagonists as a monovalent ligand, we have readily demonstrated that only group I antagonists can induce αIIbβ3-mediated Ca2+ signaling in platelets stimulated with thrombin in an aggregation-independent manner. These data suggest that LIBS expression on the β3subunit is a prerequisite for outside-in signaling through αIIbβ3.
Group I and group II antagonists induce distinct conformational changes on αIIbβ3. The ratio of ED50for PMI-1 expression/IC50 for fibrinogen binding clearly showed that the difference in the ability of PMI-1 expression between the two groups is not due to the difference in the affinities to αIIbβ3. In addition, group II antagonists are apparently more active against the binding of OP-G2 MoAb than group I antagonists. These data suggest that the binding sites of antagonists are distinct between the two groups. However, the binding of group I antagonists to αIIbβ3 that was monitored by AP5 binding was abolished by the binding of group II antagonists. These findings are consistent with the data reported by Diaz-González et al.30 Taken together, our data suggest that group I and group II antagonists interact with distinct but mutually exclusive sites on αIIbβ3. Although it has been demonstrated that RGD and H12 peptides interact with distinct but mutually exclusive sites,31 these peptides induced LIBS on both αIIb and β3 subunits. Therefore, the difference in the binding sites between group I and group II antagonists does not simply reflect the difference between RGD and H12 peptides.
Miyamoto et al32 demonstrated that, in fibroblasts, direct ligand occupancy by a monovalent ligand was not a sufficient signal for cytoskeletal protein organization. In contrast, integrin clustering without ligand occupancy induced intracellular accumulation of FAK and tensin, but not of other cytoskeletal proteins such as talin. Both ligand occupancy and integrin clustering were necessary for accumulation of talin, α-actinin, paxillin, vinculin, F-actin, and filamin.33 Although ligand occupancy would lead to LIBS expression on integrin receptors, how LIBS expression contributes to integrin outside-in signaling is not well understood. In platelets, fibrinogen binding to αIIbβ3 activated by the activating MoAb PT25-2 per se does not induce [Ca2+]i increase, even in the presence of platelet aggregation.34 In contrast, a low concentration of thrombin (0.03 U/mL) induces the two-peaked [Ca2+]i increase in platelets. The first [Ca2+]i peak is generated by the thrombin receptor, whereas the latter [Ca2+]i peak is not observed in thrombasthenic platelets and is dependent on both fibrinogen-binding to αIIbβ3 and platelet aggregation (this study and Yamaguchi et al26). Monovalent ligands such as RGDS peptide abolish the latter [Ca2+]i peak. The latter peak can be also abolished by aspirin, BM13505 (TXA2 receptor antagonist), or apyrase.35 Accordingly, the latter peak represents post-αIIbβ3 occupancy events by multivalent ligands such as fibrinogen. Both endogenous ADP release and platelet aggregation are needed for TXA2 production via αIIbβ3, which is responsible for the latter peak. As expected, both group I and group II antagonists at low concentrations abolished the latter [Ca2+]ipeak. However, group I antagonists at high concentrations induced the new second peak in thrombin-stimulated platelets, even in the absence of platelet aggregation, whereas group II did not induce it, despite LIBS expression on the αIIb subunit. Group I antagonists did not induce the second peak in thrombasthenic platelets expressing normal level of αvβ3, even at 10 μmol/L, indicating that this signal is specifically mediated by αIIbβ3. The second [Ca2+]i peak was dependent on the production of TXA2 and was abolished by aspirin, BM13505, or apyrase. Accordingly, the pathway involved in the group I antagonist-induced second [Ca2+]i peak is essentially the same as that for the fibrinogen-induced and aggregation-dependent [Ca2+]i peak. In other words, these data suggest that, in thrombin-stimulated platelets, LIBS expression induced by group I antagonists is associated with the cyclooxgenese pathway possibly through activation of phospholipase A2.36 Interestingly, in a canine coronary thrombolysis model, Murphy et al37 demonstrated that administration of a group I antagonist, Ro43-5054, increased the level of urinary 2,3-dinor-TXB2, similar to controls, whereas a group II antagonist, Ro44-9883, markedly inhibited the increase. Our in vitro data presented here may explain their in vivo data.
In the presence of thrombin stimulation, the monovalent ligand to αIIbβ3 could induce the second [Ca2+]i peak without platelet aggregation. Our data demonstrate that neither multivalency of the ligand nor platelet aggregation is essential to induce the αIIbβ3-dependent [Ca2+]i increase. It is noteworthy that the extent of LIBS expression on the β3 subunit, but not on the αIIb subunit, correlated with the level of TXB2 production. In contrast, the extent of AP5 and PMI-1 expression induced by fibrinogen was 33.7% ± 15.0% and 6.1% ± 3.7% (n = 3) of that induced by group I antagonists, respectively. Thus, group I antagonists can fully induce LIBS on αIIbβ3. These data suggest that group I antagonists-induced conformational change is needed for the second [Ca2+]i peak as an outside-in signal via αIIbβ3.
There is increasing evidence that occupancy of one integrin can also suppress the functions of other integrins (trans-dominant inhibition).38-40 Recently, Diaz-González et al30 demonstrated that Ro43-5054, but not Ro44-9883, induces this trans-dominant inhibition. Similarly to our findings, they demonstrated that the inhibitory effect on the function of the target integrin α5β1 correlated with LIBS expression in the suppressive integrin αIIbβ3.
It has been well documented that the cytoplasmic domain of the β3 subunit plays a critical role in αIIbβ3 outside-in signaling.41Truncation of the β3 subunit cytoplasmic domain as well as a certain point mutation (S752P) abolished cell spreading mediated by αIIbβ3 and fibrin clot retraction, whereas truncation of the αIIb subunit did not inhibit cell spreading.42,43 The trans-dominant inhibition of αIIbβ3 is also mediated by β3cytoplasmic domain.30 As shown by three different MoAbs against β3 LIBS here, β3 extracellular domain dramatically changed its conformation by ligand binding. It is noteworthy that anti-LIBS2 recognizes the membrane proximal region of β3. Therefore, it is likely that the conformational change detected by anti-LIBS MoAb may represent conformational changes of the β3 cytoplasmic domain.
Our data presented here demonstrate that LIBS expression of the β3 subunit could participate in outside-in signaling through this integrin during thrombogenesis and hemostasis. Antagonists for αIIbβ3 are likely to be the first anti-integrins to be widely used.44 From a therapeutic viewpoint, LIBS expression may facilitate to produce antibodies against the LIBS as a neo-epitope and cause subsequent immune thrombocytopenia.45 In addition, TXA2 is one of platelets agonists and a potent constrictor of vascular smooth muscles.46 Although LIBS expression on both subunits and the stimulation of TXA2 production by group I antagonists needs much higher concentrations than therapeutic range, we could not rule out the possibility that group I antagonists might have an adverse effect. Further study will be required to determine whether some adverse effects would be induced by group I antagonists in vivo experiment using therapeutic range, and better understanding of physiological roles of LIBS expression could contribute to the successful development of αIIbβ3antagonists.
ACKNOWLEDGMENT
The authors thank Dr Thomas J. Kunicki (Scripps Research Institute) for the MoAb AP5 and Dr Mark H. Ginsberg (Scripps Research Institute) for the MoAbs anti-LIBS1, anti-LIBS2, and PMI-1.
Supported in part by grants from the Ministry of Education, Science and Culture of Japan; the Japan Society for the Promotion of Science; and the Ryoichi Naito Foundation for Medical Research.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Yoshiaki Tomiyama, MD, The Second Department of Internal Medicine, Osaka University Medical School, 2-2, Yamadaoka, Suita 565-0871, Japan; e-mail:yoshi@hp-blood.med.osaka-u.ac.jp.


![Fig. 4. Different effects between group I and group II antagonists on [Ca2+]i changes induced by thrombin. Platelets were preincubated with 10 μmol/L of each antagonist and then stimulated with 0.03 U/mL thrombin. (A) None. (B) FK633 (group I). (C) FR169824 (group I). (D) Ro44-9883 (group II). (E) MK383 (group II). AG and Ca2+ indicate aggregation curve and trace of changes in [Ca2+]i, respectively. These results are representative of two separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/10/10.1182_blood.v92.10.3675/4/m_blod42238004x.jpeg?Expires=1769198822&Signature=BnTzNTn8vk0GhJmGX3LtOWyNpwcS-yPG6Ch~X--XZggtW2XiX40~6UovYtX4Tr2rmM5CQQaEav0WD3EZxqPVfU-UHsNJscPlph69JeKVLdDg25pYnadA65OSjaqRL8YcLNo-RJjVJRwFb0lxlzVLC2FGkJVRb5329uOUwqNIvE2XeLQ0nczvFTheNKenaD755cXv~Zky1kJp~izfFjd4H39yawREn1f4lvMxJWT4UtocxeqaerAvAI1hbOcpZ2HvpqQRFiyYdamzfAjtJKIjjgo5tfWc~VxSpmuM28JM0osvYoX3B9weiWUPqZqWhTCF-mvH76P6ERX0KcNowVmeCA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Effects of group I antagonists on the second [Ca2+]i peak in thrombasthenic platelets. Platelets obtained from a patient with Glanzmann thrombathenia were preincubated with 10 μmol/L of each group I antagonist and then stimulated with 0.03 U/mL thrombin. (A) None. (B) FK633. (C) FR169824.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/10/10.1182_blood.v92.10.3675/4/m_blod42238005x.jpeg?Expires=1769198822&Signature=QqiPGRH-T6dxw3cbb~psz2D6wUDlFP7PFsdiSn4upIbyHBvuAyWK00W00gw~NW6OJYH5KgN3mi0TEz6exB9~kQRk4wpcXYMIdhliIRoFmolYyuRIqPXK95-su7Kv3aOG6zeQQq7Nc6SvU6pBjwzk-8cAU5ypdlKx6Ib9dYvSIs3K-SVAPz4nMr5YHNp-0ZCMVJqtPGPuBYKek7YWykAZW4Eazq71RN04f8BscHSaLsmmmyseM5dnKmAI3Y9bHQzbMqrFX5I0YTW8KK1HU9cCuOQMB-jAeSMcb6I8oLtF1jxqHZOW7aekgzyZAMtgdnTqvz2jtyU3bcS0KXrELZhJVg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Inhibition of the second [Ca2+]i peak induced by group I with aspirin, BM13505, or apyrase. The second peak of [Ca2+]i induced by 10 μmol/L FK633 and 0.03 U/mL thrombin was inhibited by 100 μmol/L aspirin (cyclooxygenase inhibitor), 10 μmol/L BM13505 (TXA2receptor antagonist), or 0.5 U/mL apyrase (ADP scavenger). (a and d) 10 μmol/L FK633; (b) 10 μmol/L FK633 plus 100 μmol/L aspirin; (c) 10 μmol/L FK633 plus 10 μmol/L BM13505; (e) 10 μmol/L FK633 plus 0.5 U/mL apyrase. These results are representative of two separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/10/10.1182_blood.v92.10.3675/4/m_blod42238006x.jpeg?Expires=1769198822&Signature=lfrODOJ~nawrLLclqCEXHeanYDN-upvVyNOx6BOoBxed8VDTYRBLumz04PUgR2wFmhghIMGHf5e0~PdZw1dumzkEFiHgbvXypTOcEst6UvJDBdvlHxAa3zi3J2KumMdGKhaTRQmzm1p5f1e99x61JsfVQroNZnOG1hLlbLIENn10d2C5emWH00OtfGu8yJa~eqp1J8L240V~Mfk3oQqvjfwaF9R8LaM96lDZD-GjuC8Q7~ZuhWVVP1JDSxRSF3adytNNza5bj0g82strcSpRgUWE1qPuodYHde6DeX90pvGzMa31CZcL9MtVkxXmyO~pkCsjsCi8QFxYv4mvSJQD-A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal