Abstract
The mechanisms leading to polyclonal hypergammaglobulinemia in patients with human immunodeficiency virus (HIV) infection are not well understood. In light of the important role of interleukin-10 (IL-10) and the interaction between CD40 and CD40 ligand in the normal regulation of B-lymphocyte function and Ig production, we examined these parameters in 24 HIV-infected patients. Both plasma IL-10 levels and the percentage of CD4+ and CD8+lymphocytes expressing CD40 ligand were significantly higher in the patients than in the 10 blood donor controls. Serum IgG correlated positively with circulating IL-10 levels and the percentage of CD4+ lymphocytes expressing CD40 ligand. Furthermore, a single bolus infusion of intravenous Ig (0.4 g/kg) in 8 HIV-infected patients caused a further increase in IL-10 levels in plasma and an increase in both IL-10 and IgG production in peripheral blood mononuclear cell cultures. In another patient group (Wegener’s granulomatosis) receiving a single bolus infusion of intravenous Ig, a similar increase in plasma IL-10 levels was found, suggesting that this may be a general effect of intravenous Ig. In patients with HIV infection, our data suggest that a vicious cycle may be operative where high endogenous Ig levels may enhance IL-10 production that, in turn, leads to higher Ig production.
MOST PATIENTS WITH human immunodeficiency virus (HIV) infection have elevated serum Ig levels.1-3 The hypergammaglobulinemia is usually polyclonal and comprises several Ig isotypes.2-5 Although several studies over the years have addressed the issue,6-12 the mechanisms leading to hypergammaglobulinemia in HIV infection are still not well understood. Central to the normal B-lymphocyte activation and Ig production is a regulated interaction between CD40 ligand (CD40L; also named glycoprotein 39 or CD154) on activated T lymphocytes and CD40 on B lymphocytes.13 Also, interleukin-10 (IL-10) is important for the regulation of Ig production as a switch factor for IgG during B-lymphocyte maturation promoting plasma cell differentiation and secretion of IgG, IgM, and IgA.14-18 So far, these aspects have not been thoroughly investigated in patients with HIV infection.
In contrast to the hypergammaglobulinemia, in vitro B-lymphocyte responses to mitogens and antigens in vitro as well as in vivo responses to immunization are impaired in HIV-infected patients.1,19-21 To try to compensate for this B-lymphocyte deficiency, intravenous Ig (IVIg) preparations have been administered to HIV-infected patients in several studies. IVIg has been shown to benefit HIV-infected children with recurrent bacterial infections.22-24 In adults, data are discrepant regarding the effects of IVIg on infectious complications and mortality24-26 (and references therein). Any beneficial effects of IVIg therapy in HIV infection may be due both to antimicrobial effects of the passively transferred polyclonal Igs and to immunomodulatory effects of IVIg preparations on pathophysiological processes27-29 leading to enhanced antimicrobial immunity.
The aims of the study were to examine CD40L expression and IL-10 production in relation to Ig production in patients with HIV infection and to study the immunomodulatory effects of a single infusion of IVIg on the B-lymphocyte system in patients with HIV infection, with special emphasis on Ig secretion, CD40L expression, and IL-10 production.
MATERIALS AND METHODS
Patients.
Twenty-four HIV-seropositive patients were included in the study (21 men and 3 women; median age, 41 years; age range, 22 to 58 years). Clinically, 11 patients were classified as asymptomatic (Centers for Disease Control and Prevention [CDC] group A) and 1 patient as symptomatic (CDC group B), whereas 12 patients were classified as CDC group C (acquired immunodeficiency syndrome [AIDS]).30 Because of the immunological similarities between patients in groups CDC A and B, they are analyzed together in the following. At the time of blood sampling, 2 patients were treated for Mycobacterium avium infection, 3 patients were treated for cytomegalovirus retinitis, and 1 patient was treated for visceral leishmaniasis. Three patients had Kaposi’s sarcoma. Eleven patients received antiretroviral therapy (5 zidovudine [ZDV], 1 didanosine, 1 ZDV + lamivudine [3TC], 1 ZDV + 3TC + ritonavir, 1 ZDV + 3TC + saquinavir, 1 ZDV + 3TC + indinavir, and 1 ZDV + stavudine + indinavir). However, they had not initiated therapy or changed dosage regimen during the last 3 months.
Controls were 10 healthy, volunteer, HIV-seronegative blood donors (7 men and 3 women; median age, 39 years; age range, 23 to 59 years).
Eight of the HIV-seropositive patients (6 men and 2 women; median age, 32 years; age range, 25 to 52 years; all in CDC group A) participated in an Octagam (Octapharma, Vienna, Austria) IVIg study as detailed below. Patients with CD4+ lymphocyte counts in peripheral blood between 150 and 400 × 106/L during the last year were included in the IVIg study. No patients had shown any signs of overt infection during the last 2 months before the study. At the time of the study, 2 patients received ZDV, whereas none was receiving immunosuppressive drugs. At the time of the study, the serum level of alanine aminotransferase was less than 40 U/L and the serum creatinine level was less than 70 μmol/L in all patients.
Also, 7 patients with Wegener’s granulomatosis (median age, 35 years; age range, 23 to 66 years) who all had received a single bolus infusion of IVIg (Octagam; 0.4 g/kg) were examined. Blood samples were taken preinfusion and postinfusion at 20 and 44 hours.
IVIg study design.
The IVIg study was part of an Octagam tolerance study performed in our department as previously reported.31 All patients received a single infusion of Octagam (0.4 g/kg). Blood samples were taken preinfusion and postinfusion at 1, 20, 44, and 144 hours (6 days). The study was approved by the Regional Ethical Comitee and by the Norwegian Medicines Control Authority. Signed informed consent was obtained from all participants.
Ig preparation.
Octagam is a liquid virus-inactivated IVIg preparation (pH 4) produced from fresh frozen plasma collected in Norwegian blood banks. The final product is dispensed in sterile water containing 10% maltose (final IgG concentration, 50 g/L; final IgA and IgM concentrations, <0.1 g/L). Each portion has been tested and found negative for antibodies to HIV-1 and HIV-2 and hepatitis B and C virus. The endotoxin level in the IVIg preparation was less than 10 pg/mL (Quantitative chromogenic limulus amebocyte lysate test; BioWhittaker, Inc, Walkersville, MD). The IL-10 concentration in Octagam was 5.8 pg/mL, as determined by the high-sensitivity enzyme-linked immunosorbent assay (ELISA; see below).
Blood sampling protocol.
Blood was drawn into sterile pyrogen-free vacuum blood collection tubes (Becton Dickinson, San Jose, CA) using EDTA as anticoagulant. Blood was immediately immersed in melting ice and the tubes were centrifuged within 10 minutes (600g and 4°C for 10 minutes). Plasma was then transferred to sterile Eppendorf tubes (Treff AG, Degersheim, Switzerland) and further centrifuged at 10,000g and 4°C to obtain platelet-free plasma. Plasma was stored at −80°C in multiple aliquots until analysis. Samples were frozen and thawed only once.
Isolation and stimulation of cells.
Peripheral blood mononuclear cells (PBMCs) were obtained from heparinized blood by Isopaque-Ficoll (Lymphoprep; Nycomed Pharma AS, Oslo, Norway) gradient centrifugation within 45 minutes after blood sampling, as previously described.32 The fractions of monocytes (CD14+) and lymphocyte subsets (CD4+, CD8+, CD19+) in the isolated PBMCs were determined by immunomagnetic quantification.33 The endotoxin level in the culture medium was less than 10 pg/mL (Quantitative limulus amebocyte lysate test; BioWhittaker).
For positive selection of B lymphocytes, monodisperse immunomagnetic beads coated with anti-CD19 antibody (Dynal, Oslo, Norway) were added to PBMCs in a cell-to-bead ratio of 1:5 and incubated on a Rock-N-Roller (Labenco, Breda, The Netherlands) platform at 4°C for 30 minutes and rosetted cells were isolated by application of a samarium cobalt magnet (Dynal). To enable detachment of beads from B lymphocytes, sheep antimouse Fab (Detach-a-bead-CD19; Dynal) was added and the cells were incubated for 1 hour at room temperature on a sample mixer (Dynal).
The purity of the B lymphocytes was greater than 98% as assessed by staining of cytospins of positively selected cells by a panel of monoclonal antibodies as previously described.34 To examine the effect of non–B-lymphocyte factors on a constant number of B lymphocytes, we used a constant number of B cells in purified and mixed (PBMC) cultures. Thus, 125,000 B lymphocytes/mL or PBMCs containing 125,000 B lymphocytes/mL were cultured in RPMI 1640 with 2 mmol/L L-glutamine and 25 mmol/L HEPES buffer (GIBCO BRL, Paisley, UK) supplemented with 10% fetal calf serum (Myoclone; GIBCO) in triplicates (0.2 mL/well) in 96-well plates (Costar, Cambridge, MA) and stimulated by anti-CD40 antibody (clone EA-5; 2 μg/mL; Ancell Corp, Bayport, MN) or left unstimulated. After 7 days, supernatants were harvested and frozen at −20°C in aliquots until Ig analysis.
Quantification of IL-10.
IL-10 in plasma was quantified by ELISA (IL-10 high-sensitivity ELISA; R&D Systems, Minneapolis, MN). IL-10 in cell culture supernatants was assayed by ELISA (reagents were kindly provided by Dr S. Narula, Schering-Plough Research Institute, Kenilworth, NJ) as described elsewhere.35 The limit of detection was 0.7 pg/mL and 5 pg/mL for the high-sensitivity IL-10 (R&D) and IL-10 immunoassays, respectively. Intra-assay and interassay coefficients of variation were less than 10% for both assays.
Quantification of Igs in cell culture supernatants.
Igs in cell culture supernatants from 8 HIV-infected patients (those who participated in the Octagam study; all in CDC stage A), 10 blood donor controls, and 2 patients with Wegener’s granulomatosis were quantified by ELISA as described previously for IgA and IgM,36 with some modifications. Briefly, polystyrene microplates (Costar) were coated overnight with rabbit antihuman IgG, IgA, or IgM (Dako, Glostrup, Denmark; catalogue no. A423, A262, and A425, respectively, all at 1 μg/mL in phosphate-buffered saline [PBS; GIBCO]). This and all subsequent incubations were performed at room temperature on a shaker. Plates were blocked with bovine serum albumin (BSA; Behringwerke, Marburg, Germany) at 5 g/L in 0.01 mol/L PBS for 90 minutes. Between each of the remaining incubations, which all included BSA (5 g/L) and Tween 20 (0.1%), plates were washed four times with isotonic saline containing 0.1% Tween 20. After application of standards (IgG, IgA, and IgM from plasma; Calbiochem, La Jolla, CA) and appropriate dilutions of samples in triplicates, the following monoclonal antibodies (courtesy of T. Lea, Institute of Immunology and Rheumatology, The National Hospital, Oslo, Norway) were added for 90 minutes (anti-IgG, ascites, 1/250,000, clone B7B2; anti-IgA, ascites, 1/100,000, clone DE2C1; and anti-IgM, ascites, 1/500,000, clone IIE2). Plates were then incubated with alkaline-phosphatase–conjugated rabbit antimouse Igs (Dako; catalogue no. D314; 1/1,500) for 90 minutes and subsequently with substrate (1 mg/mL p-nitrophenyl sodium phosphate [Sigma, St Louis, MO] in diethanolamine buffer; pH 9.8). Optical density was measured at 405 nm (Labsystems Multiskan RC; Labsystems, Helsinki, Finland).
IgG2 in cell culture supernatants was quantified by ELISA (The Binding Site, Birmingham, UK) according to the manufacturer’s guidelines.
The limits of detection were 3 ng/mL for IgG and IgM and 4 ng/mL for IgA and IgG2, respectively. The intra-assay and interassay coefficients of variation were less than 8% for the IgG, IgA, and IgM assays and less than 12% for the IgG2 ELISA. All samples from each patient were measured in the same ELISA plate.
Quantification of serum Ig.
IgA, IgG, and IgM in serum were determined by a nephelometric assay in relation to a commercial standard (Behringwerke).
Cryopreservation of PBMCs.
Immunofluorescence staining and flow cytometry.
After thawing of cryopreserved PBMCs, 5 × 105 cells were washed once in PBS with 2% BSA and 0.1% sodium azide (washing buffer) and preincubated in PBS with human IgG (Octagam; 5 g/L), 5% mouse serum (Sigma), 2% BSA (Calbiochem), and 0.1% sodium azide (Sigma) for 15 minutes at room temperature. Cells were washed twice in washing buffer and incubated for 30 minutes at +4°C with monoclonal antibody conjugates. The following antibodies were used for staining: anti-CD3 (clone SK7) phycoerythrin (PE), anti-CD4 (clone SK3) peridinin chlorophyll protein (PerCP), anti-CD19 (clone 4G7) fluorescein isothiocyanate (FITC), anti-CD8 (clone SK1) PerCP (all from Becton Dickinson); anti-CD40 (clone EA-5) PE, and anti-CD40L (clone 24-31) FITC (both from Ancell). Isotype matched FITC- , PE- (Pharmingen, San Diego, CA), and PerCP- (Becton Dickinson) conjugated mouse IgGs were used as negative controls. Calibrated setup of the FACScan flow cytometer (Becton Dickinson) was performed using CaliBRITE beads (FITC, PE, and PerCP-conjugated; Becton Dickinson) and AutoCOMP software (Becton Dickinson). Ten thousand cells were acquired and analyzed by CellQuest software (Becton Dickinson). B-lymphocyte, CD4+lymphocyte, and CD8+ lymphocyte gates were set on two-parameter plots of side scatter versus CD19 (FL1), CD3 (FL2) versus CD4 (FL3), and CD3 (FL2) versus CD8 (FL3), respectively, and the percentages of CD40+ B lymphocytes and CD40L+CD4+ and CD8+ lymphocytes were determined.
Statistical analysis.
For each parameter, patients and controls were compared by the Mann-Whitney U test, whereas preinfusion and postinfusion values in the patients were compared by the Wilcoxon matched pairs test. Coefficients of correlation (r) were calculated by the Spearman rank test. The calculations were performed using the STATISTICA (StatSoft, Tulsa, OK) software package. Data are given as medians and 25th to 75th percentiles, if not otherwise stated. P values are two-sided and considered significant when P < .05.
RESULTS
Serum Ig levels.
Both patients in CDC group A/B and group C had significantly increased serum levels of both IgG and IgA compared with controls, whereas elevated IgM levels were restricted to patients in CDC group A/B (Table 1).
Ig production in cell culture.
Ig production in vitro was assessed in cell cultures from 8 HIV-infected patients (those who participated in the IVIg infusion study; all in CDC stage A) and 10 controls. Unstimulated PBMC cultures from HIV-infected patients had a significantly higher production of both IgG (∼200% increase) and IgA (∼480% increase) compared with PBMC cultures from healthy controls (Table2). Also, the IgM production tended to be higher in PBMC cultures from HIV-infected patients compared with control cultures (∼90% increase;P = .06) as shown in Table 2. Enhanced Ig production was seen both in patient and control cultures after stimulation with anti-CD40 antibody, except for IgM synthesis in control cultures (Table 2). When the ratios between anti-CD40 stimulated and unstimulated production of IgG, IgA, and IgM were calculated, no significant differences were noted when patient and control cultures were compared, as shown in Table 2.
In purified B-lymphocyte cultures from HIV-infected patients, anti-CD40–stimulated production of IgG was significantly higher (∼50% increase) compared with the control cultures (Table 2). In contrast, IgA and IgM production remained low after anti-CD40 stimulation in both patient and control cultures (Table 2). Low levels of all three Ig isotypes were found in unstimulated B-lymphocyte cultures from both patients and controls (Table 2).
T-lymphocyte membrane expression of CD40L.
A significantly higher percentage of both CD4+ and CD8+ lymphocytes from HIV-seropositive patients expressed CD40L compared with CD4+ and CD8+ lymphocytes from controls (Table 3). When analyzed separately, both CDC group A/B and group C patients had increased percentages of CD40L+ CD4+ lymphocytes compared with controls, with the highest percentage in CDC group C patients (Table 3). Elevated percentages of CD40L+ CD8+lymphocytes were restricted to the CDC group C patients, whereas the CDC group A/B patients and controls had similar percentages of CD40L+ CD8+ lymphocytes (Table 3). When comparing patients in CDC group C with and without opportunistic infections, no significant differences in the percentage of T lymphocytes expressing CD40L were noted (data not shown).
B-lymphocyte membrane expression of CD40.
CD40 membrane expression was measured on B lymphocytes from 8 patients (those participating in the IVIg study) and 10 controls. Almost all B lymphocytes from patients and controls (>95%) expressed CD40. However, as seen in Table 4, the mean fluorescence intensity (MFI) of CD40 was approximately 20% higher on B lymphocytes from patients compared with controls (P = .01).
IL-10 in plasma.
Circulating IL-10 was significantly higher in the HIV-infected patients compared with controls, as shown in Table 3. Although patients in CDC group C tended to have higher IL-10 levels than patients in CDC group A/B, this difference was not significant (P = .12). Patients in CDC group C with opportunistic infections did not have higher IL-10 levels than those without ongoing infections (data not shown).
In patients with Wegener’s granulomatosis, the median plasma IL-10 concentration was 4.7 pg/mL (range, 2.0 to 4.9 pg/mL), which was significantly higher than in the controls (P < .001).
Association between serum Igs, IL-10, and T lymphocyte CD40L expression.
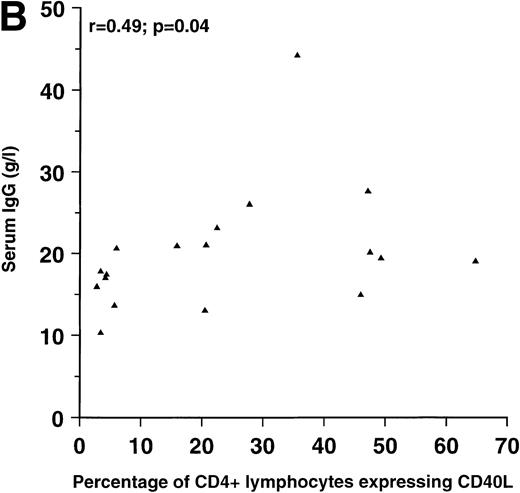
Because both IL-10 and the interaction between CD40 on B lymphocytes and CD40L on T lymphocytes have been associated with enhanced Ig production in vitro, we examined the relationship between these parameters among the HIV-seropositive patients. Significant, positive correlations were found between serum IgG on one hand and circulating IL-10 and CD40L expression on CD4+ lymphocytes on the other hand, as shown in Fig 1. No significant correlation was found between serum IgG and CD40L expression on CD8+ lymphocytes (r = .27; P = .24). The correlation coefficients between serum IgA and CD40L expression on CD4+ and CD8+ lymphocytes were .41 (P = .08) and .56 (P = .01), respectively. No significant correlations were found between serum IgA and IL-10 (r = .04), between serum IgM and IL-10 (r = .14), or between IgM and CD40L expression on CD4+ or CD8+ lymphocytes (r = .12 and r = .19, respectively).
Correlation between (A) serum IgG and IL-10 in plasma (n = 23) and correlation between (B) serum IgG and the percentage of CD4+ lymphocytes expressing CD40L (n = 18) in patients with HIV infection.
Correlation between (A) serum IgG and IL-10 in plasma (n = 23) and correlation between (B) serum IgG and the percentage of CD4+ lymphocytes expressing CD40L (n = 18) in patients with HIV infection.
Effect of IVIg infusion on plasma IL-10 concentrations.
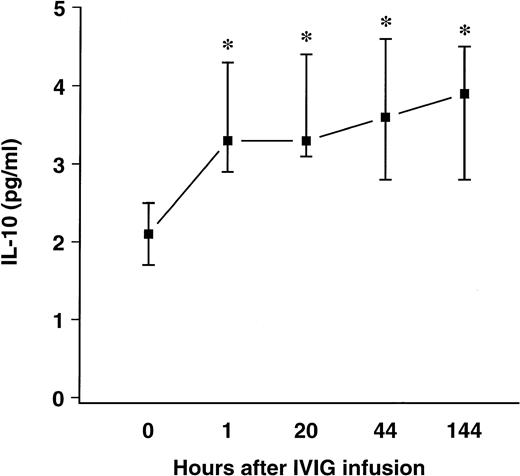
A significant increase in circulating IL-10 levels after IVIg infusion was observed in patients with HIV infection, as shown in Fig 2.
Effect of a single bolus infusion (0.4 g/kg) of IVIg on plasma IL-10 levels in 8 patients with HIV infection. IL-10 was assayed by ELISA. Data are given as medians and 25th and 75th percentiles. *P < .05 versus levels before infusion (0 hours).
Effect of a single bolus infusion (0.4 g/kg) of IVIg on plasma IL-10 levels in 8 patients with HIV infection. IL-10 was assayed by ELISA. Data are given as medians and 25th and 75th percentiles. *P < .05 versus levels before infusion (0 hours).
In patients with Wegener’s granulomatosis, a similar effect was observed as plasma IL-10 levels increased from 4.7 pg/mL (2.0 to 4.9 pg/mL) preinfusion to 7.5 pg/mL (4.1 to 7.8 pg/mL) 20 hours after infusion (P = .02 v preinfusion) and to 7.0 pg/mL (3.5 to 8.3 pg/mL) 44 hours after infusion (P = .03 vpreinfusion).
Effect of IVIg infusion on IL-10 production in mononuclear cells from HIV patients.
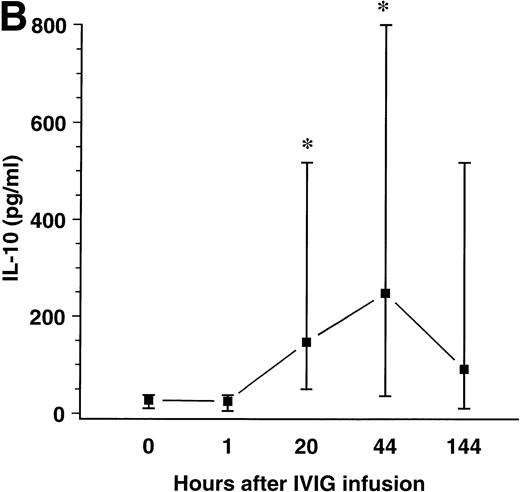
When PBMCs from the patients before and after IVIg infusion were cultured for 7 days and left unstimulated or stimulated with anti-CD40 antibody during culture, the IL-10 concentration in PBMC supernatants was significantly higher after IVIg infusion with a maximum after 44 hours (∼9-fold increase compared with preinfusion levels), as shown in Fig 3A and B. However, the profile of IL-10 production after IVIg infusion was similar in unstimulated and anti-CD40 stimulated cultures.
Effect of a single bolus infusion (0.4 g/kg) of IVIg on IL-10 production in PBMCs from 8 patients with HIV infection. PBMCs were prepared from blood samples taken at the indicated time points before and after IVIg infusion and cultured without stimulation (A) or with anti-CD40 monoclonal antibody (B). Supernatants were harvested after 7 days and assayed for IL-10 by ELISA. Data are given as medians and 25th and 75th percentiles. *P < .05 versus levels before infusion (0 hours).
Effect of a single bolus infusion (0.4 g/kg) of IVIg on IL-10 production in PBMCs from 8 patients with HIV infection. PBMCs were prepared from blood samples taken at the indicated time points before and after IVIg infusion and cultured without stimulation (A) or with anti-CD40 monoclonal antibody (B). Supernatants were harvested after 7 days and assayed for IL-10 by ELISA. Data are given as medians and 25th and 75th percentiles. *P < .05 versus levels before infusion (0 hours).
In preliminary experiments, PBMC IL-10 production did not differ significantly after 1, 2, and 7 days of culture (data not shown).
Effect of IVIg infusion on in vitro Ig production in mononuclear cells.
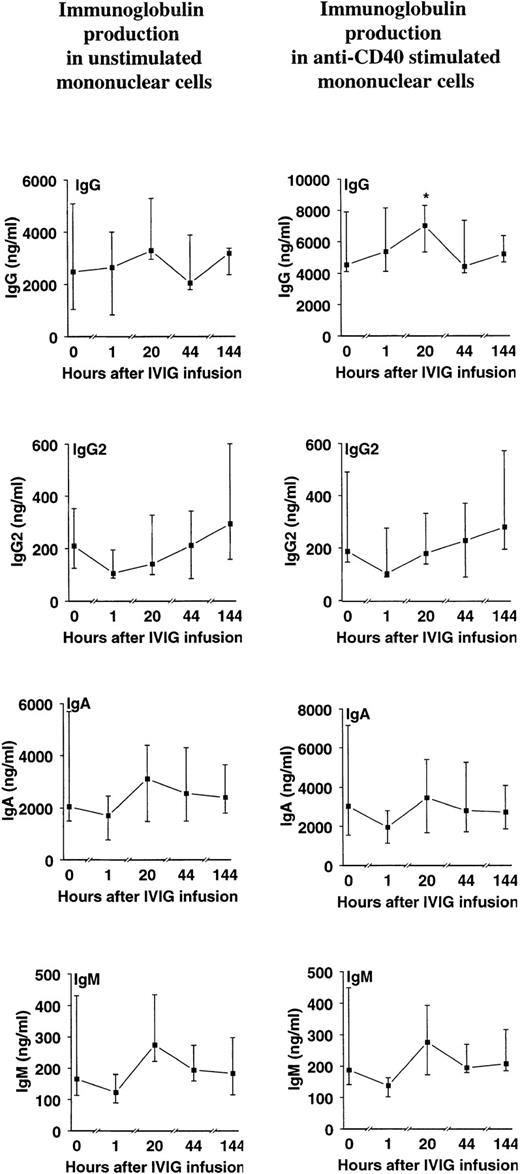
Anti-CD40 stimulated IgG production in PBMCs from HIV-infected patients increased to a maximum of 20 hours postinfusion (∼1.5-fold increase compared with preinfusion concentration; P < .05), whereas concentrations comparable to preinfusion levels were found in cultures 44 hours and 6 days postinfusion (Fig 4). No significant differences were noted for preinfusion versus postinfusion levels of anti-CD40–stimulated IgA or IgM or from unstimulated IgG, IgA, or IgM (Fig 4). No significant changes were noted for IgG2 production in PBMC cultures before and after IVIg infusion (Fig 4).
Effect of a single bolus infusion (0.4 g/kg) of IVIg on IgG, IgG2, IgA, and IgM production in PBMC cultures from 8 patients with HIV infection. PBMCs were prepared from blood samples taken at the indicated time points before and after IVIg infusion and cultured without stimulation (left panels) or stimulated with anti-CD40 monoclonal antibody (right panels). Supernatants were harvested after 7 days and assayed for Igs by ELISA. Data are given as medians and 25th and 75th percentiles. *P < .05 versus levels before infusion (0 hours).
Effect of a single bolus infusion (0.4 g/kg) of IVIg on IgG, IgG2, IgA, and IgM production in PBMC cultures from 8 patients with HIV infection. PBMCs were prepared from blood samples taken at the indicated time points before and after IVIg infusion and cultured without stimulation (left panels) or stimulated with anti-CD40 monoclonal antibody (right panels). Supernatants were harvested after 7 days and assayed for Igs by ELISA. Data are given as medians and 25th and 75th percentiles. *P < .05 versus levels before infusion (0 hours).
In unstimulated PBMC cultures from 2 patients with Wegener’s granulomatosis, IgG levels were 2,200 and 1,500 ng/mL (preinfusion) and 2,500 and 2,100 ng/mL (20 hours postinfusion). In anti-CD40–stimulated cultures, IgG levels were 4,300 and 3,300 ng/mL (preinfusion) and 4,600 and 2,900 ng/mL (20 hours postinfusion).
Effect of IVIg infusion on in vitro Ig production in B lymphocytes from HIV patients.
Ig production in unstimulated B lymphocytes increased from preinfusion levels to a maximum at 44 hours postinfusion; from 2 ng/mL (0 to 7 ng/mL) to 156 ng/mL (32 to 187 ng/mL) for IgG, from 6 ng/mL (0 to 18 ng/mL) to 56 ng/mL (44 to 146 ng/mL) for IgA, and from 0 ng/mL (0 to 4 ng/mL) to 13 ng/mL (8 to 21 ng/mL) for IgM (Fig 5). No significant alterations in anti-CD40 stimulated Ig production were noted (Fig 5).
Effect of a single bolus infusion (0.4 g/kg) of IVIg on IgG, IgA, and IgM production in purified B-lymphocyte cultures from 8 patients with HIV infection. B lymphocytes were prepared from blood samples taken at the indicated time points before and after IVIg infusion and cultured without stimulation (left panels) or stimulated with anti-CD40 monoclonal antibody (right panels). Supernatants were harvested after 7 days and assayed for Igs by ELISA. Data are given as medians and 25th and 75th percentiles. *P < .05 versus levels before infusion (0 hours).
Effect of a single bolus infusion (0.4 g/kg) of IVIg on IgG, IgA, and IgM production in purified B-lymphocyte cultures from 8 patients with HIV infection. B lymphocytes were prepared from blood samples taken at the indicated time points before and after IVIg infusion and cultured without stimulation (left panels) or stimulated with anti-CD40 monoclonal antibody (right panels). Supernatants were harvested after 7 days and assayed for Igs by ELISA. Data are given as medians and 25th and 75th percentiles. *P < .05 versus levels before infusion (0 hours).
Igs in serum from HIV patients after IVIg infusion.
The serum IgG level increased from 17.3 g/L (14.8 to 20.0 g/L) preinfusion to a maximum of 23.8 g/L (21.7 to 25.7 g/L) 1 hour after IVIg infusion; thereafter, a gradual decrease was seen, with a serum IgG level of 19.7 g/L (17.2 to 22.7 g/L) after 6 days. No changes in serum IgA or IgM concentrations were seen after IVIg infusion (data not shown).
Expression of CD40L and CD40 on lymphocytes from HIV patients after IVIg infusion.
No significant changes in CD40L expression on CD4+ or CD8+ lymphocytes was observed after IVIg infusion (data not shown). CD40 expression on B lymphocytes was always greater than 95%. The CD40 MFI on B lymphocytes did not change from preinfusion till 44 hours postinfusion, whereas the MFI was significantly reduced at 6 days postinfusion compared with the preinfusion levels (Table 4).
DISCUSSION
One of the first immunological abnormalities observed in patients with HIV infection was the elevation of circulating Ig levels, often comprising several isotypes.1-5 Previous in vitro studies have suggested various mechanisms contributing to the hypergammaglobulinemia and B-lymphocyte activation in HIV infection, such as stimulation by HIV, HIV viral proteins, IL-6, or membrane-bound tumor necrosis factor-α (TNF-α) on CD4+lymphocytes.6,9-12,38 However, the mechanisms leading to hypergammaglobulinemia in HIV infection are still not well understood.6-12 In the present study, we found a significant correlation between serum Ig levels and both the percentage of CD4+ lymphocytes expressing CD40L and plasma IL-10 levels.
Although our data do not, of course, prove that there is a causal relationship between the increased Ig production and dysregulation in T-lymphocyte CD40L expression or IL-10 metabolism in HIV infection, our findings are of particular interest in relation to present concepts of B-lymphocyte regulation and Ig secretion. Furthermore, the data suggest that an increased percentage of CD4+ lymphocytes expressing CD40L and enhanced IL-10 activity are involved in the hypergammaglobulinemia during HIV infection.
The present study is, to our knowledge, the first report of increased percentages of CD40L expression on T lymphocytes from patients with HIV infection. Among the receptor-ligand pairs that are important for contact-dependent T-B–lymphocyte collaboration, the CD40-CD40L interaction plays a central role.13 CD40L is a member of the TNF gene superfamily,13 and its importance in vivo for normal Ig secretion was shown by studies of patients with hyper-IgM syndrome having high serum levels of IgM but severely reduced levels of other isotypes due to mutations in the gene coding for CD40L.39 Mice infected with a murine retrovirus causing murine AIDS develop hypergammaglobulinema and treatment with anti-CD40L reduced their elevated Ig levels.40 Brugnoni et al41 have previously shown that, after optimal in vitro stimulation, CD40L expression of T lymphocytes was not impaired in patients with HIV infection. However, they did not study CD40L expression in unstimulated cells.41 On the other hand, Macchia et al38 did not find CD40L expression on T-lymphocyte clones that had been HIV-infected in vitro. However, this experimental in vitro system does not permit the important interplay between various cell types leading to in vivo immune activation that is probably central to the immunopathogenesis of HIV infection.42 43 Our findings of an elevated percentage of T lymphocytes expressing CD40L may well be a manifestation of this immune activation. However, when patients in CDC group C with and without ongoing opportunistic infections were compared, no difference in the percentage of T lymphocytes expressing CD40L was noted.
IL-10 is important for the regulation of Ig production, because it is a switch factor for IgG during B-lymphocyte maturation and promotes plasma cell differentiation and secretion of IgG, IgM, and IgA.14-18 With regard to IgG, IL-10 promotes a selective secretion of IgG1 and IgG3, but not of IgG2 or IgG4.14Interestingly, the IgG subclass pattern seen in patients with HIV infection is characterized by the same IgG subclass dysbalance both in serum4,44,45 and lymphoid tissue.44 We suggest that the elevated IL-10 levels found in HIV-infected patients may be of importance for their elevated Ig secretion. Elevated IL-10 levels or enhanced cellular production of IL-10 in HIV infection have been reported previously.46-52 In vitro, both HIV infection53-55 and stimulation with HIV viral proteins48,56 or mycobacterial proteins35 57lead to enhanced IL-10 production. However, in our study, patients in CDC group C with opportunistic infections did not have higher IL-10 levels than those without ongoing infections.
IVIg treatment has been used in HIV-infected patients to strengthen the antibody defense against bacterial pathogens.22-24,26 The present study demonstrates that a single IVIg infusion in vivo not only results in elevated serum level of IgG, but also induces a further enhancement of the patients own IgG production as studied in vitro. However, the enhanced IgG production did not comprise IgG2, which may be of importance for the immunity to bacterial infections in HIV-infected patients.58
Also, a marked increase in IL-10 levels, both in plasma and anti-CD40–stimulated PBMC supernatants, was observed after IVIg infusion. Previously, IVIg has been suggested to have immunosuppressive effects by increasing the level of IL-1 receptor antagonist27,59 and downregulating the TNF system.31,60,61 In particular, we have shown elsewhere that IVIg infusion in HIV-infected patients with abnormally increased TNF-α activity leads to a downregulation of the TNF system.31 However, this is the first report of increased levels of IL-10 after IVIg infusion. In addition to the effect in HIV infection, we show a similar effect of IVIg on IL-10 levels in patients with Wegener’s granulomatosis. This may suggest that the ability to increase IL-10 levels is a general IVIg effect. Thus, it may be a contributory factor to the beneficial effects of IVIg in various inflammatory and autoimmune disorders.62 63
The IVIg preparation contained a low amount of IL-10 that may have contributed to the elevation of plasma IL-10 briefly after infusion. However, administration of IL-10 has shown that this molecule has a short half-life of 2 to 4 hours.64 65 Thus, the persistently high IL-10 levels over several days cannot be due to passive transfer of IL-10. Also, the upregulated IL-10 production in PBMC culture after IVIg infusion in HIV-infected patients suggests that IVIg induces IL-10 production.
In the present study, the enhanced IL-10 production in PBMC supernatants after IVIg infusion probably contributed to the elevated Ig levels in the same cultures.
This raises the possibility of a vicious circle in HIV infection in which high IL-10 levels lead to enhanced IgG production that, in turn, further upregulates IL-10 production. However, IL-10 production was not dependent on CD40 stimulation. Several cell types may contribute to the IL-10 production in the PBMC cultures, because both T lymphocytes, B lymphocytes, and monocytes have been shown to produce IL-10.66-68
In conclusion, our findings of increased percentages of T lymphocytes expressing CD40L and elevated circulating IL-10 levels may well be of importance for the enhanced Ig production and hypergammaglobulinemia seen in HIV-infected patients. In line with these findings, a single bolus of IVIg administered to HIV-infected patients leads to elevated levels of IL-10 in vivo and in vitro, with a concomitant elevation of Ig production in cell cultures.
We suggest that the enhanced production of IL-10 as well as increased expression of CD40L on T lymphocytes may be important factors for the development of hypergammaglobulinemia in patients with HIV infection.
ACKNOWLEDGMENT
The authors thank Bodil Lunden and Lisbeth Wikeby for excellent technical assistance and Tor Lea (Institute of Immunology and Rheumatology, The National Hospital) for the monoclonal antibodies to IgA, IgG, and IgM. Recombinant IL-10 and IL-10 antibodies were kindly provided by S. Narula (Schering-Plough Research Institute).
Supported by Octapharma, Hurdal, Norway; the Norwegian Research Council; the Norwegian Cancer Society; Medinnova Foundation; Anders Jahre’s Foundation; and Odd Kåre Rabben’s Memorial Fund for AIDS research.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Fredrik Müller, MD, PhD, Section of Clinical Immunology and Infectious Diseases, Medical Department A and Research Institute for Internal Medicine, Rikshospitalet, N-0027 Oslo, Norway.