Abstract
An in vitro model consisting of endothelium grown on collagen was used to investigate how mononuclear phagocytes traverse endothelium in the basal-to-apical direction (reverse transmigration), a process that mimics their migration across vascular and/or lymphatic endothelium during atherosclerosis and resolution of inflammation, respectively. Monoclonal antibody (MoAb) VIC7 against tissue factor (TF) inhibited reverse transmigration by 77%. Recombinant tissue factor fragments containing at least six amino acids C-terminal to residue 202 also strongly inhibited reverse transmigration. TF was absent on resting monocytes but was induced on these cells after initial apical-to-basal transendothelial migration. Two additional observations suggest that TF is involved in adhesion between mononuclear phagocytes and endothelium: (1) when monocytes were incubated with lipopolysaccharide (LPS) to stimulate expression of TF before they were added to endothelium, VIC7 or soluble TF modestly inhibited their adhesion to the apical endothelial surface, each by about 35%; and (2) endothelial cells specifically bound to surfaces coated with TF fragments containing amino acids 202-219. This binding was blocked by anti-TF MoAb, suggesting that endothelial cells bear a receptor for TF. These data suggest that mononuclear phagocytes use TF, perhaps as an adhesive protein, to exit sites of inflammation.
TISSUE FACTOR (TF), a 47-kD transmembrane protein, initiates the extrinsic pathway of coagulation via formation of an enzymatic complex with factor VII/factor VIIa (factor VII[a]). Its constitutive expression by mesenchymal cells residing in the adventitial lining of blood vessels normally precludes its interaction with factor VII in plasma but allows rapid activation of coagulation when blood vessel barriers are broken.1 TF may also possess biological functions that are independent of the clotting cascade; TF is expressed in early stages of embryogenesis before factor VII(a) is present.2 In contrast to factor VII,3 TF is essential for development, as shown by inactivation of the murine TF gene.4-6 TF expressed by tumor cells promotes vascularization of the tumor, even in the presence of potent inhibitors of coagulation.7 In addition, tumor cells bearing TF display an enhanced capacity to metastasize. TF-dependent metastasis requires VII(a) proteolytic activity8 but may also depend on a role for TF that is independent of its coagulative functions. Mutation of specific residues in the cytoplasmic tail of TF prevent metastasis, even though modification of these residues does not impair the binding and activation of factor VII(a).8 9
Proinflammatory stimuli induce expression of TF on mononuclear phagocytes (MP), dendritic cells, and endothelium.1Induction of TF on circulating monocytes after activation with lipopolysaccharide (LPS) or tumor necrosis factor-α (TNF-α) is a major complication in septic shock, leading to disseminated intravascular coagulation.10 The central role of TF in septic shock is evident from the findings that neutralizing monoclonal antibodies (MoAbs) to TF block LPS-mediated intravascular coagulation in chimpanzees11 and lethality in baboons after administration of LPS.12 In another setting, expression of TF on macrophages and endothelial cells in atherosclerotic plaques13-15 may also lead to thrombosis. Our interest in mediators of inflammatory reactions, particularly those that promote resolution of such reactions, led to the present study that points to a novel role for TF in inflammation.
As acute inflammation resolves, MP are cleared from inflammatory foci by migrating to draining lymph nodes,16,17 a process that entails crossing lymphatic endothelium in the basal-to-apical direction (reverse transmigration). Reverse transmigration has also been documented in atherosclerosis,14,18-20 a form of chronic inflammation. Indeed, reverse transmigration of MP-derived foam cells across arterial endothelium may represent a mechanism by which atherosclerotic lesions can regress.14,18 A previous study using an in vitro model of a blood vessel wall showed that a majority of MP that initially migrate across endothelium to enter underlying connective tissue later exited the cultures by migrating back across the endothelium in the reverse direction.21 Because little is known about the molecular events that promote reverse transmigration in vitro or in vivo, we initially set out to identify important mediators. Our findings indicate that TF has an important role in reverse transmigration of MP. Although the exact mechanism for this is not known, we show that under conditions in which monocytes are stimulated to express TF, it contributes to the adhesion of these activated monocytes to endothelium. Moreover, we find that endothelial cells express binding sites for TF.
MATERIALS AND METHODS
Antibodies and recombinant proteins.
A panel of anti-TF MoAbs including VD8, VIC7, VIC12, IIID8, VD10, VIC6, and IVC622; anti-factor VII(a) MoAbs IVE4 and IIH223; hec7 MoAb against platelet/endothelial cell adhesion molecule-124; and hec1 MoAb against cadherin 525 were produced and characterized previously . Anti-CD14 MoAb 3C10, anti-vascular cell adhesion molecule 1 MoAb 4B9, and anti-CD3 MoAb Leu4 were gifts from Drs Samuel Wright (Merck, Rahway, NJ), John Harlan (University of Washington, Seattle), and James Young (Rockefeller University), respectively. Fluorescein isothiocyanate (FITC)-conjugated anti-CD45 MoAb was purchased from Becton Dickinson (San Jose, CA). All other MoAbs used were obtained from the VIth International Workshop on Human Leukocyte Differentiation Antigens. Goat anti-TF polyclonal antibody was purchased from American Diagnostica (Greenwich, CT). Purified, nonlipidated fragments of soluble recombinant human TF representing various regions of the extracellular domain, expressed in Escherichia coli, were prepared as described.26 Additional soluble recombinant TF was the generous gift of Drs Yale Nemerson and Arabinda Guha (Mt Sinai Medical Center, New York, NY). Reagents used in transmigration experiments, including MoAbs, were tested for LPS using the limulus amebocyte lysate assay purchased from BioWhittaker, Inc (Walkersville, MD).
Transendothelial migration assays.
In vitro cultures to mimic a blood vessel wall were prepared using a minor variation of a well-characterized method.27 In brief, HUVEC were grown on type I collagen gels in microtiter wells and were maintained in Medium 199 (M199) containing 20% fetal bovine serum (FBS) or 20% heat-inactivated human serum. FBS without detectable levels of LPS was obtained from HyClone Laboratories (Logan, UT). In some experiments, collagen gels were polymerized in the presence of 2.5 × 10-4% FITC-conjugated, 0.5-μm diameter polystyrene microspheres from Polysciences (Warrington, PA) before adding HUVEC.
For transmigration experiments, freshly isolated peripheral blood mononuclear cells (PBMC)27 or PBMC cultured for 4 hours in 10% FBS/M199 with or without addition of 2 ng/mL of LPS were resuspended in 0.1% HSA/M199 and added to HUVEC cultures that had been confluent for at least 3 days. Monocytes, but not lymphocytes, transmigrate across unstimulated endothelial monolayers by 1 hour of coincubation.27 In some experiments, HUVEC monolayers were pretreated with 10 ng/mL recombinant human TNF-α (Genzyme; Cambridge, MA) for 4 hours before adding monocytes. For experiments involving TNF-activated endothelium, PBMC were further purified to yield 70% monocytes by negative selection as follows: PBMC were incubated with 1 μg/mL Leu4 MoAb for 30 minutes on ice and then magnetic beads conjugated to goat anti-mouse IgG, purchased from Dynal (Lake Success, NY), were added at a ratio of 5 beads/cell and incubated with gentle inversion at 4°C for 30 additional minutes. CD3+T-lymphocytes and free magnetic beads were depleted using a strong magnet.
For reverse transendothelial migration assays, PBMC were incubated with endothelium for 1 or 2 hours to allow accumulation of monocytes in the subendothelial collagen. Then cultures were rinsed in M199 to remove nonmigrated cells (ie, lymphocytes), 20% FBS/M199 with or without addition of MoAbs or polymyxin B (Sigma Chemical Co, St Louis, MO) was added, and incubation was continued. At 24-hour intervals until the end of the experiment, cultures were rinsed in M199 to remove nonadherent MP that may have accumulated in the apical compartment by reverse transmigration, and medium was replenished. Experiments included six replicates per parameter tested.
Experiments were analyzed by using one or both of the following methods: (1) Cultures were fixed in glutaraldehyde, stained with eosin and methylene blue, and the number of MP beneath the endothelium were counted in five high-power fields during an en face microscopic examination using Nomarski interference optics.25 To confirm whether MP in the cultures were adherent to the apical surface of the endothelial monolayer or present beneath the endothelium, some cultures were embedded in JB-4 glycol methacrylate (Polysciences), transverse sections (2-μm) were prepared, and the disposition of MP relative to the endothelium was determined. (2) An automated technique was used to evaluate total cell number by quantitating total DNA/well using the dye Yo-Pro-1 from Molecular Probes (Eugene, OR), which fluoresces after binding DNA. MP adherent to the apical surface of the endothelium were removed from wells before evaluation with Yo-Pro-1 by washing the cultures twice in cold Hanks’ balanced salt solution (HBSS) containing 1 mmol/L EGTA and then twice more in plain HBSS. Plates containing subendothelial MP and endothelium were frozen and thawed, incubated for 1 hour at 37°C after addition of 100 μL distilled water/well, and frozen and thawed once more before addition of 100 μL of Yo-Pro-1 (4 μmol/L in Tris buffer [10 mmol/L] containing NaCl [2 mol/L] and EDTA [1 mmol/L]). Fluoresence was measured using a Cytofluor 2350 fluorescence measurement system from PerSeptive Biosystems (Framingham, MA). The intensity of fluorescence in wells containing only endothelium was subtracted from the intensity of fluorescence in wells containing both MP and endothelium in the same assay plate, allowing an assessment of the relative number of MP beneath the endothelium. Percent reverse transmigration was calculated as the percentage decrease in the number of MP beneath the endothelial monolayer compared with the number that initially accumulated below the endothelium at 2 hours of incubation.
Chemotaxis assay.
Solutions of FMLP (Sigma) or soluble recombinant TF, prepared in 0.1% HSA/M199, were placed in the lower compartments of a 48-well chemotaxis chamber from Neuroprobe, Inc (Cabin John, MD) in the presence or absence of added MoAb. MP resuspended in 0.1% HSA/M199, with or without addition of MoAb, were placed in the upper compartment of the chambers, which was separated from the lower compartment by a cellulose nitrate filter (Neuroprobe, Inc) permeated with 5.0-μm pores or an 8.0-μm filter coated with type I bovine collagen. The chambers were incubated for 90 minutes at 37°C and then fixed and stained with hematoxylin.28 Five 400× fields per filter were evaluated microscopically to determine the number of MP that had migrated to depths of 25 and 50 μm within the filter. At these depths, significant numbers of MP (20 to 50 cells/field) were found in positive controls, but few in negative controls. For coating filters with collagen, individual filters were submerged in a solution of monomeric type I bovine collagen (1.7 mg/mL) purchased from Celtrix (Palo Alto, CA) and incubated at 37°C for 20 minutes to allow polymerization. Excess polymerized collagen was removed from the surface of the filter by rotating it gently in M199.
In other experiments, collagen gels in 96-well plates were equilibrated with FMLP (10-7 mol/L), then rinsed for 10 minutes in M199 lacking FMLP. This technique allows for the development of a gradient of FMLP that increases with increasing depth of collagen. Reverse transmigrated MP were applied to these gels in the presence or absence of anti-TF MoAb or control MoAb. Cultures were incubated for 2 hours, then rinsed and (1) fixed or (2) processed for cell quantitation using Yo-Pro-1 as described above. In fixed samples, the depth of MP penetration into the gel was assessed.
Flow cytometry.
Before staining for flow cytometry, endothelial cells and MP residing on/within collagen gels were separated from this matrix by solubilizing the collagen using 1 mg/mL collagenase (Worthington, Freehold, NJ) at 37°C for 25 minutes. Cell suspensions were resuspended in 5% human serum/M199. MoAbs against surface antigens were added to the suspensions at 10 μg/mL and incubated on ice for 30 minutes. Cells were washed twice in 0.1%HSA/HBSS, incubated in 1:100 dilution of FITC-conjugated goat anti-mouse IgG (Dako; Carpinteria, OR) for 30 minutes at 4°C, and finally washed twice in 0.1%HSA/HBSS. Fluorescence intensity was measured using a Becton Dickinson FACScan, in which 10,000 events were collected per sample.
Clotting assay.
A one-stage clotting assay was conducted essentially as previously described.22 Cell samples and human plasma (100 μL each) were mixed and warmed for 1 minute at 37°C, followed by addition of 25 mmol/L CaCl2 (100 μL). The number of seconds that elapsed until the formation of a visible clot was measured. Using bovine lung thromboplastin (ICN, Costa Mesa, CA) as a standard, a linear curve was obtained across the tested concentration range of 0.1 to 1,000 mU procoagulant activity (PCA; log-log plot). For clotting assays conducted on cells cultured in collagen gels, intact collagen gels were removed from the microtiter wells and mixed with human plasma before starting the clotting assay. Alternatively, cultures were frozen and thawed. Then cells were lysed and solubilized by successive addition of 15 mmol/L 1-O-n-octyl-β-D-glucopyranoside purchased from Boehringer Mannheim (Indianapolis, IN; 30 μL) and HEPES-buffered saline solution (70 μL). Thus, treated cultures were removed from microtiter wells for assessement of PCA. The latter procedure enhanced the detection of PCA in cultures by approximately two-fold.
Cell adhesion assay.
Ninety-six well enzyme-linked immunosorbent assay (ELISA) plates from Nunc (Wiesbaden, Germany) were coated overnight with 5 μg/mL TF fragments or bovine serum albumin (BSA) in carbonate buffer (15 mmol/L Na2CO3, 33 mmol/L NaHCO3, pH 9.5). The plates were washed two times with phosphate-buffered saline (PBS; pH 7.6), and thereafter blocked with 0.2% BSA in PBS for 30 minutes at 37°C. HUVEC (passage 3 to 5) were harvested and resuspended in Endothelial Cell Basal Medium (PromoCell, Heidelberg, Germany) with Supplement Pack (PromoCell; contains FBS, endothelial cell growth supplement, human epidermal growth factor, human basic fibroblast growth factor, HC-500, gentamicin, amphotericin B). For some samples, this medium also included 50 μg/mL anti-TF MoAb or 50 μg/mL control MoAb. Cells were seeded (20,000 cells/well) onto plates for 1, 2, or 5 hours, as indicated. Nonadherent cells were removed by two washes in PBS. Adherent cells were quantified by measuring the enzymatic cleavage of the tetrazolium salt XTT to formazan by mitochondrial succinate-tetrazolium reductase (Boehringer Mannheim, Germany). The color reaction was measured at 450 nm. A reference curve was established by incubating different cell numbers on a separate plate from the time when the cells were seeded to the plates.
Statistics.
The Mann-Whitney U test was used for statistical analysis, using SPSS software.
RESULTS
In vitro model to study reverse transendothelial migration.
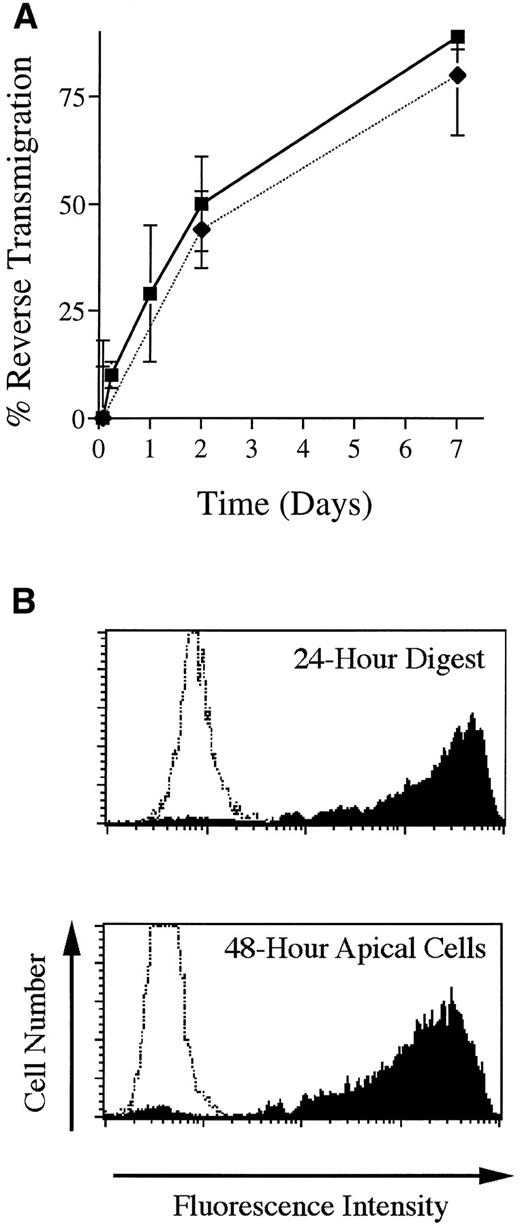
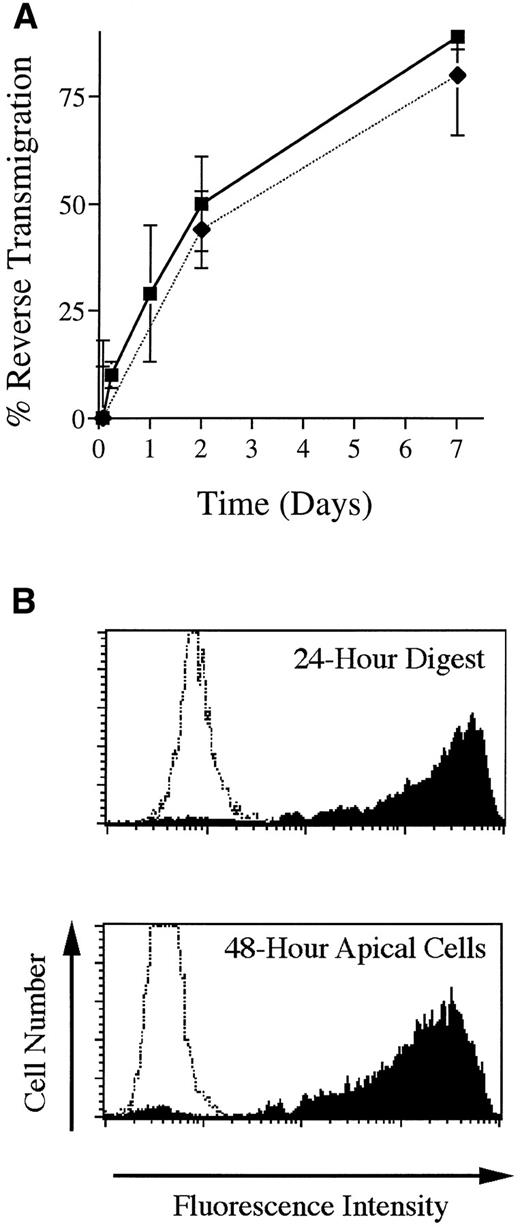
The in vitro model used herein, consisting of HUVEC grown on reconstituted bovine type I collagen, has been well characterized with respect to the initial diapedesis of monocytes to enter the underlying collagen.25,27 The subsequent exit of MP from HUVEC cultures by basal-to-apical migration back across the endothelium (reverse transmigration) has been investigated previously using a different model in which HUVEC are grown on amniotic stroma.21 Likewise, in the present model, MP that enter the subendothelial collagen later exit the cultures by retraversing the endothelium with a t1/2 of 48 hours (Fig 1A). The endothelial monolayer remained intact throughout these experiments. A direct comparison between two methods that were used to analyze reverse transmigration experiments yielded similar results (Fig 1A).
Basal-to-apical transendothelial migration of MP. (A) Unstimulated endothelial cells grown on collagen were incubated with PBMC for 2 hours, then rinsed to remove nonadherent cells. Some cultures were then processed for analysis; others were incubated further for as long as 7 days. Two methods were used to analyze reverse transmigration experiments: visual enumeration of MP beneath endothelial monolayers using Nomarski interference optics (filled squares) and assessment of MP content in cultures using the DNA-binding dye Yo-Pro-1 (filled diamonds). Percent reverse transmigration is defined as the percentage decrease in the number of MP beneath the endothelium, relative to the number of subendothelial MP at 2 hours. (B) Some cultures were prepared that contained FITC-conjugated beads embedded in the collagen. Using flow cytometry, the uptake of these beads by MP that migrated into the collagen was assessed at 24 hours, after the cells were removed from the collagen with collagenase, and compared to the extent of beads associated with MP that accumulated in the apical compartment of parallel cultures between 24 and 48 hours of incubation. Profiles represent histograms from a representative experiment of MP collected from fluorescent bead–containing cultures (shaded) or MP from cultures without beads (unshaded).
Basal-to-apical transendothelial migration of MP. (A) Unstimulated endothelial cells grown on collagen were incubated with PBMC for 2 hours, then rinsed to remove nonadherent cells. Some cultures were then processed for analysis; others were incubated further for as long as 7 days. Two methods were used to analyze reverse transmigration experiments: visual enumeration of MP beneath endothelial monolayers using Nomarski interference optics (filled squares) and assessment of MP content in cultures using the DNA-binding dye Yo-Pro-1 (filled diamonds). Percent reverse transmigration is defined as the percentage decrease in the number of MP beneath the endothelium, relative to the number of subendothelial MP at 2 hours. (B) Some cultures were prepared that contained FITC-conjugated beads embedded in the collagen. Using flow cytometry, the uptake of these beads by MP that migrated into the collagen was assessed at 24 hours, after the cells were removed from the collagen with collagenase, and compared to the extent of beads associated with MP that accumulated in the apical compartment of parallel cultures between 24 and 48 hours of incubation. Profiles represent histograms from a representative experiment of MP collected from fluorescent bead–containing cultures (shaded) or MP from cultures without beads (unshaded).
To show further that MP in this system were indeed retraversing the endothelium in the basal-to-apical direction, we set up cultures in which the collagen was polymerized in the presence of FITC-conjugated microspheres. HUVEC were grown to confluence on this matrix, and monocytes were added to the cultures and allowed to transmigrate. After 24 hours, some cultures containing subendothelial MP were resuspended by digestion of the matrix with collagenase, and the MP were analyzed by flow cytometry to determine the extent to which they had engulfed the fluorescent beads. In parallel cultures that were not digested, MP that accumulated in the apical compartment by reverse transmigration were collected in the time interval between 24 and 48 hours and also analyzed by flow cytometry. The MP collected from the apical compartment at 48 hours had the same profile of fluorescence as those found beneath the collagen at 24 hours (Fig 1B), indicating that the MP had migrated beneath the endothelium, phagocytized the fluorescent beads, and later exited the matrix by retraversing the endothelium.
Role of tissue factor in reverse transendothelial migration.
A variety of MoAbs were tested in reverse transendothelial migration assays to search for molecules that mediate the interactions between MP and endothelium during this process. Control experiments analyzed whether MoAbs added to the apical side of endothelial monolayers could penetrate these monolayers and bind to subendothelial antigens. Indeed, FITC-conjugated MoAb against CD45, added to the apical aspect of endothelial cultures just after monocytes had migrated beneath them, successfully penetrated the endothelial barrier to bind to the CD45 antigen on the monocytes within 3 hours. This was determined by digesting MoAb-treated cultures and examining the fluorescence intensity of subendothelial monocytes by flow cytometry.
Even though MoAbs were thereby shown to have access to subendothelial antigens, MoAbs against an extensive list of molecules that are known to mediate binding between leukocytes and endothelium during apical-to-basal transmigration revealed none that is essential for reverse transmigration of MP for as long as 48 hours. For example, neutralizing MoAbs to E-selectin,21 vascular cell adhesion molecule 1,21 and platelet/endothelial cell adhesion molecule 129 have no effect on reverse transmigration. Neutralizing MoAb to intercellular adhesion molecule 1 or the β2 subunit of integrins prevents reverse transmigration of MP across interleukin-1 (IL-1)β-stimulated HUVEC in the first 12 hours.21 However, when assays are conducted for 24 hours or longer in the presence of these MoAbs, no inhibition of reverse transmigration is observed using either cultures of HUVEC grown on amniotic stroma (G.J. Randolph and M.B. Furie, unpublished observations, June 1995) or in the culture system used herein (Fig 2 and data not shown).
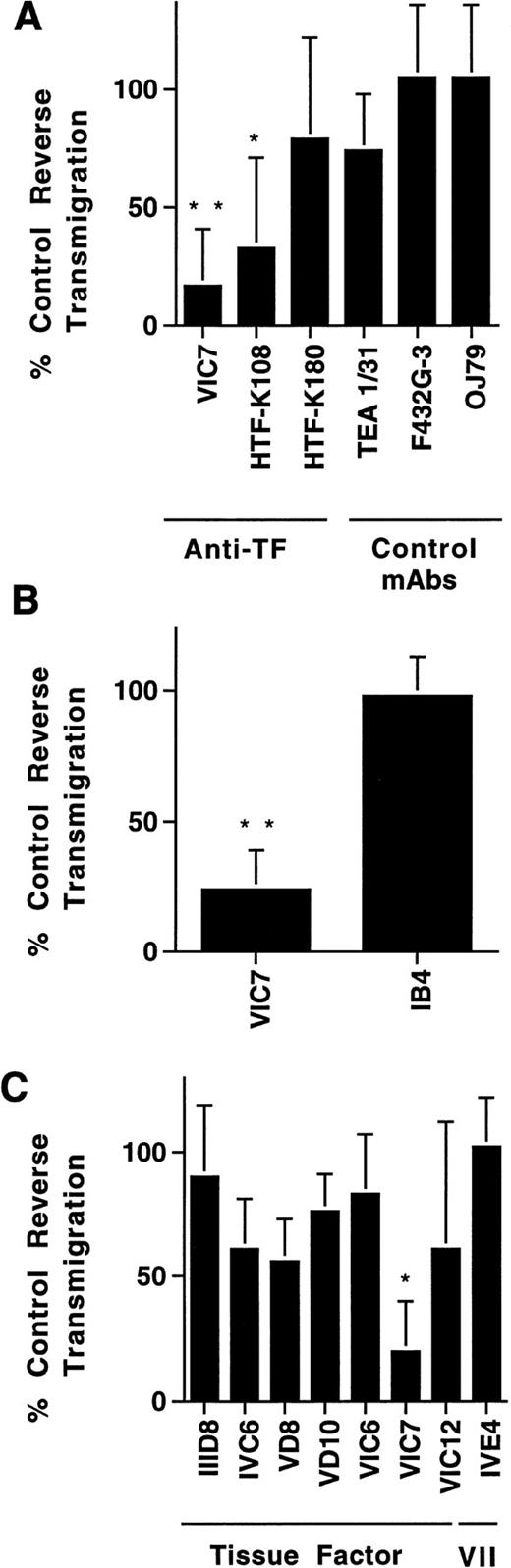
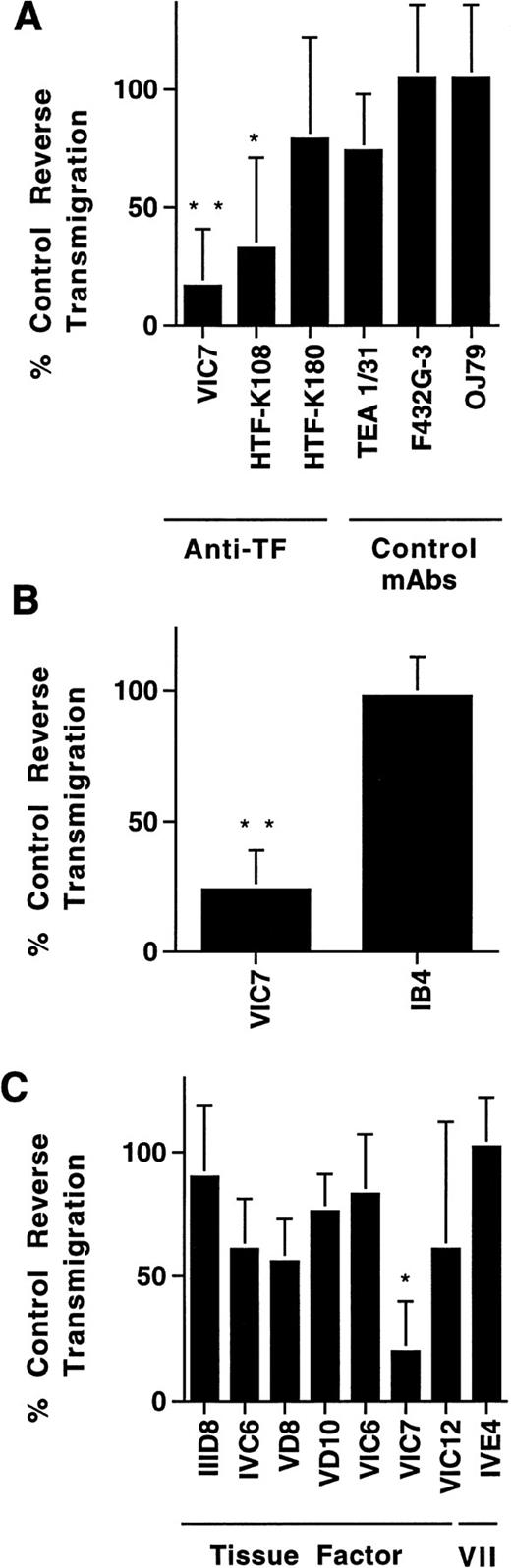
Effect of MoAbs against TF on reverse transendothelial migration of MP. PBMC were incubated with unstimulated HUVEC grown on collagen gels for 2 hours to allow accumulation of monocytes in the subendothelial collagen. Then cultures were washed to remove nonadherent cells, and fresh medium with or without MoAbs (20 μg/mL) was added. After 24 hours, the cultures were rinsed, and MoAb preparations were replenished. Cocultures of MP and HUVEC were incubated for a total of 48 hours, and then analyzed for reverse transmigration. Data are plotted relative to the percentage of reverse transmigration observed in the absence of added MoAb. Anti-TF MoAbs VIC7, HTF-K108, and HTF-K180 (all IgG1), and a variety of other IgG1 MoAbs, including TEA 1/31 against cadherin 5, F432G-3 against S-endo-4, and OJ79 against MUC18, were tested in a single screening experiment (A). The effect of anti-TF MoAb VIC7 was compared with that of anti-CD11/CD18 MoAb IB4 in three experiments (B). Another group of anti-TF MoAbs and an anti–factor VII MoAb were also evaluated in three experiments (C). Statistical differences relative to controls with no MoAb are denoted as (*), P < .02; (**), P < .005.
Effect of MoAbs against TF on reverse transendothelial migration of MP. PBMC were incubated with unstimulated HUVEC grown on collagen gels for 2 hours to allow accumulation of monocytes in the subendothelial collagen. Then cultures were washed to remove nonadherent cells, and fresh medium with or without MoAbs (20 μg/mL) was added. After 24 hours, the cultures were rinsed, and MoAb preparations were replenished. Cocultures of MP and HUVEC were incubated for a total of 48 hours, and then analyzed for reverse transmigration. Data are plotted relative to the percentage of reverse transmigration observed in the absence of added MoAb. Anti-TF MoAbs VIC7, HTF-K108, and HTF-K180 (all IgG1), and a variety of other IgG1 MoAbs, including TEA 1/31 against cadherin 5, F432G-3 against S-endo-4, and OJ79 against MUC18, were tested in a single screening experiment (A). The effect of anti-TF MoAb VIC7 was compared with that of anti-CD11/CD18 MoAb IB4 in three experiments (B). Another group of anti-TF MoAbs and an anti–factor VII MoAb were also evaluated in three experiments (C). Statistical differences relative to controls with no MoAb are denoted as (*), P < .02; (**), P < .005.
Screening of a large panel of MoAbs (>100) submitted to the VIth International Workshop on Human Leukocyte Differentiation Antigens revealed two MoAbs against TF that inhibited reverse transmigration strongly for at least 48 hours (Fig 2A). In comparison, 55 other isotype-matched MoAbs tested, including HTF-K180 against TF, had little to no effect. A MoAb against p-glycoprotein (MRK16; IgG2a) also specifically inhibited reverse transmigration.29 Anti-TF MoAb VIC7 impeded reverse transmigration by 77 ± 22% in 12 experiments. A direct comparison of the effect of VIC7 against a neutralizing MoAb to the integrin β2 subunit, IB4, revealed no inhibition by the latter in the same three experiments in which VIC7 prevented reverse transendothelial migration by 78 ± 15% (Fig 2B).
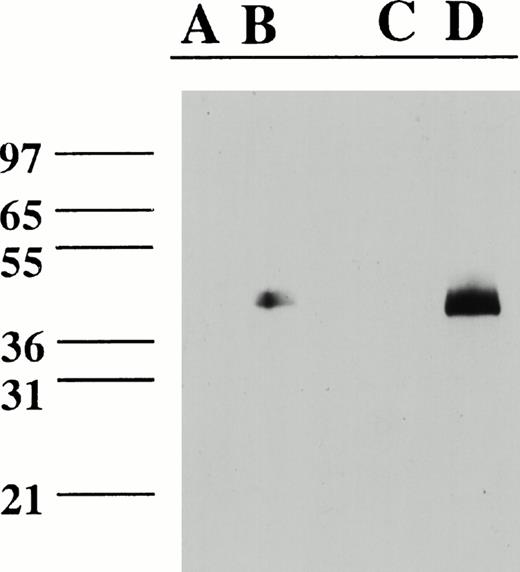
Examination of a larger panel of anti-TF MoAbs showed that VIC7 was by far the most potent inhibitor of reverse transmigration, although a few others inhibited modestly (Fig 2C). In contrast, anti–factor VII(a) MoAbs IVE4 or IIH2 did not impede reverse transmigration (Fig 2C). None of the MoAbs affected the total number of live cells in the cultures, as assessed by adding the number of reverse-transmigrated MP collected from above the endothelium to the number that remained beneath the endothelium. All of the MoAbs used in the panel of anti-TF MoAbs recognize 47-kD purified TF apoprotein in immunoblotting analysis and are effective inhibitors of PCA.22 Epitope mapping studies show that the epitopes for VIC7, VD10, IVC6, and VIC6 include recognition of at least some of the amino acids between 181-214 or 175-202, whereas IIID8, VD8, and VIC12 recognize epitopes that are contained in the first 25 amino acid residues of TF.26Because VIC7 was the most potent inhibitor in the panel, its specificity for TF was examned further by immunoblotting cell extracts prepared from LPS-stimulated monocytes and HUVEC. VIC7 recognized a single band of 47 kD in the LPS-stimulated cells, but not in the unstimulated cell extracts, consistent with the induction of TF by LPS (Fig 3).
Immunoblot of HUVEC and monocyte cell extracts with VIC7. Lanes were loaded with cell extracts from unstimulated HUVEC (A); LPS-stimulated HUVEC (B); freshly isolated, unstimulated monocytes (C); and LPS-stimulated monocytes (D). Lanes contained approximately 40 mg (HUVEC) or 20 mg (monocytes) total protein. A blank lane separates (B) and (C).
Immunoblot of HUVEC and monocyte cell extracts with VIC7. Lanes were loaded with cell extracts from unstimulated HUVEC (A); LPS-stimulated HUVEC (B); freshly isolated, unstimulated monocytes (C); and LPS-stimulated monocytes (D). Lanes contained approximately 40 mg (HUVEC) or 20 mg (monocytes) total protein. A blank lane separates (B) and (C).
Most MoAbs in this panel contained levels of LPS at approximately 0.2 to 1.0 endotoxin U/mL at a MoAb concentration of 20 μg/mL. When tested in the present study, another control MoAb IH8 against carcinoembryonic antigen, containing 1.0 endotoxin U/mL, did not prevent reverse transmigration. Exogenous addition of LPS to cultures at concentrations as high as 5 endotoxin (E) U/mL also did not block (data not shown).
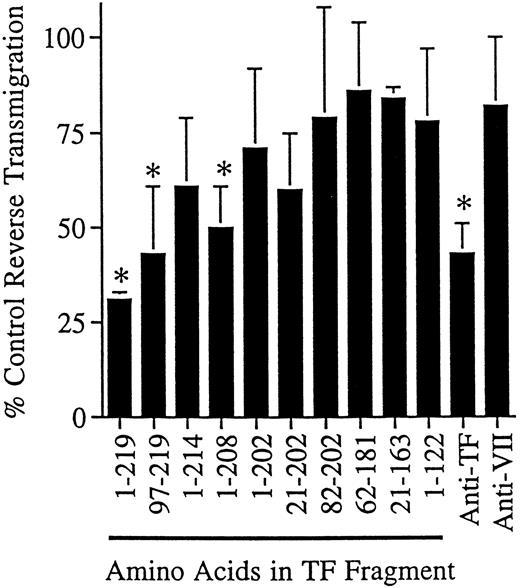
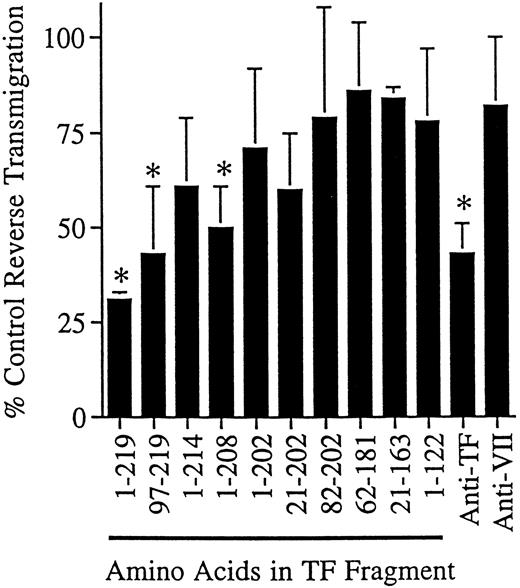
We also conducted reverse transmigration assays in the presence of soluble, recombinant forms of human TF representing the complete amino acid sequence of the extracellular region or fragments thereof, expressed in E coli and used in delipidated form. When the fragments were used at 5 to 20 μg/mL and assayed at 24-hour intervals, greater than 50% of the soluble recombinant TF fragment (residues 1-219) could be recovered in the supernatant of the cultures, as quantitated by ELISA,22 indicating that the fragments were not degraded or consumed during incubation. Soluble TF impeded reverse transmigration by 69 ± 2% in eight independent experiments (Fig 4). Only fragments containing amino acid residues carboxyl to residue 202 blocked reverse transmigration effectively (Fig 4). This finding agrees well with the location of the epitope for VIC7, which is between amino acids 181-214.26
Effect of soluble TF fragments on reverse transendothelial migration of MP. A 48-hour reverse transmigration assay was conducted as described in Fig 2. After 2 hours of incubation during which monocytes accumulated beneath endothelial cell cultures, medium containing anti-TF MoAb VIC7 (10 μg/mL), anti-VII MoAb IIH2 (10 μg/mL), soluble TF representing the full extracellular domain (1-219), or partial fragments thereof was added to the cultures (5 μg/mL). Data are derived from three to eight independent experiments. Statistical differences relative to controls with no MoAb are denoted as (*), P < .05.
Effect of soluble TF fragments on reverse transendothelial migration of MP. A 48-hour reverse transmigration assay was conducted as described in Fig 2. After 2 hours of incubation during which monocytes accumulated beneath endothelial cell cultures, medium containing anti-TF MoAb VIC7 (10 μg/mL), anti-VII MoAb IIH2 (10 μg/mL), soluble TF representing the full extracellular domain (1-219), or partial fragments thereof was added to the cultures (5 μg/mL). Data are derived from three to eight independent experiments. Statistical differences relative to controls with no MoAb are denoted as (*), P < .05.
Expression of tissue factor in MP/HUVEC cocultures.
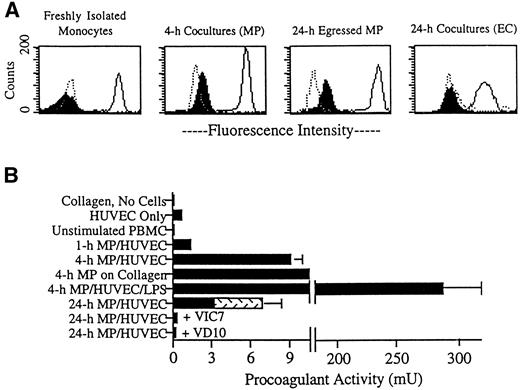
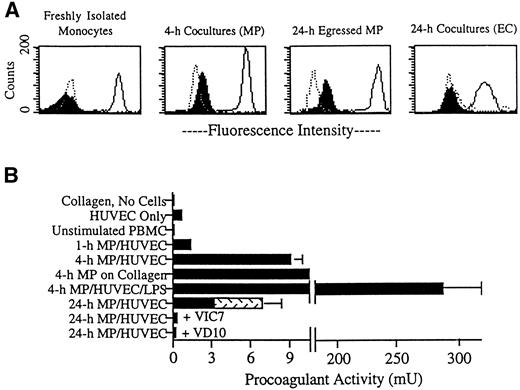
We next sought to examine the expression of TF in MP/HUVEC cocultures. TF was not detected on freshly isolated monocytes, as examined by flow cytometry (Fig 5A). At various time points after transmigration, cells were separated from the collagen by digestion with collagenase and then incubated with MoAbs for analysis. TF was induced on the surfaces of MP by 4 hours of coculture with endothelium (Fig 5A), but was not detected on MP after 1 hour of coincubation (not shown). After 24 hours, TF remained present on MP residing in subendothelial collagen (not shown), as well as on MP that had egressed from the cultures by reverse transmigration (Fig 5A). These 24-hour egressed MP did not require digestion for removal from the cultures (Fig 5A). However, as a control, some 24-hour egressed MP were treated with collagenase. This treatment did not change the expression of TF (not shown). In contrast to its expression on MP, TF was not observed on endothelial cell surfaces at any time (Fig 5A). In most experiments, staining for TF was done using the MoAb VD10 against TF. However, similar staining patterns were observed using the other MoAbs to TF.
Expression of TF in MP/HUVEC cocultures. (A) Endothelial cells cultured alone or endothelial cells coincubated with MP for 4 or 24 hours were treated thereafter with collagenase to prepare single-cell suspensions. For other samples, reverse-transmigrated MP were collected from the apical surface of intact cocultures (24-hour egressed MP). The latter did not require collagenase for removal. Aliquots of these suspensions were stained for flow cytometry using MoAbs against vascular cell adhesion molecule 1 (4B9; negative control for MP), cadherin 5 (hec 1; positive control for HUVEC), CD14 (3C10; positive control for MP, negative control for HUVEC), and TF (VD10). Dotted line profiles represent respective negative controls, solid lines indicate positive controls, and filled profiles correspond to cells stained for TF. MP and HUVEC were separated for analysis by gating on their distinct forward-scatter and side-scatter profiles. (B) The presence of TF in the cultures was also assessed by measuring PCA. Plots represent the mean ± SD of PCA in three to six individual 96-well cultures. Data are representative of four experiments. After incubation of monocytes with plain collagen gels or endothelial monolayers grown on collagen for 1 hour in the absence of added MoAb, cultures were washed to remove nonadherent lymphocytes. Then 20% FBS/M199 was added, with inclusion of LPS (1 ng/mL), VIC7 (20 μg/mL), or VD10 (20 μg/mL) in some samples. Individual cultures contained approximately 50,000 HUVEC on a 50-μL collagen gel and, when present, about 50,000 MP. Accordingly, PCA shown for unstimulated PBMC represents the activity observed in 50,000 peripheral blood monocytes. At 24 hours, PCA derived from cells remaining in the collagen gel was assessed separately from PCA in reverse-transmigrated MP collected from the same cultures. PCA from reverse-transmigrated MP is indicated by the stippled portion of the bar. For some samples, the SD was too low to be visible in the constructed graphs. PCA detected in HUVEC cultured alone was statistically increased over PCA in mock cultures of collagen gels lacking cells (collagen was coated with fibronectin and incubated in 20% FBS/M199 in similar fashion as the other cultures), P < .005. PCA detected in 4-hour and 24-hour cocultures of MP and HUVEC in the absence of added MoAb was significantly greater than the activity in either HUVEC alone or unstimulated PBMC, P < .005.
Expression of TF in MP/HUVEC cocultures. (A) Endothelial cells cultured alone or endothelial cells coincubated with MP for 4 or 24 hours were treated thereafter with collagenase to prepare single-cell suspensions. For other samples, reverse-transmigrated MP were collected from the apical surface of intact cocultures (24-hour egressed MP). The latter did not require collagenase for removal. Aliquots of these suspensions were stained for flow cytometry using MoAbs against vascular cell adhesion molecule 1 (4B9; negative control for MP), cadherin 5 (hec 1; positive control for HUVEC), CD14 (3C10; positive control for MP, negative control for HUVEC), and TF (VD10). Dotted line profiles represent respective negative controls, solid lines indicate positive controls, and filled profiles correspond to cells stained for TF. MP and HUVEC were separated for analysis by gating on their distinct forward-scatter and side-scatter profiles. (B) The presence of TF in the cultures was also assessed by measuring PCA. Plots represent the mean ± SD of PCA in three to six individual 96-well cultures. Data are representative of four experiments. After incubation of monocytes with plain collagen gels or endothelial monolayers grown on collagen for 1 hour in the absence of added MoAb, cultures were washed to remove nonadherent lymphocytes. Then 20% FBS/M199 was added, with inclusion of LPS (1 ng/mL), VIC7 (20 μg/mL), or VD10 (20 μg/mL) in some samples. Individual cultures contained approximately 50,000 HUVEC on a 50-μL collagen gel and, when present, about 50,000 MP. Accordingly, PCA shown for unstimulated PBMC represents the activity observed in 50,000 peripheral blood monocytes. At 24 hours, PCA derived from cells remaining in the collagen gel was assessed separately from PCA in reverse-transmigrated MP collected from the same cultures. PCA from reverse-transmigrated MP is indicated by the stippled portion of the bar. For some samples, the SD was too low to be visible in the constructed graphs. PCA detected in HUVEC cultured alone was statistically increased over PCA in mock cultures of collagen gels lacking cells (collagen was coated with fibronectin and incubated in 20% FBS/M199 in similar fashion as the other cultures), P < .005. PCA detected in 4-hour and 24-hour cocultures of MP and HUVEC in the absence of added MoAb was significantly greater than the activity in either HUVEC alone or unstimulated PBMC, P < .005.
The presence of TF activity in cultures of MP and HUVEC was also evaluated using a one-stage clotting assay (Fig 5B). PCA was not detected in freshly isolated PBMC. In contrast, a low, but statistically significant, level of PCA was measured in endothelial cell cultures (0.7 ± 0.2 mU in HUVEC cultured on collagen, compared with <0.1 mU in collagen gels without cells). PCA was increased by an order of magnitude when the HUVEC monolayers were cocultured with MP. An increase in PCA of similar magnitude was also observed when monocytes were incubated with collagen gels (containing no detectable LPS) in the absence of endothelium (Fig 5B; 4-hour MP on collagen). This upregulation of PCA was sustained for at least 24 hours. At 24 hours, nearly half of the total PCA was recovered in the population of reverse-transmigrated MP (Fig 5B, stippled portion of bar in 24-hour cocultures). This population was comprised of 15,000 to 17,000 MP of the approximately 50,000 total MP that initially migrated into the collagen in each culture. In cultures that received MoAbs just after monocytes transmigrated into the subendothelial matrix (1 hour), PCA subsequently detected at 24 hours was inhibited by greater than 90% by all anti-TF MoAbs (Fig 5B, VIC7 and VD10 are shown) and by 50% using anti-VII(a) MoAbs. These data show that all anti-TF MoAbs had equivalent access to and retention in MP/HUVEC cocultures. Because anti-VII(a) MoAb did not completely inhibit PCA, we cannot eliminate a role for VII(a)-mediated activity in reverse transmigration. However, among the anti-TF MoAbs, there was no correlation between inhibition of PCA and their effect on reverse transmigration. Addition of LPS to the cocultures at 1 hour increased PCA by nearly 300-fold (Fig 5B). More than 99% of this activity was inhibited by preincubating LPS-treated cultures with VIC7 or VD10 before conducting the clotting assay (not shown).
Role of tissue factor in interactions between mononuclear phagocytes and endothelium.
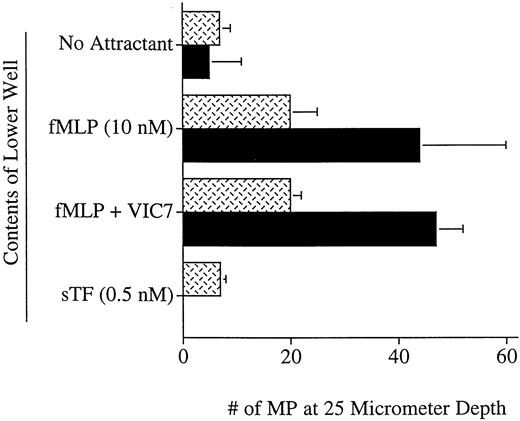
TF, including nonlipidated recombinant soluble TF, possesses chemotactic activity for smooth muscle cells.30 TF on MP might also be required for general motility or adhesion. Therefore, chemotaxis assays measuring migration into collagen gels or migration across uncoated or collagen-coated cellulose nitrate filters were conducted. Freshly isolated monocytes or reverse-transmigrated MP failed to migrate chemotactically toward concentrations of soluble TF ranging from 1 pmol/L to 1.000 nmol/L, including 0.5 nmol/L (Fig 6), which is optimally chemotactic for smooth muscle cells,30 and 800 nmol/L (not shown), which was maximally effective at blocking reverse transmigration. Moreover, migration toward FMLP in nitrocellulose filters was unaffected by anti-TF MoAb VIC7 (Fig 6). FMLP-stimulated penetration of MP into collagen gels, prepared as for transmigration assays but without endothelium, also was not affected by MoAb VIC7 or soluble recombinant TF (data not shown). These data indicate that TF is neither chemotactic for MP nor essential for their migration on or through collagen.
Chemotaxis assay using soluble TF and anti-TF MoAb. Lower wells of blind-well chambers were filled with control medium (No Attractant) or medium containing 10 nmol/L FMLP, 10 nmol/L FMLP + 10 μg/mL anti-TF MoAb VIC7, or 0.5 nmol/L soluble TF. Reverse-transmigrated MP collected from the apical aspect of 24-hour cocultures with endothelium were resuspended in control medium with or without added VIC7 (VIC7 was included in the apical compartment when it was also used in the basal compartment) and added to the upper chamber of chemotaxis wells. Stippled bars depict results obtained using uncoated 5.0-μm cellulose nitrate filters; solid bars show results using 8.0-μm collagen-coated filters. Data are representative of six experiments.
Chemotaxis assay using soluble TF and anti-TF MoAb. Lower wells of blind-well chambers were filled with control medium (No Attractant) or medium containing 10 nmol/L FMLP, 10 nmol/L FMLP + 10 μg/mL anti-TF MoAb VIC7, or 0.5 nmol/L soluble TF. Reverse-transmigrated MP collected from the apical aspect of 24-hour cocultures with endothelium were resuspended in control medium with or without added VIC7 (VIC7 was included in the apical compartment when it was also used in the basal compartment) and added to the upper chamber of chemotaxis wells. Stippled bars depict results obtained using uncoated 5.0-μm cellulose nitrate filters; solid bars show results using 8.0-μm collagen-coated filters. Data are representative of six experiments.
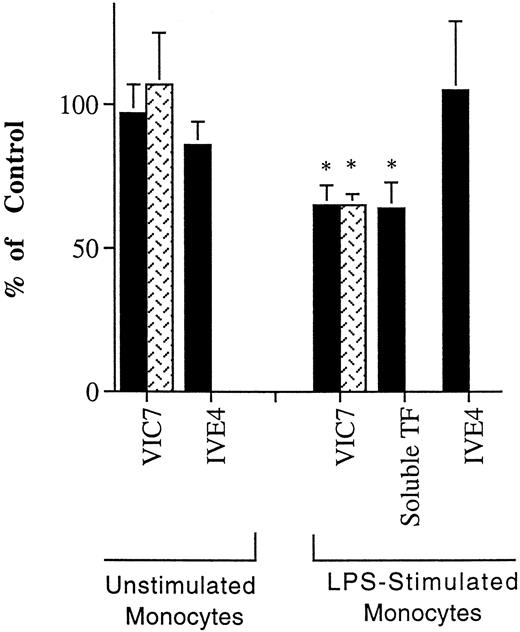
Other experiments suggest that TF has a role in adhesion. The effect of MoAb VIC7 on the initial interactions of monocytes with the apical surface of the endothelium was evaluated using resting monocytes, which do not express TF, and monocytes induced to express TF by stimulation with LPS. VIC7 inhibited the adhesion of LPS-stimulated, but not resting, monocytes to unstimulated or TNF-activated HUVEC by 35 ± 7% in four experiments. LPS-stimulated monocytes that were not prevented from adhering in the presence of MoAb VIC7 migrated across the endothelium, as observed by microscopic examination. These data suggest that TF is involved in adhesion but does not affect subsequent steps in apical-to-basal transendothelial migration. Recombinant soluble TF inhibited this adhesion similarly, by 36 ± 9% in three experiments (Fig 7).
Effect of anti-TF MoAb on adhesion of unstimulated and LPS-stimulated monocytes to endothelium. Unstimulated monocytes or LPS-stimulated monocytes were incubated with resting (black bars) or TNF-activated (stippled bars) HUVEC grown on collagen for 1 hour in medium without MoAb, or medium containing anti-TF MoAb VIC7, anti-factor VII MoAb IVE4 (tested using unstimulated HUVEC only) at 10 μg/mL, or soluble recombinant TF (tested using unstimulated HUVEC only; 20 μg/mL). The extent of binding was compared with the number of monocytes that bound in the absence of added MoAb. The reduced binding of LPS-stimulated monocytes observed in the presence of VIC7 or recombinant TF is statistically different from the controls without MoAb, P < .02 (*).
Effect of anti-TF MoAb on adhesion of unstimulated and LPS-stimulated monocytes to endothelium. Unstimulated monocytes or LPS-stimulated monocytes were incubated with resting (black bars) or TNF-activated (stippled bars) HUVEC grown on collagen for 1 hour in medium without MoAb, or medium containing anti-TF MoAb VIC7, anti-factor VII MoAb IVE4 (tested using unstimulated HUVEC only) at 10 μg/mL, or soluble recombinant TF (tested using unstimulated HUVEC only; 20 μg/mL). The extent of binding was compared with the number of monocytes that bound in the absence of added MoAb. The reduced binding of LPS-stimulated monocytes observed in the presence of VIC7 or recombinant TF is statistically different from the controls without MoAb, P < .02 (*).
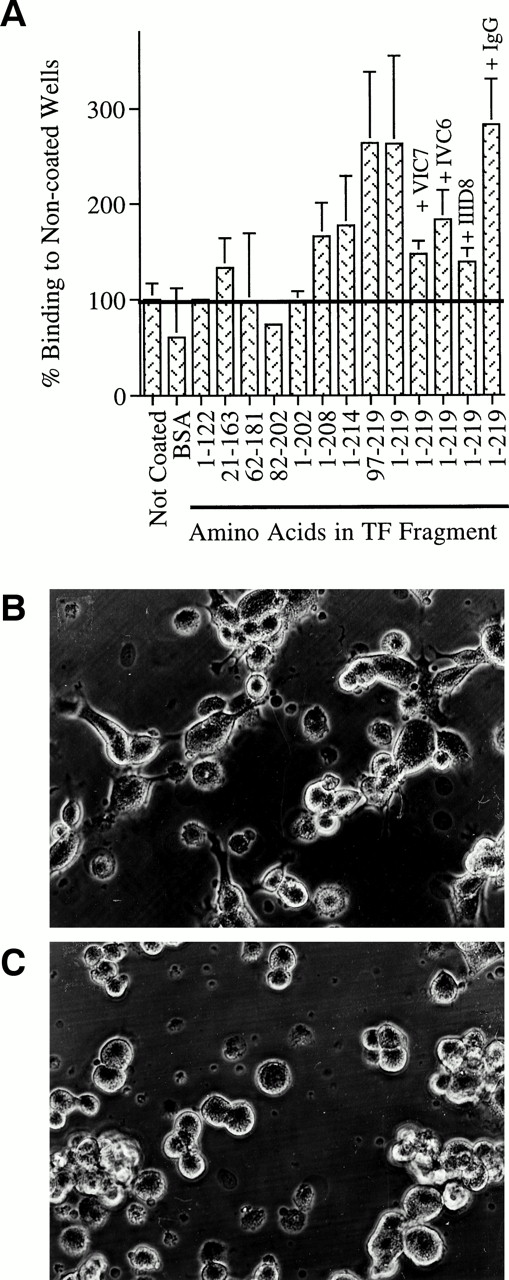
The former experiments are consistent with a model in which MP-expressing TF use it to bind to a ligand on the endothelium. Therefore, experiments were conducted to explore whether such a ligand might exist. Soluble TF fragments were immobilized in microtiter wells. ELISA plates were used for these studies, instead of traditional tissue culture-treated plates, because these plates support optimal protein binding but little background adhesion of HUVEC. Unstimulated HUVEC were added to wells coated with TF or control proteins in the presence or absence of anti-TF MoAb for 2 hours. After 2 hours of incubation, endothelial cell adhesion to TF fragments containing amino acid residues 202-219 was greater than their binding to control surfaces or to TF fragments lacking these residues (Fig 8A). Spreading of HUVEC during the first 2 hours was observed on surfaces coated with TF fragments 97-219 (Fig 8B) or 1-219 (not shown), but to a much lesser extent on surfaces coated with BSA (not shown) or the TF fragment spanning amino acids 1-122 (Fig 8C). Together, these data identify a region of TF between amino acid residues 202-219 that supports accelerated adhesion and spreading of endothelial cells. Endothelial cell binding to control surfaces was significantly lower than binding to fragments containing this region of TF, until after more than 5 hours of incubation when cells adhered well to all surfaces. Together, these data suggest that endothelial cells express binding sites for TF.
Binding of endothelium to surfaces coated with fragments of soluble TF. Fragments representing different regions of TF were immobilized in microtiter wells. Endothelial cells were added in the presence or absence of anti-TF MoAb (VIC7, IVC6, IID8) or control MoAb (IgG). Cultures were incubated for 2 hours, washed, and the number of adherent cells was quantitated. The extent of binding to fragments was compared with the extent of binding to noncoated wells or wells coated with BSA (A). Photomicrographs of endothelial cells incubated for 2 hours in wells coated with a TF fragment representing amino acids 97-219 (B) or a fragment representing amino acids 1-122 (C). Photographs were taken before cultures were washed to remove nonadherent cells. These illustrate that TF fragment 97-219 supports spreading of EC, but fragment 1-122 does not. Original magnification × 150. Results are the mean of at least three independent experiments that were conducted using six replicates per parameter tested. HUVEC binding to fragments 1-219 and 97-219 was increased to a statistically signifcant degree over binding to control surfaces, P < .05. The block in binding to the full-length fragment by MoAb VIC7 and IID8 was also significantly lower than binding without addition of MoAb or in the presence of control IgG, P < .05.
Binding of endothelium to surfaces coated with fragments of soluble TF. Fragments representing different regions of TF were immobilized in microtiter wells. Endothelial cells were added in the presence or absence of anti-TF MoAb (VIC7, IVC6, IID8) or control MoAb (IgG). Cultures were incubated for 2 hours, washed, and the number of adherent cells was quantitated. The extent of binding to fragments was compared with the extent of binding to noncoated wells or wells coated with BSA (A). Photomicrographs of endothelial cells incubated for 2 hours in wells coated with a TF fragment representing amino acids 97-219 (B) or a fragment representing amino acids 1-122 (C). Photographs were taken before cultures were washed to remove nonadherent cells. These illustrate that TF fragment 97-219 supports spreading of EC, but fragment 1-122 does not. Original magnification × 150. Results are the mean of at least three independent experiments that were conducted using six replicates per parameter tested. HUVEC binding to fragments 1-219 and 97-219 was increased to a statistically signifcant degree over binding to control surfaces, P < .05. The block in binding to the full-length fragment by MoAb VIC7 and IID8 was also significantly lower than binding without addition of MoAb or in the presence of control IgG, P < .05.
The fragments that promoted adhesion of HUVEC parallel those that block reverse transmigration (compare Fig 8A to Fig 4). All anti-TF MoAbs added to these incubations (VIC7, IVC6, and IID8), recognizing different epitopes of TF, inhibited HUVEC binding to immobilized, full-length TF (Fig 8A). However, because IVC6 and IID8 recognize regions of TF that do not include amino acids 202-219,26 in contrast to VIC7, their inhibitory effect in this assay must occur by steric hindrance.
DISCUSSION
Using an in vitro model, we have identified a role for TF in basal-to-apical transendothelial migration of MP. Monoclonal antibody VIC7 against TF and soluble recombinant TF each strongly inhibited this process, termed reverse transmigration, for at least 48 hours. Identification of TF as a long-term mediator of reverse transmigration in vitro will permit future exploration in vivo to determine whether the molecular mechanism of basal-to-apical transendothelial migration in vitro is predictive of the molecular events that mediate reverse transmigration processes in vivo. Although the endothelial cells used in this model are of vascular origin, this reverse transmigration model appears to mimic at least some important aspects of lymphatic clearance. Namely, a recent study has shown that p-glycoprotein, another molecular mediator of reverse transmigration in this model, also mediates lymphatic trafficking of human epidermal dendritic cells and T lymphocytes.29 Moreover, reverse-transmigrated MP collected from our cultures express phenotypic characteristics that are similar to cells recovered from lymph.31 That venular endothelial cells appear to mimic at least some aspects of lymphatic vessels in our cultures may be related to the known loss of specialized function in cultured endothelial cells32 and to the lack, in our model, of other typical cellular features of a blood vessel wall, including pericytes and smooth muscle cells. As in our cultures, lymphatic vessels in vivo are not surrounded by interstitial cells,33 as are blood vessels. Experiments are currently under way to determine whether TF mediates reverse transmigration across lymphatic endothelium and whether it mediates ablumenal-to-lumenal migration of macrophage-derived foam cells across arterial endothelium in atherosclerosis.
The expression of TF in atherosclerotic lesions is consistent with a potential role in reverse transmigration from these lesions. As observed using immuno-electron microscopy on atherosclerotic lesions in pigeons, TF is expressed on macrophage-derived foam cells projecting between endothelial cells and into the arterial lumen as they are apparently migrating from the subendothelium.14 However, it is not expressed by normocholesterolemic MP. Interestingly, the authors of this study note that ablumenal-to-lumenal transendothelial migration of foam cells appears to be associated with regression of atherosclerotic lesions and that this regression is, in turn, associated with a transient increase in PCA on the lesions.14
In general agreement with the present study, others have previously reported induction of TF on monocytes cocultured with endothelium.34-36 Our experiments suggest that interactions of MP with collagen is sufficient to induce TF expression. We observed a sustained, albeit relatively low, expression of TF for at least 24 hours. These kinetics are similar to those observed by Collins and coworkers.35 However, others describe expression of TF that peaks at a high level at 4 hours but is greatly reduced by 24 hours.34,36 Perhaps interactions of MP with the collagenous matrix itself results in signals that modify the kinetics of TF expression. Sustained expression of TF also appears to occur in vivo, as it is observed on resting peritoneal murine macrophages37 and is a marker for differentiation of monocytes to alveolar macrophages in rabbits.38
Because not all anti-TF MoAbs that inhibit coagulation block reverse transmigration, the role of TF in reverse transmigration may be independent of its procoagulative functions. However, we were not able to inhibit PCA fully with anti-VII(a) MoAbs, leaving open the possibility that VII(a) may play a role in this migration. Although the exact mechanism by which TF mediates reverse transmigration is not known, our experiments imply that TF on MP is involved in an adhesive interaction. First, we found no evidence that TF acted as a chemoattractant for MP, nor did anti-TF MoAb inhibit their migration through collagen. Second, the finding that both anti-TF MoAb and soluble TF inhibit reverse transmigration is consistent with a model in which the binding of endogenous TF on MP to a putative endothelial ligand is prevented by anti-TF MoAb and is competed with by soluble recombinant TF. Third, we observed that, under conditions in which monocytes express TF, anti-TF MoAb and soluble TF each inhibited adhesion to the apical surface of the endothelial cells to a modest but significant extent. Finally, we found that HUVEC would bind to soluble TF fragments containing at least amino acid residues 202-208. Fragments lacking these amino acids did not support adhesion. Best adhesion was observed using fragments that extended to residue 219. These data indicate that binding sites for TF are present on endothelium.
Fragments of TF that blocked reverse transmigration closely paralleled those that supported endothelial cell adhesion. However, whereas only MoAb VIC7 inhibited reverse transmigration strongly, all MoAbs tested inhibited adhesion of HUVEC to soluble TF. Based on their epitope specificities, only MoAb VIC7 could be binding directly to the critical epitope to block adhesion of HUVEC to soluble TF. The other MoAbs clearly blocked adhesion of HUVEC to TF by steric hindrance. Because reverse transmigration assays are lengthy (48 hours), in contrast to endothelial cell binding assays (2 hours), only a MoAb that binds directly to the functional epitope with high affinity might be expected to show inhibitory activity. Such a rationale might explain why MoAb VIC7, but not other MoAbs, effectively inhibits reverse transmigration.
The effect of anti-TF MoAb on adhesion of LPS-stimulated monocytes to the apical surface of endothelium is more modest than the inhibitory effect of the MoAb on reverse transmigration. Possibly, an endothelial cell ligand for TF is more abundantly expressed on the ablumenal surface. Alternatively, there may be a difference in the number of adhesive interactions available in the two circumstances. Reverse transmigration may be mediated by very few adhesion molecules, whereas monocytes can use multiple adhesive mechanisms to bind to the apical surface of endothelium, so that antagonizing any single pathway does not lead to inhibition of a large magnitude.39 Under conditions in which circulating monocytes would be activated to express TF, including exposure to LPS, many of these adhesive cascades that mediate binding of monocytes to endothelium are activated, and the effect of inhibiting only one involving TF would not likely be extensive. Nevertheless, the finding that expression of TF promotes the adhesion of cells to the apical (lumenal) surface of endothelium suggests that this phenomenon may have pathophysiologic consequences in addition to its role in reverse transendothelial migration.
In models of metastasis, tissue factor has a dual role. One of these roles clearly requires its procoagulant activity,8 but the other is dependent on residues in the cytoplasmic tail8,9,40 and may be independent of prothrombinase activity. Ligation of TF results in binding of its cytoplasmic tail to actin binding protein 280. This interaction, in turn, facilitates adhesion and spreading of TF+ cells, possibly via interaction of a TF-VII(a) complex with cell surface- or matrix-bound tissue factor pathway inhibitor (TFPI) or TFPI-2.40 On the other hand, functional VII(a) may not be required for actin binding protein recruitment, because a similar result can be obtained with surrogate ligands.40 Plasminogen is a newly described ligand for TF, which binds to cellular TF or TF apoprotein.41 This binding interaction occurs at a site distinct from the VII(a) binding region of TF and results in activation of plasminogen to plasmin. Both TFPI42 and plasminogen41 are presented on the endothelial cell surface where they might serve as appropriate ligands for mediating reverse transmigration. Thus, the possibility that these molecules or an as-yet-undescribed ligand for TF mediates reverse transmigration awaits future exploration.
ACKNOWLEDGMENT
We thank Drs Yale Nemerson and Arabinda Guha for generously providing recombinant tissue factor and Drs Samuel Wright, John Harlan, and James Young for gifts of MoAbs. We are indebted to the staff at Mt Sinai Medical Center and the New York Blood Center for supplying umbilical cords, and thank Ronald Liebman, Tricia Greene, and Elizabeth Polizzi for assistance with endothelial cell culture. We extend additional thanks to Dr Nemerson for critical reading of the manuscript.
Supported by NRSA grant HL09722 to G.J.R, and grant HL46849 to W.A.M., who is an Established Investigator of the American Heart Association.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Gwendalyn J. Randolph, PhD, 1300 York Ave, Department of Pathology, Cornell University Medical College, New York, NY 10021; e-mail: GJRandol@mail.med.cornell.edu.