To the Editor:
A novel hematopoietic stem and progenitor cell marker, monoclonal antibody (MoAb) AC133, was recently published by Miraglia et al1 and Yin et al2 in the December 15, 1997 issue of Blood. AC133 recognizes only CD34 bright and CD38− subsets of human progenitor cells including colony-forming unit granulocyte macrophage (CFU-GM) needed for short-term engraftment and probably the severe combined immunodeficient (SCID) mouse repopulating cell.3 Investigators could detect AC133 in a majority of CD34+ cases of acute leukemia, suggesting that it may be an early marker for human progenitor cells. In contrast, they could not detect AC133 in the CD34− leukemic blasts of 1 patient with acute myelogenous leukemia (AML) or in any of 5 human AML cell lines, although expression was found in 3 nonhematopoietic cell lines of human origin.
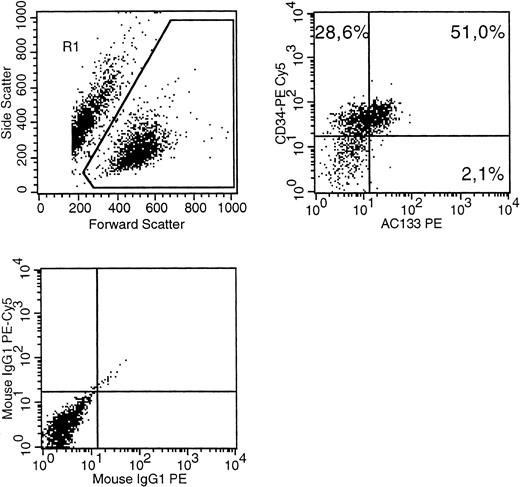
We investigated several human cell lines by multiparameter immunophenotyping using AC133-PE (Miltenyi, Bergisch Gladbach, Germany) and CD34-Cy5, CD90-FITC (Immunotech, Marseille, France). We found that a majority of cells of MUTZ-2 coexpressed CD34 and AC133 (Fig 1). Mutz-2 is a cell line derived from AML and displays an FAB:M2 morphology.4 This is the first known human hematopoietic cell line expressing AC133, representing a possible additional tool for experiments concerning the function of the AC133 receptor.
A MUTZ-2 cell sample was stained with CD34(PE-Cy5), AC133(PE) as well as isotype control antibodies conjugated with PE-Cy5 or PE. (Top left) The gate on forward versus side scatter (1) contained the leukemic blast cell population (10% probability dot plot). (Top right) Expression of CD34 and AC133 on gated (R1) cells is shown. (Bottom left) The pattern of IgG-PE-Cy5 and IgG-PE isotype MoAb expression on the R1 population.
A MUTZ-2 cell sample was stained with CD34(PE-Cy5), AC133(PE) as well as isotype control antibodies conjugated with PE-Cy5 or PE. (Top left) The gate on forward versus side scatter (1) contained the leukemic blast cell population (10% probability dot plot). (Top right) Expression of CD34 and AC133 on gated (R1) cells is shown. (Bottom left) The pattern of IgG-PE-Cy5 and IgG-PE isotype MoAb expression on the R1 population.
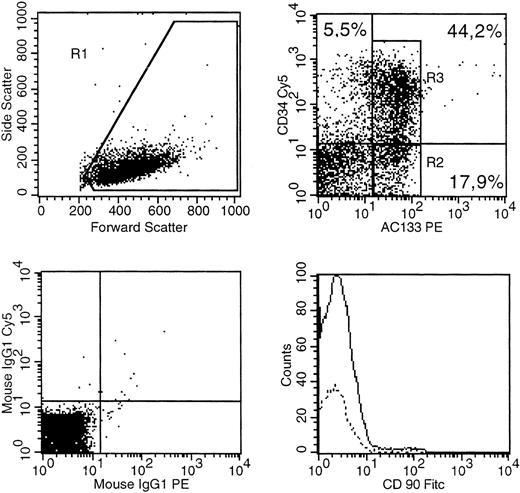
A bone marrow sample (53-year-old male AML:M4) was stained with CD34(PE-Cy5), AC133(PE). CD90 (FITC) as well as isotype control antibodies conjugated with PE-Cy5, PE, FITC. (Top left) The gate on forward versus side scatter (1) contained the leukemic blast cell population (10% probability dot plot). (Top right) Expression of CD34 and AC133 on gated (R1) cells is shown. (Bottom right) Expression of CD90 in R2 (dotted line) versus R3. (Bottom left) The pattern of IgG-PE-Cy5 and IgG-PE isotype MoAb expression on the R1 population.
A bone marrow sample (53-year-old male AML:M4) was stained with CD34(PE-Cy5), AC133(PE). CD90 (FITC) as well as isotype control antibodies conjugated with PE-Cy5, PE, FITC. (Top left) The gate on forward versus side scatter (1) contained the leukemic blast cell population (10% probability dot plot). (Top right) Expression of CD34 and AC133 on gated (R1) cells is shown. (Bottom right) Expression of CD90 in R2 (dotted line) versus R3. (Bottom left) The pattern of IgG-PE-Cy5 and IgG-PE isotype MoAb expression on the R1 population.
We also characterized expression of CD34 and AC133 in bone marrow blasts from 10 patients with AML. None of the six patients with a pure CD34 negative blast population had detectable levels of AC133+ blasts. On the other hand, we saw a 53-year-old male AML patient with blasts of FAB:M4 morphology and a high percentage of bone marrow infiltration (>90% leukemic blasts) who had a mixed population of CD34+ and CD34− blasts (Fig2). As expected, we found coexpression of CD34 and AC133 in 44% of the blast population. Only a very small population of leukemic blasts was AC133− and CD34+. Interestingly, we could find a significant blast population (18%) in which expression of AC133 was not accompanied by expression of CD34 or CD90 (Thy-1). This first report of a CD34−CD90−AC133+ leukemic blast population could represent clonal diversity of the blast population. On the other hand, it may suggest a possible differentiation pathway, wherein CD34−CD90−AC133+ cells give rise to CD34+AC133+ cells. Alternatively, the CD34+AC133+ blasts could be progenitors of the CD34−AC133+ cells seen in this patient. Therefore, our finding of a CD34−AC133+ blast population raises questions about the significance of AC133 expression in AML. A more detailed investigation of the role of AC133 in AML differentiation pathways needs to be done.