Abstract
Serine phosphorylation of bcl-2 has been reported after treatment of cells with protein kinase C, okadaic acid, taxol, and other chemotherapeutic agents that attack microtubules. We report here that bcl-2 is phosphorylated on serine in acute myeloblastic leukemia (AML) blasts exposed to all trans retinoic acid (ATRA). Two-dimension gels (isoelectric focusing followed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis [SDS-PAGE]) disclosed a novel acidic isoform of bcl-2 in ATRA-treated blast cells from a continuous line and from two AML patients; when the cell lysates were digested with λ-phosphatase, bcl-2 reverted to the control position, indicating that it was phosphorylated. Metabolic labeling experiments using32Pi showed that, while control bcl-2 was labeled, incorporation was greatly increased when cells were treated with ATRA. A comparison of bcl-2 from blasts treated with ATRA or taxol showed that bcl-2 was phosphorylated on serine in cells treated with either agent; however, both qualitative and quantitative differences were seen. Qualitatively, the phosphorylated isoform from taxol-treated cells was slightly larger than the native isoform and could be distinguished on 10% to 20% SDS-polyacrylamide gradient gels, while the phosphorylated bcl-2 after ATRA ran as a single band on gradient gels at the same position as control bcl-2. Quantitatively, all bcl-2 from ATRA-treated cells was in the phosphorylated isoform, while after taxol, both phosphorylated and native bcl-2 was present; incorporation of 32Pi into bcl-2 was stimulated to greater extent in ATRA-treated compared with taxol-treated cells. We used immunoprecipitation experiments to ask if bcl-2 phosphorylated after ATRA or taxol had altered capacity to dimerize with bax. No change in dimerization was demonstrated. We conclude that: bcl-2 is phosphorylated on serine after treatment of AML blasts with ATRA; bcl-2 phosphorylation after ATRA is different from that seen after taxol; bcl-2 phosphorylated after either agent retains capacity to dimerize with bax. The ATRA or taxol-induced phosphorylation of bcl-2 can also be seen in blast cells obtained from AML patients.
© 1998 by The American Society of Hematology.
CANCER CELLS RESPOND to many chemotherapeutic agents by programmed cell death or apoptosis.1-4 Apoptosis is regulated by a number of proteins or phospholipids that either promote cell death or protect against it.2,5,6 These regulators may affect the outcome of chemotherapy by changing the probabilities that cells will recover or complete apoptosis. Members of the bcl-2 family are prominent among the regulators of apoptosis.7-9 The family contains members that protect against cell death, such as bcl-210 and bcl-xL11; others, such as bax12 and bad13 promote apoptosis. Members of the bcl-2 family share two homology regions, BH1 and BH2, that allow them readily to form homo and heterodimers.14 Dimerization between bcl-2 family members with life and death promoting functions has been proposed as a mechanism for regulating apoptosis after injury, including exposure to chemotherapeutic agents.9 12
Posttranslational modification of bcl-2 family members by phosphorylation may also be important in the regulation of apoptosis. Haldar et al15 reported phosphorylation of bcl-2 after treatment with okadaic acid and suggested that the function of the protein was inhibited. Observations of bcl-2 phosphorylation after taxol treatment16 17 provided a link with chemotherapy, as inhibition of bcl-2 function might increase cell kill.
Phosphorylation may affect function of bcl-2 or bcl-xL by altering the capacity of the proteins to form dimers or act independently of dimerization. The three-dimensional structure of bcl-xL18 provides support for the latter possibility. The structure consists of two hydrophobic α-helices surrounded by amphipathic helices. In addition to these highly structured elements, both bcl-xL and bcl-2 have large unstructured protein loops. Chang et al19 have shown that deletion of this protein loop increases the antiapoptotic activity of bcl-xL and blocks okadaic acid-induced phosphorylation; heterodimerization with bax is not changed. Ito et al20have shown the phosphorylation of serine at position 70 of bcl-2 is required for its capacity to protect NSF/N1.H7 mouse myeloid cells from death after interleukin-3 (IL-3) deprivation, but does not alter its capacity to dimerize with bax. Thus, the studies of Chang et al and Ito et al provide examples of regulation of bcl-2 family members that are independent of dimerization.
We report here that serine phosphorylation of bcl-2 is seen in acute myeloblastic leukemia (AML) blast cells of the continuous line OCI/AML-5 and blasts obtained from two patients with AML. The work was undertaken because we had observed that treatment of AML blasts with all trans retinoic acid (ATRA) often increases their sensitivity to cytosine arabinoside (ara-C), a major chemotherapeutic drug used in the treatment of AML.21-23 In a search for mechanism, we found that ATRA decreased the expression of bcl-2 mRNA and the stability of the protein in OCI/AML-2 and OCI/AML-5 cells.24 25
We asked if the reduced half-life of bcl-2 seen after ATRA treatment might be associated with posttranslational modification of the protein. Two dimensional gels of lysates from cells treated with ATRA disclosed a novel acidic bcl-2 isoform. After digestion with λ-phosphatase, the acidic isoform became indistinguishable from the native bcl-2 from control cells, indicating that it was a phosphorylated form of the protein. We compared phosphorylated bcl-2 from AML cells treated with ATRA or taxol. Serine phosphorylation was seen after either agent, but quantitative and qualitative differences were seen in the phosphorylated bcl-2. Metabolic labeling with orthophosphate confirmed that bcl-2 phosphorylation was increased in AML blasts cells treated with either ATRA or taxol. Neither agent changed the capacity of bcl-2 to form dimers with bax, as measured in immunoprecipitation experiments. The work provides a further example of posttranslational modification of bcl-2 that is not associated with change in its capacity for dimerization.
MATERIALS AND METHODS
Cell culture.
The human leukemia cell line OCI/AML-5 established in this laboratory was used in the experiments. The cell line was grown in α-minimal essential medium (α-MEM) (GIBCO, Burlington, Canada) supplemented with 10% heat-inactivated fetal calf serum (FCS) (Sigma, St Louis, MO) (growth medium) and 10% medium conditioned by the bladder carcinoma line 5637 (5637-CM); the cells were incubated at 37°C in an atmosphere of 5% CO2 in air. Cells were harvested in the logarithmic growth phase for experiments. Cryopreserved blast cells from two patients were used, selected because Western blot analysis showed high bcl-2 levels. The cells were thawed and cultured in suspension in 5637-CM for 48 hours, then recultured, and exposed to either ATRA or taxol.
Reagents and antibodies.
ATRA (Sigma) was first dissolved in 100% ethanol at a concentration of 10−2 mol/L, and the solution further diluted to the final concentrations in the culture. The monoclonal antibodies against human bcl-2 used in the experiment are: (1) bcl-2 124 (DACO, Mississauga, Ontario, Canada); (2) 6C8, a gift from Dr S.J. Korsmeyer (Washington University, St Louis, MO). Rabbit polyclonal antibax (N-20) was purchased from Santa Cruz (Santa Cruz, CA) or was a gift from Dr D.W. Andrews (McMaster University, Hamilton, Ontario, Canada). 32Pi was obtained from Dupont (Mississauga, Ontario, Canada).
Western blot and immunoprecipitation.
Western blots were used to measure bcl-2 protein. Briefly, cells at a concentration of 5 × 106 cells/mL were lysed in lysis buffer (10 mmol/L tris, pH 7.4, 0.15 mol/L NaCl, 5 mmol/L EDTA, 1 mmol/L phenylmethyl sulfonyl fluoride [PMSF], 2 mg/mL aprotinin, 1% Triton X-100) for 1 hour at 4°C with freshly added protease inhibitors. The nuclei were removed by centrifugation. A total of 100 μg protein from each sample was solubilized with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer, and electrophoresed through 12.5% SDS-polyacrylamide gel for about 2 hours at room temperature. For immunoblotting, the proteins separated by SDS-PAGE were electrotransferred onto nitrocellulose filters (Amersham, Oakville, Canada). The filters with the proteins were blocked with phosphate-buffered saline (PBS) containing 2% nonfat milk and 0.1% Tween-20 (Sigma) and incubated with primary antibody for 2 hours. The filters were then incubated with horseradish peroxidase (HRP)-labeled secondary antibodies (Amersham) for another 2 hours. The filters were developed using enhanced chemiluminescent (ECL) detection systems (Amersham). For immunoprecipitation, the lysates from untreated and treated cells were precleared by adding 2 μg hamster IgG for anti–bcl-2 or rabbit IgG for anti-bax; 20 μL 50% (vol/vol) protein G-agarose was added for 1 hour at 4°C, followed by centrifugation to remove the protein G-agarose pellets. Specific antibody against human bcl-2 (6C8) or against bax (N-20) was added to the lysate and incubated for 2 hours, followed by incubation with 20 μL of 50% protein G-agarose for another 2 hours to capture the immunoprecipitates. The immunoprecipitates were then separated by 12.5% SDS-PAGE and electrotransferred onto nitrocellulose filters (Amersham). The bcl-2 protein on the filters was detected by the immunostaining method as described above for Western blotting. For gradient gels, equivalent amounts of protein (100 μg) from each cell lysate were separated by 10% to 20% gradient SDS-PAGE. After electrophoresis, the proteins were transferred to nitrocellulose filters, and Western blotting was performed as described above.
Two-dimensional (2D) gel analysis of bcl-2 protein.
2D gel electrophoresis was used to study the heterogeneity in bcl-2 protein from the leukemia cells. Proteins were first separated on the basis of charge by isoelectric focusing and then by size, using SDS-PAGE. Briefly, lysates from the cells treated under a number of conditions were separated by isoelectric focusing (IEF) using tube gels (4% acrylamide, 9.2 mol/L urea, 5% ampholyte, pH 4-8, 2% NP-40) (Resolyte, from BDH, Ltd, Poole, UK), with a Mini 2-D Electrophoresis Cell from Bio-Rad (Bio-Rad Laboratories Ltd, Mississauga, Ontario, Canada). IEF ran at 500 V for 6 hours. After the first-dimension IEF, the tube gels were removed from the glass tubes and loaded onto slab gels (12.5% SDS-polyacrylamide gel) for electrophoresis in the second dimension at 100 V for 2 hours. The proteins separated by SDS-PAGE were electrotransferred onto nitrocellulose filters (Amersham) and bcl-2 protein was detected by immunostaining as described for Western blotting. The 2D gels were reproducible for each lysate, but small variations were observed between lysates. Therefore, in every experiment, bacterial-synthesized bcl-2 protein isolated from recombinant Escherichia coli cells as a carboxy terminus truncated human bcl-2 cloned into the expression vector pSPGEX (pSPGEXbcl-2), obtained from Dr D. Andrews (McMaster University, Hamilton, Ontario, Canada) was added (2.5 μL of cell lysate) to serve as a marker. The marker helped in the orientation of bcl-2 isoforms and facilitated comparison between lysates. The identification of individual isoforms was proven by demonstrating their presence in mixtures with lysates containing other isoforms.
Phosphatase treatment.
Recombinant λ phosphatase (λ-PPase) (New England Biolabs, Beverly, MA) was used to release phosphate groups from serine, threonine, and tyrosine residues. Lysates containing 200 μg protein from each sample were diluted with λ-PPase buffer (50 mmol/L Tris-HCl [pH8.0], 5 mmol/L dithiothreitol [DTT], 2 mmol/L MnCl2, and 100 μg/mL bovine serum albumin [BSA]) to a final volume of 100 μL. A total of 1,000 U of λ-PPase was added to each reaction for 2 hours at 30°C and the samples were separated subsequently by 2D gel.
Metabolic labeling.
For in vivo metabolic labeling with 32PiOCI/AML-5 cells were cultured in α-MEM supplemented with 10% heat-inactivated FCS and 10% 5637-CM. One hour before labeling, medium was removed and the cells were washed twice with Tris-buffered saline (TBS). The cells were resuspended in phosphate-free MEM (Sigma) supplemented with 5% dialyzed FCS at a concentration of 1 × 106 cells/mL and incubated at 37°C for 30 minutes.32Pi (Dupont) was added to the culture at a final radioactivity of 1 mCi/mL in the presence of 10-7 mol/L ATRA or 10−6 mol/L taxol. The cells were incubated at 37°C for another 15 hours. The orthophosphate-labeled cells were washed three times with TBS and lysed with lysis buffer. Radiolabeled lysate containing 1 mg protein from each sample was immunoprecipitated with anti–bcl-2 antibody (6C8), and the precipitates were separated by 12.5% SDS-PAGE and subsequently electrotransferred onto polyvinylidene difluoride (PVDF) filters (Dupont, Boston, MA). The filters with radiolabeled bcl-2 protein were first exposed to a phosphor imaging screen (Molecular Dynamics, Sunnyvale, CA) for detection of phosphorus and then stained with anti Bcl-2 antibody (bcl-2 124; DACO, Mississauga, Ontario, Canada). The bcl-2 protein on the filters was then detected by immunostaining as described above for Western blotting. For in vitro metabolic labeling, cells were treated with either ATRA or taxol for 24 hours, harvested, lysed in lysis buffer with freshly added protease inhibitors, and immunoprecipitated using specific hamster antihuman bcl-2 (6C8) antibody for 2 hours, followed by incubation with 20 μL of 50% protein G-agarose for another 2 hours to capture the immunoprecipitates. For the kinase reaction, the immunoprecipitates were washed, resuspended in 50 μL buffer containing 30 mmol/L Tris-Cl pH7.5, 10 mmol/L MgCl2, 2 mmol/L MnCl2, 50 μCi/mL γ-32P-adenosine triphosphate (ATP) (3,000 Ci/mmol/L; Amersham) and incubated at 30°C for 30 minutes. The reactions were terminated by adding 50 μL SDS-sample loading buffer and heating at 100°C for 5 minutes. The precipitates were then electrophoresed through 12.5% SDS-polyacrylamide gel and electrotransferred onto PVDF filters for autoradiography. In each procedure, to control for loading, after autoradiography, the filters were stained with anti–bcl-2 antibody.
Analysis of phosphorylation of amino acids.
We used phosphoamino acid analysis26 27 to determine which amino acids are phosphorylated in AML cells exposed to ATRA or taxol. The cells were incubated overnight in phosphate-free medium with 5% dialyzed FCS and 32Pi (1 mCi/mL) in the presence of ATRA (10−7 mol/L) or taxol (10−6 mol/L). The cells were lysed and the lysates were immunoprecipitated with anti–bcl-2 antibody (6C8) and the immunoprecipitates separated by SDS-PAGE. The separated proteins were transferred to a PVDF membrane. After autoradiography, the radiolabeled bcl-2 band was excised, digested with N-tosyl-L-phenylalanine chloromethyl ketone (TPCK)-trypsin, lyophilized, and treated with 6 N HCl for 1 hour at 110°C and dried. Residual HCl was removed with water by lyophilization. The phosphoamino acids were then identified by 2D electrophoresis first in buffer N-tosyl-L-phenylalanine chloromethyl ketone (TPCK) pH 1.9 and then in buffer pH 3.5 in a thin-layer chromatography plate; radioactive amino acids were visualized by autoradiography. These were compared with the position of ninhydrin-stained control phosphoamino acids. The same methods were used to detect phosphorylation of amino acids obtained by in vitro labeling (see above).
RESULTS
Phosphorylation of bcl-2 in ATRA-treated OCI/AML-5 cells.
Bcl-2 in OCI/AML-5 cells treated with ATRA becomes less stable.24 We used 2D gel electrophoresis to determine whether this change in stability was associated with a posttranslational modification of the protein. OCI/AML-5 cells were grown in 10−7 mol/L ATRA for 24 hours. The cells were lysed and the protein separated by IEF, followed by size separation, as described in Materials and Methods; the results of a replicated experiment are shown in Fig 1. The bacterially synthesized protein serves as a marker and is indicated in all gels by an arrow. Comparing the result of control lysate to ATRA-treated lysate, there is an apparent shift of bcl-2 to a more acidic isoform. This shift was confirmed by mixing lysates of control and ATRA-treated cells (data not shown). Phosphorylation is one form of posttranslational modification that can produce such a shift. To determine whether phosphorylation was responsible for the shift, lysate from ATRA-exposed cells, was treated with λ-phosphatase, and analyzed by 2D electrophoresis. The acidic isoform was not evident in phosphatase-treated cells; bcl-2 protein was in the same position as the native protein (Fig 1). The difference in isoelectric point of the phosphatase-treated and untreated samples was confirmed by mixing lysate from ATRA-treated cells with an aliquot of the same lysate that had been digested with λ-phosphatase; the 2D gel of the mixture is shown at the bottom of Fig 1. Three distinct bcl-2 reacting spots are seen; that shown by the arrow is the bacterially synthesized protein. The spot just to the left of this marker is from the phosphatase-treated lysate and is in the same position as bcl-2 from control cells. The most acidic bcl-2 isoform is derived from the ATRA-treated lysate. Together, these studies verify that ATRA treatment induces a change in bcl-2 protein and that this change is due to phosphorylation.
2D gels; upper left is the bacterially synthesized marker protein, shown throughout the figures as an arrow. Upper right is a 2D gel of a control lysate from OCI/AML-5 cells. Middle left is 2D gel of a lysate from ATRA-treated cells, showing the acidic isoform. Middle right is a 2D gel of an aliquot of lysate from ATRA-treated cells, digested with λ-phosphatase. It may be compared with the 2D control gel directly above it in the figure. The 2D gel at the bottom of the figure was made from a mixture of the two lysates above it; it shows the marker, a spot at the position of the native protein, but derived from λ-phosphatase–digested lysate from ATRA-treated cells and an acidic isoform, derived from the ATRA-treated cells.
2D gels; upper left is the bacterially synthesized marker protein, shown throughout the figures as an arrow. Upper right is a 2D gel of a control lysate from OCI/AML-5 cells. Middle left is 2D gel of a lysate from ATRA-treated cells, showing the acidic isoform. Middle right is a 2D gel of an aliquot of lysate from ATRA-treated cells, digested with λ-phosphatase. It may be compared with the 2D control gel directly above it in the figure. The 2D gel at the bottom of the figure was made from a mixture of the two lysates above it; it shows the marker, a spot at the position of the native protein, but derived from λ-phosphatase–digested lysate from ATRA-treated cells and an acidic isoform, derived from the ATRA-treated cells.
Comparison of phosphorylation induced by ATRA or taxol.
Taxol and other drugs acting on microtubules have been reported to induce phosphorylation of bcl-2; the phosphorylated bcl-2 isoform from taxol-treated cells is recognized as a slower migrating band in 10% to 20% gradient polyacrylamide gels.15-17 28 To determine whether ATRA-induced phosphorylation is seen in such gels, OCI/AML-5 cells were treated with ATRA or taxol for 24 hours and the lysates separated on 10% to 20% gradient gels and probed for bcl-2 (Fig 2A). A slower mobility band was evident in the taxol-treated cells, but not the ATRA-treated or control cells. We confirmed that the shift induced by taxol was due to phosphorylation by treating lysates with λ-phosphatase. As expected, this resulted in loss of the slow mobility form of bcl-2.
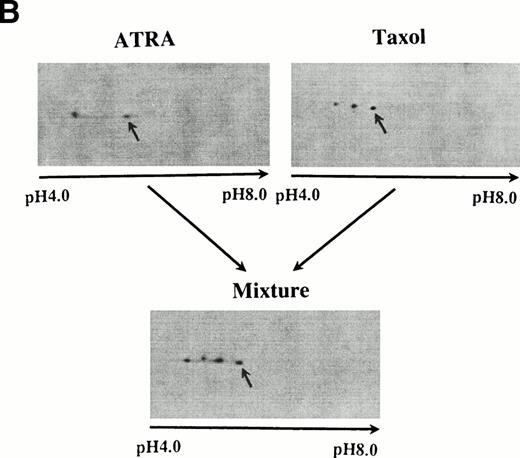
(A) At the top is a 10% to 20% gradient gel showing the presence of a slower-moving isoform of bcl-2 from cells treated with taxol (middle lane). The control (left lane) and lysate from ATRA-treated cells show only a single band (right lane). The bottom is 2D gels from taxol-treated cell lysate (left) and the lysate after digestion with λ-phosphatase. These conditions permit the demonstration of the slightly larger than control molecular weight of the phosphorylated bcl-2 isoform seen after taxol. (B) Lysates from ATRA- and taxol-treated cells (above) were mixed and the mixture run as a 2D gel (below). The experiment shown is representative of two replicates.
(A) At the top is a 10% to 20% gradient gel showing the presence of a slower-moving isoform of bcl-2 from cells treated with taxol (middle lane). The control (left lane) and lysate from ATRA-treated cells show only a single band (right lane). The bottom is 2D gels from taxol-treated cell lysate (left) and the lysate after digestion with λ-phosphatase. These conditions permit the demonstration of the slightly larger than control molecular weight of the phosphorylated bcl-2 isoform seen after taxol. (B) Lysates from ATRA- and taxol-treated cells (above) were mixed and the mixture run as a 2D gel (below). The experiment shown is representative of two replicates.
These results indicate that a difference may exist between phosphorylation of bcl-2 after treatment by ATRA or taxol. To determine whether this is the case, we compared taxol- and ATRA-induced phosphorylation of bcl-2 by 2D gel analysis. Bcl-2 from taxol-treated cells appeared as two spots in addition to the marker, one at the location of the native protein and a second, more acidic, and slightly larger in size; this latter spot was lost after treatment of the lysate with phosphatase (Fig 2B). The position of the novel isoform in taxol-treated cells appears to be less acidic than in ATRA-treated cells. This was confirmed by mixing lysates from ATRA- and taxol-treated cells and then separating the proteins on a 2D gel (Fig2A). Four spots, representing bcl-2 isoforms, are seen. The most basic form is the bacterially expressed bcl-2 (denoted by an arrow). The two isoforms just to the left of the marker are derived from the taxol lysate and represent the native isoform and a phosphorylated isoform. The most acidic isoform is derived from the ATRA-treated cells. Taken together, these experiments indicate that there is both a qualitative and quantitative difference in the phosphorylation of bcl-2 induced by taxol compared with ATRA.
Phosphorylation of bcl-2 in primary AML blast cells.
The above experiments were performed with the cell line OCI/AML-5; identical results were seen in a second cell line, OCI/AML-2 (data not shown). To show that the ATRA- and taxol-induced phosphorylation of bcl-2 is not restricted to cell lines, we cultured the cryopreserved AML blast cells from two patients for 48 hours in growth medium and 5637-CM. The cells were then treated with taxol (10−6mol/L) or ATRA (10−7 mol/L) for 24 hours, lysates prepared, and analyzed by 2D gel electrophoresis. As evident in Fig 3, the changes in bcl-2, seen in the primary blast cells, are the same as those seen in the leukemic cell lines.
2D gels of lysates from blast cells from an AML patient. Control is seen at the top; the middle shows gels from cells treated with ATRA (left) and taxol (right). At the bottom is the 2D gel of a mixture of these two lysates. Similar results were obtained using the blast cells from a second patient.
2D gels of lysates from blast cells from an AML patient. Control is seen at the top; the middle shows gels from cells treated with ATRA (left) and taxol (right). At the bottom is the 2D gel of a mixture of these two lysates. Similar results were obtained using the blast cells from a second patient.
Metabolic labeling.
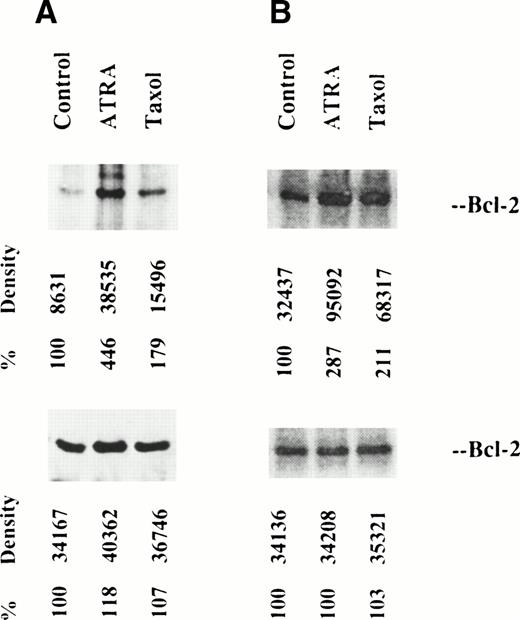
We looked for direct evidence of bcl-2 phosphorylation by measuring incorporation of radiophosphorus into the protein, either in vivo or in vitro as described in Materials and Methods. The labeled immunoprecipitates were separated by 12.5% PAGE, transferred to a PVDF filter, and visualized by radioautography. Figure 4 shows the radioautographs; some phosphorylation of native bcl-2 was seen, but this increased in cells treated with either ATRA or taxol. The 26 kD phosphoprotein was identified as bcl-2 by comparison with Western blots. Both in vivo and in vitro, bcl-2 from ATRA-treated cells was more highly phosphorylated than bcl-2 from taxol-treated cells. A second 30-kD phosphorylated protein is observed in in vivo labeled, ATRA-treated cells, but not in controls or taxol-treated cells.
Metabolic labeling of bcl-2 from OCI/AML-5 cells in vivo (left) and in vitro (right). Radioautographs of labeled protein from control, ATRA-treated and taxol-treated cells, separated by 12.5% SDS-PAGE are shown at the top. Densitometer readings are shown. At the bottom, Western blots of the lysates, stained with anti–bcl-2, are shown. Such Western blots, prepared after 12.5% SDS-PAGE, show bcl-2 from taxol-treated cells only as a single band.
Metabolic labeling of bcl-2 from OCI/AML-5 cells in vivo (left) and in vitro (right). Radioautographs of labeled protein from control, ATRA-treated and taxol-treated cells, separated by 12.5% SDS-PAGE are shown at the top. Densitometer readings are shown. At the bottom, Western blots of the lysates, stained with anti–bcl-2, are shown. Such Western blots, prepared after 12.5% SDS-PAGE, show bcl-2 from taxol-treated cells only as a single band.
Phosphoamino acid analysis.
32Pi labeling of bcl-2 both in vivo and in vitro was used to identify the phosphorylated amino acids, as described in Materials and Methods. The results of the in vivo labeling are shown in Fig 5 as 2D electrophoresis on thin layer chromatography with the label detected by autoradiography. The positions of three phosphoamino acids (P-serine, P-threonine, and P-tyrosine), as detected by ninhydrin staining, are shown. Lysates were prepared from controls and cells treated either with ATRA or taxol. In all three conditions, only phosphorylation of serine was observed. The same result was obtained when bcl-2 was labeled in vitro (data not shown). The finding that serine is phosphorylated after taxol is in agreement with results in the literature.28
Phosphoamino acid analysis to show phosphorylation of bcl-2 on serine. The figure shows 2D electrophoresis on thin layer chromatography with the label detected by autoradiography. The cells were incubated overnight with 32Pi as controls or with ATRA or taxol. Cell lysates were prepared and immunoprecipitated with anti–bcl-2 antibody (6C8) and the immunoprecipitates were separated by SDS-PAGE and transferred to PVDF membrane. After autoradiography, the radiolabeled bcl-2 band was excised, digested with TPCK-trypsin, lyophilized, treated with 6 N HCl for 1 hour at 110°C and dried. The phosphoamino acids were then identified by 2D electrophoresis first at pH 1.9 and then at pH 3.5. The positions of the radioactive amino acids were compared with ninhydrin-stained control amino acids, as indicated in each panel of the figure. The same methods were used to detect phosphorylation of amino acids obtained by in vitro labeling. Using both methods, only serine phosphorylation was detected.
Phosphoamino acid analysis to show phosphorylation of bcl-2 on serine. The figure shows 2D electrophoresis on thin layer chromatography with the label detected by autoradiography. The cells were incubated overnight with 32Pi as controls or with ATRA or taxol. Cell lysates were prepared and immunoprecipitated with anti–bcl-2 antibody (6C8) and the immunoprecipitates were separated by SDS-PAGE and transferred to PVDF membrane. After autoradiography, the radiolabeled bcl-2 band was excised, digested with TPCK-trypsin, lyophilized, treated with 6 N HCl for 1 hour at 110°C and dried. The phosphoamino acids were then identified by 2D electrophoresis first at pH 1.9 and then at pH 3.5. The positions of the radioactive amino acids were compared with ninhydrin-stained control amino acids, as indicated in each panel of the figure. The same methods were used to detect phosphorylation of amino acids obtained by in vitro labeling. Using both methods, only serine phosphorylation was detected.
Dimerization of bcl-2 and bax.
Haldar et al28 found that bcl-2 phosphorylated after treatment with ATRA did not dimerize with bax. Based on this, they proposed that the function of bcl-2 was inhibited. We took two different approaches to ask whether phosphorylation of bcl-2 affects dimerization with bax. First, lysates from controls, taxol-, or ATRA-treated cells were immunoprecipitated with antibody against bax. The immunoprecipitates were separated by electrophoresis on a gradient gel and the resulting filters probed with antibody against bax or bcl-2. Bcl-2 protein coprecipitated with bax in all three cases. In the taxol-treated lysate, both isoforms of bcl-2 were brought down, indicating that bax is dimerized with phosphorylated bcl-2 (Fig 6). In the ATRA-treated cells, we also infer that bax is dimerized with phosphorylated bcl-2, as by 2D gel analysis, virtually all of the bcl-2 is in a phosphorylated form (Fig1).
Dimerization of bcl-2 and bax. Lysates from control cells and cells treated with ATRA or taxol were immunoprecipitated with anti–bcl-2 (A) or with anti-bax (B). The immunoprecipitates were separated by 10% to 20% SDS-PAGE gel electrophoresis, transferred to nitrocellulose, and stained with bcl-2. It is evident that both normal and phosphorylated bcl-2 formed heterodimers with bax.
Dimerization of bcl-2 and bax. Lysates from control cells and cells treated with ATRA or taxol were immunoprecipitated with anti–bcl-2 (A) or with anti-bax (B). The immunoprecipitates were separated by 10% to 20% SDS-PAGE gel electrophoresis, transferred to nitrocellulose, and stained with bcl-2. It is evident that both normal and phosphorylated bcl-2 formed heterodimers with bax.
Second, because this result differed from that of Haldar et al, we tried to determine whether there was a quantitative difference in the amount of bax associated with bcl-2. For these experiments, lysates from control, taxol- or ATRA-treated cells were immunoprecipitated with antibody against bcl-2. Protein from the pellet and the supernatant was separated by SDS-PAGE and transferred to nitrocellulose. The resulting membranes were then probed for the presence of bcl-2 in the pellet and bax in the supernatant. All of the bcl-2 was present in the pellet from samples after immunoprecipitation with anti–bcl-2; blc-2 protein could not be demonstrated in the supernatants by Western blot (data not shown). After precipitation with anti–blc-2, the amounts of bax in the supernatants were similar, regardless of how the cells were treated (Fig 7). These experiments demonstrate that phosphorylation of bcl-2 by taxol or ATRA does not result in the release of bax from bcl-2.
10% to 20% gradient gels after bcl-2 immunoprecipitation of lysates from OCI/AML-5 cells, as controls or after treatment with ATRA or taxol. The panel at the top shows the immunoprecipitates, stained with anti–bcl-2. The bottom panels show the supernatants, stained with anti-bax. It is seen that treatment of the OCI/AML-5 cells did not change the amount of bax that did not coprecipitate with bcl-2.
10% to 20% gradient gels after bcl-2 immunoprecipitation of lysates from OCI/AML-5 cells, as controls or after treatment with ATRA or taxol. The panel at the top shows the immunoprecipitates, stained with anti–bcl-2. The bottom panels show the supernatants, stained with anti-bax. It is seen that treatment of the OCI/AML-5 cells did not change the amount of bax that did not coprecipitate with bcl-2.
DISCUSSION
There are two major findings described in this report. First, we report that bcl-2 becomes phosphorylated on serine after treatment with ATRA. Second, phosphorylation of bcl-2 after ATRA is different from that observed in cells treated with taxol.
Phosphorylation of bcl-2 was shown using 2D gels of lysates from ATRA-treated blast cells. After ATRA, bcl-2 became more acidic; after digestion of lysates from ATRA-treated cells with λ-phosphatase, 2D gels showed bcl-2 in the same position as the control native isoform. This observation showed that the acidic bcl-2 isoform is phosphorylated. Metabolic labeling of bcl-2 with32Pi or γ-32P-ATP showed increased incorporated isotope in bcl-2 of ATRA-treated cells compared with controls, confirming directly that ATRA leads to the phosphorylation of bcl-2.
Bcl-2 is phosphorylated in cells treated in culture with okadaic acid or taxol.15,17 The phosphorylated isoform after such treatment can be detected in gradient gels as a novel band with decreased mobility. Similar bands are seen when bcl-2 is treated with c-Jun N-terminal kinase (JNK) in vitro.29 Electrophoresis of lysates from ATRA-treated blast cells through 10% to 20% polyacrylamide gels failed to show a second band. Because of this difference between phosphorylation after ATRA and taxol, we undertook a direct comparison of the effects of the two agents. As expected from the published results, taxol-induced phosphorylation increased the apparent size of bcl-2 protein; the phosphorylated isoform was seen as an extra band in gradient gels (Fig 3). This change in mobility was shown to be due to phosphorylation indirectly by demonstrating the sensitivity of the acidic isoform to λ-phosphatase and directly by showing incorporation of phosphorus into the bcl-2 protein, although stimulation of phosphorylation was less after taxol than after ATRA. Further, 2D gels of lysates from taxol-treated cells showed both the native isoform and a novel acidic isoform, which was sensitive to λ-phosphatase. Taken together these data show both qualitative differences (comparison of size in gradient gels and 2D gels) and quantitative differences (complete conversion of the native isoform to the phosphorylated isoform after ATRA compared with retention of the native isoform after taxol, and increased metabolic labeling in ATRA-treated cells compared with taxol-treated cells). Parallel phosphoamino acid analysis of ATRA- and taxol-treated cells showed that both are phosphorylated on serine (Fig 5). Therefore, the differences between phosphorylated bcl-2 in ATRA-treated compared with taxol-treated cells cannot be explained by phosphorylation of different amino acids; different sites or extent of phosphorylation or both remain as possible reasons for the observed differences. Experiments are in progress directed towards this important issue.
The role of bcl-2 phosphorylation in the regulation of apoptosis remains unclear. Conflicting reports are available concerning bcl-2 phosphorylation. First, descriptions of the influence of bcl-2 phosphorylation on its capacity to form dimers with other family members are not consistent. Both of the reports of Zha et al30 and Haldar et al17 show phosphorylation modifying the function of bcl-2 family members by changing patterns of dimerization. In contrast, Ito et al20 and Chang et al19 found that phosphorylation of bcl-2 did not affect dimerization. Our studies provide support for the latter in that we found that bcl-2 phosphorylated after either ATRA or taxol has unchanged capacity to form heterodimers with bax. The discrepancy between our taxol result and that of Haldar et al may be explained by differences in the cellular systems or immunoprecipitation methods used in the two studies. Regardless, our studies provide a further example of posttranslational modification of bcl-2 by phosphorylation that does not change its capacity to form heterodimers with bax.
Second, controversy exists as to whether phosphorylation increases or diminishes the antiapoptotic activity of bcl-2 family members. Mutants of bad that abolish phosphorylation are very effective in promoting apoptosis, as these are incapable of dimerization; mutants with reduced, but not eliminated, phosphorylation sites show intermediate apoptotic capacity.30 In contrast, Ito et al20report that phosphorylation is required for antiapoptoic activity. We have only correlative evidence for the consequence of ATRA-induced phosphorylation. ATRA reduces clonogenicity in AML cell lines in proportion to the presence of its receptor31; ATRA-treated OCI/AML-5 and OCI/AML-2 cells are sensitized to ara-C after exposure to ATRA.21 ATRA may have many effects on leukemic cells; nonetheless, loss of clonogenicity and increased ara-C sensitivity might be the consequence, in part, of decreased bcl-2 function. The reduced stability of bcl-2 protein in ATRA-treated cells is consistent with altered function. The bcl-2 phosphorylation after ATRA is a posttranslational modification that is associated with a shortened half-life and may contribute to a change in its function, perhaps through degradation. If this view is correct, our data would agree with those who propose that bcl-2 phosphorylated after taxol is less able to prevent cell death.16 17 It remains a possibility that, if phosphorylation is a regulator of bcl-2 function, its effect might be different, depending on extent and sites of phosphorylation and upon the cellular context.
ATRA is an important biologic agent in the treatment of promyelocytic leukemia32,33 and is showing promise in the early results of a randomized clinical trial as part of a chemotherapeutic regimen for acute myeloblastic leukemia without the t(15;17).34 We have shown that ATRA treatment led to phosphorylation of bcl-2 in cells from two AML patients. The response of fresh cells to ATRA is heterogeneous, when clonogenic cell survival or sensitization to ara-C are the end-points; we expect that phosphorylation of bcl-2 after ATRA will also vary from patient-to-patient. There exists an opportunity, therefore, to test for an association between phosphorylation of bcl-2 and response in trials that include ATRA. If such an association were found, it would provide convincing evidence of the functional significance of bcl-2 phosphorylation and might help to select for patients who would benefit from the addition of ATRA to a therapeutic regimen.
Supported by the National Cancer Institute of Canada and the Medical Research Council of Canada.
Address reprint requests to E.A. McCulloch, MD, 610 University Ave, Toronto, Ontario M5G 2M9, Canada; e-mail:mcculloch@oci.utoronto.ca.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.