Abstract
Despite the increasing use of cytokines to circumvent the acute dose-limiting myelotoxicity of cancer treatment, little is known about the combined effects of cytotoxic agents and cytokines on the primitive stem cells responsible for long-term hematopoiesis. In an experimental model, we administered cytotoxic agents that have variable effects on primitive stem cells in C57BL/6 (B6)-mice. Mice received six every-other-week doses of cyclophosphamide (CY, 84 mg/kg), VP-16 (24 mg/kg) + cisplatinum (2.4 mg/kg), carboplatinum (50 mg/kg), chlorambucil (12 mg/kg), BCNU (13.2 mg/kg), or TBI (80 cGy). Granulocyte colony-stimulating factor (G-CSF; 250 μg/kg/day) was administered subcutaneously twice daily on days 3 to 6 after each dose of the cytotoxic agent. Comparison with animals receiving the cytotoxic agent alone was made to investigate the effects of G-CSF on long-term hematopoiesis. Hematopoiesis was measured 20 weeks after the last dose of the cytotoxic agent by assessment of peripheral blood counts, marrow cellularity, progenitor cell content (colony-forming units-spleen; CFU-S), and primitive stem cell number (long-term repopulating ability and day 28 and day 35 cobblestone area-forming cell [CAFC] frequencies). Exposure to cytotoxic agents alone resulted in a significant decrease in primitive stem cells (as measured by repopulating units [RU] and day 28 and day 35 CAFC content) in animals given carboplatinum, chlorambucil, BCNU, and TBI, but not in animals treated with cyclophosphamide or VP-16 and cisplatinum. The addition of G-CSF resulted in a significant decrease in stem cell content when compared with no G-CSF administration in animals treated with chlorambucil, BCNU, or TBI. Thus, G-CSF administered after repeated exposure to cytotoxic agents, appeared to damage the primitive stem cell compartment when used in combination with agents known to damage primitive stem cells. These results, although obtained in an experimental model, should raise concerns for the indiscriminate use of G-CSF in the clinic.
© 1998 by The American Society of Hematology.
THE MAJORITY OF PATIENTS undergoing autologous transplantation for lymphoma and multiple myeloma receive stem cells previously exposed to cytotoxic agents. These patients are at risk for impaired hematopoiesis. The risk appears to correlate with the intensity and number of cycles of prior multiagent chemotherapy.1-3 The greater the exposure to cytotoxic agents, the higher the risk.
There has been increasing use of cytokines to circumvent the acute dose-limiting myelotoxicity of many cancer treatments and bone marrow transplantation (BMT).4,5 However, little is known about the effects of cytokines on the primitive stem cells, which provide for long-term hematopoietic support. In theory, the use of cytokines could be either beneficial or detrimental. Excessive stimulation of primitive stem cell proliferation by cytokines may lead to loss of primitive stem cells and premature bone marrow failure (as with kit-ligand [KL] given before and after 5-fluorouracil [5-FU]6,7), whereas selective stimulation of later progenitors may have no effect, or even a protective effect on primitive stem cells. A previous study in mice has suggested that administration of granulocyte-macrophage colony-stimulating factor (GM-CSF) or granulocyte colony-stimulating factor (G-CSF) to speed recovery from repeated doses of high dose cyclophosphamide (350 mg/kg) appears to damage primitive stem cells.8 This study demonstrated that primitive stem cell function was impaired in groups given G-CSF or GM-CSF when assessed by serial transplantation into lethally irradiated recipients 7 weeks after the last cycle of cyclophosphamide.
The current experiments were designed to further study the effect of G-CSF on hematopoietic stem cell populations after repeated drug exposure and to explore potential mechanisms of cytokine-induced primitive stem cell damage. A number of different cytotoxic agents were evaluated to determine whether the effects of G-CSF were independent of the direct effect of cytotoxic agents on the marrow.
MATERIALS AND METHODS
Mice.
Male C57BL/6J (B6)-mice purchased from Jackson Laboratories (Bar Harbor, ME) were used as recipients of cytotoxic agents and cytokines and for competitive repopulation studies. Male congenic C57BL/6J-Gpi-1a/Gpi-1a (B6-Gpi-1a) mice, purchased from Jackson Laboratories, were used as a source of normal untreated marrow in the competitive repopulation assays.
Cytotoxic agent studies.
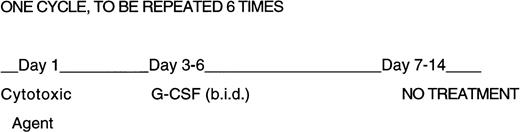
Experiments were designed to expose groups of 3-month old male B6-mice to six every-other-week intraperitoneal (IP) doses of each cytotoxic agent (in a total volume of 0.2 mL/mouse). rhuG-CSF (Amgen Inc, Thousand Oaks, CA) was administered twice daily on days 3 to 6 after cytotoxic agent administration as shown in Fig 1. The dose of each cytotoxic agent was determined as the amount which resulted in a fractional day 8 colony-forming units-spleen (CFU-S) survival of 0.37 (Do) at 24 hours after a single IP administration.9 This equivalent endpoint for each agent was selected because the effects on progenitor cells appears to account for the dose-limiting toxicity of cytotoxic agents in the clinic.
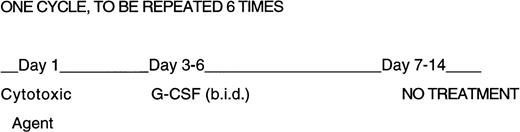
Design of repeated cytotoxic agent and G-CSF administration. Mice were given six 2-week cycles of cytotoxic agents, half of the mice received G-CSF twice daily on days 3 through 6 at 250 μg/kg/day. No treatment was given on days 7 through 14.
Design of repeated cytotoxic agent and G-CSF administration. Mice were given six 2-week cycles of cytotoxic agents, half of the mice received G-CSF twice daily on days 3 through 6 at 250 μg/kg/day. No treatment was given on days 7 through 14.
The cytotoxic agents used were cyclophosphamide (Bristol Myers, Princeton, NJ; 84 mg/kg), VP-16 (etoposide; Bristol Myers; 24 mg/kg) combined with cisplatinum (Bristol Myers; 2.4 mg/kg), chlorambucil (Sigma, St Louis, MO; 12 mg/kg), carboplatinum (Sigma; 50 mg/kg), BCNU (1,3-bis(2-chloroethyl)-1-nitrosourea; Bristol Myers; 13.2 mg/kg), and total body irradiation (TBI; 80 cGy given at a dose-rate of 0.93 Gy/minute from a 137Cs-source (Gamma Cell 40, Atomic Energy of Canada, Ottawa, Canada). Saline was used in control mice. Cyclophosphamide was chosen because of its limited effect on primitive stem cells at conventional doses. Less is known about the effects of VP-16 and cisplatinum on primitive stem cells; this combination was selected because of its common use in the clinic. Carboplatinum and chlorambucil have intermediate effects on primitive stem cells; BCNU and TBI were selected because these agents are known to permanently damage the stem cell compartment.10-12 Because doses of the cytotoxic agents were chosen to have mild acute effects on the hematopoietic system, no severe neutropenia was observed as a result of these treatments. Hence, very few deaths occurred before the time of scheduled sacrifice (20 weeks after the last dose of each cytotoxic agent). Only three of 194 mice died after completion of six cycles, but before 20 weeks (one mouse treated with BCNU alone, one with BCNU+G-CSF, and one mouse treated with chlorambucil [CAM] alone). In addition, no differences were found in white blood cell (WBC) counts at the time of the next cytotoxic agent administration to indicate that there was no ongoing neutropenia (data not shown). The objective of this study was to compare G-CSF effects under conditions of stem cell damage without acute drug-related lethality.
Half of the mice were treated with rhuG-CSF (250 μg/kg/day, administered in two equal doses, subcutaneously, for 4 days 8 to 10 hours apart, starting 2 days after each cytotoxic agent). The rhG-CSF doses used in mice are usually up to 50-fold higher than in humans, at least in part due to limited cross-species reactivity. Studies in mice have shown that 20-fold higher dose of G-CSF (when compared with human studies) given for up to 14 days are required for enhanced neutrophil recovery after 5-FU13,14 or TBI.15
After the sixth dose of each cytotoxic agent, animals were rested to allow recovery of peripheral blood counts before testing for hematopoietic reserve. Although full recovery of counts was anticipated at approximately 1 month after administration of each cytotoxic agent, we chose a longer interval for assessment of hematopoietic stem cell reserve to assess for permanent damage. Previous murine studies have shown that after BMT it can take up to 4 months for stable hematopoiesis to occur.16 17 Therefore, we chose a time of 20 weeks after administration of the last cytotoxic agent to analyze animals for WBC counts, marrow cellularity, marrow progenitor cell content (day 8 CFU-S content), and primitive stem cell content (long-term competitive repopulating ability [LTRA] in vivo and day 28 and day 35 cobblestone area-forming cell [CAFC] content in vitro). Using these endpoints we could measure if there was permanent damage to the marrow.
Competitive repopulation in vivo.
The competitive repopulation (CR) assay measures the long-term repopulating ability of a test cell population relative to normal bone marrow cells in vivo.18 For determination of CR, varying numbers of bone marrow or blood test cells (5 × 105to 4 × 106) from B6-Gpi-1b mice were mixed with a constant number (5 × 105 to 1 × 106) of control B6-Gpi-1a marrow cells. The mixtures were injected into groups of five to six lethally irradiated B6-Gpi-1b recipients. Absence of endogenous marrow repopulation was determined by injecting one group (Gpi-1b) with control cells only (Gpi-1a). Recipients were killed at 6 months after BMT and the ratios of test (Gpi-1b) to control (Gpi-1a) cells determined by electrophoresis of peripheral blood erythrocytes.19 The number of repopulating units (RU), a measure of long-term repopulating cells, was calculated according to the formula:
where C is the number of RU in control (Gpi-1a) marrow and 1 RU is defined as the repopulating ability of 105 normal bone marrow cells.20
CAFC assay in vitro.
In vitro determination of hematopoietic stem and progenitor cell frequencies was performed by limiting dilution analysis (LDA) of CAFC in microcultures according to methods previously described,21-23 with some modifications.7Cultures were scored at days 28 and 35 by scanning each well under an inverted microscope for the presence of cobblestone areas (CA). CA are colonies of immature hematopoietic cells (at least six cells per colony) residing within a preestablished FBMD-1 (provided by Dr Steve Neben, Genetics Institute, Cambridge, MA) stromal layer. The proportion of negative wells at each dilution was used in a Poisson-based LDA calculation to determine the CAFC frequency.23,24 It has been reported that in vitro CAFC (day 28 or day 35) show considerable overlap in function with in vivo repopulating cells, but the precise relation between these subsets is not known.22,23 25 In the current experiments, we chose to use both the in vivo and in vitro assays.
Statistics.
To test differences between treatment groups for statistical significance, P values were calculated with the Student’st test assuming unequal variances of the two variables. The Poisson-based LDA calculation for CAFC frequencies also provides a 95%, 99%, and 99.9% confidence interval.23 24 These intervals were used to determine significant differences as P< .05 (P > .01), P < .01 (P > .001) andP < .001, respectively. Cytotoxic agent-treated mice were compared with saline-treated controls and G-CSF–treated mice were compared with mice receiving the cytotoxic agent alone.
RESULTS
Blood and marrow counts and progenitor cell and stem cell content in mice previously exposed to cytotoxic agents.
B6 mice that received six cycles of cyclophosphamide, VP-16+cisplatinum, carboplatinum, chlorambucil, BCNU, TBI, or saline (controls) were killed at 20 weeks after completion of the cytotoxic agent administration for assessment of long-term hematopoietic parameters. Results of WBC, bone marrow cellularity (BMC), and CFU-S content per hind limb (HL) are listed in Table 1. Stem cell numbers (CAFC-28/HL, CAFC-35/HL, and RU/HL) are shown in Table2. There was no difference in long-term hematopoiesis in mice receiving cyclophosphamide or VP-16 and cisplatinum compared with saline controls.
Carboplatinum- and chlorambucil-treated mice had moderate decreases in long-term hematopoietic stem cell reserve as demonstrated by decreases in marrow cellularity (carboplatinum, P = .04), CFU-S/HL (carboplatinum, P = .03), CAFC-28/HL (carboplatinum, P< .05, and chlorambucil: P < .05), CAFC-35/HL (carboplatinum, P < .05, and chlorambucil, P < .05), and RU/HL (chlorambucil, P < .01) when measured 5 months after the last dose of carboplatinum or chlorambucil.
BCNU- and TBI-treated mice had the greatest decrease in long-term hematopoietic stem cell reserve as demonstrated by decreases in nearly all hematopoietic parameters including WBC (TBI, P = .03), marrow cellularity (BCNU, P = .02), CFU-S/HL (BCNU, P< .01, and TBI, P < .01), CAFC-35/HL (BCNU, P < .01), and RU/HL (BCNU, P < .01, and TBI, P < .01) when measured 5 months after the last dose of BCNU or TBI. Animals showed decreases in primitive stem cell content before manifesting decreases in blood or marrow cellularity.
Blood and marrow counts and progenitor cell and stem cell numbers in mice previously exposed to cytotoxic agents: The effect of G-CSF administration.
The addition of G-CSF to control, cyclophosphamide-treated, VP-16 + cisplatinum-treated, and carboplatinum-treated mice had only a very modest effect on long-term hematopoietic stem cell reserve (see Tables 3, 4, and 5). G-CSF administration did not result in a significant decrease in progenitor or primitive stem cell content compared with the cytotoxic agent administration without G-CSF.
However, G-CSF administration appeared to adversely effect marrow stem cell reserve when administered after chlorambucil, BCNU, or TBI, cytotoxic agents that by themselves have a detrimental effect on marrow reserve. G-CSF + chlorambucil administration resulted in a loss of long-term hematopoietic stem cell reserve as demonstrated by decreases in CAFC-28/HL (P < .001) and RU/HL (P < .01) compared with chlorambucil alone. An even greater loss in stem cell reserve was seen with G-CSF administered after BCNU or TBI, as demonstrated by decreases in marrow cellularity (TBI, P = .05), CFU-S/HL (BCNU, P < .01, and TBI, P < .01), CAFC-28/HL, (BCNU, P < .001, and TBI, P < .001), CAFC-35/HL (TBI, P < .05), and RU/HL (BCNU, P< .01, TBI, P = .01) compared with BCNU or TBI alone.
DISCUSSION
G-CSF increasingly has been used to reduce the severity of treatment-related neutropenia after high dose chemotherapy or BMT26,27; reviewed by Welte et al.28 G-CSF is thought to act mainly on granuloid precursors; little is known about the long-term effects of G-CSF on the more primitive hematopoietic stem cells.
In one of the few reports evaluating the effects of G-CSF on hematopoietic stem cells, Hornung and Longo8 demonstrated enhancement of primitive stem cell damage by the addition of G-CSF to high dose cyclophosphamide (350 mg/kg) given in six every other weekly cycles. Seven weeks after the last dose of cyclophosphamide, marrow was obtained for serial transplantation into lethally irradiated recipients. After three serial transplants, mice given G-CSF (or GM-CSF) and high dose cyclophosphamide demonstrated long-term marrow stem cell damage by deficiency in generating hematopoietic progenitors and CFU-S, and in marrow repopulating ability compared with mice treated with cyclophosphamide alone. In the current study, cyclophosphamide administered at lower doses (84 mg/kg) with or without G-CSF did not appear to damage primitive stem cells. This is in agreement with previous reports that cyclophosphamide has a greater detrimental effect on progenitor cells than on the more primitive stem cells.9 29-31 The fourfold higher doses used in the Hornung study resulted in high animal mortality when repeated in our laboratory (unpublished data) suggesting that cyclophosphamide may result in a loss of marrow reserve only at doses that cause life-threatening myelosuppression.
Rather than studying the effect of dose intensification, we varied the use of G-CSF after administration of cytotoxic agents given at a constant dose. In this way we could study the direct effect of the G-CSF rather than the combined effect of the dose intensification combined with the G-CSF. The agents and doses chosen were equally toxic to the CFU-S day 8 population, but varied in their effects on the more primitive stem cells. As progenitor and peripheral blood numbers are dose-limiting for the clinical use of cytotoxic agents, while primitive stem cell numbers appear important for long-term marrow reserve, the experiments were designed to model clinical practice while testing for the long-term consequences of treatment. Progenitor and stem cell numbers were compared at 20 weeks after the end of six consecutive 2-week cycles of cytotoxic agents to provide a long-term measure of marrow damage.
This study demonstrates that G-CSF administered after multiple doses of cytotoxic agents appears to impair long-term hematopoiesis and marrow stem cell reserve. G-CSF had no significant adverse effect when administered to control, cyclophosphamide or VP-16, and cisplatinum-treated mice; animals that had demonstrated little detriment in long-term repopulating ability without the use of G-CSF. G-CSF added to carboplatinum also showed no additional adverse effect despite the moderate stem cell deficit seen with this cytotoxic agent alone. However, when G-CSF was given after chlorambucil, BCNU, or TBI, all agents known to damage primitive stem cells, additional loss in primitive stem cell capacity was seen. These effects were most apparent at the primitive stem cell level (CAFC-28/35 and RU), indicating a permanent loss in marrow stem cell reserve with the addition of G-CSF to each cytotoxic agent. This deficit also led to reduced progenitor cell numbers, but had less effect on WBC and marrow counts suggesting that peripheral blood and marrow cellularity were maintained even when there was long-term loss of marrow reserve. The results confirm that normal peripheral counts are a poor indicator of marrow stem cell reserve after exposure to cytotoxic agents.32
There is evidence that although the marrow stem cell compartment has considerable reserve potential, it is limited in proliferative and self-renewal capacity. Over 30 years ago, there was data to suggest that stem cells have limited doubling capacity.33,34Experimentally, limited self-renewal and proliferative capacity of stem cells has been demonstrated through serial transplantation of marrow into lethally irradiated recipients35-37; through exposure of marrow to radiation,38,39 to combinations of cytokines and cytotoxic agents,6,8 or to cytotoxic agents that damage early stem cells40-43 and through the ability of proliferative stress to considerably reduce marrow reserve.32,39 44 Based on this evidence, the most likely mechanism for G-CSF–induced damage of primitive stem cells after multiple doses of cytotoxic agents is the increased proliferation of stem cells in response to G-CSF at the expense of self-renewal. G-CSF–driven differentiation of progenitor cells into mature granuloid lineages would leave the subsequent recovery of the precursor pool to be recruited from primitive stem cells. In the current experiments, where the cytotoxic agent dose was calculated to be equally toxic to the progenitor cell pool (CFU-S), the adverse effect of G-CSF was seen only in animals with cytotoxic agent-induced damage to primitive stem cells. This suggests that mice with limited marrow reserve are the most susceptible to the repeated use of G-CSF; this appears to result from increased proliferative stress on an already damaged primitive stem cell compartment.
Other possible mechanisms for G-CSF–induced stem cell damage include: (1) G-CSF may damage primitive stem cells directly. This appears unlikely because when G-CSF was combined with cyclophosphamide, VP-16+cisplatinum or saline, no permanent reduction in stem cell numbers was observed compared with the use of these agents without G-CSF. (2) G-CSF may cause damage to stromal cells leading to a reduction in the supportive capacity of the hematopoietic microenvironment, or it may reduce adherence of stem cells to the stroma leading to their release into the circulation. Migration of stem cells from the marrow to the peripheral blood and/or spleen by the combined effects of cytotoxic drugs and G-CSF45 would have affected our primitive stem cell measurements, which were restricted to the bone marrow. However, 20 weeks after a mobilization protocol, blood and marrow stem cell levels will most certainly have returned to normal levels. It has been shown that marrow stem cell numbers are normal 2 to 7 weeks after mobilization protocols including G-CSF in mice or baboons.45-47 (3) G-CSF may activate the cycling of primitive stem cells making them more susceptible to the effects of a subsequent cytotoxic agent exposure. This appears to occur with cytotoxic agents that affect cycling cells, such as the combination of 5-FU and kit-ligand.6,7,48 49 There is less support for this possible mechanism in the current study because the drugs that showed the largest effects on stem cells (BCNU, TBI) are not cell cycle-specific.
Additional work is needed to further characterize the mechanisms for and the circumstances under which cytokines such as G-CSF (cytokine dose, number and duration of cycles) damage primitive hematopoietic stem cells. However, data from these experiments suggest that G-CSF, when administered after multiple courses of a cytotoxic agent, may damage marrow self-renewal capacity. This damage appears to occur under circumstances where the self-renewal capacity of the marrow is already compromised.
ACKNOWLEDGMENT
The authors would like to thank Dr Samuel Hellman (A.N. Pritzker Distinguished Professor, University of Chicago) for critically reviewing the manuscript.
Supported by the Grant No. RO1 CA 10941-26 and P50 HL54785-01 from the National Institutes of Health, Bethesda, MD.
Address reprint requests to Ronald van Os, PhD, Joint Center for Radiation Therapy, Department of Radiation Oncology, Harvard Medical School, 330 Brookline Ave, Boston, MA 02215; email:RVANOS@speedy.jcrt.harvard.edu.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.