Abstract
Attempts to expand repopulating hematopoietic cells ex vivo have yielded only modest amplification in stem cell numbers. We now report that expression of an exogenous human multi-drug resistance 1 (MDR1) gene enables dramatic ex vivo stem cell expansion in the presence of early acting hematopoietic cytokines. Bone marrow cells were transduced with retroviral vectors expressing either the MDR1 gene or a variant of human dihydrofolate reductase (DHFR), and then expanded for 12 days in the presence of interleukin-3 (IL-3), IL-6, and stem cell factor. When these cells were injected into nonirradiated mice, high levels of long-term engraftment were only seen with MDR1-transduced grafts. To verify that expansion of MDR1-transduced repopulating cells had occurred, competitive repopulation assays were performed using MDR1 expanded grafts. These experiments showed progressive expansion of MDR1-transduced repopulating cells over the expansion period, with a 13-fold overall increase in stem cells after 12 days. In all of the experiments, mice transplanted with expanded MDR1-transduced stem cells developed a myeloproliferative disorder characterized by high peripheral white blood cell counts and splenomegaly. These results show that MDR1-transduced stem cells can be expanded in vitro using hematopoietic cytokines without any drug selection, but enforced stem cell self-renewal divisions can have adverse consequences.
HEMATOPOIETIC STEM CELLS are characterized by their ability to sustain lympho-myeloid hematopoiesis over the lifetime of an animal. This process of blood formation requires both stem cell divisions that produce lineage-committed progenitors, and self-renewal divisions to maintain the stem cell pool in vivo. The cellular controls for these developmental decisions remain largely unknown, and are a major area of focus in stem cell biology. Elucidation of these mechanisms would be interesting not only at a basic level, but also for application in bone marrow transplantation (BMT) and in hematopoietic cell gene therapy. In particular, the ability to expand repopulating stem cells ex vivo could be used to generate hematopoietic grafts from low numbers of cells and could enhance the efficiency by which stem cells can be modified with genetic vectors.
The identification of a large number of hematopoietic cytokines over the past decade has led to testing whether appropriate culture conditions can be derived for stem cell amplification. To date, no single cytokine has been identified with the ability to promote stem cell self-renewal. In the mouse, the combination of interleukin-3 (IL-3), IL-6, and stem cell factor (SCF) has been shown to enhance retroviral transduction of murine stem cells, presumably by inducing nondifferentiative stem cell divisions.1,2 However, other studies have shown that ex vivo culture in cytokine-containing media can quantitatively diminish the repopulating ability of murine BM grafts.3-5 The modest threefold expansion of murine stem cells obtained using a combination of SCF, Flt-3 ligand, and IL-116 suggests that the particular cytokines used in culture are an important variable. In contrast, more mature progenitor populations such as the murine colony-forming unit-spleen (CFU-S) and colony-forming unit in culture (CFU-C) are capable of extensive proliferation in a variety of culture conditions.7 8
Analogous to the data obtained in the murine system, human hematopoietic stem cells are also refractory to ex vivo expansion despite the relative ease in expanding myeloid CFU-C. Clonogenic myeloid progenitors derived from CD34+ human peripheral blood cells can be extensively expanded in cytokine-containing cultures.9 In vitro expansion has also been shown for the more primitive long-term culture-initiating cell (LTC-IC).10-12 However, it remains controversial whether the LTC-IC assay is a true indicator of human repopulating cells.13-15 A better surrogate may be the severe combined immunodeficient (SCID) mouse repopulating cell (SRC). Recent work has shown that high concentrations of Flt-3 ligand and SCF, together with other cytokines, result in a modest expansion of SRC over 4 days, but that these cultures are depleted after 9 days.16 Therefore, it can be seen that further progress is needed to achieve high levels of ex vivo stem cell amplification.
In this study, we describe an approach that leads to large increases in murine repopulating cells cultured ex vivo. We have found that transduction with a retroviral vector expressing the human multidrug resistance 1 (MDR1) gene allows a greater than 1 log expansion of murine repopulating cells grown in the presence of IL-3, IL-6, and SCF for 12 days. This expansion results in high levels of engraftment in nonirradiated mice, and reconstitution with very small volume fractions of donor marrow in myeloablated recipients. Interestingly, mice transplanted with these expanded cells developed a myeloproliferative syndrome, leading to severe leukocytosis and splenomegaly. These results show the overexpression of the human MDR1 gene product, P-glycoprotein (P-gp), alters the ability of stem cells to respond to hematopoietic cytokines in culture, and suggests a role for expression of endogenous P-gp in the regulation of stem cell growth and differentiation.
MATERIALS AND METHODS
Retroviral producer cell lines and vector constructs.
The Harvey (Ha)MDR1 and HaDHFRL22Y vectors and ecotropic producer cell lines were generated as described previously.17,18 The MDR1 cDNA encodes a protein with the wild-type glycine at amino acid 185.19 This MDR1 cDNA has also been modified as previously described17 to reduce aberrant splicing of viral RNA.20 The DHFRL22YcDNA contains a leucine to tyrosine mutation at codon 22 (L22Y) that greatly increases resistance to the antifolate trimetrexate.18
Retroviral-mediated BM cell transduction.
BM cells were flushed from both hind limbs of either C57/Bl6 (C57) or B6.C-H1/BY (HW80) congenic mouse strains (day −4) and prestimulated for 48 hours in Dulbecco's modified essential medium (DMEM; BioWhittaker, Walkersville, MD) supplemented with 15% fetal bovine serum, 100 U/mL penicillin, and 100 ng/mL streptomycin (P/S; GIBCO-BRL, Gaithersburg, MD). Growth factors were also included in the suspension culture at the following concentrations: 20 ng/mL murine IL-3 (Amgen, Thousand Oaks, CA), 50 ng/mL human IL-6 (Amgen), and 50 ng/mL murine SCF (Amgen and R & D Systems, Minneapolis, MN). After prestimulation (day –2), cells were cocultured on irradiated (1,500 rads) GP + E86 ecotropic producer cell lines for 48 hours in the presence of the same growth factor combination but also with added 6 μg/mL polybrene (Sigma, St Louis, MO).
Ex vivo culture and expansion of myeloid progenitors.
After transduction (day 0), cells were cultured in the presence of the growth factor combination described above. Cells were resuspended at 1 × 106 cells/mL every 3 days for at least 12 days of expansion. Aliquots of cells were removed for CFU-C analysis at various time points. The percentage of drug-resistant progenitors was calculated by plating cells in methylcellulose (Stem Cell Technologies, Vancouver, Canada) in the presence of selective concentrations of drugs. MDR1-transduced progenitors were resistant to 50 nmol/L Taxol (Bristol-Myers Squibb Co, Princeton, NJ) and dihydrofolate reductase (DHFR)-transduced progenitors were resistant to 25 to 50 nmol/L trimetrexate. These concentrations of trimetrexate and taxol completely eliminated nontransduced background colonies when plated in methylcellulose. Colonies derived from clonogenic progenitors were counted after 7 days. Trimetrexate-glucuronate was received as the base from the Drug Synthesis and Chemistry Branch, Developmental Therapeutics Program, Division of Cancer Treatment, National Cancer Institute (Bethesda, MD).
Nonirradiated recipient BMTs.
During BMT into nonirradiated recipients, mice received 5 daily intravenous injections with either MDR1- or DHFR-transduced BM cells (total of 40 to 100 × 106 cells over the 5-day period). Later each day, mice also received an intraperitoneal injection with trimetrexate (130 mg/kg) and the nucleoside transport inhibitor nitrobenzylmercaptopurine riboside phosphate (NBMPR-P; 20 mg/kg). NBMPR-P was included to block thymidine salvage and thereby augment trimetrexate toxicity in myeloid progenitor cells. After this 5-day treatment course, the presence of donor hemoglobin (Hb) was monitored in recipient mice beginning at 1 week and followed for 8 to 14 months. C57/Bl6 donor mice have a single Hb pattern while HW80 have a diffuse Hb pattern when separated on cellulose acetate gels (Helena Laboratories, Beaumont, TX). These Hb patterns were subsequently used for characterization of engraftment.
Competitive repopulation assays.
Expanded MDR1 transduced cells were mixed with expanded DHFRL22Y transduced cells or with freshly harvested marrow at a ratio defined by the percent of the original donor hind limb volume. Cells were mixed thoroughly and injected via the tail vein into lethally irradiated (925 to 1,000 rads) recipient mice. Beginning at 10 weeks posttransplant, Hb patterns were analyzed by electrophoresis on cellulose acetate gels to calculate the relative proportions of single and diffuse donor Hb in reconstituted mice. The results of these analyses were quantitated by densitometry using an AlphaImager 2000 documentation and analysis system (Alpha Innotech Corp, San Leandro, CA).
Secondary BMTs.
BM was obtained from primary transplant recipients 10 to 24 weeks after transplant and injected via the tail vein into lethally irradiated secondary HW80 recipients (925 to 1,000 rads). Secondary transplanted mice received at least 5 × 106 BM cells each. Hb electrophoresis patterns were monitored in secondary recipients after reconstitution (8 to 10 weeks). Secondary CFU-S were obtained from recipient mice 12 days after injection of 1 to 5 × 104 cells.
Southern blot analysis.
Genomic DNA was prepared from CFU-S colonies by proteinase K digestion in the presence of sodium dodecyl sulfate, phenol/chloroform extraction, and ethanol precipitation. Ten to 20 μg of DNA was restricted with EcoR1 and separated on a 1% agarose gel by electrophoresis. Gels were blotted overnight onto Hybond N+nylon membrane (Amersham, Arlington Heights, IL), UV crosslinked, and hybridized with a full-length [32P]-labeled MDR1 cDNA. Blots were washed extensively at 65°C, exposed overnight, and autoradiographic images were obtained using a phosphorimager (Molecular Dynamics, Sunnyvale, CA). [32P]dCTP was obtained from Amersham (Arlington Heights, IL).
Polymerase chain reaction (PCR) assay for replication-competent retrovirus.
Peripheral blood leukocyte DNA from mice with the myeloproliferative disorder was used as a template for PCR. [32P]-labeled deoxycytidine triphosphate (dCTP; 800 mCi/mmol per liter; Amersham) was included in the reaction mixture to increase the sensitivity of the assay. Primers specific for the 3′ end of pol and the 5′ end of env regions of the helper virus genome were used to detect replication-competent retrovirus (RCV).21 PCR was performed under the following conditions: 94°C, 1.5 minutes' denaturation; 55°C, 1.0 minute annealing; 72°C, 1.5 minutes' extension; 28 cycles. As an internal control, β-globin oligos were used under the same cycle parameters.22
Stem cell expansion calculation.
The dose of expanded marrow used for transplant was calculated as a percent of the original hind limb volume used to start the culture. For example, if a culture was initiated with cells obtained from both tibias and femurs from a single mouse, the total stem cell content on day 0 was defined as 1 hind limb volume. In this case, the total cells present after 12 days of expansion would still represent 1 hind limb volume, even if the absolute number of cells expanded 100-fold. This calculation facilitates measurement of changes in the total stem cell content of the cultures, as determined by competitive repopulation assays. Because of the large increases in the total cell number over time, the percent hind limb volume represented by a given number of cells significantly decreased over the 12-day culture period.
RESULTS
Ex vivo expansion of retrovirally transduced murine myeloid progenitors.
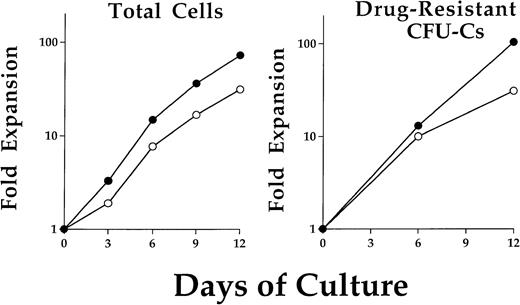
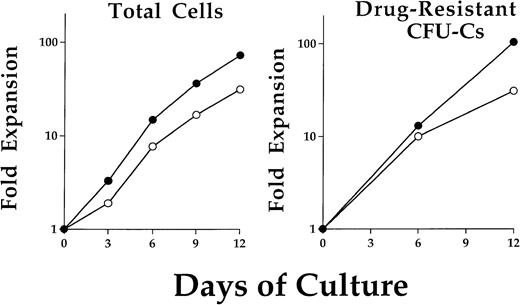
BM cells were obtained from a single C57/Bl6 (C57) mouse and prestimulated for 48 hours in the presence of IL-3, IL-6, and SCF. Cells were then transduced by coculture on either of two retroviral producer cell lines. The two Harvey murine sarcoma virus vectors contained either the stably transmitted human MDR1 cDNA17or a resistance-conferring DHFR mutant which is referred to as DHFRL22Y.18 Immediately after transduction, cells were put into liquid suspension culture at a time referred to as day 0 of expansion. The average progenitor transduction efficiencies on day 0 were: MDR1 Taxol-resistant (40.3% ± 10.2%), DHFR trimetrexate-resistant (39.6% ± 17.8%). Total cells expanded logarithmically over the 12 days in culture (Fig 1, left). At 6-day intervals, an aliquot of cells was removed and plated in semisolid media to assay for myeloid progenitor colony formation. The relative percentage of drug-resistant progenitors remained constant throughout the 12 days in culture. Examples of representative expansions are shown for both MDR1- and DHFR-transduced drug-resistant progenitors (Fig 1, right). Typical expansions yielded an approximate 100-fold increase in the content of progenitors and total cells by 2 weeks and did not differ significantly between the two vectors.
Expansion kinetics for total cells and drug-resistant progenitors after retroviral transduction. Cells were maintained in liquid suspension cultures with addition of IL-3, IL-6, and SCF. A typical cell expansion is shown for cells from either MDR1 (•) or DHFR (○) cocultures (left). No significant difference in cell expansion was noted between these two groups. Cells were removed on day 0, 6, and 12 for clonogenic progenitor assay in methylcellulose. Selective concentrations of Taxol or trimetrexate were used to determine MDR1 or DHFR expressing drug-resistant progenitor cells, respectively. The progenitor population was found to expand at a similar rate as the total cells.
Expansion kinetics for total cells and drug-resistant progenitors after retroviral transduction. Cells were maintained in liquid suspension cultures with addition of IL-3, IL-6, and SCF. A typical cell expansion is shown for cells from either MDR1 (•) or DHFR (○) cocultures (left). No significant difference in cell expansion was noted between these two groups. Cells were removed on day 0, 6, and 12 for clonogenic progenitor assay in methylcellulose. Selective concentrations of Taxol or trimetrexate were used to determine MDR1 or DHFR expressing drug-resistant progenitor cells, respectively. The progenitor population was found to expand at a similar rate as the total cells.
Long-term engraftment of MDR1-transduced hematopoietic stem cells in nonirradiated recipient mice.
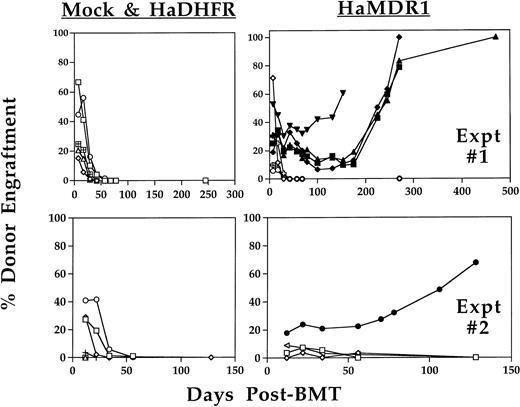
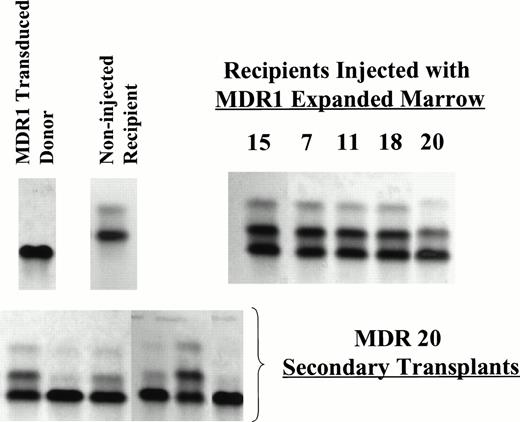
MDR1- or DHFR-transduced and expanded cells were injected into mice with the initial aim of testing whether short-term engraftment of drug-resistant progenitors would be protective against antifolate-induced myelosuppression. HW80 recipient mice were injected daily for 5 days with 12- to 16-day expanded cells at cumulative cell doses of 40 to 100 × 106 cells per mouse. Several hours later each day, recipient mice were treated with trimetrexate in combination with the nucleoside transport inhibitor nitrobenzylmercaptopurine riboside phosphate (NBMPR-P), a drug which increases the hematopoietic toxicity of trimetrexate. After transplant, the proportion of single Hb from C57 donor origin was monitored in the recipient mice beginning at 1 week and continued through greater than 1 year after injection (Fig 2). Partial reconstitution with donor cells was seen at varying levels with both vectors as early as 1 week after the final cell injection. However, this engraftment was only transient in mice receiving DHFR- or mock-transduced marrow (0 of 16; from two separate expansion experiments). By contrast, 5 of 12 mice which received MDR1-transduced marrow showed long-term engraftment stable up to at least 6 months after transplant and up to 14 months for the latest time point obtained. Representative Hb electrophoresis profiles for engrafted recipients showed the presence of C57 donor reconstitution at time points 5 to 7 months after injection (Fig3). In addition, secondary transplant recipients using mouse no. 20 as a donor showed a range of donor engraftment from 50% to 100% in secondary mice, indicating reconstitution with primitive long-term repopulating cells of C57 donor origin. Southern blot analysis on DNA from BM, spleen, thymus, and peripheral blood confirmed multilineage engraftment (data not shown). Expression of P-glycoprotein was seen by flow cytometry analysis of red blood cells from all four mice engrafted from experiment 1 at time points greater than 10 weeks following transplant (data not shown). Because the donor to recipient ratio of 1:12 was highly unfavorable for long-term engraftment, the high levels of engraftment obtained suggested that a large stem cell expansion had occurred ex vivo.
Long-term analysis of engraftment with donor BM in nonirradiated recipients. HW80 recipient mice were injected for 5 consecutive days with transduced BM cells (C57) that had been expanded in culture for 12 to 16 days. Later the same day, mice were treated with trimetrexate (130 mg/kg) and NBMPR-P (20 mg/kg). Beginning at 1 week posttransplant, donor C57 Hb levels were quantitated by electrophoresis on cellulose acetate gels. Persistent engraftment was only seen in mice receiving expanded BM cells transduced with MDR1 (5 of 12). Engrafted mice included: MDR 7 (▪), MDR 11 (▴), MDR 18 (⧫), MDR 20 (▾), and from the second experiment MDR 15 (•). No stable engraftment was seen with mock-transduced (0 of 8) or DHFR-transduced (0 of 8) expanded BM. Engraftment levels in mice receiving mock-transduced BM (+ symbol) were low to undetectable, thus many of these symbols are not seen on the graph. Mice without evidence of long-term engraftment are indicated with open symbols. Shown are mice from two separate expansion experiments.
Long-term analysis of engraftment with donor BM in nonirradiated recipients. HW80 recipient mice were injected for 5 consecutive days with transduced BM cells (C57) that had been expanded in culture for 12 to 16 days. Later the same day, mice were treated with trimetrexate (130 mg/kg) and NBMPR-P (20 mg/kg). Beginning at 1 week posttransplant, donor C57 Hb levels were quantitated by electrophoresis on cellulose acetate gels. Persistent engraftment was only seen in mice receiving expanded BM cells transduced with MDR1 (5 of 12). Engrafted mice included: MDR 7 (▪), MDR 11 (▴), MDR 18 (⧫), MDR 20 (▾), and from the second experiment MDR 15 (•). No stable engraftment was seen with mock-transduced (0 of 8) or DHFR-transduced (0 of 8) expanded BM. Engraftment levels in mice receiving mock-transduced BM (+ symbol) were low to undetectable, thus many of these symbols are not seen on the graph. Mice without evidence of long-term engraftment are indicated with open symbols. Shown are mice from two separate expansion experiments.
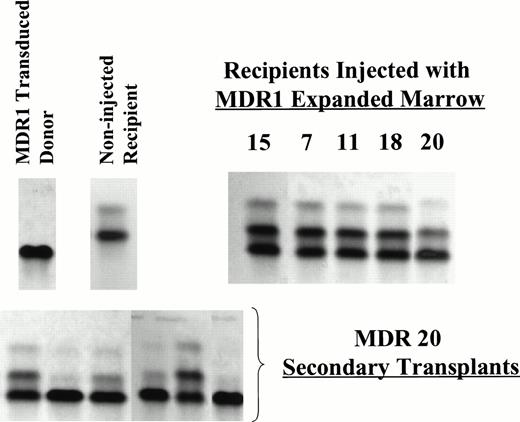
Representative Hb electrophoresis gels from nonirradiated mice engrafted with expanded BM. C57 BM was used as the donor marrow for transduction and expansion. Recipient mice were HW80. The differing Hb patterns for donor and recipient are indicated (upper left). Primary recipients shown are MDR 7, 11, 18 (7 months post BMT), MDR 20 (5 months post-BMT) from experiment 1 and MDR 15 (3 months post-BMT) from experiment 2. Secondary irradiated recipients (HW80) were transplanted with marrow cells from MDR 20 (below) demonstrating persistence of donor engraftment 8 weeks after secondary transplant.
Representative Hb electrophoresis gels from nonirradiated mice engrafted with expanded BM. C57 BM was used as the donor marrow for transduction and expansion. Recipient mice were HW80. The differing Hb patterns for donor and recipient are indicated (upper left). Primary recipients shown are MDR 7, 11, 18 (7 months post BMT), MDR 20 (5 months post-BMT) from experiment 1 and MDR 15 (3 months post-BMT) from experiment 2. Secondary irradiated recipients (HW80) were transplanted with marrow cells from MDR 20 (below) demonstrating persistence of donor engraftment 8 weeks after secondary transplant.
Quantitation of expanded MDR1-transduced hematopoietic stem cells by competitive repopulation assays.
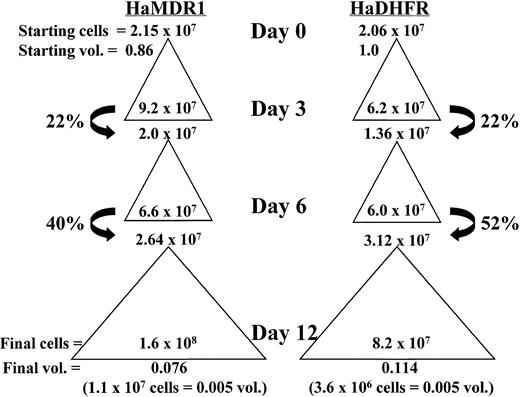
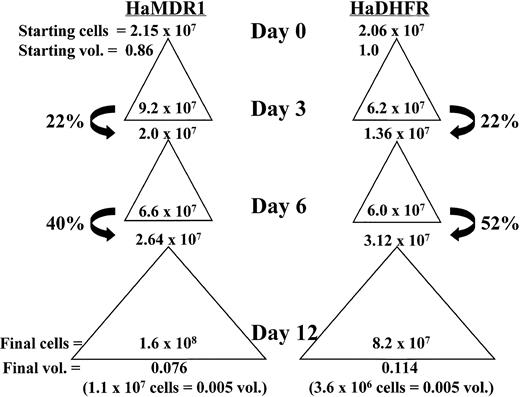
To examine whether stem cell expansion was responsible for the high levels of engraftment obtained in the nonirradiated model, a third expansion was analyzed using a competitive repopulation assay. MDR1- and DHFR-transduced cells were expanded over a 12-day period as described above and competed either against fresh BM or against each other after injection into lethally irradiated recipient mice. Figure 4 illustrates an example of a typical expansion and indicates how percent hind limb volumes were calculated. Beginning at 10 weeks, the levels of reconstitution in recipient mice were analyzed by Hb electrophoresis (Fig 5). MDR1-expanded cells were competed against fresh BM cells at a 0.005/0.25 hind limb volume ratio. If the expansion had no effect on the absolute number of stem cells, this ratio should give 2% reconstitution with the C57 donor pattern. The actual observed value was 26% donor reconstitution, indicating that a 13-fold expansion in the overall content of repopulating cells had occurred. By contrast, when DHFR-expanded marrow was mixed with fresh marrow at the same ratio (middle), no engraftment could be detected from the expanded cells. Finally, when MDR1-expanded cells were mixed with an equal volume of DHFR-expanded marrow, the MDR1 graft completely outcompeted DHFR marrow, indicating a much greater stem cell content in the MDR1 expanded graft (right).
Total cell expansion and percent hind limb volume calculation of cultured cells from a representative experiment. Starting cell numbers before expansion are indicated at the top of each triangle and represent either donor volume fractions (vol.) of 0.86 or 1.0 for HaMDR1 or HaDHFR, respectively. Total cell numbers expanded during each culture period and this expansion is represented by a triangle. The cell numbers before replating on days 3, 6, and 12 are indicated inside the base of each triangle. On days 3 and 6 only a fraction of the cells were replated (arrows) while the rest were discarded. This resulted in a decrease in the fraction donor volume remaining. Thus, on day 12 the fraction donor hind limb volume remaining was very low (bottom of each column) despite the significant increase in cell number relative to day 0. The number of cells injected into individual mice, and the corresponding donor volumes they represent, are shown below in parentheses.
Total cell expansion and percent hind limb volume calculation of cultured cells from a representative experiment. Starting cell numbers before expansion are indicated at the top of each triangle and represent either donor volume fractions (vol.) of 0.86 or 1.0 for HaMDR1 or HaDHFR, respectively. Total cell numbers expanded during each culture period and this expansion is represented by a triangle. The cell numbers before replating on days 3, 6, and 12 are indicated inside the base of each triangle. On days 3 and 6 only a fraction of the cells were replated (arrows) while the rest were discarded. This resulted in a decrease in the fraction donor volume remaining. Thus, on day 12 the fraction donor hind limb volume remaining was very low (bottom of each column) despite the significant increase in cell number relative to day 0. The number of cells injected into individual mice, and the corresponding donor volumes they represent, are shown below in parentheses.
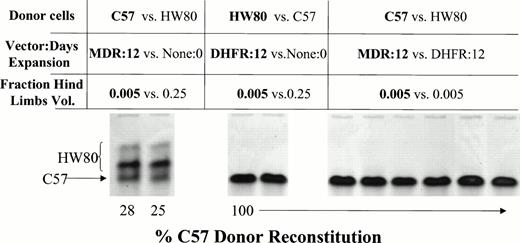
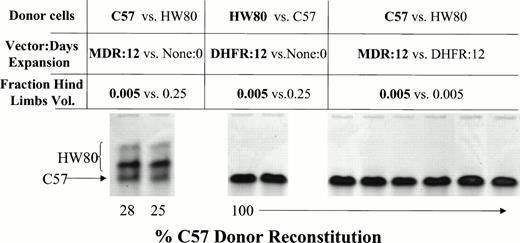
Competitive repopulation assay to determine the relative stem cell content of ex vivo–expanded BM versus fresh nonexpanded marrow. C57 donor BM cells were transduced with the HaMDR1 vector. HW80 donor BM cells were transduced with the HaDHFR vector. Cells were expanded for 12 days in culture and then combined at the indicated hind limb volumes. 0.005 hind limb volumes of expanded cells were competed against 0.25 hind limb volumes of fresh competing marrow. MDR1 expanded cells competed effectively against fresh HW80 marrow despite the unfavorable donor volume ratio (left). DHFR-expanded marrow was completely outcompeted by fresh C57 marrow (middle). When MDR1 (C57) cells were competed against DHFR (HW80) cells at equal hind limb volumes, mice reconstituted solely with MDR1 marrow, indicating a much greater stem cell content (right; mouse identification numbers are 5, 6, 7, 8, 9, and 10).
Competitive repopulation assay to determine the relative stem cell content of ex vivo–expanded BM versus fresh nonexpanded marrow. C57 donor BM cells were transduced with the HaMDR1 vector. HW80 donor BM cells were transduced with the HaDHFR vector. Cells were expanded for 12 days in culture and then combined at the indicated hind limb volumes. 0.005 hind limb volumes of expanded cells were competed against 0.25 hind limb volumes of fresh competing marrow. MDR1 expanded cells competed effectively against fresh HW80 marrow despite the unfavorable donor volume ratio (left). DHFR-expanded marrow was completely outcompeted by fresh C57 marrow (middle). When MDR1 (C57) cells were competed against DHFR (HW80) cells at equal hind limb volumes, mice reconstituted solely with MDR1 marrow, indicating a much greater stem cell content (right; mouse identification numbers are 5, 6, 7, 8, 9, and 10).
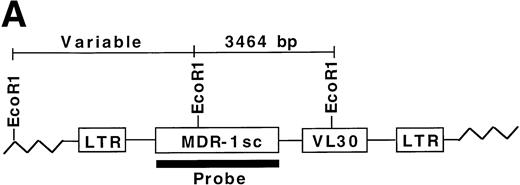
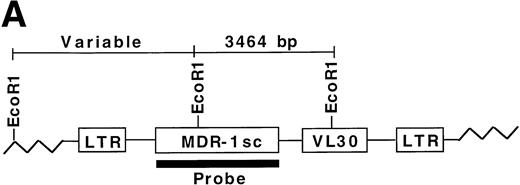
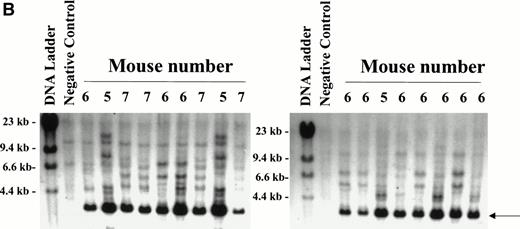
All expanded stem cells contain MDR1 proviral DNA.
To determine whether all engrafted donor cells were transduced with the MDR1 vector, secondary day 12 CFU-S obtained from recipients of MDR1 expanded grafts were analyzed for proviral sequences. Genomic DNA from individual CFU-S was digested with EcoR1 and probed with a full-length MDR1 probe (Fig 6A). A total of 88/88 CFU-S from 7 primary recipients (6 MDR1 v DHFR mice from competitive repopulation experiment 1, and MDR no. 15 from nonirradiated experiment 2) were shown to be positive by Southern blot for the MDR1 provirus, giving a band of the expected size (3,464 bp). Shown are 17 representative CFU-S analyzed by Southern blot (Fig 6B). These results indicate that only MDR1-transduced stem cells were expanded in culture. Integration-site patterns were also analyzed to determine if the stem cell expansion was polyclonal. Five separate clones could be seen in CFU-S from three mice receiving cells from the same expansion pool. In addition, some individual primary mice showed engraftment with multiple clones (CFU-S from mouse no. 6). This result indicates that expansion of repopulating stem cells in culture was polyclonal and strongly links the presence of the retroviral transgene and expansion of primitive stem cells.
Secondary CFU-S analysis for HaMDR1-transduced primitive hematopoietic cells. At time points from 10 to 24 weeks posttransplant, primary recipients from MDR1 versus DHFR competitive repopulation mice (n = 6) and MDR 15 were killed and BM cells were injected into secondary recipients. Day 12 CFU-S were obtained and DNA was prepared for Southern blot analysis. (A) DNA was restricted with EcoR1 and hybridized with a full-length MDR1 probe that allowed for simultaneous identification of a known fragment size and integration-site analysis. (B) A band of the expected size (3,464 bp, arrow) was seen in all CFU-S examined (88 of 88) from seven individual primary mice. Seventeen representative CFU-S are shown from primary mice nos. 5, 6, and 7 from Fig 5. Negative controls included CFU-S from mice transplanted with untransduced BM and showed two bands presumably from the endogenous MDR genes. Multiple integration patterns could be seen in CFU-S from mice receiving cells from the same expansion pool. Mouse no. 6 in particular showed engraftment with multiple stem cell clones.
Secondary CFU-S analysis for HaMDR1-transduced primitive hematopoietic cells. At time points from 10 to 24 weeks posttransplant, primary recipients from MDR1 versus DHFR competitive repopulation mice (n = 6) and MDR 15 were killed and BM cells were injected into secondary recipients. Day 12 CFU-S were obtained and DNA was prepared for Southern blot analysis. (A) DNA was restricted with EcoR1 and hybridized with a full-length MDR1 probe that allowed for simultaneous identification of a known fragment size and integration-site analysis. (B) A band of the expected size (3,464 bp, arrow) was seen in all CFU-S examined (88 of 88) from seven individual primary mice. Seventeen representative CFU-S are shown from primary mice nos. 5, 6, and 7 from Fig 5. Negative controls included CFU-S from mice transplanted with untransduced BM and showed two bands presumably from the endogenous MDR genes. Multiple integration patterns could be seen in CFU-S from mice receiving cells from the same expansion pool. Mouse no. 6 in particular showed engraftment with multiple stem cell clones.
Expansion of repopulating cells increases with period of time in culture.
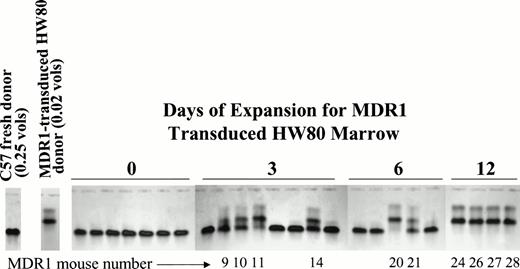
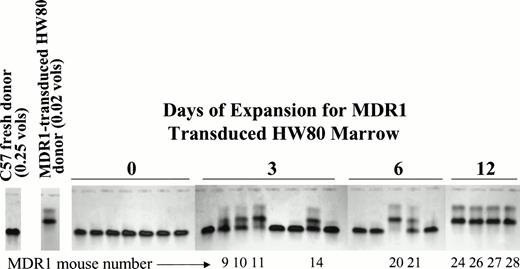
To determine the kinetics of stem cell expansion during culture, a fourth stem cell expansion was performed. For this experiment, the donor background was switched to eliminate any possibility that engraftment was related to the donor origin. HW80 BM cells were transduced with the MDR1 retrovirus and 0.02 donor volumes were competed against 0.25 donor volumes of fresh C57 marrow cells after 0, 3, and 6 days of expansion. After 12 days, the cell dose was reduced in half to 0.01 donor volumes of MDR1 versus 0.125 donor volumes fresh BM, while keeping the same ratio of cells. Engraftment with MDR1 marrow was only seen after at least 3 days of expansion and increased progressively with time in culture (Fig 7). By day 12, MDR1-expanded marrow grafts completely outcompeted fresh marrow despite the 12.5-fold dilution at the time of transplant. These results confirm a progressive increase in stem cell numbers occurring over the 12-day culture period.
Kinetic analysis of HaMDR1 transduced stem cell expansion. BM cells were transduced with the HaMDR1 retrovirus and expanded for the indicated time periods. HaMDR1 cells (0.02 hind limb volumes/HW80 background) were combined with fresh competed marrow (0.25 hind limb volumes/C57 background) and injected into lethally irradiated mice. Unexpanded MDR1 BM (day 0) did not result in reconstitution with MDR1-transduced cells. However, expansion for 3 to 12 days resulted in a progressive increase in the percentage of engraftment of MDR1 (HW80) marrow. The data shown are for mice analyzed 9 months after transplant. Identification numbers for mice engrafted with expanded BM are indicated below each lane. Due to the large number of cells on day 12, mice were injected with 0.01 hind limb volumes of HaMDR1 cells versus 0.125 hind limb volumes of fresh C57 cells.
Kinetic analysis of HaMDR1 transduced stem cell expansion. BM cells were transduced with the HaMDR1 retrovirus and expanded for the indicated time periods. HaMDR1 cells (0.02 hind limb volumes/HW80 background) were combined with fresh competed marrow (0.25 hind limb volumes/C57 background) and injected into lethally irradiated mice. Unexpanded MDR1 BM (day 0) did not result in reconstitution with MDR1-transduced cells. However, expansion for 3 to 12 days resulted in a progressive increase in the percentage of engraftment of MDR1 (HW80) marrow. The data shown are for mice analyzed 9 months after transplant. Identification numbers for mice engrafted with expanded BM are indicated below each lane. Due to the large number of cells on day 12, mice were injected with 0.01 hind limb volumes of HaMDR1 cells versus 0.125 hind limb volumes of fresh C57 cells.
A myeloproliferative disorder results from stem cell expansion.
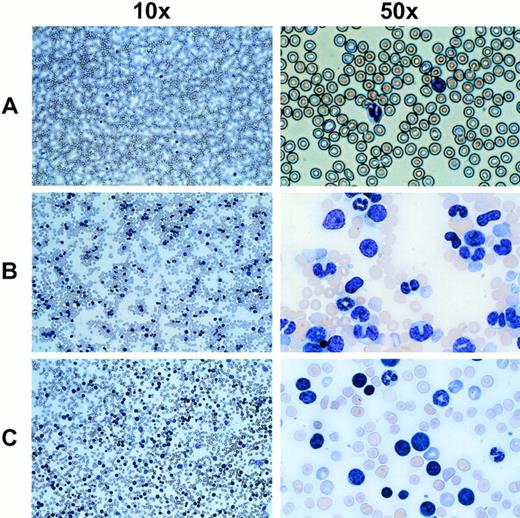
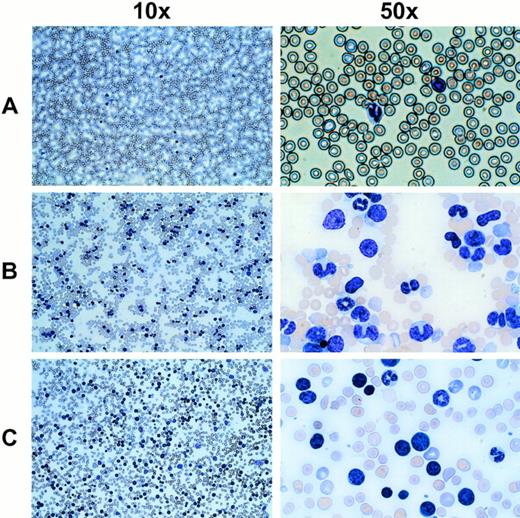
In mice receiving MDR1-expanded grafts, there was a prolonged 3- to 4-month period of abnormal leukocyte counts fluctuating from normal levels to approximately 30,000/μL. As an example, 8 of 10 mice numbered in Fig 7 showed a rapid increase in leukocyte counts at 5 to 7 months after transplant. In most cases, the acute phase of leukocyte increase was extremely rapid and increased by as much as 10-fold within a few days. White blood cell counts ranged from 100,000 to 450,000 cells/μL for MDR1 mice (P < .001) which developed the disorder (n = 24). Analysis of Wright-stained blood smears showed a relative increase in an abnormal cell population (Fig 8). Immunophenotyping of the abnormal population showed a high percentage of GR-1+ and MAC-1+ cells (data not shown), consistent with the granulocytic morphology of the majority of these cells. In a few instances (4 of 24 mice), blasts were seen in the peripheral blood consistent with progression into leukemia (Fig 8C). These cells did not stain positively for any lineage marker (data not shown). The disease was found to be transplantable into secondary recipients, which rapidly developed the same leukocytosis (data not shown), even in some cases where the primary donor mouse had normal blood counts at the time of secondary transplant. Integration-site analysis on peripheral blood DNA from mice with the myeloproliferative disorder showed multiple transduced clones (data not shown), indicating that the disorder was not strictly monoclonal in origin.
Wright-stained peripheral blood smears from mice displaying an abnormal cell population and elevated leukocyte counts. (A) A normal untransplanted mouse blood smear at the indicated magnifications is shown in the top panel. (B) The middle panel shows the most common phenotype seen with MDR1 expanded cells, which includes a large number of cells with a granulocytic morphology. (C) In a few cases, a leukemic blast phenotype was seen (4 of 24 mice), indicating possible transformation from a myeloproliferative disorder into a less differentiated leukemia.
Wright-stained peripheral blood smears from mice displaying an abnormal cell population and elevated leukocyte counts. (A) A normal untransplanted mouse blood smear at the indicated magnifications is shown in the top panel. (B) The middle panel shows the most common phenotype seen with MDR1 expanded cells, which includes a large number of cells with a granulocytic morphology. (C) In a few cases, a leukemic blast phenotype was seen (4 of 24 mice), indicating possible transformation from a myeloproliferative disorder into a less differentiated leukemia.
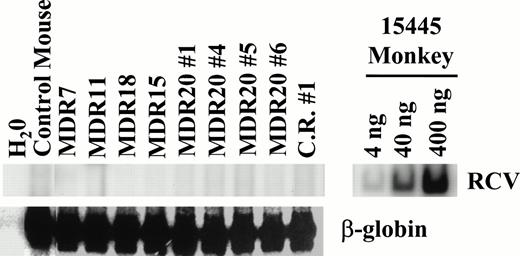
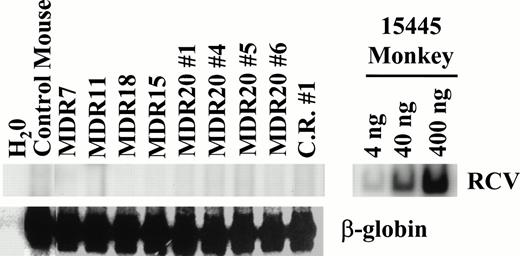
Replication-competent retrovirus (RCR) assays were performed extensively on both transduced cell lines and plasma from mice with the myeloproliferative disorder to rule out wild-type virus as the cause of the disorder. A very sensitive PCR assay for helper virus did not detect the helper genome but was highly positive when using positive control monkey DNA23,24 (Fig9). In addition, marker rescue assays on Mus dunni cells25eliminated the possibility of contamination with retroviruses of a wide host range (data not shown). These complementary data indicate that the stem cell expansion and myeloproliferative disorder are not due to a contamination of helper virus.
Assay for RCR. PCR was performed on peripheral blood leukocyte DNA obtained from mice with a myeloproliferative disorder. DNA from an infected monkey 15445 served as a positive control for PCR and demonstrates the sensitivity of the assay (upper right). For test samples, 200 ng of template DNA was used for PCR with either oligos specific for RCV or for β-globin that served as an internal control. No helper genome was detectable in all samples tested. At the time of analysis all mice had elevated leukocyte counts except for MDR11. C.R. no. 1 is from the competitive repopulation experiment 1 (MDR1 vfresh marrow). MDR20 nos. 1, 4, 5, and 6 represent secondary transplanted mice from MDR20.
Assay for RCR. PCR was performed on peripheral blood leukocyte DNA obtained from mice with a myeloproliferative disorder. DNA from an infected monkey 15445 served as a positive control for PCR and demonstrates the sensitivity of the assay (upper right). For test samples, 200 ng of template DNA was used for PCR with either oligos specific for RCV or for β-globin that served as an internal control. No helper genome was detectable in all samples tested. At the time of analysis all mice had elevated leukocyte counts except for MDR11. C.R. no. 1 is from the competitive repopulation experiment 1 (MDR1 vfresh marrow). MDR20 nos. 1, 4, 5, and 6 represent secondary transplanted mice from MDR20.
In addition to the elevated peripheral leukocyte count, the number of clonogenic myeloid progenitors in the blood and spleen increased dramatically. Typical progenitor concentrations in the peripheral blood of a normal animal were 1 to 4 CFU-C per 105 cells. Progenitor counts in MDR1 mice ranged from 57 to 1,290 CFU-C per 105 cells. Splenomegaly was also seen in mice with the myeloproliferative disorder. Spleen weights ranged from 483 to 834 mg compared with 106 ± 48 mg for normal mice. The progenitor content in the spleen was concomitantly increased from an average of 11 per 105 to 180 per 105 cells. Expression of P-gp could be detected in both normal and abnormal leukocytes, but expression levels varied from mouse to mouse (data not shown). Also, the levels of drug-resistant progenitors in the blood and spleen were variable. Taxol-resistant progenitors in mice ranged from 1.1% to 6.7% in the blood and as high as 34.7% in the spleen. BM cellularity was found to be normal in all mice examined.
Despite the abnormal hematologic phenotype, most mice appeared asympotmatic even with the highest white blood cell counts. Analysis of the BM showed no increase in the proportion of myeloblasts. In addition, the mouse karyotype was normal and there were no chromosome translocations present in peripheral blood metaphases from two mice examined. Peripheral blood cells from several diseased mice were also injected into SCID mice without the development of tumors. These data are consistent with a prolonged period of abnormal myeloproliferation with transformation to leukemia in only a minority of mice.
DISCUSSION
In summary, we have shown that culturing MDR1-transduced BM cells for 12 days in IL-3, IL-6, and SCF results in a dramatic polyclonal expansion of repopulating cells. It is important to note that no drug selection was used either during the in vitro expansion or in vivo after transplant. This expansion was absolutely dependent on transduction with the MDR1 vector and was not seen with the control DHFR vector or mock-transduced cells. Furthermore, the MDR1 provirus was present in 100% of CFU-S from mice transplanted with expanded marrow, indicating that only MDR1-transduced stem cells were expanded during culture. This latter finding suggests that the calculated 13-fold expansion of total repopulating cells within the culture is an underestimate of actual expansion of transduced stem cells. If only 20% of the stem cells were initially transduced, a typical frequency obtained in murine retroviral-mediated gene transfer experiments, then the 13-fold increase in overall repopulating activity indicates a 65-fold expansion of transduced stem cells. Compared with previous studies of ex vivo stem cell expansion,6 the MDR1-facilitated expansion is of much greater magnitude and can be sustained for significantly longer periods of time in culture.
These results show that the MDR1 gene product, P-gp, can influence self-renewal decisions in repopulating stem cells. This finding is consistent with previous studies showing that MDR1 vectors enhance serial transplantation in mice, presumably by causing stem cell expansion in vivo.26 The mechanism by which MDR1 vectors facilitate stem cell expansion is unknown. Most previous attempts to induce stem cell amplification ex vivo have resulted in a quantitative depletion of repopulating cells.4,27 This loss can be caused either by induction of differentiation28or by initiation of apoptosis.29 One possibility is that P-gp is modulating an apoptotic mechanism that normally holds cytokine-induced self-renewal divisions in check. There is accumulating evidence that P-gp can protect against apoptosis in a number of systems.30-32 The mechanism for this anti-apoptotic effect has been related to P-gp–mediated changes in the intracellular concentration of ceramide or the membrane distribution of phosphatidylserine.33,34 The specific substrates that are being modulated within stem cells are unknown but will be of great interest. Considering that various mouse and human P-gps have different substrate affinity profiles,19,35 it is important to note that the effects we have observed could be specific to the MDR1 cDNA used in our vector. In particular, it is known that the amino acid residue at codon 185 is an important determinant of substrate specificity. The cDNA used in these experiments has a glycine residue at position 185,17 rather than the common valine substitution. Therefore, transduction with the MDR1 vector may act by introducing a new substrate affinity that is not endogenously present in murine stem cells. Alternately, vector transduction could act by simply increasing the overall level of P-gp expression in stem cells, as has been previously described.20
Another possibility is that constitutive expression of the exogenously introduced MDR1 gene may prevent stem cell differentiation, leading to stem cell amplification in culture. The potential role of P-gp expression in maintaining the pluripotentiality of stem cells is suggested by the sharp downregulation of endogenous P-gp expression during myeloid development.36 In fact, P-gp expression is a relatively specific marker for hematopoietic stem cells and has been used in a number of stem cell isolation protocols.37-40Therefore, it is plausible to speculate that enforced expression of P-gp throughout development could partially block myeloid differentiation. The association of persistent P-gp expression in some cases of acute myelogenous leukemia is consistent with this interpretation.41-43 To the contrary, the lack of an obvious phenotype in mice bearing genetic disruptions of the endogenous MDR1-like genes is an argument against P-gp having a role in hematopoietic development.44-46 Given that the stem cell mass in these mice has not been specifically studied, a perturbation in the stem cell compartment cannot be ruled out at this time. Alternately, another endogenous P-gp-like transporter could be providing a redundant function in stem cells from these knockout mice.
The other main finding in our studies was that the expanded grafts caused a myeloproliferative syndrome in transplanted mice. This disorder was only seen in association with stem cell expansion, and directly correlated with the number of days of ex vivo culture. Therefore, these results are not inconsistent with prior studies showing that unexpanded MDR1-transduced cells can be safely used in both preclinical models22,26,47 and in phase I clinical trials.48 49 Based on these findings, we hypothesize that the myeloproliferative syndrome is a consequence of the ex vivo expansion rather than any direct result of the MDR1 vector per se. The rapid self-renewal divisions induced by this system may lead to stem cell replication errors. If this interpretation is correct, it could indicate that any method that enforces rapid stem cell replication in vitro has the potential to result in secondary genetic damage. Although we saw no adverse effects in mice transplanted with unexpanded cells, our results raise questions about the safety of using MDR1 vectors for clinical myeloprotection strategies. The fact that some mice developed a myeloproliferative syndrome with as little as 3 days of expansion suggests that shorter periods of culture with MDR1 vectors in the presence of early acting cytokines could result in a low incidence of this complication.
Ultimately, elucidation of the mechanisms leading to MDR1-mediated stem cell expansion and myeloproliferation could provide important insights regarding the molecular control of stem cell self-renewal divisions. As a first step, it will be important to determine if the observed effects are specific to the vector configuration. We have seen several cases of the myeloproliferative syndrome using another Harvey-based vector (data not shown) that expresses both the MDR1 and DHFR cDNAs.17It is interesting to note that the Harvey murine sarcoma vector contains endogenous rat VL30 sequences50 that could be necessary for the proliferative effects. This question can be addressed by constructing alternate MDR1 vectors that lack these sequences. However, the VL30 elements are clearly not sufficient for inducing the phenotype, as shown by the lack of stem cell amplification using the Harvey-based DHFR vector. It will also be important to determine whether stem cell expansion and the myeloproliferative disorder are invariably linked. If these two effects can be segregated, perhaps by titrating P-gp expression through transcriptionally regulated vectors, or by decreasing the proliferative rate with alternate cytokine mixtures, then a modification of this system could be used for BMT and gene therapy applications.
ACKNOWLEDGMENT
The authors thank Sarah Wilkinson and James Allay for technical assistance. We also thank Susan Ragsdale and John Cunningham for assistance with mouse karyotyping and cytogenetics. We are grateful to Elio Vanin for providing RCR+ control monkey DNA and Mus dunni/G1Na cells for use in assays for detecting helper virus.
Supported in part by National Heart, Lung, and Blood Institute Program Project Grant No. P01 HL 53749, The James S. McDonnell Foundation Grant No. 94-50, US Public Health Service Grant No. P01 CA 31922, Cancer Center Support Grant No. P30 CA 21765, and the American Lebanese Syrian Associated Charities (ALSAC).
Address reprint requests to Brian P. Sorrentino, MD, Department of Biochemistry and Hematology/Oncology, St Jude Children's Research Hospital, 332 N Lauderdale, Memphis, TN 38105; e-mail:brian.sorrentino@stjude.org.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.