Abstract
Activity of the c-jun N-terminal kinase (JNK) has been shown in hematopoietic cells transformed by p210 BCR-ABL. However, analysis has not been reported for hematopoietic cells on the consequences of this activity for c-jun promoter regulation within its distinctive proximal 8-base consensus CRE-like element, an element linked to JNK-mediated increase in c-jun transcription. In the present study, regulation of the proximal c-jun promoter was studied in murine myeloid cells transformed by p210 BCR-ABL. Promoter regulation in p210 BCR-ABL transformed cells was compared with regulation of the promoter in nontransformed interleukin-3 (IL-3)–dependent parental cells. The composition of nuclear AP-1 proteins contained within cells with p210 BCR-ABL, and their binding to the c-jun promoter proximal CRE-like element, was compared with the composition and binding of AP-1 proteins in IL-3–treated parental cells without p210 BCR-ABL. The present analysis found fivefold increased c-jun transcription occurring in p210 BCR-ABL transformed murine myeloid cells possessing a corresponding magnitude of increased kinase activity of JNK, compared with IL-3–stimulated parental cells. Augmented JNK activity was accompanied by increased nuclear abundance of c-jun and c-fos proteins that bound specifically to the proximal c-jun promoter CRE element. Also, representative human leukemic cell lines expressing p210 BCR-ABL and possessing abundant kinase activity of JNK, when compared with parental cells that were deficient in JNK activity, had increased c-jun and c-fosproteins. Finally, to show the relevance of these observations in model systems, we studied blast cells from patients with Philadelphia chromosome–positive acute leukemic transformation, and observed comparable activities of JNK catalysis and c-jun/AP-1 protein relative to the cell lines that possessed p210 BCR-ABL and JNK activity. These studies provide a basis for investigating the set of downstream genes which augmented c-jun/AP-1 activity enlists in the process of transformation by p210 BCR-ABL.
EXPRESSION OF THE nuclear-localized product of the c-jun early response gene is required for cellular transformation by the p21ras oncogene and for the cellular proliferation and migration occurring during early embryogenesis, as shown by a model of c-jun knockout in mice.1,2 In addition, disruption of the cytoplasmic-to-nuclear signal transduction cascade targeted for c-jun transcriptional control is also embryo-lethal.3 Such disruption can be accomplished by genomic knockout of the gene encoding the dual specificity serine/tyrosine kinase, SEK1. SEK1 is a major immediate upstream regulator of the serine/theonine kinase called c-jun N-terminal kinase (JNK) or stress-activated protein kinase (SAPK).4SEK1 activates JNK by phosphorylating threonine and tyrosine residues within an amino acid sequence motif containing threonine separated from tyrosine by a proline residue, TPY.5
Active JNK exerts its serine kinase function on substrate with specificity for the N-terminal regions of c-jun (serines 63 and 73) and for ATF.5,6 JNK also phosphorylates a domain within the Elk-1 (ets-related) transcription factor.7Phosphorylation of these transcription factors by JNK augments their interaction with the basal transcriptional machinery, and, hence, enhances their transactivating potential on a collection of genes relevant for cellular transformation. In the case of the phosphorylated c-jun protein, the most important promoter target may be that of the c-jun gene itself.8 JNK-mediated posttranslational modification on the c-jun protein leads to augmented c-jun/AP-1–driven c-jun transcription in a positive autoregulatory loop.5 8
It has been proposed that upstream signals emanating from either p21ras or p21rac, in their guanosine triphosphate (GTP)-bound active forms, can activate JNK and thus lead to augmented c-jun transcription.9-12 This function of oncogenic p21ras was the basis for initial purification and characterization of JNK in the laboratory of Karin,9 whereas the JNK/SAPK enzyme was characterized by others for its stress functions.13
Our laboratory observed strong activity of p210 BCR-ABL (the nonreceptor tyrosine kinase oncogene product of the Philadelphia chromosome that is an initiating cause of chronic granulocytic leukemia) for maintaining the activation state of p21ras in its GTP-bound form.14 In consideration of this, it may not be surprising that another laboratory observed high activity of JNK conferred by p210 BCR-ABL.15 Most importantly, nonfunctional c-jun mutants, expressed in cells along with p210 BCR-ABL, exerted dominant-negative activity for cellular transformation by p210 BCR-ABL.15 Therefore, full characterization of the mode of c-jun transcriptional regulation in cells with p210 BCR-ABL, and the role of JNK in establishing the content of nuclear AP-1 proteins, is important to further understanding of the pathophysiology of chronic granulocytic leukemia, CGL.
As part of a previous characterization of the consequences of JNK activity within p210 BCR-ABL transformed fibroblasts, strong transcriptional activity of a 7-base consensus AP-1 binding enhancer/promoter element derived from the collagenase gene was observed, and was dependent on the cellular activity of p21ras, on MEKK, an upstream regulator of SEK1, and on JNK itself.15However, JNK-mediated increase in c-jun transcription is a function of regulatory events on the distinctive 8-base consensus CRE-like element of the proximal c-jun promoter, which is capable of binding other AP-1 factors, including ATF. In this report we studied the activity and regulation of the proximal c-jun promoter in model myeloid cell lines that differ only in expression of p210 BCR-ABL. In addition, the occurrence of high activity of JNK, associated with abundant c-jun/AP-1 protein, was shown in primary hematopoietic cells in blast transformation of Philadelphia chromosome–positive leukemia.
MATERIALS AND METHODS
Cells and cell culture.
The growth factor–independent murine myeloid cell line transformed with an expression vector for p210 BCR-ABL, H7 Bcr-Abl.A54, has been described previously.14 These cells were maintained in McCoy's medium supplemented with 10% fetal calf serum (FCS). The parental interleukin-3 (IL-3)–dependent cell line NFS/N1.H7 (H7) was passaged in McCoy's medium plus 10% FCS supplemented with conditioned medium from the WEHI-3 cell line as a rich source of IL-3. The human chronic granulocytic leukemia blast cell line, K562, was obtained from the Cell Depository of the American Type Culture Collection (ATCC, Rockville, MD), as was the myelocytic leukemia cell line, HL-60. The human factor–dependent cell line M07e was passaged in Iscove's modified Dulbecco's medium (IMDM) plus 20% FCS with the addition of granulocyte-macrophage colony stimulating factor (GM-CSF). A transformed factor-independent variant of M07, arising as a result of transfection with p210BCR-ABL, M07p210 BCR-ABL, was used.16 It was passaged in IMDM plus 20% FCS. Blast cells from the bone marrow of patients with blast transformation of Philadelphia chromosome–positive leukemia were obtained at the time of diagnostic testing after informed consent. These cells were diluted 1:4 with Hanks' balanced salt solution and layered onto Ficoll-Hypaque (Pharmacia, Uppsala, Sweden). After centrifugation for 30 minutes at 1,200 rpm, the buoyant fraction was isolated and then washed with phosphate-buffered saline (PBS) before processing. Those cells to be assayed directly in kinase assays (see below) were washed in ice-cold PBS with 1 mmol/L sodium orthovanadate (Na3VO4). The cell pellet was lysed in sample buffer (20 mmol/L TrisHCl, pH 8.0, 137 mmol/L NaCl, 10% glycerol, 1 mmol/L phenylmethyl sulfonyl fluoride [PMSF], 1 μg/mL aprotonin, 2 mmol/L EDTA, 10 μg/mL leupeptin, 1 mmol/L orthovanadate, 1% Triton-X100). Bone marrow samples used for analysis were restricted to those with greater than 80% blast cells in the diagnostic aspirate, or with greater than 60% in the diagnostic aspirate with further enrichment following mononuclear cell isolation on the gradient.
Nuclear protein extraction and gel mobility shift analysis.
For nuclear protein isolation, cells were incubated in medium with reduced serum content (2% FCS) overnight, either in the presence of IL-3 (for H7 parental cells) or in the absence of IL-3 (H7 Bcr-Abl.A54). The method used for isolation was a modification of that reported by Dignam et al, and has been described.17 Using this method, nuclear proteins were also isolated from M07e and M07p210 BCR-ABL taken from their respective culture medium. For isolation of DNA binding proteins from primary leukemia samples (mononuclear cells containing >80% blast cells), a high-salt buffer containing 1% NP-40 was used according to the method described.18 19 The gel mobility shift reaction mixture contained 5 to 15 μg of the nuclear protein extract, 0.1 to 0.5 ng of the proximal c-junpromoter (−110/CTF site to +40 BamHI fragment) end-labeled with 32P-ATP in a binding buffer (4 mmol/L HEPES pH 7.9, 40 mmol/L KCl, 10 mmol/L MgCl2, 4% glycerol, 1 mmol/L dithiothreitol [DTT]) along with nonspecific competitor DNA, polydIdC. Specific competitor DNA was added in 100- to 200-fold excess as required. Antibodies, when used, were added at 2 μg per reaction. Reaction contents were electrophoresed on 5% polyacrylamide gels in 0.5× Tris borate EDTA buffer at room temperature. In other gel mobility shift reactions, a consensus 7-base collagenase AP-1 binding site contained within a 36-bp oligonucleotide was used, and was purchased from Promega (Madison, WI).
Antibodies.
The following antibodies were used for supershifting (gel mobility assays) or immunoblotting of proteins Western transferred onto nitrocellulose filters after sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE): anti–c-jun/AP-1 (N), anti-JNK (C-17), anti-junB (N-17), anti–ATF-1, ATF-2 (C-19), anti–c-fos (4), and anti-junD (329). These antibodies were obtained from Santa Cruz Biotechnology Santa Cruz, CA).
Immunoblotting.
Cytosolic or nuclear proteins were resolved on 12% SDS polyacrylamide gels and transferred to a nitrocellulose filter by a semi-dry Western blotting method (BioRad, Hercules, CA). The filter was preblocked in 5% blocking agent (BA) (BioRad) in TBS-T (Tris-buffered saline, 0.5% Tween 20) at 4°C for 16 hours. The blots were incubated with the primary antibody at 0.5 μg/mL in TBS-T/5% BA at room temperature for 2 hours. After extensive washing, the blots were incubated with the appropriate horseradish peroxidase–conjugated secondary antibody at a dilution of 1:2,000 in TBS-T/5% BA at room temperature for 2 hours. After further extensive washing the results were visualized by enhanced chemiluminescence (ECL). The blots were stripped according to the manufacturer's protocol (Amersham, Arlington Heights, IL) and subsequently reprobed with the remaining antibodies.
Kinase assay of JNK.
Proteins (25 to 200 μg) were incubated with 2 μg of a GST fusion protein of the N-terminal region of c-jun (amino acids 5-89) coupled to agarose beads. These reactants were brought to a final volume of 50 μL in a phosphotyrosine lysis buffer and incubated with gentle rocking at 4°C for 5 hours to allow binding of endogenous JNK to substrate protein as described (solid-phase assay).20 This mixture was washed extensively by pelleting and resuspension, and the beads were finally resuspended in 10 μL distilled water. Next, 20 μL of 2× kinase buffer containing 50 μCi/mL 32P-γ-ATP (1 μCi per reaction) was added, and the reaction was incubated at 30°C for 20 minutes. In some experiments, cell lysates were first immunoprecipitated with anti-JNK coupled to protein A, and then immune precipitates were washed and immune complex kinase assays were performed with substrate. The reactions were stopped by adding 2× volume of Laemmli sample buffer (BioRad) and the reaction contents were boiled and electrophoresed on 12% SDS polyacrylamide gels. The gel was then dried and subjected to autoradiography. Control reactions were performed with GST-coated beads or with a mutant GST–c-jun (5-89) with serines 63,73 converted to leucine.20
Transient transfections and reporter activity assays.
The c-jun promoter fragment −132/+170 cloned in front of a CAT reporter vector was a gift of Michael Karin, PhD (University of California San Diego).8 From this vector, the proximal promoter element containing sequences at −110 bp 5′ of the CTF site to +40 was obtained by polymerase chain reaction (PCR) using the HindIII-Pst I fragment as template.8The PCR product was cloned into a non-TA tailed cloning vector (pNOTA; Invitrogen). Subsequently, a 150-bp HindIII-Kpn I fragment was used for directional cloning into the promoterless pXP2 luciferase reporter vector, to yield 5′ CTF–c-junluciferase. A derivative vector with specific mutation of the AP-1 site within 5′ CTF -c-jun, converting the AP-1 consensus sequence from wild-type of GTGACATCA to an mAP-1 sequence of GATGCACCA, was prepared by a combinatorial PCR technique described previously.17 The incorporated change was verified by sequencing. Both parental and the p210 BCR-ABL transformed cell line were transfected by methodology described.17 In brief, the cells were resuspended in Ca2+/Mg2+-free PBS containing 20 mmol/L HEPES. Vector DNA was added at 0.5 μg/106 cells for the luciferase vector plus the constitutively-active RSV β-galactosidase to serve as an internal control for transfection efficiency at 1 μg/106 cells. The cell/DNA mixture was incubated on ice for 10 minutes, then exposed to a pulse of 350 V at 500 μF, delivered over 10 to 13 ms (BioRad gene pulser with capacitance extender). After further incubation on ice for 10 minutes, the cells were resuspended in McCoy's medium plus 10% FCS, and in the case of the factor-dependent parental cell line additional supplementation by 5% WEHI-3 conditioned medium. The cells were placed in incubation for 18 hours, and then cytosolic extracts were prepared for measurement of luciferase activity using a Bechthold luminometer according to methods recommended by the assay kit (Promega). Data from three independent experiments are reported as relative mean luciferase activity/β-galactosidase × 100, from equal numbers of cells of H7 parental or p210 BCR-ABL transformed cells. Wild-type 5′ jun luciferase activity in p210 BCR-ABL transformed cells was on average 10,000 to 20,000 luminometer units.
RESULTS
Correspondence between augmented kinase activity of JNK and c-jun transcriptional activity in p210 BCR-ABL transformed cells.
We have previously reported that myeloid cells transformed by p210 BCR-ABL overexpress c-jun mRNA compared with their IL-3–stimulated parental cells, assayed by Northern blot analysis.14,21 This increase in steady-state c-junmRNA in the cell line Bcr-Abl.A54, compared with IL-3–treated H7 parental cells, was on the order of fourfold to fivefold.14 21 Therefore, we wondered whether JNK activity and c-jun transcription rates might be correspondingly increased in the transformed cells, supporting their involvement in a linear pathway to the accumulation of c-jun mRNA.
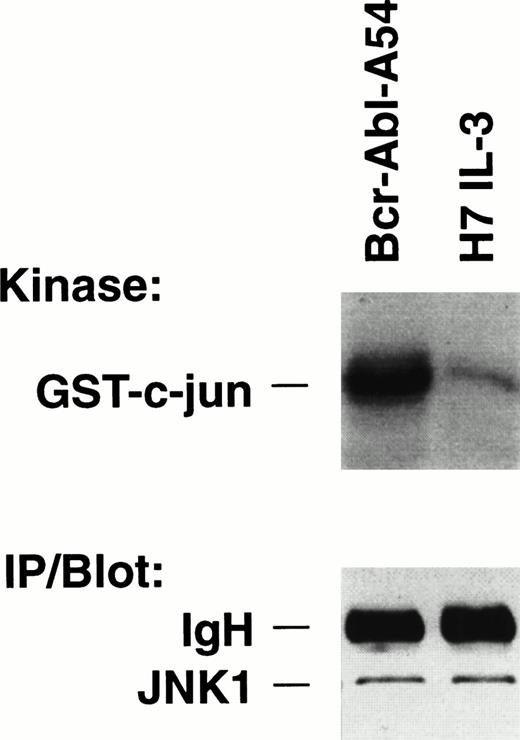
Activity assays for JNK were performed from the extracts of IL-3–treated H7 parental cells and of Bcr-Abl.A54 cells (Fig 1). There was significantly greater incorporation of radioactive phosphate into the GST–c-jun(5-89) substrate in reactions of Bcr-Abl.A54 cell extract compared with H7 cell extract by a factor of fourfold to fivefold (Fig 1). Activity of the kinase was absent or markedly reduced on the control substrate GST–c-jun (delta 63, 73) (data not shown). By contrast to this difference, the cellular abundance of JNK enzyme in the two samples was identical (Fig 1, bottom).
Increased kinase activity of JNK within cells transformed by p210 BCR-ABL (Bcr-Abl.A54) compared with IL-3–treated parental cells. (Top) Equal quantities (50 μg) of nuclear proteins isolated from the respective cells growing in medium (in the case of H7 parental cells including 5% WEHI CM as a source of IL-3) were placed in side-by-side solid-phase JNK activity assays. The reaction contents were then boiled and subjected to SDS-PAGE followed by autoradiography. JNK reactions were also performed at a range of cytosol protein concentrations with the same result. (Bottom) One hundred micrograms of each cell lysate was immunoprecipitated with anti-JNK antibody, then immunoblotted for JNK. The same result was obtained by direct immunoblotting.
Increased kinase activity of JNK within cells transformed by p210 BCR-ABL (Bcr-Abl.A54) compared with IL-3–treated parental cells. (Top) Equal quantities (50 μg) of nuclear proteins isolated from the respective cells growing in medium (in the case of H7 parental cells including 5% WEHI CM as a source of IL-3) were placed in side-by-side solid-phase JNK activity assays. The reaction contents were then boiled and subjected to SDS-PAGE followed by autoradiography. JNK reactions were also performed at a range of cytosol protein concentrations with the same result. (Bottom) One hundred micrograms of each cell lysate was immunoprecipitated with anti-JNK antibody, then immunoblotted for JNK. The same result was obtained by direct immunoblotting.
Next we studied the consequences of these differences in JNK activity as they might dictate c-jun regulation on the 5′ c-jun proximal promoter element. In preparation for functional studies of the importance of the c-jun/AP-1 binding site in transcriptional assays, it was necessary to establish c-junbinding to the promoter fragment in gel mobility shift assay (Fig 2). Extract from Bcr-Abl.A54 cells was found to form two complexes of distinct mobility with the radiolabeled 5′ c-jun fragment, and these complexes were specific because they were totally competed by the unlabeled self-probe in 100-fold excess (Fig 2). A similar molar excess of a commercial 7-base collagenase AP-1/TRE site oligonucleotide caused marked reduction in band-shifts (Fig 2).
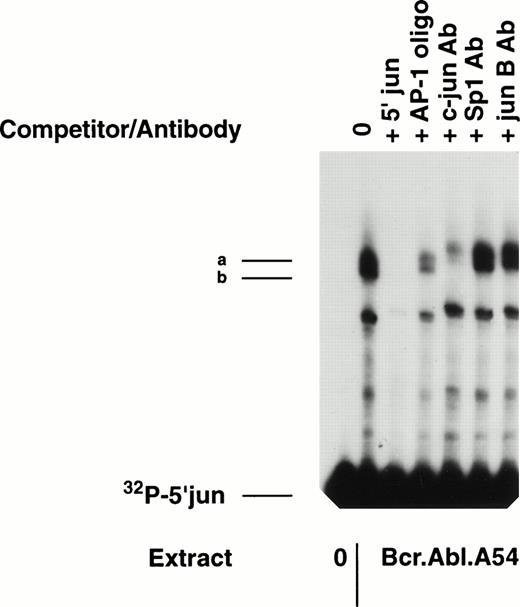
Gel mobility shift reaction with 5′ c-junprobe shows two distinct complexes containing c-jun protein. A gel mobility shift assay was performed with nuclear extracts (10 μg) from Bcr-Abl.A54 cells. Cold-competition with nonradioactive self probe (100×) or with nonradioactive commercial oligonucleotide containing a 7-base TRE was accomplished. Specific supershifting of the band-shift complexes (a, b) was shown with c-jun antibody, but not by Sp1 or jun B antibodies.
Gel mobility shift reaction with 5′ c-junprobe shows two distinct complexes containing c-jun protein. A gel mobility shift assay was performed with nuclear extracts (10 μg) from Bcr-Abl.A54 cells. Cold-competition with nonradioactive self probe (100×) or with nonradioactive commercial oligonucleotide containing a 7-base TRE was accomplished. Specific supershifting of the band-shift complexes (a, b) was shown with c-jun antibody, but not by Sp1 or jun B antibodies.
To confirm the presence of c-jun binding protein within the two distinct band-shift complexes, antibody specific for c-jun was added to the binding reactions to look for supershift or neutralization (Fig 2). Reproducibly, the c-jun antibody caused supershifting of both complexes and, additionally, near-total neutralization of the faster mobility complex (complex b) (Fig 2). As a control, antibodies against neither Sp1 nor junB effected these results.
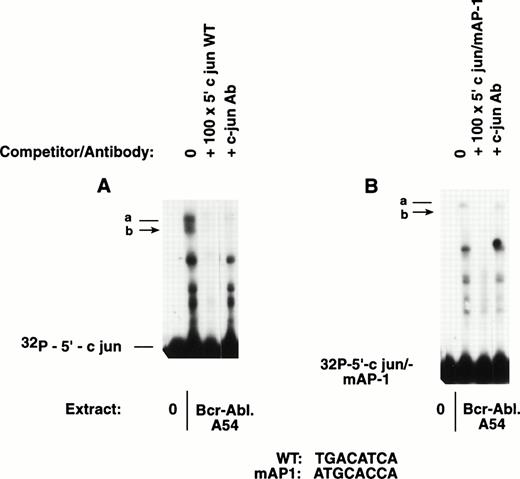
Site-directed mutation within the 5′ c-jun proximal promoter was undertaken by PCR technology, and the fragment containing the mutant core element (GATGCACCA) was tested in gel mobility shift reactions alongside the wild-type 5′ c-jun fragment. There was binding activity for c-jun/AP-1 by extract from Bcr-Abl.A54 cells. The mutant probe radiolabeled to a comparable level as wild-type probe did not elicit the expected band-shift (Fig 3).
The 5′ c-jun promoter fragment containing a mutation disrupting the AP-1 consensus fails to bind c-jun/AP-1. Side-by-side mobility shift reactions were performed with the same nuclear extract from Bcr-Abl.A54 cells and 5′ c-jun probes (150-bp inserts) labeled to a comparable activity. (A) On the left, the wild-type c-jun probe formed two band-shift complexes, which were cold-competed with nonradioactive self-probe, and were neutralized by c-jun antibody. (B) On the right, the gel mobility shift performed with the 150-bp 5′ c-jun probe containing a mutated AP-1 core sequence failed to bind c-jun. A faint band-shift complex was formed that was competed with nonradioactive self-probe that also lacked AP-1 consensus. This faint band-shift was not affected by the c-junantibody dilution that completely neutralized c-jun/AP-1 complexes in the companion reaction.
The 5′ c-jun promoter fragment containing a mutation disrupting the AP-1 consensus fails to bind c-jun/AP-1. Side-by-side mobility shift reactions were performed with the same nuclear extract from Bcr-Abl.A54 cells and 5′ c-jun probes (150-bp inserts) labeled to a comparable activity. (A) On the left, the wild-type c-jun probe formed two band-shift complexes, which were cold-competed with nonradioactive self-probe, and were neutralized by c-jun antibody. (B) On the right, the gel mobility shift performed with the 150-bp 5′ c-jun probe containing a mutated AP-1 core sequence failed to bind c-jun. A faint band-shift complex was formed that was competed with nonradioactive self-probe that also lacked AP-1 consensus. This faint band-shift was not affected by the c-junantibody dilution that completely neutralized c-jun/AP-1 complexes in the companion reaction.
Next, a series of transcriptional assays involving transient transfection of the 5′ c-jun luciferase versus the mAP-1 5′ c-jun luciferase reporter vectors were performed to compare AP-1 transacting capacity for the c-jun promoter within Bcr-Abl.A54 cells versus H7 parental cells (grown in IL-3). There was 30-fold activation of the 5′ c-jun luciferase vector above background control in the Bcr-Abl.A54 cells, and the AP-1 site mutant reporter vector was essentially inactive (Table 1). By contrast, 5′-c-jun–mediated transcriptional activity within H7 parental cells was fivefold lower than in Bcr-Abl.A54 when both values were controlled for β-galactosidase activity, which was exactly comparable within the two cell types (Table 1).
Composition of AP-1 proteins binding to the proximal CRE element of 5′ c-jun in cell lines differing in p210 BCR-ABL. It was of interest to distinguish the binding interactions occurring on the jun promoter in cells transformed by p210 BCR-ABL compared with IL-3–dependent parental cells. Gel mobility shift was performed involving side-by-side analysis of extracts from H7 parental cells compared with Bcr-Abl.A54 cell extract (Fig 4). Repeatedly, it was observed that Bcr-Abl.A54 cell extracts were unique in their content of the double band-shift complex containing a unique faster mobility complex (complex b) that was supershifted and largely neutralized with antibodies specific for c-jun and for c-fos (Fig 4). As noted previously, antibody to junB did not cause any supershifting, which is consistent with the results of other immunoblot experiments for the absence of junB protein in these extracts (data not shown). On the other hand, addition of an antibody against ATF2 (known by immunoblot experiments [see below] to be a major constituent of the extracts) was not effective in supershifting either mobility shift band, indicating ineffectiveness of this antibody preparation (Fig 4).
Gel mobility shift experiment involving 5′ c-jun probe and comparing nuclear extracts of H7 parental cells grown in IL-3 versus Bcr-Abl.A54 cells. Equal quantities of nuclear extract from the two cell lines were placed in a gel mobility shift reaction as noted above, without or with the addition of antibody against c-jun or c-fos, or against ATF-2 or junB. Another reaction was performed with each extract along with the inclusion of 100-fold excess of nonradioactive 5′ c-junDNA (self) probe to establish specificity of the band-shift. Note absence of complex (b) in H7 parental cell extracts, and the supershifting/depletion of complex (b) in Bcr-Abl.A54 cells extracts by either antibody against c-jun or c-fos.
Gel mobility shift experiment involving 5′ c-jun probe and comparing nuclear extracts of H7 parental cells grown in IL-3 versus Bcr-Abl.A54 cells. Equal quantities of nuclear extract from the two cell lines were placed in a gel mobility shift reaction as noted above, without or with the addition of antibody against c-jun or c-fos, or against ATF-2 or junB. Another reaction was performed with each extract along with the inclusion of 100-fold excess of nonradioactive 5′ c-junDNA (self) probe to establish specificity of the band-shift. Note absence of complex (b) in H7 parental cell extracts, and the supershifting/depletion of complex (b) in Bcr-Abl.A54 cells extracts by either antibody against c-jun or c-fos.
By contrast to this result with extract from Bcr-Abl.A54 cells, parental H7 cell extract had somewhat lower overall binding to the radiolabelled 5′ c-jun probe. This extract was characterized by complete absence of the faster mobility band-shift complex that had been easily neutralized by the c-jun and c-fos antibodies (Fig 4). In addition, the single band-shift complex formed by H7 cell extract was poorly affected in terms of neutralization or supershift by inclusion of antibodies against c-jun and c-fos, suggesting deficiency within this AP-1 band-shift of antigenically recognizable c-jun and c-fos proteins (Fig 4).
Immunoblot experimentation was undertaken to confirm the content of c-jun and c-fos and to investigate the presence of ATF-2 within these extracts. Equal amounts of nuclear extract from H7 parental cells, either IL-3–deprived or IL-3–deprived and –restimulated, and from Bcr-Abl.A54 cells were subjected to electrophoresis by SDS-PAGE and then immunoblotting (Fig 5). It was observed that Bcr-Abl.A54 cells had equal amount of ATF-2 protein compared with IL-3 stimulated H7 cells (Fig 5).
Comparative immunoblot of nuclear protein extracts from H7 parental cells versus Bcr-Abl.A54 cells shows increased c-jun and c-fos in cells transformed by p210 BCR-ABL.
Comparative immunoblot of nuclear protein extracts from H7 parental cells versus Bcr-Abl.A54 cells shows increased c-jun and c-fos in cells transformed by p210 BCR-ABL.
On the other hand, Bcr-Abl.A54 cells had greater quantities of c-jun and c-fos than H7 cells subjected to IL-3 stimulation (Fig 5). Interestingly, IL-3 stimulation of H7 cells increased the abundance of c-fos and ATF-2, as previously reported,22 but IL-3 stimulation did not increase, and actually reduced somewhat, the amount of detectable c-junprotein (Fig 5). Considering the composition of the band-shift complexes formed on the 5′ c-jun promoter described above, and also taking into consideration the results of other investigators,23 24 it is likely that the slower mobility band-shift (complex a) from extracts of Bcr-Abl.A54 as well as the sole complex from H7 cells may be composed of c-jun and ATF-2, whereas the faster mobility complex is likely to be uniquely composed of c-jun and c-fos.
Human acute leukemia cells containing the Philadelphia chromosome have strong constitutive kinase activity of JNK and contain abundant c-jun/AP-1 that binds to DNA. The scientific relevance of the above findings depends on the existence of a similar state of control that exists in primary human leukemia cells containing the BCR-ABL gene in a Philadelphia chromosome (Ph+). The human K562 is a standard cell line model derived from a patient with Ph+ CGL in blast phase. We studied the activity of JNK within K562 as compared with a “naive” leukemic cell line, HL-60, not possessing a known tyrosine kinase oncogene of the class of BCR-ABL as a negative control (Fig 6).
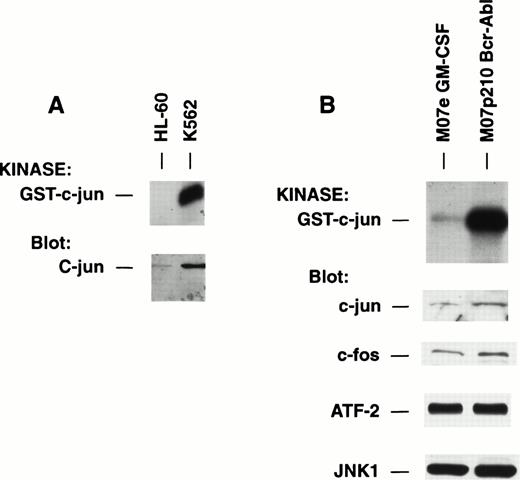
Comparative analysis of human leukemia cell lines differing in expression of p210 BCR-ABL for JNK activity. (A) Equal quantities of cell lysate from HL-60 versus K562, a p210 BCR-ABL transformed cell line, were placed into solid-phase JNK activity assay or were immunoblotted for expression of c-jun. (B) Equal quantities of cell lysate from M07e cells growing in GM-CSF versus M07p210 cells were placed into solid-phase JNK activity assay or were immunoblotted for expression of c-jun, c-fos, ATF-2, or JNK. Note absence of difference in abundance of JNK (isoform 1) or ATF-2 between cells, but major increase in c-jun (2.8-fold by densitometry) and c-fos (2.4-fold) proteins in M07p210 that mirrors increase in kinase activity of JNK.
Comparative analysis of human leukemia cell lines differing in expression of p210 BCR-ABL for JNK activity. (A) Equal quantities of cell lysate from HL-60 versus K562, a p210 BCR-ABL transformed cell line, were placed into solid-phase JNK activity assay or were immunoblotted for expression of c-jun. (B) Equal quantities of cell lysate from M07e cells growing in GM-CSF versus M07p210 cells were placed into solid-phase JNK activity assay or were immunoblotted for expression of c-jun, c-fos, ATF-2, or JNK. Note absence of difference in abundance of JNK (isoform 1) or ATF-2 between cells, but major increase in c-jun (2.8-fold by densitometry) and c-fos (2.4-fold) proteins in M07p210 that mirrors increase in kinase activity of JNK.
K562 had strong constitutive JNK activity, but HL-60 did not possess measurable activity (Fig 6A). K562 had abundant c-jun protein but HL-60 had only a very small amount of c-jun (Fig 6A). Similarly, we compared the activity of JNK within M07p210 BCR-ABL transformed cells versus GM-CSF–dependent parental M07e cells as a stricter control for possible cell lineage background effects independent of p210 BCR-ABL. Again, there was more than fourfold to fivefold greater JNK activity within p210 BCR-ABL–containing cells (Fig 6B). There was a corresponding twofold to threefold greater amount of c-jun and c-fos proteins accompanying p210 BCR-ABL and its augmented JNK activity (Fig 6B). On the other hand, like the situation that obtained in the comparison of IL-3–treated H7 versus Bcr-Abl.A54, the two cell lines had exactly the same amounts of ATF-2 (Fig 6B). Further, in the case of the related M07e/M07p210 BCR-ABL paired cell lines, we showed that differences in the absolute abundance of the JNK enzyme could not explain the magnitude of difference in JNK activity and the resulting c-jun protein expression because JNK (the JNK1 isoform) was expressed equally (Fig 6B).
JNK activity assay was also performed on cell lysates from patients with acute leukemic blast transformation associated with the Philadelphia chromosome. Cell lines were included as controls: HL-60 cell lysate was lacking in JNK activity, K562 had strong JNK activity (Fig 7). However, kinase activity of JNK within sample no. 2224 from a patient with a Ph+ acute leukemia was even greater than activity within K562 (Fig 7). Another distinct (no. 2116) sample from a patient with Ph+ acute lymphocytic leukemia also showed strong kinase activity of JNK at a level equal to K562 (data not shown). In contrast to the constitutive kinase activity of JNK observed in these samples was absence of activity within a series of “good prognosis” acute leukemia cases studied (Cripe et al, in preparation; see also below).
JNK activity assay on lysates of cell lines and a leukemic blast crisis bone marrow sample from a patient with a transformed myeloproliferative disorder containing a Philadelphia chromosome. Both the human cell line model of acute leukemia formed in the presence of a Philadelphia chromosome (K562) and the primary patient sample containing Philadelphia chromosome show strong constitutive JNK activity. Another Ph+ acute leukemia (not shown) also demonstrated a similar level of activity.
JNK activity assay on lysates of cell lines and a leukemic blast crisis bone marrow sample from a patient with a transformed myeloproliferative disorder containing a Philadelphia chromosome. Both the human cell line model of acute leukemia formed in the presence of a Philadelphia chromosome (K562) and the primary patient sample containing Philadelphia chromosome show strong constitutive JNK activity. Another Ph+ acute leukemia (not shown) also demonstrated a similar level of activity.
The antibody against human c-jun is poorly active in supershift reactions. Therefore, we wished to more accurately determine relative levels of c-jun and c-fos composing an AP-1 species that is capable of binding DNA, within the extracts of the K562 cell line, the patient sample no. 2271 (relapse specimen from same patient as sample no. 2224), and also within the murine model cell line, Bcr-Abl.A54. A gel mobility shift reaction was performed with the 7-base collagenase TRE, which binds only to c-jun/c-fosAP-1 (as opposed to binding also with jun/ATF) (Fig 8). Another gel mobility shift reaction was simultaneously set up with an oligonucleotide probe representing the consensus CTF box-binding CTF/NF-1 factor site (Fig8A). This was used as a binding control for a constitutive CTF factor whose abundance is not expected to differ significantly among these extracts. We observed relatively equal binding by extracts from these distinct BCR-ABL–containing cells to the radiolabeled CTF probe (Fig8A, right). By contrast, there was a definite increase in c-jun/AP-1 within extract from patient sample no. 2271 (relapse specimen no. 2224) compared with the positive control human cell line K562 and Bcr-Abl.A54 (Fig 8A). This result was confirmed in another independent experiment, and specificity of the binding reaction was shown by competition of the binding with 100-fold excess of nonradioactive collagenase TRE DNA (Fig 8B).
Gel mobility shift reactions using whole cell extracts from cell lines and patient samples prepared on a small scale by high-salt extraction demonstrate c-jun/AP-1 binding activity. (A) Extracts from the Ph+ model cell lines K562, Bcr-Abl.A54 (the murine cell line expressing p210 BCR-ABL), and patient sample no. 2271 were placed in gel mobility shift reactions with commercial oligonucleotide for the 7-base collagenase TRE (left) or a CTF/NF-1 oligonucleotide, which was used to control for protein loading by assay of a constitutively abundant transcription factor. Note relative abundance of c-jun/AP-1 binding activity. (B) Increased c-jun/AP-1 binding activity present in the patient sample is specific. On the left, another gel mobility shift reaction was performed with the 7-base collagenase TRE(AP-1) oligonucleotide including an extract, also prepared by the same high-salt extraction protocol, from a cell line (T47 breast cancer) known to be deficient in c-jun/AP-1 activity (HL-60 lysates could not be prepared by this protocol because of extensive autodigestion that could not be effectively inhibited). On the right, specificity of binding by extract from patient sample no. 2271, as well as from lysate of patient no. 2306 (known to harbor constitutive JNK activity), are shown to be specific because of cold-competition of their band-shift complexes formed with the collagenase TRE oligonucleotide by nonradioactive collagenase TRE at 100-fold excess. Note that the intensity of band-shift complexes formed is proportionately reduced after decay of the probe subsequent to labeling.
Gel mobility shift reactions using whole cell extracts from cell lines and patient samples prepared on a small scale by high-salt extraction demonstrate c-jun/AP-1 binding activity. (A) Extracts from the Ph+ model cell lines K562, Bcr-Abl.A54 (the murine cell line expressing p210 BCR-ABL), and patient sample no. 2271 were placed in gel mobility shift reactions with commercial oligonucleotide for the 7-base collagenase TRE (left) or a CTF/NF-1 oligonucleotide, which was used to control for protein loading by assay of a constitutively abundant transcription factor. Note relative abundance of c-jun/AP-1 binding activity. (B) Increased c-jun/AP-1 binding activity present in the patient sample is specific. On the left, another gel mobility shift reaction was performed with the 7-base collagenase TRE(AP-1) oligonucleotide including an extract, also prepared by the same high-salt extraction protocol, from a cell line (T47 breast cancer) known to be deficient in c-jun/AP-1 activity (HL-60 lysates could not be prepared by this protocol because of extensive autodigestion that could not be effectively inhibited). On the right, specificity of binding by extract from patient sample no. 2271, as well as from lysate of patient no. 2306 (known to harbor constitutive JNK activity), are shown to be specific because of cold-competition of their band-shift complexes formed with the collagenase TRE oligonucleotide by nonradioactive collagenase TRE at 100-fold excess. Note that the intensity of band-shift complexes formed is proportionately reduced after decay of the probe subsequent to labeling.
Unlike the situation in cell lines that are matched in a parental:progeny relationship such as H7:A54 and M07e:M07p210, comparison of patient samples implies a distinctive cellular background. Other features of the blast cells might also be relevant to the distinction of c-jun/JNK activity levels, irrespective of the presence of p210 BCR-ABL signaling from upstream. Relevant questions on this point include whether all leukemic cells may have upstream signaling pathways activated that facilitate JNK-mediated c-jun expression, but pathway output is simply limited by JNK enzyme levels. To answer this question, leukemic samples with known activity of JNK in solid-phase assay, and whose levels of c-junprotein were independently established, were studied for JNK expression by immunoblot and immune complex kinase assay after immunoprecipitation (Fig 9).
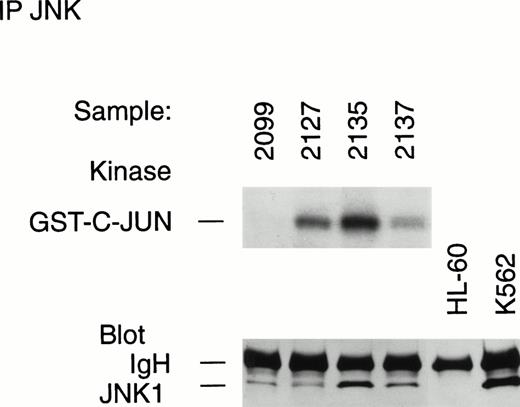
Variations in JNK activity between different leukemia samples is not explained by the absolute level of cellular enzyme expression. Lysates from leukemic samples registering negative (nos. 2099, 2127, 2137) or positive (no. 2135) in the solid-phase JNK assay were immunoprecipitated and immunoblotted to show the presence of JNK protein in the lysates. Half of each immunoprecipitate was placed in an immune complex kinase JNK assay. Note the inactivity of immunoprecipitated JNK1 within no. 2099, despite the presence of a similar protein amount as no. 2127. Also note the apparent absence of immunoprecipitable JNK in the HL-60 cell line.
Variations in JNK activity between different leukemia samples is not explained by the absolute level of cellular enzyme expression. Lysates from leukemic samples registering negative (nos. 2099, 2127, 2137) or positive (no. 2135) in the solid-phase JNK assay were immunoprecipitated and immunoblotted to show the presence of JNK protein in the lysates. Half of each immunoprecipitate was placed in an immune complex kinase JNK assay. Note the inactivity of immunoprecipitated JNK1 within no. 2099, despite the presence of a similar protein amount as no. 2127. Also note the apparent absence of immunoprecipitable JNK in the HL-60 cell line.
Leukemia samples studied included the sample no. 2135 from an NK-phenotype ANLL FAB-type M2 without p210 BCR-ABL. The blast cells of this marrow sample demonstrated strong JNK activity and very abundant c-jun protein, at a level comparable with Ph+ blast crisis cells (no. 2271) (see below). Also included were samples no. 2127, 2099, and 2137, which showed reduced content of JNK enzyme by direct immunoblotting, and which were negative for kinase activity of JNK in the solid-phase assay (not shown). These samples were immunoprecipitated with anti-JNK and half of the immunoprecipitate was immunoblotted and the other half was placed in immune complex kinase assay. Immunoprecipitates of the control cell lines were also immunoblotted (Fig 9).
All of the immunoprecipitated samples had demonstrable expression of JNK1 except HL-60, which was lacking in JNK (Fig 9). However, the relative abundance of JNK as detected by immunoblot was not the primary determinant of JNK activity in the aliquot subjected to kinase assay. Immunoprecipitated samples no. 2099 and 2127 had comparable levels of JNK1, but, among these, sample no. 2099 did not demonstrate JNK enzyme activity (Fig 9). On the other hand, sample 2127 had greater JNK enzyme activity than sample 2137, whose content of JNK1 protein was greater still (Fig 9). These results show that different leukemic samples contain variable levels of JNK enzyme, but the enzyme activity is largely independent of the quantitative level of JNK protein. Importantly, we also determined in an independent immunoprecipitation experiment that cell samples from the Ph+ leukemia case with strong JNK activity presented above (nos. 2224/2229/2271) had exactly the same content of JNK1 as did sample no. 2099, whose JNK activity was profoundly diminished (Fig 9, and data not shown).
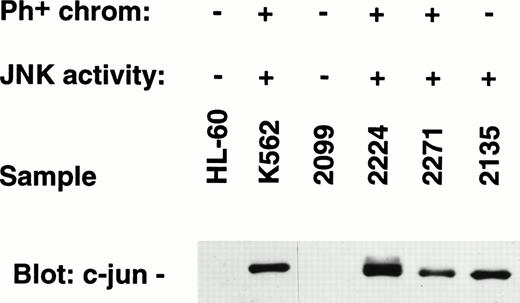
Immunoblotting was also performed to confirm the abundance of c-jun within extract from Ph+-patient samples nos. 2224 and 2271 (relapse specimen), and the unrelated leukemia sample characterized by constitutive JNK activity (no. 2135) (Fig 10). On the other hand, there was an absence of c-jun protein within the JNK inactive patient sample no. 2099, as well as in the negative control cell line, HL-60 (Fig 10).
Western blots prepared from SDS-PAGE electrophoresis of primary leukemia cell sample extracts were blotted for expression of c-jun protein. c-jun protein was shown in samples known to possess constitutive JNK enzyme activity.
Western blots prepared from SDS-PAGE electrophoresis of primary leukemia cell sample extracts were blotted for expression of c-jun protein. c-jun protein was shown in samples known to possess constitutive JNK enzyme activity.
DISCUSSION
Activation of a p21ras pathway downstream of p210 BCR-ABL, a nonreceptor tyrosine kinase oncogene that is the best characterized of a class of leukemia oncogenes, has received much attention.14-16 25-27 However, it is less well understood what number of consequences static p21ras activation has for cellular homeostasis. One of these consequences of p21ras activity is a transformation program that has been suggested to involve the activity of JNK, the N-terminal c-jun kinase, and, as a consequence, upregulation of c-jun within cells.
Previously, JNK was identified as an effector in the p21ras pathway of transformation initiated by p210 BCR-ABL.15 In consequence of BCR-ABL–induced JNK activity, abundant AP-1 transactivating capacity was identified in cells, which was inhibited by dominant-negative versions of p21ras, MEKK, JNK, as well as by dimerization-defective and DNA-binding domain-defective versions of c-jun.15 However, several of these events were studied in fibroblasts, and the relative consequence of JNK activity differences imparted by p210 BCR-ABL on c-jun gene regulation in myeloid cells was not reported. In addition, it has not been reported whether these changes documented in model cell lines are also observed in clinical cases of acute leukemia associated with the Philadelphia chromosome.
The results of our investigation suggest a scenario by which stable expression of pBCR-ABL and constitutive upregulation of JNK activity results in augmented c-jun expression. Augmentation of the enzyme activity of JNK within murine myeloid cells in the presence of stable overexpression of p210 BCR-ABL acutely affects increased transcription of the c-jun gene, and binding to the proximal CRE element of the c-jun gene with more c-jun and c-fos proteins follows (Fig 5). When this novel AP-1 complex composed of c-jun and c-fos is abundant, 5′ c-jun transcription, which is highly dependent on this binding, is upregulated fourfold to fivefold (Table 1). Devary et al23 and Herr et al24 have also demonstrated that the proximal CRE element of 5' c-jun is unique in its binding of c-jun/c-fos AP-1, and is the site responsive to signals conferred by JNK.
In the model systems we studied, parental cells without the oncogenic p210 BCR-ABL and growing under the influence of IL-3 or GM-CSF had lower JNK activity, which was associated with a lower stable concentration of c-jun and c-fos (Figs 5 and 6). Recently, stimulation of factor-dependent cell lines with cytokines was noted to acutely activate JNK and p38/RK MAP kinases, but in each case the level of activation was low and quite transient.28,29In addition, whereas ATF-2 is a substrate for p38 RK, c-jun is not.30 In keeping with these results, c-jun is not an abundant transcript induced by IL-3 or GM-CSF in factor-dependent myeloid cell lines.14,21 31-34 These data raise the question of what components of AP-1 are present in the “normal” growth factor–dependent cells without the high level kinase activity of JNK that characterizes p210 BCR-ABL, when they are growth factor stimulated.
In our model systems, ATF-2 was an abundant jun/ATF factor present in extracts of IL-3–treated H7 cells or GM-CSF–treated M07e cells, as determined by immunoblotting. Unfortunately, none of the ATF antibodies we tested (ATF-1,-2 antibodies from various suppliers) was effective in supershift/neutralization analysis of gel mobility-shift bands, so that the participation of this protein in DNA binding could not be addressed. Also, poor recognition of the sole band-shift complex in the IL-3–stimulated H7 parental cells by c-jun antibody suggests that another jun family member may be in a dimeric complex with ATF-2 to the exclusion of c-jun (Fig 4). Immunoblot experiments not shown here suggest that the most abundantjun family protein in these IL-3–treated parental H7 cells isjunD (data not shown). On the other hand, junB has been found in abundance in IL-3–stimulated FDC-P cells.32
It might be queried whether, in addition to static p21ras activation, another mechanism exists that promotes such strong kinase activation of JNK in the presence of p210 BCR-ABL. Recently, the rac-specific guanine nucleotide exchange factor, vav, was found to be activated by tyrosine phosphorylation, leading to rac-dependent JNK activity.34,35 This occurs when active GTP-bound rac binds to MEKK1.36 When p210 BCR-ABL is expressed in myeloid cells, vav is known to be heavily tyrosine-phosphorylated and bound to p210 BCR-ABL, which would be the initiation point of such a cascade.37 In contrast to the vav-rac mechanism downstream from p210 BCR-ABL, a mechanism by which active, GTP-bound p21ras directly initiates the JNK cascade is less well defined but may also involve MEKK1 binding.38 In addition, a Grb-2 adaptor interaction can activate the Ste-20 homologue, hematopoietic-specific kinase 1 (HPK1) to initiate MLK3 and SEK1 activity en route to JNK in a parallel pathway to p21ras.39
Additionally, our data show that heightened JNK activity, increased c-jun expression, and c-jun/AP-1 binding to DNA also characterized primary leukemic blast cells from patients with Ph′-mediated leukemias (Figs 7-10). Indeed, such leukemias may exhibit a state of resistance to induction chemotherapy regimens containing the oxidative damage-inducing drug adriamycin, because of the participation of gene products transcriptionally upregulated in a c-jun/AP-1–dependent manner which buffer oxyradicals, and which also mediate glutathione conjugation and export of oxyradical metabolites and xenobiotics. Among these genes/gene products are metallothionein IIA; γ-glutamyl cysteine synthase, the rate-limiting enzyme for de novo synthesis of glutathione40-45; as well as the enzymes, glutathione-S-transferase and quinone reductase.46-50
In keeping with this hypothesis, we have measured the pools of reduced and oxidized glutathione in the paired murine cell lines H7 versus Bcr-Abl.A54, and have observed significant augmentation of the cellular content of total glutathione in A54 cells with p210 BCR-ABL (data not shown). The origin of this adaptive response may relate to the cellular requirement to counteract peroxidative byproducts downstream of active p21ras.51 Interestingly, it was recently observed in model cell lines that the anti-apoptotic mechanism conferred by p210 BCR-ABL exists upstream of, and protects against, mitochondrial release of cytochrome C and activation of caspases, and may partly involve bcl-xL.52 It has been observed by others that intracellular thiol pools are additive with bcl protein levels in the capacity of upstream “buffering” and protection of mitochondrial integrity.53 54
Another aspect of our data is that certain acute leukemic blast cell samples, in addition to those that express p210 BCR-ABL, may also exhibit constitutive kinase activity of JNK and abundant levels of c-jun protein. Our preliminary experience in examining approximately 20 cases of adult acute myeloid leukemias on diagnostic marrows obtained before treatment shows that relapsed and secondary leukemias commonly exhibit constitutive JNK activity, whereas de novo, “good prognosis” acute myeloid leukemias occurring in young adults are more likely to lack JNK activity, and to fail to express c-jun protein (eg, see sample no. 2099, Figs 6 and 9). The feature distinguishing acute leukemic samples that display constitutive JNK activity and c-jun protein versus those samples that lack c-jun protein in the absence of constitutive JNK activity cannot be explained simply on levels of JNK enzyme(s) expression. Rather, we hypothesize that the majority of acute leukemias which exhibit constitutive kinase activity of JNK may be those that emit strong upstream tyrosine kinase signals, or those that contain activated small ras/rho-family G proteins. On the other hand, we have preliminary evidence that dominant suppression of JNK may account for absence of JNK activity in some samples (not shown).
In summary, a pathway from the tyrosine kinase oncogene p210 BCR-ABL to JNK-mediated c-jun transcriptional expression in myeloid leukemias is demonstrated here. Constitutive activation of the JNK pathway in a range of myeloid leukemias may confer important functional characteristics that determine prognosis. We are currently pursuing this hypothesis.
E.A.W. and L.D.C. contributed equally to this work.
Supported in part by a Leukemia Society of America Translational Research Grant, and a Merit Review Award from the Department of Veterans Affairs, and by Grant No. DHP-124 from the American Cancer Society to H.S.B.; and by National Institutes of Health Grant No. R01 CA 42533 to A.S.K. L.D.C. and H.S.B. receive support from the Joseph and Gloria Boone Leukemia Research Fund of the Indiana University Foundation.
Address reprint requests to H. Scott Boswell, MD, Indiana University School of Medicine, Medical Research Four Bldg, Room 202, 1044 W Walnut St, Indianapolis, IN 46202.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.