Abstract
The Epstein-Barr virus (EBV)-encoded latent membrane proteins, LMP1 and LMP2, are consistently expressed by the malignant Hodgkin/Reed-Sternberg (HRS) cells of EBV-associated Hodgkin's disease (HD). Cytotoxic T lymphocyte (CTL) responses to both of these proteins have been shown in the blood of EBV-seropositive individuals, yet in HD the apparent failure of the CTL response to eliminate HRS cells expressing LMP1 and LMP2 in vivo has given rise to the suggestion that HD may be characterized by the presence of defects in antigen processing/presentation or in CTL function. This study has used immunohistochemistry to show high-level expression of major histocompatibility complex (MHC) class I molecules by the HRS cells of EBV-associated HD and either low level or absence of expression of MHC class I molecules on HRS cells of EBV-negative tumors. In addition, HRS cells expressed high levels of transporter-associated proteins (TAP-1, -2), irrespective of the presence of latent EBV infection. These results suggest that global downregulation of MHC class I molecules does not account for the apparent ability of EBV-infected HRS cells to evade CTL responses, but may be important in the understanding of EBV-negative disease.We have also sequenced an epitope in LMP2A (CLGGLLTMV) that is restricted through HLA A2.1, a relatively common allele in Caucasian populations, and showed that this epitope is wild type in a small group of EBV-associated HLA A2.1-positive HD tumors. This result may be relevant to proposed immunotherapeutic approaches for EBV-positive HD patients that target CTL epitopes.
THE EPSTEIN-BARR VIRUS (EBV) is a human herpesvirus that infects approximately 95% of the world's adult population.1 EBV is generally carried as a clinically silent infection but is strongly implicated in the pathogenesis of a number of human malignancies including African Burkitt's lymphoma,2,3 nasopharyngeal carcinoma,4,5 and more recently, Hodgkin's disease (HD). EBV DNA has been detected in the tumor samples of HD patients by Southern blotting and polymerase chain reaction (PCR) analysis6-9 and a variety of studies have localized viral DNA10 and virus gene products11-13 to the malignant Hodgkin/Reed-Sternberg (HRS) cells of HD. Furthermore, using EBV terminal repeat probes, the EBV genomes within individual cases have been shown to be monoclonal in origin, suggesting that monoclonal proliferation occurred after EBV infection.7,8,10,14 The frequent occurrence of abnormally elevated titers of antibodies against EBV antigens before the onset of overt disease and the detection of impaired cellular responses to EBV in HD patients have led to the hypothesis that a defective control of EBV-infected cells might be important in the pathogenesis of a proportion of HD tumors.15-19 EBV-infected HRS cells are characterized by a pattern of viral protein expression that is restricted to EBNA1, LMP1, and LMP2.12,20-23 Although the dominant CTL responses seem to be directed to epitopes in proteins of the EBNA3 family, LMP1 and LMP2 have been shown to be targets for autologous cytotoxic T lymphocytes (CTLs) in vitro.24-27The apparent failure of the immune system to eliminate HRS cells expressing potential targets for CTLs has provided further support for immunologic dysfunction in EBV-associated HD.
One possible mechanism of CTL escape in EBV-associated HD may be downregulation of major histocompatibility complex (MHC) class I molecules on HRS cells. CTLs recognize endogenously processed virus proteins only when presented at the cell surface as peptides bound to MHC class I molecules. A reduction or the absence of MHC class I expression can therefore render virus-infected cells resistant to CTL destruction. Such a mechanism has been shown previously for some virus-associated tumors, including EBV-associated Burkitt's lymphoma (BL) and a proportion of human papilloma virus (HPV)-infected cervical carcinomas.28-31 In the case of HPV-positive cervical carcinomas, MHC class I downregulation was associated with the loss of function of one of the transporter-associated proteins (TAP-1).32 33
It has been shown that amino acid substitutions at key positions within peptides presented by MHC class I molecules may reduce or completely abrogate CTL responses. For example, two HLA A11-restricted CTL epitopes (residues 399-408 and 416-424) from the EBNA3B protein are regularly mutated in EBV strains from Southeast Asia,34 35and memory CTL responses specific for the variant epitopes have not been detectable in HLA A11-positive Chinese donors infected with the mutated viruses. It is possible that similar mechanisms might also account for the failure of CTLs to eliminate EBV-infected HRS cells.
Therefore, the aim of this current study was to investigate the expression of MHC class I and TAP molecules in both EBV-positive and EBV-negative HD. The identification of an epitope in LMP2(CLGGLLTMV) that is restricted through HLA A2.1, a relatively common allele in Caucasian populations, provides an ideal target to study the possibility that epitope variations in EBV genes expressed in tumor tissues might be responsible for CTL escape.36
MATERIALS AND METHODS
Preparation of specimens.
Paraffin-wax sections and paraffin-wax–embedded tissue blocks from 39 cases of HD were available from the pathology files of the Johns Hopkins Hospital, Baltimore, MD; and the Queen Elizabeth Hospital, Birmingham, UK. From tissue blocks 4-μm paraffin sections were prepared and all sections were deparaffinized and washed in Tris-buffered saline (TBS), pH 7.6. Cryostat sections were also prepared from selected samples. Hematoxylin and eosin–stained sections of HD tumors were reviewed and subtyped according to the Rye classification system.
Detection of latent EBV infection.
In situ hybridization for the detection of the Epstein-Barr virus early RNAs (EBERs) was performed according to standard methods.37Positive controls for EBER in situ hybridization included paraffin-wax sections of lymphoblastoid cell lines (LCL) grown as solid tumors in severe combined immunodeficiency (SCID) mice (LCL-SCID tumors) and a known EBER-positive HD case. U6 and sense control probes were included in all runs.
Immunohistology.
Immunohistochemistry for LMP1 and LMP2 was also performed on selected cases to confirm the presence of latent EBV infection in HRS cells. Immunostaining for MHC class I and TAP-1 was performed on acetone-fixed frozen and paraffin-wax sections of HD tissues and on a variety of cell lines (Table 1), including the HD lines HDLM2, L428, HS445, and Km-H2. HD cell lines and selected HD biopsies were also stained for TAP-2. Immunohistochemistry for TAP-1 and TAP-2 was additionally performed on acetone-fixed cytospin preparations of the BL lines, Mutu I (group I BL) and Mutu III (group III BL).
The demonstration of some antigens in paraffin material required microwave pretreatment in 0.1 mol/L citrate buffer, pH 6.0. The optimum microwave pretreatment times were determined for each antigen and are listed in Table 2. After pretreatment, endogenous peroxidase activity was blocked in 0.3% hydrogen peroxide in methanol and sections transferred to TBS.
Sections were then incubated in primary antibodies at optimum dilutions previously determined for each antibody (Table 2). All primary antibodies were diluted in 10% normal sheep serum. Mouse monoclonal antibodies were detected either using the peroxidase-based Dako Duet system (Dako Ltd cat no: K492; Dako Ltd, Glostrup, Denmark) or the standard alkaline phosphatase and alkaline phosphatase (APAAP) method. The rat monoclonal antibody to LMP2 was detected using rabbit anti-rat immunoglobulins (Dako Ltd cat no: Z455) at a dilution of 1:400, followed by the Dako Catalyzed Signal Amplification system (Dako Ltd cat no:K0620).
Positive controls for LMP1 and LMP2 immunostaining consisted of paraffin-wax sections of LCL-SCID tumors. Positive controls for MHC class I, TAP-1, and TAP-2 included paraffin-wax and frozen sections of human tonsil. Negative controls for immunohistochemistry consisted of consecutive test sections in which the primary antibody was replaced with nonimmune serum of the same IgG subclass. Full details of antibodies used are given in Table 2.
Epitope sequencing.
A series of frozen HD biopsies were initially studied to determine their EBV and MHC status. The presence of latent EBV infection was determined as described above. HLA A2.1-positive tumor samples were identified in APAAP immunohistochemical assays using the MA2.1 antibody (Table 2). A variety of HLA A2.1-positive cell lines (both homozygous and heterozygous) and HLA A2.1-negative cell lines (Table 1) were used as controls in these immunohistochemical assays. Both acetone-fixed cytospin preparations and frozen sections of SCID tumors generated from these lines were tested.
HLA A2.1 and EBV-positive HD samples identified in this way were subjected to DNA extraction and PCR sequencing to determine the sequence of the LMP2A epitope, CLGGLLTMV, previously identified to be restricted through HLA A2.1. Sequences were compared with wild-type (B95.8) DNA. To obtain sufficient DNA from HD biopsies for sequencing it was necessary to extract DNA from agarose gels for reamplification. Primers, D5639LY and D5640LY (Fig 1A), were used in both PCR amplification and sequencing steps. Sequencing was performed twice in both directions on all samples.
(A) LMP2A sequence showing position of epitope and primers used in the PCR and sequencing analyses. (B) Autoradiograph of sequencing gel showing wild-type LMP2 sequence.
(A) LMP2A sequence showing position of epitope and primers used in the PCR and sequencing analyses. (B) Autoradiograph of sequencing gel showing wild-type LMP2 sequence.
RESULTS
Detection of latent EBV gene expression.
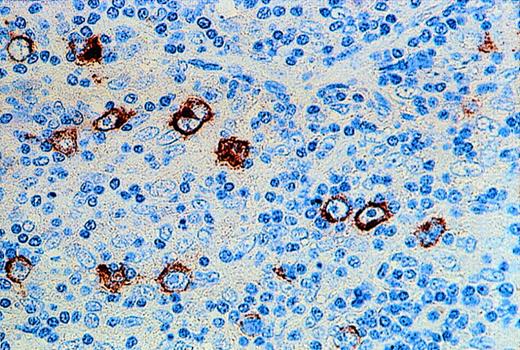
EBER expression was shown in HRS cells of 18 of 39 examined HD cases. The expression of LMP1 in all EBER-positive HD cases was confirmed by immunohistochemistry. LMP2 protein was also detectable in the cytoplasm of HRS cells in 16 of 16 EBV-positive HD cases (Fig 2).
Immunohistochemical demonstration of LMP2 expression by HRS cells in a case of EBV-positive HD.
Immunohistochemical demonstration of LMP2 expression by HRS cells in a case of EBV-positive HD.
MHC class I staining.
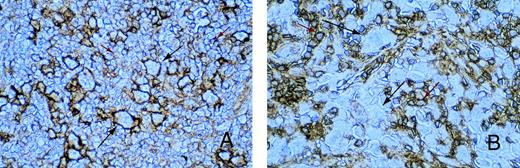
In all tissue sections, the presence of MHC class I-positive cells served as an internal control. Expression of MHC class I was mainly confined to the cell membrane of positively stained cells. Of 17 cases of EBER-positive HD analyzed, 13 showed the presence of MHC class I expression on HRS cells. However, MHC class I expression was detectable on HRS cells in only 4 of 21 cases of EBV-negative HD. In most EBV-positive HD samples, HRS cells could be easily identified because the intensity of class I staining was often stronger than on surrounding reactive cells (Fig 3A). This was in contrast to the majority of EBV-negative HD tumors, where HRS cells were either not stained or only weakly stained for class I (Fig3B). Class I expression was not related to subtype, and similar results were obtained on paraffin and frozen sections of HD tumors. In all cases, reactivity of surrounding reactive cells could be distinguished from staining on HRS cells. The HD cell lines, HDLM2, Km-H2, and HS445, all showed strong expression of MHC class I, whereas the HD cell line, L428, was consistently negative in all assays (not shown).
Immunohistochemistry for MHC class I expression. Long black arrows show class I expression on HRS cells (A) or its absence (B). The intensity of class I staining on surrounding small reactive cells (short red arrows) is less than that of the HRS cells in (A) and greater than that of the HRS cells in (B).
Immunohistochemistry for MHC class I expression. Long black arrows show class I expression on HRS cells (A) or its absence (B). The intensity of class I staining on surrounding small reactive cells (short red arrows) is less than that of the HRS cells in (A) and greater than that of the HRS cells in (B).
Immunohistochemistry for TAP-1 and TAP-2.
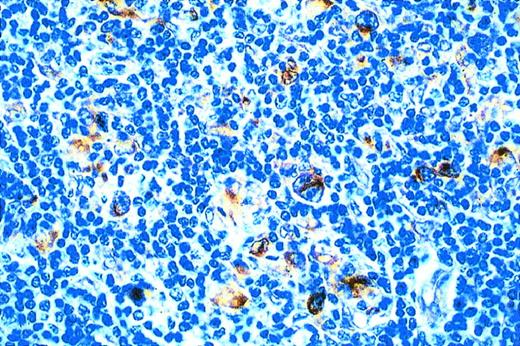
On control tonsil tissue, germinal center cells were generally not stained for TAP-1 and TAP-2, with the exception of occasional positive cells. The interfollicular cells were most strongly stained for both markers and the squamous epithelium, when present, was also positively stained (data not shown). Similar results were obtained on both frozen and paraffin-wax sections. Cell preparations (both acetone-fixed cytospins and pelleted paraffin-wax sections) of Mutu I and Mutu III cell lines expressed both TAP-1 and TAP-2, although staining for both markers was noticeably weaker in Mutu I cells when compared with the Mutu III line. All the HD cell lines tested (HDLM2, L428, HS445, and Km-H2) were strongly stained for both TAP-1 and TAP-2. Frozen and paraffin wax-embedded HD tumors were also analyzed for TAP-1 and TAP-2 expression. With the exception of two HD cases, HRS cells showed strong staining for TAP-1 (Fig 4), irrespective of the presence of latent EBV infection in these cells. Selected HD cases were also stained for TAP-2. In all cases, HRS cells showed strong staining for TAP-2 (not shown).The results of EBER in situ hybridization, MHC I, and TAP-1 immunohistochemical assays are summarized in Table 3.
Epitope sequencing.
A series of LCLs prepared from HLA A2.1-positive and -negative donors (Table 1) initially served as controls for the MA2.1 antibody. All cell lines from A2.1-positive donors reacted strongly with this antibody in APAAP assays, and all A2.1-negative cell lines, as expected, were nonreactive (data not shown).
Of the 20 HD biopsies available as frozen material, 9 contained EBER-positive HRS cells, and a further 6 of these were HLA A2.1 positive by immunohistochemistry. These 6 biopsies were subjected to PCR analysis to determine the sequence of the A2.1-restricted LMP2A-derived CTL epitope, CLGGLLMTV (Fig 1B). All 6 HD tumors showed wild-type epitope sequence.
DISCUSSION
There are strong indications that the outgrowth of EBV-infected tumors in vivo is dependent on the pattern of viral antigens expressed by the tumor and also the level of immunocompetence of the host. For example, the role of immunosurveillance in limiting the proliferation of EBV-infected cells in vivo is clearly illustrated by the development of EBV-positive lymphoproliferative disease in immunocompromised individuals.38-41 These lymphoproliferations may be regarded as the in vivo counterpart of LCLs. Indeed, these lesions often regress when immunosuppressive therapy is withdrawn or reduced.42,43 Many of these lesions express the full spectrum of EBV latent genes, with high cell surface expression of ICAM1 and LFA3 providing a wide range of potential targets for the immune system.44 It generally has been assumed that the sensitivity of these lesions to EBV-specific CTLs may be similar to LCLs. Evidence supporting this assumption has recently been provided by adoptive transfer experiments in which donor lymphocytes were used as a source of virus-specific CTLs to treat EBV lymphoproliferative disease in bone marrow transplant recipients.45 46
In contrast to posttransplant lymphoproliferative disease and related conditions, HD arises in relatively immunocompetent hosts. The consistent expression of LMP1 and LMP2 in EBV-positive HD3,12,20,21 and the demonstration of effective CTL responses to epitopes derived from these proteins in vitro36,47,48 implies that defects in the antigen processing/recognition machinery may be a feature of these tumors. The mechanisms accounting for the failure of CTLs to eliminate EBV-infected HRS cells have not been established yet, although MHC class I downregulation by HRS cells, as originally demonstrated by Poppema and Visser,49 has been suggested as a possible mechanism.
This study has shown high-level expression of MHC class I molecules on the surface of HRS cells in EBV-associated HD cases, and much lower levels of expression or the absence of expression on HRS cells in EBV-negative samples, and is in accordance with the results of a previous study.50 These results suggest that MHC class I downregulation is unlikely to account for the failure of CTLs to eliminate the tumor cells, at least in EBV-positive cases. The mechanism responsible for downregulation or absence of MHC molecules in EBV-negative HD has yet to be determined. Unlike cervical carcinomas, where downregulation of MHC class I was associated with low-level expression of TAP molecules,32 downregulation of TAP molecules was not shown in this present study. The high-level expression of MHC class I molecules on EBV-positive HRS cells potentially could be mediated by LMP1 expression. LMP1 has been shown to upregulate class I expression when transfected into EBV-negative B-cell lines51 and epithelial cells (Dawson C. and L.S.Y., unpublished observations). LMP1 also has been shown to increase the stimulatory capacity of EBV-negative BL lines in allogeneic mixed lymphocyte cultures,51 to upregulate the expression of adhesion molecules,52 and to enhance the presentation of endogenous and exogenous antigens.53 54
Another possible mechanism of CTL escape is suggested by the finding that memory CTL responses specific for variant epitopes in two HLA A11-restricted CTL epitopes (residues 399-408 and 416-424) from the EBNA3B protein were not detectable in HLA A11-positive Chinese donors infected with strain variants.34,35 This present study has established that a recently identified epitope in LMP2 that is restricted through HLA A2.155 is wild type in a small series of HLA A2-positive HD tumors, suggesting that mutation in virus epitope sequences may not be a common feature of EBV-positive HD, at least in this group of patients. Similar data showing that this same epitope is not commonly mutated has been presented by Bryden et al.56 The lack of mutation may have important implications for potential CTL therapy for HD patients, particularly because the CTL response to this epitope previously has been shown to be reactive against a range of virus isolates, including type-1 and type-2 Caucasian, Southeast Asian, New Guinean, and African isolates. Some of these isolates contained amino acid substitutions within the epitope, but were nevertheless recognized by CTLs raised against the B95.8 virus.36 The finding of similar, functionally conserved epitopes restricted through relatively common HLA alleles may be important for the future development of CTL therapy for EBV-associated HD.
Recent studies have shown that the HD cell line, HDLM2, is able to process and present epitopes from LMP1 and LMP2 in the context of multiple class I alleles including HLA A2 and is sensitive to lysis by EBV-specific CTLs.48 Furthermore, using autologous fibroblasts infected with a vaccinia recombinant encoding LMP2 as a target, the same authors were able to identify and expand LMP2-specific CTLs from the peripheral blood of an HD patient. However, recent data have pointed to the possibility of local suppression of EBV-specific immunity in the vicinity of the tumor that may confound certain immunotherapeutic strategies in HD. This was first suggested by a comparative study of EBV-specific responses in patients with EBV-positive and EBV-negative HD which failed to show virus-specific cytotoxicity in the tumor-infiltrating lymphocytes of six EBV-positive cases.57 However, CTL precursors were detected in the blood of one of the EBV-positive patients. A possible explanation of this finding was reported by Herbst et al,58 who showed that significantly higher proportions of EBV-positive HD cases contained interleukin-10 (IL-10)-expressing tumor cells when compared with EBV-negative tumors, suggesting that IL-10 production by HRS cells may be responsible for local downregulation of the CTL response in EBV-positive cases.58
The results of this present study provide some encouragement for the pursuit of CTL therapy for EBV-associated HD. However, further work is required to establish if the microenvironment of EBV-positive HRS cells is responsible for the inhibition of virus-specific CTLs and whether this effect is likely to confound immunotherapeutic strategies targeted at EBV-positive HD patients.
NOTE ADDED IN PROOF
Similar data regarding MHC class I and TAP expression in Hodgkin's disease have recently been presented.67
R.F.A. is a Leukemia Society Scholar.
Supported by the Cancer Research Campaign and the National Institutes of Health Grants No. PO1 CA15396 and PO1 CA69266.
Address reprint requests to Richard F. Ambinder, MD, PhD, Johns Hopkins Oncology Center, 418 N Bond St, Baltimore, MD 21231.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.