Abstract
To clarify a molecular defect responsible for moderate IIbβ3 deficiency, we examined two unrelated patients, MT and MS, suffering from type II and type I Glanzmann thrombasthenia (GT), respectively. Sequence analysis of polymerase chain reaction (PCR) fragments derived from platelet mRNA showed a single A→C substitution at nucleotide (nt) 2334 leading to a Gln747→ Pro in IIb in both patients. Allele-specific restriction enzyme analysis (ASRA) of genomic DNA demonstrated that patient MT was homozygous for the Gln747→Pro substitution and patient MS was compound heterozygous for this substitution and for an RNA splice mutation at the consensus sequence of the splice acceptor site of exon 18 (AG→AA). Furthermore, ASRA showed that, among 17 unrelated Japanese GT patients, this Gln747→Pro substitution was detected in 4 patients, including MT and MS (homozygous, 2 patients; heterozygous, 2 patients). Cotransfection of Pro747IIb and β3 constructs into 293 cells resulted in moderate reduction in the amount of IIbβ3 within the transfected cells as well as on the cell surface. However, Pro747IIbβ3 bound the ligand mimetic monoclonal antibody (MoAb) PAC-1 after activation of IIbβ3 by the MoAb PT25-2, suggesting that the mutant IIbβ3 possesses the ligand-binding function. The association between the mutant proIIb and β3 was not disturbed. Surface labeling and pulse chase study showed that the Gln747→Pro substitution moderately impaired both intracellular transport of the IIbβ3 heterodimers to the Golgi apparatus and endoproteolytic cleavage of proIIb into heavy and light chains. By contrast, replacement of Gln747 with Ala by mutagenesis did not impair IIbβ3expression on the cell surface. These results suggest that the presence of Pro, rather than the absence of Gln, at amino acid residue 747 on IIb is responsible for moderate IIbβ3 deficiency.
© 1998 by The American Society of Hematology.
INTEGRIN αIIbβ3 (platelet GPIIb-IIIa), a calcium-dependent heterodimeric complex, is a prototype integrin that functions as a physiologic receptor for fibrinogen and von Willebrand factor and plays a crucial role in normal hemostasis and platelet aggregation.1-3 The importance of this integrin has been well documented by the clinical features of Glanzmann thrombasthenia (GT), a rare autosomal recessive bleeding disorder characterized by a quantitative or qualitative abnormality of αIIbβ3.4
Analysis of cultured human leukemic and megakaryocytic cell lines has led to a better understanding of the key steps for αIIbβ3 biosynthesis.5-7 The αIIb subunit is synthesized as a single-chain precursor, proαIIb, that associates with the β3subunit within the endoplasmic reticulum of cells. The proαIIbβ3 complex is then transported to the Golgi apparatus, where αIIb undergoes sugar modification and endoproteolytic cleavage into heavy and light chains. After these processing events within the Golgi apparatus, the mature αIIbβ3 complex is rapidly transported to the cell surface. Classically, GT can be divided into three subgroups according to the amount of αIIbβ3: type I has a severe αIIbβ3 deficiency (<5% of normal), type II has a moderate αIIbβ3deficiency (10% to 20% of normal), and a variant has normal to near normal levels of a dysfunctional αIIbβ3(50% to 100% of normal).4 To date, more than 30 mutations in either the αIIb or β3 gene responsible for the thrombasthenic phenotype have been identified.8,9However, most of the reported mutations are responsible for severe αIIbβ3 deficiency (type I GT). Among these, the single amino acid substitutions have been especially informative in defining precise structural domains of integrins that play a role in the biosynthesis and/or function. For example, Gly242 → Asp (Gly273 → Asp)10 and Gly418 → Asp11 in αIIb have been characterized in type I GT; these were highly conserved residues adjusted to the first calcium binding domain and flanking the fourth calcium binding domain of αIIb, respectively. By contrast, the molecular basis for moderate αIIbβ3 deficiency (type II GT) remains obscure. Four mutations have been reported: Leu183 → Pro12 and Arg327 → His13,14 in αIIb; Leu117 → Trp15 and Cys374 → Thr16 in β3.
We have recently demonstrated that the amount of αIIb is much lower than that of β3 in a number of Japanese GT patients.17 Our data suggest that the molecular defect may exist more often in the αIIb gene than in the β3 gene in Japanese GT patients. In this study, we describe a new single amino acid substitution (Gln747 → Pro) in αIIb responsible for moderate αIIbβ3 deficiency in 4 unrelated GT patients. Among them, patient MT (type II) was homozygous for the Gln747 → Pro substitution and patient MS (type I) was compound heterozygous for this substitution and a RNA splice mutation.
MATERIALS AND METHODS
Patients.
Patient MT, the product of nonconsanguineous parents, was a 40-year-old Japanese woman who had a life-long history of moderate mucocutaneous bleeding. Hematological examinations showed a prolonged bleeding time and absence of platelet aggregation in response to ADP, epinephrine, and collagen, but a normal response to ristocetin. Clot retraction was normal. She was patient no. 7 in our previous report and was classified as type II GT.17 Patient MS, the product of nonconsanguineous parents, was a 44-year-old Japanese woman who was also diagnosed as a typical case of GT. Clot retraction was slightly impaired (38%; normal values, 48% to 68%). Patients MT and MS were unrelated.
Antibodies.
Rabbit polyclonal antisera specific for αIIbβ3 and murine monoclonal antibodies (MoAbs) AP2 (αIIbβ3-specific MoAb) were generously provided by Dr Thomas J. Kunicki (Scripps Research Institute, La Jolla, CA).18 AP3 (β3-specific MoAb) was a generous gift from Dr Peter Newman (The Blood Center of Southeastern Wisconsin, Milwaukee, WI).19 PAC-1 (a ligand mimetic MoAb) binds specifically to activated αIIbβ3 and was kindly provided by Dr Sanford Shattil (Scripps Research Institute).20 PT25-2 (αIIbβ3-specific MoAb) activates αIIbβ3 and was a kind gift from Drs Makoto Handa and Yasuo Ikeda (Keio University, Tokyo, Japan).21TP80 (αIIb-specific MoAb) and MOPC21 were purchased from Nichirei (Tokyo, Japan) and Sigma Chemical (St Louis, MO), respectively.
Synthetic ligand.
FK633(N-(N-{4-(4-Amidinophenoxy)butyl}-a-L-aspartyl-L-valine), a peptidomimetic antagonist specific for αIIbβ3, was generously provided by Dr Jiro Seki (Fujisawa Pharmaceutical Co, Osaka, Japan).22
Immunoblot assay and flow cytometry.
Immunoblot assay using rabbit polyclonal antisera specific for αIIbβ3 and flow cytometric analysis using various MoAbs were performed as previously described.23 24The amount of αIIb and β3 was semiquantified by densitometry using a CS 9000 dual-wavelength flying spot scanner (Shimadzu Corp, Kyoto, Japan).
Amplification and analysis of platelet RNA.
Total cellular RNA of platelets was isolated from 30 mL of whole blood and αIIb or β3 mRNA was specifically amplified by reverse transcription-polymerase chain reaction (RT-PCR), as previously described.24 The primers for the amplification of αIIb mRNA and conditions for RT-PCR were described elsewhere.24 The following primers were constructed based on the published sequence of β325 and used for the first-round PCR of β3 mRNA: IIIa1, 5′-CGGCCCCGGCCGCTCTGGGTGACTG-3′ (sense, nucleotide [nt] −15-10); IIIa2, 5′-CAACTCTTCAGGGAGGTCACG-3′ (antisense, nt 1147-1127); IIIa3, 5′-GAGCTCATCCCAGGGACCAC-3′ (sense, nt 1015-1034); and IIIa4, 5′-CACTGACTCAATCTCGTCACGGC-3′ (antisense, nt 1974-1952). IIIa7 and IIIa8 were described elsewhere.24 The following nested primers were used for the second-round PCR: IIIa1-Sal I, 5′-CTGTCGACGCGCTGGGGGCGCTG-3′ (sense, nt 8-30; mismatched sequences were underlined); IIIa2-Sph I, 5′-GGGCATGCACGCACTTCCAGCTC-3′ (antisense, nt 1137-1114); IIIa3-Sal I, 5′-GGGTCGACAGTTGGGGTTCTGTC-3′ (sense, nt 1027-1049); IIIa4-Sph I, 5′-GACGCATGCTCGTCACGGCAGTAACG-3′ (antisense, nt 1945-1970); IIIa7-Sal I, 5′-CTAGTCGACCAATGGGCTGCTGTG-3′ (sense, nt 1749-1772); and IIIa8-Sph I, 5′-GGCGCATGCTGATAATGATCTGAG-3′ (antisense, nt 2376-2353).
Nucleotide sequences of PCR products and subcloned cDNA fragments were determined by using Taq DyeDeoxy Terminator Cycle Sequencing Kit (Applied Biosystems, Foster City, CA).
Allele-specific restriction enzyme analysis (ASRA).
Amplification of the region around exon 23 of the αIIbgene was performed by using primers IIbE23, 5′-CAGGTCTAACTTCAGTGTGGC-3′ (sense, nt 13134-13154 in the αIIb gene), and IIbE24, 5′-CAGGATGTAGAGCAGGTC-3′ (antisense, nt 13761-13744), using 250 ng of DNA as a template.26 The first-round PCR products were reamplified using primers IIbE23 and IIbE24Pvu II, 5′-CTCTCACCCTCGCAGCTCAGCT-3′ (antisense, nt 13355-13334; mismatched sequences were underlined). PCR products were then digested with restriction enzyme Pvu II. For the amplification of the region around exon 18 of the αIIb gene, amplified DNA fragments using primers IIbE16, 5′-GAGGTCGACTTACGTCTTTTGC-3′ (sense, nt 9324-9344), and IIbI18, 5′-GGGTTACATTGTGACTTGGCAC-3′ (antisense, nt 10048-10027), were reamplified using nested primers IIbE17A, 5′-ATGCCGAGCTGCAGCTG-3′ (sense, nt 9501-9517), and IIbI18. PCR products were digested with Avr II. The resulting fragments were electrophoresed in a 6% polyacrylamide gel.
Construction of αIIb expression vectors.
The αIIb and β3 cDNA constructs were cloned into a mammalian expression vector pcDNA3 (Invitrogen Corp, San Diego, CA) and generously provided by Dr Peter Newman. To construct the expression vectors containing the 2334A (wild-type [WT]) or 2334C (Pro747) form of αIIb cDNA, PCR-based cartridge mutagenesis was performed. The 1,184-bp region (nt 1988-3171) of platelet αIIb cDNA from patient MS, who was heterozygous for 2334A and 2334C, was amplified by RT-PCR using primers IIb5 and IIb8. Then, second-round amplification was performed using 1 μL of the first-round PCR products as a template with nested primers IIb5A, 5′-CCAGATAGGAATCGCGATG-3′ (sense, nt 2185-2203), and IIb8Xba I, 5′-CCTTCTAGAATAGTGTAGGCTGCACC-3′ (antisense, nt 3148-3123; mismatched sequence was underlined) and Vent Polymerase (New England Biolabs, Beverly, MA). The amplified fragments were digested withRsr II and Xba I, and the resulting 823-bp fragments (nt 2318-3140) were extracted using GeneClean II kit (Bio 101, La Jolla, CA). The 2,367-bp fragment extending from the beginning of the open reading frame to nt 2317 was obtained by digesting the full-length of αIIb cDNA with HindIII andRsr II. These two fragments were double-inserted into the pcDNA3 digested with HindIII and Xba I. Single clones that encode A or C at nt 2334 were selected by PCR followed byPvu II digestion. The selected clones were characterized by sequence analysis to verify the absence of any other substitutions and the proper insertion of the PCR cartridge into the vector.
To generate an Ala747αIIb construct, we performed the site-directed mutagenesis by PCR. We synthesized mismatched sense primer IIb747Ala, 5′-CCGGTCCGGGCAGAGGCCGCAGTG-3′ (sense, nt 2315-2338; mismatched sequences were underlined) and performed PCR using full-length αIIb cDNA as a template and primers IIb747Ala and IIb8Xba I. PCR products were digested withRsr II and Xba I. The 823-bp fragments (nt 2318-3140) were shuttled into pcDNA3 as described above. The mutant clones were characterized by sequence analysis to verify the absence of any other substitutions and the proper insertion of the PCR cartridge into the vector.
The wild-type or mutant αIIb construct was cotransfected into 293 cells with wild-type β3 construct by the calcium phosphate method, as previously described.27 The cells were cultured in Dulbecco’s modified medium (DME) with 10% heat-inactivated fetal calf serum (FCS).
Surface labeling of the transfected cells.
Surface proteins of the transfected cells were biotinylated, and immunoprecipitation using MoAbs was performed as previously described.27
Metabolic label with [35S] methionine and pulse chase.
Metabolic labeling of transfected cell was performed 1 day after transfection, as previously described.27 The cells were incubated with 0.2 mCi/mL of [35S]-methionine for 120 minutes. For pulse chase study, the cells were incubated with 0.4 mCi/mL of [35S]-methionine for 30 minutes and the medium was then changed to DME/10% FCS with 50 μg/mL of nonradioactive methionine. Cells were equally divided into five dishes and chased after 0, 2, 4, 8, and 24 hours, respectively. Immunoprecipitation was performed as previously described.27
RESULTS
Immunoblot analysis.
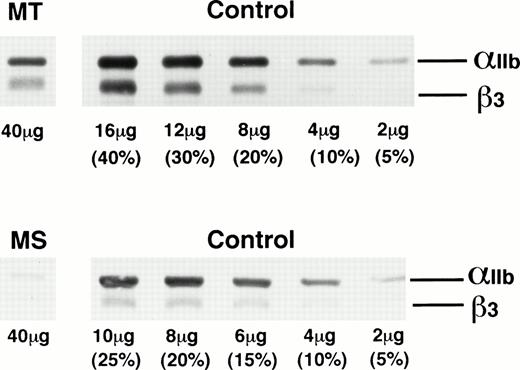
We first analyzed platelet proteins from patients MT and MS in an immunoblot assay under nonreducing (not shown) and reducing conditions (Fig 1). Various amounts of platelet proteins obtained from three normal subjects were also examined to obtain a standard curve. In patient MT, the amounts of αIIb and β3 were 15% and 22% of control, respectively, whereas in patient MS, αIIb and β3 were 4% and 8%, respectively. Abnormal αIIb or β3, such as a premature form of αIIb, was not detected under nonreducing and reducing conditions in either patient. From these data, MT was classified as type II GT and MS as type I GT.
Immunoblot analysis of platelet proteins from patients MT and MS using anti-IIbβ3 antibodies. Platelet proteins from GT patients MT and MS and various amounts of control platelet proteins from three normal subjects were electrophoresed on 7.5% polyacrylamide gel under reducing conditions and transferred to a nitrocellulose membrane. IIb and β3 were detected with a 1:10,000 dilution of rabbit anti-IIbβ3 antibodies. The amount of proteins electrophoresed is indicated at the bottom of each line.
Immunoblot analysis of platelet proteins from patients MT and MS using anti-IIbβ3 antibodies. Platelet proteins from GT patients MT and MS and various amounts of control platelet proteins from three normal subjects were electrophoresed on 7.5% polyacrylamide gel under reducing conditions and transferred to a nitrocellulose membrane. IIb and β3 were detected with a 1:10,000 dilution of rabbit anti-IIbβ3 antibodies. The amount of proteins electrophoresed is indicated at the bottom of each line.
Nucleotide sequence analysis of αIIb cDNA from MT and MS.
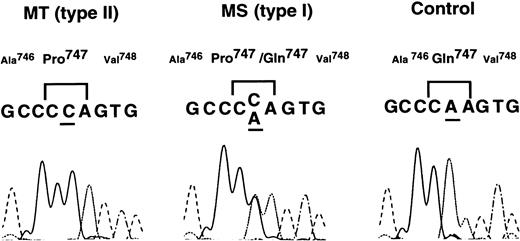
To identify the molecular defect in patients MT and MS, platelet mRNA was isolated from these patients and normal controls. The whole coding regions of αIIb and β3 cDNA were amplified by RT-PCR. Examination of nucleotide sequences of the PCR fragments using an ABI 373A DNA sequencer (Applied Biosystems, Foster City, CA) showed a single A → C substitution at nt 2334 in αIIb cDNA that leads to a Gln747 → Pro substitution in exon 23 of αIIb (Fig 2). Patient MT appeared homozygous for the 2334A → C substitution. The homozygosity of the substitution was confirmed by nucleotide sequence analysis of PCR fragments from genomic DNA (data not shown). No other nucleotide substitution was detected in either αIIb or β3 cDNA from patient MT. Patient MS was heterozygous for the 2334A → C substitution.
Nucleotide sequence analysis of IIb cDNA from patients MT and MS. Nucleotides of IIb cDNA from patients MT and MS and normal control were amplified by RT-PCR. The amplified fragments were directly examined using Taq DyeDeoxy Terminator Cycle Sequencing kit, and samples were run and analyzed on an ABI 373A DNA sequencer.
Nucleotide sequence analysis of IIb cDNA from patients MT and MS. Nucleotides of IIb cDNA from patients MT and MS and normal control were amplified by RT-PCR. The amplified fragments were directly examined using Taq DyeDeoxy Terminator Cycle Sequencing kit, and samples were run and analyzed on an ABI 373A DNA sequencer.
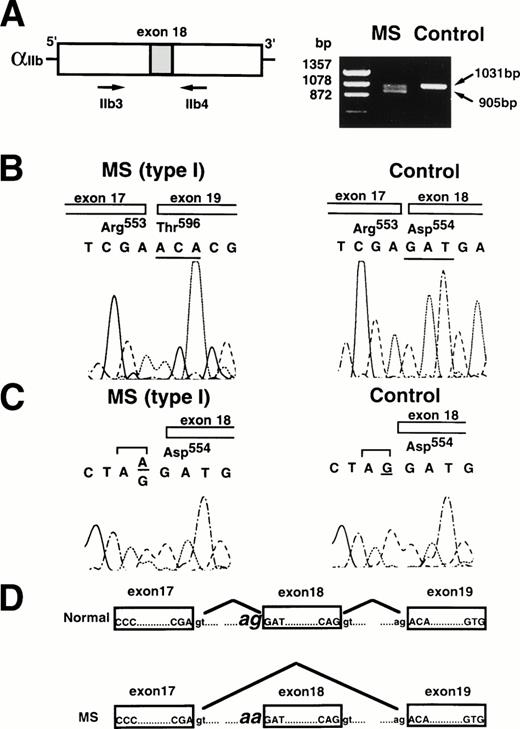
Because patient MS was heterozygous with severe deficiency, another genetic defect in the αIIb gene was sought. Electrophoretic analysis of the first round of RT-PCR fragments using primers IIb3 and IIb4 in patient MS showed that two different-sized cDNAs were amplified: an expected size (1,031 bp) and a smaller size (∼900 bp) (Fig 3). Each cDNA fragment was subcloned into pUC19, and nucleotide sequences were analyzed. Sequence analysis of the smaller-sized fragment showed that a 126-bp region corresponding to the whole nucleotide sequence of exon 18 was deleted (Fig 3). No abnormality existed in the nucleotide sequence of the expected-sized fragment. The flanking region of exon 18 of the αIIb gene was then amplified from genomic DNA of patient MS as well as control by PCR using primers IIbE16 and IIbI18. Nucleotide sequence showed an AG → AA substitution at the consensus splice acceptor site (−1) of exon 18 (Fig 3). No other nucleotide substitution was detected in either αIIb or β3 cDNA in patient MS. Thus, MS appeared to be heterozygous for the 2334A → C substitution and the G → A substitution at the splice acceptor site of exon 18 in the αIIb gene.
Analysis of IIb cDNA and the IIb gene in patient MS. (A) Amplification of IIb cDNA from patient MS by RT-PCR. Two hundred fifty nanograms of total cellular RNA from MS or a normal control was amplified by RT-PCR using primers IIb3 and IIb4. The PCR products were electrophoresed on 1.5% agarose gel. (B) Nucleotide sequence analysis of IIb cDNA from patient MS. The cDNA PCR fragments were subcloned into pUC19, and nucleotides were sequenced. (C) Nucleotide sequence analysis of the IIb gene from patient MS. Nucleotide of the IIb gene from patient MS or a normal control was amplified by PCR using primers IIbE16 and IIbI18 and sequenced. (D) Schematic diagram indicates the mechanism of exon18 skipping of the platelet IIb mRNA.
Analysis of IIb cDNA and the IIb gene in patient MS. (A) Amplification of IIb cDNA from patient MS by RT-PCR. Two hundred fifty nanograms of total cellular RNA from MS or a normal control was amplified by RT-PCR using primers IIb3 and IIb4. The PCR products were electrophoresed on 1.5% agarose gel. (B) Nucleotide sequence analysis of IIb cDNA from patient MS. The cDNA PCR fragments were subcloned into pUC19, and nucleotides were sequenced. (C) Nucleotide sequence analysis of the IIb gene from patient MS. Nucleotide of the IIb gene from patient MS or a normal control was amplified by PCR using primers IIbE16 and IIbI18 and sequenced. (D) Schematic diagram indicates the mechanism of exon18 skipping of the platelet IIb mRNA.
ASRA.
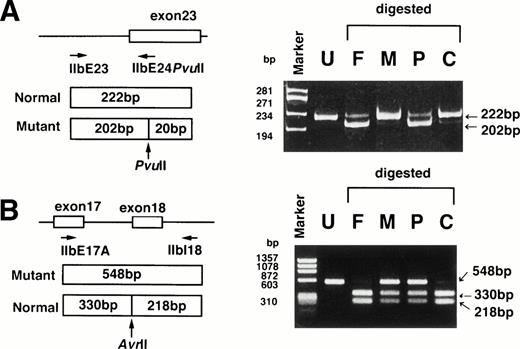
To confirm that patient MS was a compound heterozygote, exon 23 and exon 18 with their flanking regions were amplified by PCR, followed by digestion with Pvu II and Avr II, respectively. A restriction site for Pvu II would be created by the2334A → C substitution and a restriction site forAvr II would be abolished by the G → A substitution. ASRA clearly indicated that the A → C substitution in exon 23 was derived from the patient’s father and that the G → A substitution at the splice acceptor site of exon 18 was derived from the mother (Fig4). These data confirmed that patient MS was a compound heterozygote. ASRA further confirmed that patient MT was homozygous for the A → C substitution in exon 23 (data not shown). Using ASRA, we examined the presence of the 2334A → C substitution in 15 other unrelated Japanese GT patients (type I, 8 cases; type II, 7 cases) and 20 control subjects. This substitution was present in 2 type II GT patients who were homozygote and heterozygote, respectively (data not shown). None of control subjects had this substitution.
ASRA. (A) The region around exon 23 of the IIb gene was amplified by PCR using primers IIbE23 and IIbE24Pvu II, followed by digestion with PvuII. The A → C substitution creates a restriction site forPvu II and yields 202-bp and 20-bp fragments. The resulting fragments were electrophoresed in a 6% polyacrylamide gel. (B) The region around exon 18 of the IIb gene was amplified by PCR using primers IIbE17A and IIbI18, followed by digestion withAvr II. Avr II digestion of the PCR products yields 330-bp and 218-bp fragments in the normal allele. The G → A substitution abolished a restriction site for Avr II. The resulting fragments were electrophoresed in a 1.5% agarose gel. F, M, P, and C denote DNA from the patient’s father, mother, patient (MS), and control, respectively. Undigested PCR fragment from the control is also shown (U). Marker: ◊X174 digested with Hae III.
ASRA. (A) The region around exon 23 of the IIb gene was amplified by PCR using primers IIbE23 and IIbE24Pvu II, followed by digestion with PvuII. The A → C substitution creates a restriction site forPvu II and yields 202-bp and 20-bp fragments. The resulting fragments were electrophoresed in a 6% polyacrylamide gel. (B) The region around exon 18 of the IIb gene was amplified by PCR using primers IIbE17A and IIbI18, followed by digestion withAvr II. Avr II digestion of the PCR products yields 330-bp and 218-bp fragments in the normal allele. The G → A substitution abolished a restriction site for Avr II. The resulting fragments were electrophoresed in a 1.5% agarose gel. F, M, P, and C denote DNA from the patient’s father, mother, patient (MS), and control, respectively. Undigested PCR fragment from the control is also shown (U). Marker: ◊X174 digested with Hae III.
Effect of Gln747 → Pro substitution on αIIbβ3expression.
To examine whether the 2334A → C substitution leading to Gln747 → Pro substitution (Pro747) in αIIb might be responsible for type II GT, we constructed an expression vector that contained the wild-type or mutant Pro747 form of αIIb. Each vector was cotransfected with the wild-type β3 cDNA into 293 cells.
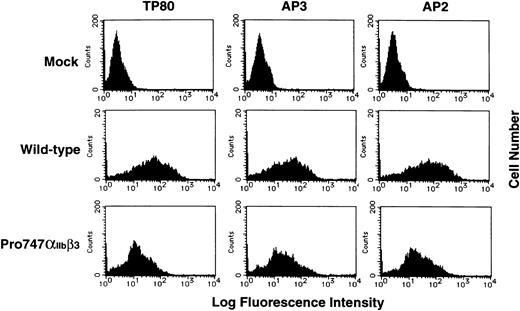
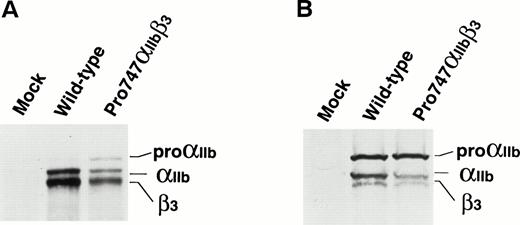
RNA blot analysis showed that the efficiency of transfection between the wild-type and the mutant Pro747αIIb was essentially the same (data not shown). Flow cytometric analysis using the αIIb-specific MoAb, TP80; the β3-specific MoAb, AP3; and the αIIbβ3 complex-specific MoAb, AP2, showed that the level of mutant Pro747αIIbβ3 expression was moderately reduced compared with wild-type αIIbβ3expression (Fig 5). Immunoprecipitation of surface-labeled transfected cells using AP2 MoAb also showed that the amount of Pro747αIIbβ3 complex was moderately reduced compared with wild-type and that the molecular weight of the mutant αIIb was the same as the wild-type (Fig 6A). Interestingly, in the mutant Pro747αIIbβ3 transfected cells, a significant amount of a premature form of αIIb(proαIIb) was precipitated by AP2 MoAb. These data indicate that proαIIb could be expressed and complexed with β3 on the surface of the mutant Pro747αIIbβ3 transfected cells. Densitometric analysis showed approximately 20% of normal levels of αIIb (proαIIb + αIIb) and approximately 29% of normal levels of β3 expressed on the surface of the Pro747αIIbβ3transfectants (mean of 2 separate experiments). Employing immunoblot assay using polyclonal antisera specific for αIIbβ3, we also examined the amount of αIIbβ3 in transfected cells. Again, the mature forms of Pro747αIIb and β3 in mutant transfected cells were moderately reduced compared with wild-type transfected cells (≈30% of normal levels of αIIb, ≈48% of normal levels of β3, n = 2; Fig 6B). However, the amount of proαIIb was not reduced in mutant transfected cells (≈100% of normal levels of proαIIb, n = 2; Fig 6B). These data indicate that the 2334A → C substitution leads to moderate reduction in the amount of αIIbβ3 within the transfected cells as well as on the cell surface.
Flow cytometric analysis of IIbβ3 on the transfected cell surface. Recombinant IIb cDNA containing the 2334A → C substitution subcloned into pcDNA3 was cotransfected with recombinant wild-type β3 cDNA in 293 cells. The transfected cells were incubated with TP80 (IIb-specific MoAb), AP3 (β3-specific MoAb), or AP2 (IIbβ3 complex-specific MoAb) for 30 minutes on ice and washed once, and bound antibodies were detected by FITC-conjugated goat F(ab′)2 antimouse IgG. Results are expressed as histograms of cell number (linear scale) on the ordinate versus fluorescence intensity (log scale) on the abscissa.
Flow cytometric analysis of IIbβ3 on the transfected cell surface. Recombinant IIb cDNA containing the 2334A → C substitution subcloned into pcDNA3 was cotransfected with recombinant wild-type β3 cDNA in 293 cells. The transfected cells were incubated with TP80 (IIb-specific MoAb), AP3 (β3-specific MoAb), or AP2 (IIbβ3 complex-specific MoAb) for 30 minutes on ice and washed once, and bound antibodies were detected by FITC-conjugated goat F(ab′)2 antimouse IgG. Results are expressed as histograms of cell number (linear scale) on the ordinate versus fluorescence intensity (log scale) on the abscissa.
Expression of IIb containing the Gln747 → Pro (Pro747) mutation in transfected cells. (A) Immunoprecipitation analysis of biotin surface-labeled transfected cells. Wild-type or the mutant Pro747 form of IIb cDNA was cotransfected with wild-type β3 cDNA into 293 cells. The transfected cells were surface labeled with biotin 2 days after transfection. Immunoprecipitation was then performed using AP2 (IIbβ3 complex-specific MoAb). Precipitates were separated by 6% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions. After transferring to a nitrocellulose membrane, precipitated proteins were detected by chemiluminescence. (B) Immunoblot analysis of transfected cells. The transfected cells were lysed and separated by 6% SDS-PAGE under reducing conditions 2 days after transfection. After transferring to a nitrocellulose membrane, IIb and β3 were detected with polyclonal anti-IIbβ3 antisera.
Expression of IIb containing the Gln747 → Pro (Pro747) mutation in transfected cells. (A) Immunoprecipitation analysis of biotin surface-labeled transfected cells. Wild-type or the mutant Pro747 form of IIb cDNA was cotransfected with wild-type β3 cDNA into 293 cells. The transfected cells were surface labeled with biotin 2 days after transfection. Immunoprecipitation was then performed using AP2 (IIbβ3 complex-specific MoAb). Precipitates were separated by 6% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions. After transferring to a nitrocellulose membrane, precipitated proteins were detected by chemiluminescence. (B) Immunoblot analysis of transfected cells. The transfected cells were lysed and separated by 6% SDS-PAGE under reducing conditions 2 days after transfection. After transferring to a nitrocellulose membrane, IIb and β3 were detected with polyclonal anti-IIbβ3 antisera.
Effect of Pro747 mutant on αIIbβ3biosynthesis.
To elucidate the mechanism of impaired expression of the mutant αIIbβ3, we examined the association between the mutant Pro747proαIIb and β3. Transfected cells were labeled with [35S]-methionine for 2 hours; immunoprecipitation using TP80 MoAb or AP3 MoAb was then performed. Densitometric analysis of the immunoprecipitate showed that the β3/(proαIIb + mature αIIb) ratios were essentially the same between wild-type and the mutant transfected cells. They were 0.66 (wild-type) and 0.67 (mutant) using TP80 and 1.93 (wild-type) and 1.91 (mutant) using AP3 (n = 2; Fig 7A). These results demonstrated that the association of the mutant proαIIbwith β3 was the same as that of wild-type proαIIb. The densitometric analysis also showed that wild-type and the mutant transfected 293 cells synthesized β3 in excess compared with proαIIb and that approximately 70% of labeled β3 was still in the free form.
Effect of the Pro747 mutant on IIbβ3 biosynthesis. (A) Association of wild-type or the Pro747 mutant proIIb with wild-type β3. Wild-type or the Pro747 mutant IIbβ3 transfected cells were labeled with 0.2 mCi/mL of [35S]-methionine for 120 minutes and total cellular lysates were prepared. Subunit association was assessed by immunoprecipitation with TP80 (IIb-specific MoAb) or AP3 (β3-specific MoAb). Precipitates were separated by 6% SDS-PAGE under reducing conditions. (B) Pulse chase analysis of the stability of wild-type and Pro747 mutant proIIb subunit. Wild-type or Pro747IIb cDNA was transfected into 293 cells without the wild-type β3 cDNA, and cells were labeled with 0.4 mCi/mL of [35S]-methionine for 30 minutes and chased with media containing 50 μg/mL of nonradioactive methionine for various periods of time, as indicated. Immunoprecipitation was performed using TP80. Precipitates were separated by 6% SDS-PAGE under reducing conditions. (C) Pulse chase analysis of wild-type or the Pro747 mutant IIbβ3 in transfected cells. Wild-type or Pro747 IIbβ3 transfected cells were labeled with 0.4 mCi/mL of [35S]-methionine for 30 minutes and chased with media containing 50 μg/mL of nonradioactive methionine for various periods of time, as indicated. Immunoprecipitation was performed using TP80. Precipitates were separated by 6% SDS-PAGE under reducing conditions. This figure shows a representative of six separate experiments. (D) Densitometric analysis of the kinetics of biosynthesis of IIbβ3 shown in (C). The bands corresponding to proIIb (□), IIb (◊), and β3 (○) were analyzed by scanning densitometry. The results were normalized relative to dye-front band at each lane.
Effect of the Pro747 mutant on IIbβ3 biosynthesis. (A) Association of wild-type or the Pro747 mutant proIIb with wild-type β3. Wild-type or the Pro747 mutant IIbβ3 transfected cells were labeled with 0.2 mCi/mL of [35S]-methionine for 120 minutes and total cellular lysates were prepared. Subunit association was assessed by immunoprecipitation with TP80 (IIb-specific MoAb) or AP3 (β3-specific MoAb). Precipitates were separated by 6% SDS-PAGE under reducing conditions. (B) Pulse chase analysis of the stability of wild-type and Pro747 mutant proIIb subunit. Wild-type or Pro747IIb cDNA was transfected into 293 cells without the wild-type β3 cDNA, and cells were labeled with 0.4 mCi/mL of [35S]-methionine for 30 minutes and chased with media containing 50 μg/mL of nonradioactive methionine for various periods of time, as indicated. Immunoprecipitation was performed using TP80. Precipitates were separated by 6% SDS-PAGE under reducing conditions. (C) Pulse chase analysis of wild-type or the Pro747 mutant IIbβ3 in transfected cells. Wild-type or Pro747 IIbβ3 transfected cells were labeled with 0.4 mCi/mL of [35S]-methionine for 30 minutes and chased with media containing 50 μg/mL of nonradioactive methionine for various periods of time, as indicated. Immunoprecipitation was performed using TP80. Precipitates were separated by 6% SDS-PAGE under reducing conditions. This figure shows a representative of six separate experiments. (D) Densitometric analysis of the kinetics of biosynthesis of IIbβ3 shown in (C). The bands corresponding to proIIb (□), IIb (◊), and β3 (○) were analyzed by scanning densitometry. The results were normalized relative to dye-front band at each lane.
The fate of the recombinant proteins was further examined in pulse-chase experiments. First, we examined the stability of the mutant Pro747proαIIb. The αIIb transfected cells were pulsed with [35S]-methionine for 30 minutes, chased with unlabeled methionine for various periods of time, and then immunoprecipitated using TP80 MoAb. As shown in Fig 7B, Pro747 mutation did not affect the stability of the proαIIb subunit. Next, to examine the effect of this mutation on the kinetics of αIIbβ3 complex formation, wild-type or Pro747αIIb was cotransfected with wild-type β3. Pulse-chase experiments showed that the mutant proαIIb was clearly detected at 24 hours postchase and was more stable than wild-type proαIIb when assembled with β3. The mutant Pro747proαIIb was cleaved into heavy and light chains. However, this process was moderately impaired compared with wild-type proαIIb (Fig7C and D).
Expression of site-directed Ala747αIIbmutant on 293 cells.
To further examine the role of the Gln residue at amino acid 747 of αIIb on αIIbβ3 expression, we introduced a Gln747 → Ala mutation (Ala747) by PCR-based site-directed mutagenesis. The mutant Ala747 form of αIIbcDNA was cotransfected with wild-type β3 cDNA into 293 cells. Flow cytometric analysis using AP2 MoAb showed that the level of surface expression of Ala747αIIbβ3 complex on the transfected cells was almost the same as wild-type αIIbβ3 complex (mean fluorescence intensity: 81.9 for wild-type and 92.3 for Ala747αIIbβ3, n = 2; Fig8). These data indicate that the Gln747 → Ala mutation does not impair αIIbβ3expression.
Effect of Gln747 → Ala substitution on IIbβ3 expression and PAC-1 binding to wild-type and mutant IIbβ3 activated with PT25-2 MoAb. The IIb cDNA containing the Gln747 → Ala substitution was cotransfected with wild-type β3 cDNA into 293 cells. The transfected cells were incubated with AP2 or PT25-2 for 30 minutes on ice and washed once, and bound antibodies were detected by FITC-conjugated goat F(ab′)2 antimouse IgG. For PAC-1 binding, PT25-2–treated or FK633-treated 293 cells were incubated with FITC-labeled PAC-1 for 30 minutes on ice. Results are expressed as histograms of cell number (linear scale) on the ordinate versus fluorescence intensity (log scale) on the abscissa.
Effect of Gln747 → Ala substitution on IIbβ3 expression and PAC-1 binding to wild-type and mutant IIbβ3 activated with PT25-2 MoAb. The IIb cDNA containing the Gln747 → Ala substitution was cotransfected with wild-type β3 cDNA into 293 cells. The transfected cells were incubated with AP2 or PT25-2 for 30 minutes on ice and washed once, and bound antibodies were detected by FITC-conjugated goat F(ab′)2 antimouse IgG. For PAC-1 binding, PT25-2–treated or FK633-treated 293 cells were incubated with FITC-labeled PAC-1 for 30 minutes on ice. Results are expressed as histograms of cell number (linear scale) on the ordinate versus fluorescence intensity (log scale) on the abscissa.
PAC-1 binding to wild-type and mutant αIIbβ3.
Because the mutant Pro747αIIbβ3 receptors were expressed at substantial levels on the surface of transfected cells, we then examined the binding of the ligand-mimetic MoAb PAC-1 in the presence of the activating MoAb PT25-2. Negative control for the PAC-1 binding was obtained using FK633, a peptidomimetic antagonist specific for αIIbβ3. As shown in Fig 8, PAC-1 could bind to both Pro747αIIbβ3 and Ala747αIIbβ3 in the presence of PT25-2. The PAC-1 binding to activated αIIbβ3 was dependent on the PT25-2 binding and the PAC-1/PT25-2 binding ratios were 1.28 and 0.97 for Pro747αIIbβ3 and Ala747αIIbβ3, respectively, which were normalized relative to the ratio for wild-type. These data suggest that ligand binding function of Pro747αIIbβ3 and Ala747αIIbβ3 is not disturbed.
DISCUSSION
In this report, we described a new point mutation (2334A → C) leading to Gln747 → Pro amino acid substitution in αIIb that is responsible for moderate αIIbβ3 deficiency (type II phenotype) in 6 of the 34 possibly mutant chromosomes in 17 unrelated Japanese GT patients. In addition, a G → A mutation at the consensus sequence of the splice acceptor site of exon 18 of the αIIb gene that is likely to be responsible for the exon 18 skipping in αIIb cDNA was also found. The exon 18 skipping leads to an in-frame deletion of 42 amino acids in the extracellular domain of αIIb. Together with the Pro747 mutation, the deletion of exon 18 contributed to the severe reduction in αIIbβ3 expression (in type I GT patient MS).
We demonstrated that the Pro747 substitution in αIIb was not a naturally occurring polymorphism of αIIb. ASRA showed that none of 20 control subjects possessed this substitution. Mammalian expression vectors encoding the mutant Pro747 form of αIIb were constructed and cotransfected with wild-type β3 cDNA into 293 cells. Both flow cytometric and immunoprecipitation analysis using anti-αIIbβ3 MoAbs demonstrated that the Pro747 substitution directly leads to moderate reduction in αIIbβ3 expression on the cell surface (20% to 30% of wild-type). The impairment of reactivity with a panel of MoAbs was not due to disruption of their epitopes, because immunoblot analysis using polyclonal anti-αIIbβ3antisera clearly showed reduction in the total amount of αIIbβ3 in transfected cells. Recently, it has been demonstrated that the Leu183 → Pro mutation in αIIb leads to both quantitative and qualitative abnormalities in αIIbβ3.12However, the Gln747 → Pro mutation did not impair the ligand-binding function. These results demonstrate that the Pro747 mutation only leads to a quantitative abnormality.
Characterization of the two mutations (Asp242 and Asp418) flanking the calcium-binding domains of αIIb responsible for type I GT indicates that these relatively well-conserved flanking sequences are not essential for assembly of the αIIbβ3heterodimer; rather, they are critical for the proper folding of αIIbβ3 to be transported to the Golgi apparatus, where αIIb cleavage occurs.10,11However, the Pro747 mutation was far from the calcium-binding domains. By means of protein analysis of the proteolytic fragments of isolated αIIbβ3 heterodimers, Calvete et al28 have demonstrated that three regions of the αIIb heavy chain were involved in interaction with β3: amino acids 486-553, 696-734, and 780-814. Because amino acid 747 was close to the 696-734 region, we examined whether the Pro747 mutation might impair assembly of αIIbβ3 heterodimers. Immunoprecipitation analysis using metabolically labeled transfected cells clearly indicated that assembly of the mutant Pro747proαIIb and β3 normally occurred. In contrast to the Asp242 and Asp418 mutations, pulse chase studies demonstrated that some of the mutant Pro747proαIIbβ3 complexes underwent endoproteolytic cleavage of proαIIb into heavy and light chains. These data suggest that some αIIbβ3heterodimers can be transported to the Golgi apparatus. Thus, the Pro747 mutation does not completely prevent intracellular transport of the heterodimers to the Golgi apparatus.
Recently, Kolodziej et al29 demonstrated that the endoproteolytic cleavage of proαIIb occurs on the carboxyl side of dibasic Arg858-Arg859. They also demonstrated that the failure of cleavage does not prevent expression of αIIbβ3 on the cell surface. Immunoprecipitation using AP2 MoAb of biotin-labeled surface proteins showed that, in addition to mature αIIb, a small amount of proαIIb was expressed on the Pro747 mutant transfected cells. Pulse-chase studies demonstrated that the stability of the mutant Pro747proαIIb was increased only when assembled with β3. These data suggest that the mutant proαIIb complexed with β3 also impairs cleavage of αIIb to some extent, probably due to the induction of a conformation in αIIb that is less favorable to protease activity. Because the cleavage of proαIIb into heavy and light chains is not critical for surface expression of αIIbβ3,29our data suggest that the Pro747 mutation impairs intracellular transport of αIIbβ3 to the Golgi apparatus as well. Although our transfection experiments demonstrated that the mutation led to the expression of a significant amount of proαIIb on the transfected cells, proαIIbwas not detected in platelets from patients MT and MS even in the immunoblot assay. This is probably due to differences between transfected cells and platelets that circulate for 10 days with no new mRNA being made. In contrast to our patients, Jung et al30 have reported a type I GT patient whose platelets contained no normal αIIb, but did show a trace amount of a premature form of αIIb in an immunoblot assay.30 One of the genetic defects of their patient was an RNA splicing mutation leading to skipping of exon 26 that contains the endoproteolytic cleavage site of αIIb.31However, this abnormal αIIb could not be expressed on the platelet surface. Because exon 26 skipping led to loss of 42 amino acids as well as the cleavage site, it is likely that the mutation altered the conformation of αIIbβ3heterodimer sufficient to prevent intracellular transport in their case.
We replaced Gln747 with Ala by mutagenesis to examine the role of Gln747 on αIIbβ3 expression. In contrast to the Pro747 mutation, Ala747 substitution did not impair αIIbβ3 expression. These data suggest that the presence of Pro747, rather than the absence of Gln747, is critical for moderately impaired αIIbβ3 expression. Pro is an uncharged amino acid known to disrupt secondary protein structures, and a number of naturally occurring mutations creating a Pro residue leading to impaired expression or function of proteins have been reported.12,32-34 In GT patients, it has been well documented that the Ser752 → Pro mutation in the cytoplasmic domain of β3 leads to a variant GT phenotype.32Pro752 mutation makes αIIbβ3 incapable of being activated by intracellular signals and decreases its capacity to mediate cell spreading.35,36 However, replacement of Ser752 with Ala had no adverse effects on αIIbβ3-mediated cell spreading.37 Kahn et al38 have demonstrated that replacement of Gly242 residue with the nonpolar amino acids Ala or Val had no effect on αIIbβ3 expression, whereas replacement with the negatively charged Glu, positively charged Lys, or nonpolar Pro caused intracellular retention of αIIbβ3.
In summary, we have described a novel point mutation, Gln747 → Pro in αIIb responsible for type II GT. The mutation moderately impaired the intracellular transport of αIIbβ3 heterodimers to the Golgi apparatus and endoproteolytic cleavage of proαIIb into heavy and light chains. Our in vitro studies suggest that the impairment of αIIbβ3 is likely due to the presence of the Pro747 residue, rather than to the absence of Gln747. Molecular genetic examination of additional GT patients should provide further insight into the structural requirements for αIIbβ3expression, as well as differences between type I and type II GT phenotypes.
ACKNOWLEDGMENT
The authors thank Dr Thomas J. Kunicki for the rabbit polyclonal antisera specific for αIIbβ3 and MoAb AP2; Dr Peter Newman for MoAb AP3 and the αIIb and β3 cDNA cloned into a mammalian expression vector pcDNA3; Dr Sanford Shattil for MoAb PAC-1; and Drs Makoto Handa and Yasuo Ikeda for MoAb PT25-2.
Supported in part by grants from the Ministry of Education, Science and Culture; the Japan Society for the Promotion of Science; and the Ryoichi Naito Foundation for Medical Research.
Address reprint requests to Yoshiaki Tomiyama, MD, The Second Department of Internal Medicine, Osaka University Medical School, 2-2 Yamadaoka, Suita Osaka 565-0871, Japan; e-mail:yoshi@hp-blood.med.osaka-u.ac.jp.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


![Fig. 7. Effect of the Pro747 mutant on IIbβ3 biosynthesis. (A) Association of wild-type or the Pro747 mutant proIIb with wild-type β3. Wild-type or the Pro747 mutant IIbβ3 transfected cells were labeled with 0.2 mCi/mL of [35S]-methionine for 120 minutes and total cellular lysates were prepared. Subunit association was assessed by immunoprecipitation with TP80 (IIb-specific MoAb) or AP3 (β3-specific MoAb). Precipitates were separated by 6% SDS-PAGE under reducing conditions. (B) Pulse chase analysis of the stability of wild-type and Pro747 mutant proIIb subunit. Wild-type or Pro747IIb cDNA was transfected into 293 cells without the wild-type β3 cDNA, and cells were labeled with 0.4 mCi/mL of [35S]-methionine for 30 minutes and chased with media containing 50 μg/mL of nonradioactive methionine for various periods of time, as indicated. Immunoprecipitation was performed using TP80. Precipitates were separated by 6% SDS-PAGE under reducing conditions. (C) Pulse chase analysis of wild-type or the Pro747 mutant IIbβ3 in transfected cells. Wild-type or Pro747 IIbβ3 transfected cells were labeled with 0.4 mCi/mL of [35S]-methionine for 30 minutes and chased with media containing 50 μg/mL of nonradioactive methionine for various periods of time, as indicated. Immunoprecipitation was performed using TP80. Precipitates were separated by 6% SDS-PAGE under reducing conditions. This figure shows a representative of six separate experiments. (D) Densitometric analysis of the kinetics of biosynthesis of IIbβ3 shown in (C). The bands corresponding to proIIb (□), IIb (◊), and β3 (○) were analyzed by scanning densitometry. The results were normalized relative to dye-front band at each lane.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/8/10.1182_blood.v92.8.2750/5/m_blod42033007w.jpeg?Expires=1766180928&Signature=Mb7Ew8XGJidlHF6KaiOZP6W55nN4UZQwMDizoRqoDf8jZlyxddih7I04GBC6rzn-GO5g~zaz9Vw9J2fXfvgmlUzp~jgtxujjbYbSynAB0VR7e41YFNrQBCYML-1L0hlGd2GBahoVF-4qEFA-6Fo5QAAcyBIVim2Z81INwC3SXy72bkxM~7o9Ev5imI9O0CEfflqkqe3JWIxhHtb60zApfBvvFftnp2~-QKHOKl8oyCpn2IkiN0gh5Svpkyruk--yZVNM9MrG4726-eR6WnEcxTWT~13yuH~if4M5rKE254iIKiTozQtcVbwerqxBHWkwqbksoY69GIFegbFLJDMCAA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)