Abstract
The effect of lipopolysaccharide (LPS) on endothelial cells is a key component of the inflammatory response seen in Gram-negative sepsis. LPS does not cause death of cultured human endothelial cells. However, when the expression of new proteins is inhibited by cycloheximide, microvascular endothelial cells in culture undergo apoptosis. This finding suggests that LPS induces apoptotic and antiapoptotic pathways, with the antiapoptotic response being dependent on the synthesis of new proteins. Concurrent activation of apoptotic and antiapoptotic pathways has previously been documented for tumor necrosis factor (TNF). In the case of TNF, the antiapoptotic signal has been attributed to at least two cytoprotective proteins: the Bcl-2 homologue, A1, and the zinc-finger protein, A20. In this study, we demonstrate that both these molecules are induced in microvascular endothelial cells by LPS. Enforced overexpression of either A1 or A20 inhibits LPS and cycloheximide-initiated apoptosis. Induction of A1 and A20 does not require synthesis of intermediary proteins, but is dependent on the presence of soluble CD14. In addition, we show that inhibition of signaling by the transcription factor, NF-κB, blocks accumulation of A1 and A20 mRNA. Taken together, our findings suggest that LPS directly induces expression of the cytoprotective proteins, A1 and A20, via a CD14-dependent pathway requiring activation of NF-κB.
© 1998 by The American Society of Hematology.
THE INCIDENCE OF Gram-negative sepsis has increased dramatically through the latter half of the 20th century. Lipopolysaccharide (LPS) is a critical glycolipid component of the outer wall of Gram-negative bacteria.1 Many of the proinflammatory and procoagulant effects of Gram-negative bacteria are elicited by LPS.2,3 Monocytes, neutrophils, and endothelial cells are critical for the initiation and potentiation of the inflammatory response, although several cell types contribute.4 The effects of LPS on endothelial cells are diverse and have been demonstrated in animal and human studies in vivo as well as in vitro.4 Some of the effects on endothelium include upregulation of adhesion molecules, chemokine release, increased expression of tissue factor, and decreased expression of protein C and antithrombin III.4
Apoptosis is a form of cell death requiring the activation of cysteine proteases termed caspases.5 Endothelial apoptosis has been associated with several disorders over the last few years.6-8 In many cases, the significance of endothelial apoptosis in the etiology of disease is not clear. However, in certain situations, endothelial apoptosis may be a primary pathogenic event. For instance, in scleroderma it is felt that the skin lesions are the result of endothelial apoptosis due to anti-endothelial cell antibodies.6
Similarly, endothelial damage plays a crucial role in the pathogenesis of septic shock due to Gram-negative bacteria.9 However, at the concentrations of LPS measured in patients’ sera during sepsis, there is minimal to no direct toxicity of LPS on cultured human umbilical vein endothelial cells.10 In contrast, prevention of new gene expression results in significant amounts of endothelial death.10 Although umbilical vein endothelial cells are a well-characterized model, much of the effect of LPS occurs at the level of the microvasculature.9 11 Thus, we were interested in determining whether human microvascular endothelial cells (HMEC-1) showed the same resistance to LPS-induced toxicity as large vessel endothelium.
We have previously shown that tumor necrosis factor (TNF) induces the upregulation of the Bcl-2 homologue, A1, which can, in turn, prevent the apoptotic events mediated by TNF.12,13 Others have demonstrated similar findings for the zinc finger protein, A20, in murine fibroblasts.14 However, whether LPS can directly induce these molecules in HMEC-1 has not been reported. We hypothesized that, similar to TNF, LPS may induce A1 and A20 in microvascular endothelial cells.
In this report, we demonstrate that, in the presence of the protein synthesis inhibitor, cycloheximide (CHX), LPS induces microvascular endothelial cell apoptosis. However, LPS does not cause endothelial death in the absence of CHX. We demonstrate further that LPS can upregulate mRNA of the antiapoptotic molecules A1 and A20, as well as A20 protein levels. These molecules can inhibit the apoptotic pathway that is concomitantly induced by LPS. This induction of A1 and A20 is a direct LPS effect that is dependent on the presence of soluble CD14, but is independent of TNF or interleukin-1 (IL-1) signaling. Finally, we show that induction of these cytoprotective molecules by LPS requires activation of the transcription factor NF-κB.
MATERIALS AND METHODS
Reagents.
LPS and CHX were purchased from Sigma (St Louis, MO) and TNF from R&D Systems (Minneapolis, MN). FLAG-epitope antibody (M2) was from IBI (New Haven, CT), rabbit polyclonal anti-A20 antibody was a gift of V. Dixit (Genentech, Inc, San Francisco, CA), neutralizing anti-CD14 antibody (60bd) was a gift of R. Todd (University of Michigan, Ann Arbor, MI), and the IgG2a isotype control was from Sigma. The neutralizing anti-TNF antibody (clone 195) was purchased from Boehringer Mannheim (Indianapolis, IN) and IL-1 receptor antagonist (IL-1RA) was obtained from R&D Systems. The horseradish peroxidase-conjugated secondary antibodies used for immunoblotting were purchased from Bio-Rad Laboratories (Hercules, CA).
Cell culture.
The HMEC-1 human dermal microvascular endothelial cell line15 was provided by the Center for Disease Control and Prevention (Atlanta, GA) and was cultured in RPMI 1640 medium supplemented with 10% fetal calf serum and 20 μg/mL bovine brain extract. Cells were maintained at 37°C in 5% CO2.
Viability assay.
For viability assays, transduced or wild-type HMEC-1 cells were seeded on 96-well plates at a density of 15,000 cells/well. On the following day, cells were incubated in LPS (concentrations as indicated) and/or CHX (50 μg/mL). At various time points, viable cell numbers were estimated by 3-[4′,5-dimethylthiazol-2-yl]-2,5 diphenyltetrazolium bromide (MTT) assay.16 Briefly, medium was removed and replaced with medium containing 1 mg/mL MTT (Sigma) and incubated for 5 hours. The medium was then aspirated and the formazan product was solubilized with dimethylsulfoxide. Absorbance at 630 nmol/L was subtracted (background absorbance) from absorbance at 570 nmol/L for each well. Viability was expressed as a proportion of cells incubated in complete medium. In our laboratory, HMEC-1 cells cultured in complete medium show greater than 98% viability as measured by trypan blue exclusion or flow cytometry.
Oligonucleosomal banding.
Oligonucleosomal banding was demonstrated by harvesting of total cellular DNA as previously described.17 Briefly, after the treatment indicated, cells were harvested, washed with phosphate-buffered saline (PBS), and lysed in 50 mmol/L Tris, pH 7.5, 10 mmol/L EDTA, 0.5% Triton-X-100, and 0.5 mg/mL of proteinase K for 2 hours at 50°C. Samples were then extracted twice with phenol/chloroform/isoamyl alcohol and precipitated with ethanol. The pellet was resuspended in Tris-EDTA and 10 μg/mL RNase A, and the DNA was separated on a 2% agarose gel.
Northern analysis.
HMEC-1 cells were stimulated, as described, with LPS or TNF for various times as indicated. Total cellular RNA (10 μg) was separated on agarose-formaldehyde gels, blotted onto nitrocellulose filters, and hybridized overnight with random-primed 32P-labeled probes as indicated. The final washing conditions were 0.1× SSC, 0.1% sodium dodecyl sulfate (SDS) for 15 minutes at room temperature. Blots were stripped in boiling water before reprobing. A 3′ probe was used to identify the approximately 1-kb human A1 transcript, and a 300-bp HindIII fragment of A20 was used to identify the approximately 4.5-kb A20 mRNA. A β-actin probe hybridizing to a 2-kb transcript was used to confirm equivalent loading of RNA samples.
Gene transfer.
Generation of HMEC-FLAG-A1, HMEC-Bcl-XL, and HMEC-Neo cells has previously been described.13 HMEC-A20 and HMEC-IκBmt cells were constructed using previously described methods.18 Briefly, the coding region of A20 (provided by V. Dixit) or FLAG-IκBmt (provided by D. Ballard, Vanderbilt University, Nashville, TN) was ligated into an engineeredXho I site (A20) or the HindIII/Hpa I (FLAG-IκBmt) site of the replication-deficient retroviral vector pLNCX (provided by A.D. Miller, Fred Hutchinson Cancer Research Center, Seattle, WA).19 The viral long terminal repeat drives expression of NeoR, whereas the cytomegalovirus promoter drives transgene expression. Generation of packaging cell lines was performed as described.13 18 The pLNCA20 construct or pLNCIκBmt was transiently transfected into the ecotropic packaging line, PE501, by calcium-phosphate precipitation. Viral supernatants were harvested and used to transduce the amphotropic line PA317 in the presence of 4 μg/mL of polybrene. Retrovirus-producing cell lines were obtained by selection in 750 μg/mL G418 (Life Technologies, Gaithersburg, MD). Retroviral supernatants from the PA317 cell lines were used to transduce HMEC-1 cells. After selection in 200 μg/mL of G418 and expansion, transduced HMEC-1 cells were used in survival assays. Polyclonal HMEC-1 lines were used to avoid artifacts due to retroviral integration site.
Immunoblotting.
Total cellular extracts were prepared from HMEC-1 cells by lysing 105 cells in 20 mmol/L Tris, 137 mmol/L NaCl, 1% Triton-X-100, 1 mmol/L AEBSF, 10 μg/mL leupeptin, and 10 μg/mL aprotinin. Protein was fractionated by 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) gels and electrotransferred onto nitrocellulose membranes over 1 hour at 4°C. Filters were blocked overnight with PBS containing 5% skim milk. Immunostaining steps were performed in PBS with 3% bovine serum albumin (BSA) and 0.05% Tween 20 at room temperature. Filters were incubated with primary antibody for 2 hours and secondary antibody for 1 hour. Filters were washed in PBS and 0.05% Tween 20 four times for 10 minutes between each step and were developed by chemiluminescence.
RESULTS
LPS induces HMEC-1 apoptosis in the presence of CHX.
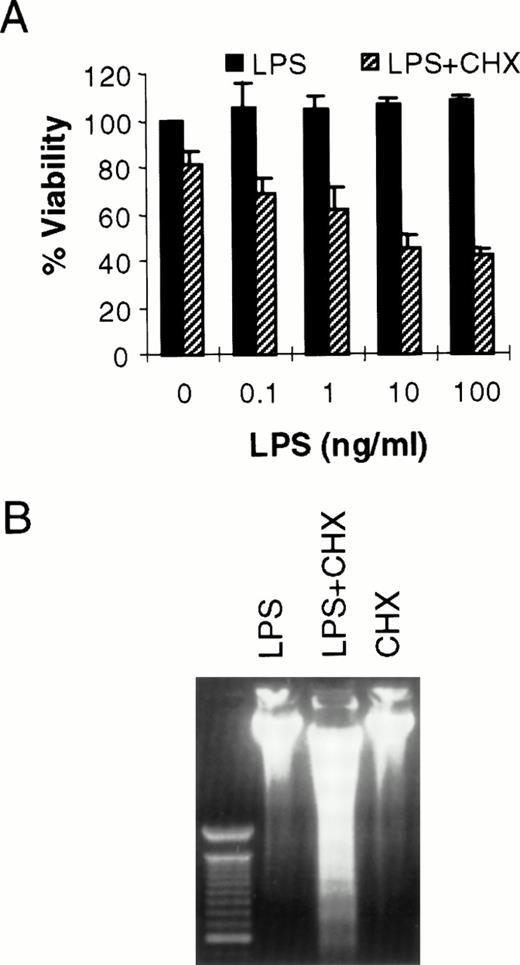
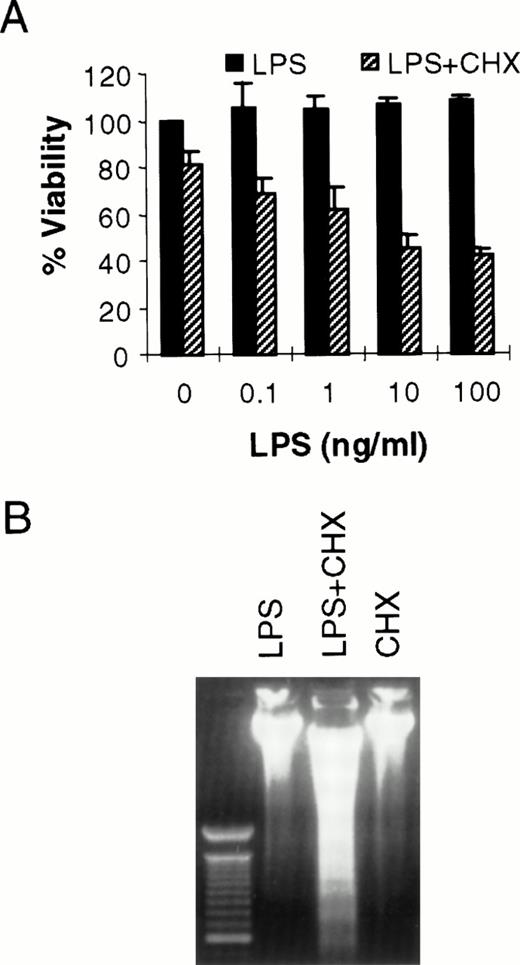
It has been shown previously that LPS can cause toxicity of large vessel endothelium when protein or mRNA expression is blocked by CHX or actinomycin D, respectively.10 LPS alone does not kill human umbilical vein endothelial cells.10 However, in bovine or ovine large vessel endothelium, LPS can directly cause cell death.20,21 Others have reported LPS-induced toxicity of human pulmonary arterial endothelial cells.22 Because the microvasculature plays a key role in the pathogenesis of sepsis,11 we were interested in determining the effect of LPS in HMEC-1. As seen in Fig 1A, LPS alone does not kill HMEC-1. However, when protein synthesis is blocked, there is a dose-dependent toxicity of LPS on HMEC-1 cells. The mode of cell death in this model is apoptotic, as demonstrated by the oligonucleosomal fragmentation of chromosomal DNA (Fig 1B). This finding is consistent with our studies demonstrating the activation of caspases in HMEC-1 by LPS in the presence of CHX (Choi et al22a). Others have also shown the activation of caspase-1 by LPS in human umbilical vein endothelial cells.23
LPS initiates endothelial apoptosis only in the presence of CHX. (A) HMEC-1 cells were treated with LPS for 15 hours in the presence or absence of CHX (50 μg/mL). Each data point is the proportion of viable cells with respect to cells treated with medium only. Results are the means + SD of three experiments. (B) HMEC-1 cells were treated with LPS (100 ng/mL) only, LPS and CHX (50 μg/mL), or CHX only for 15 hours and harvested DNA was separated on an ethidium bromide-stained agarose gel. The lane on the left is a 100-bp molecular weight marker.
LPS initiates endothelial apoptosis only in the presence of CHX. (A) HMEC-1 cells were treated with LPS for 15 hours in the presence or absence of CHX (50 μg/mL). Each data point is the proportion of viable cells with respect to cells treated with medium only. Results are the means + SD of three experiments. (B) HMEC-1 cells were treated with LPS (100 ng/mL) only, LPS and CHX (50 μg/mL), or CHX only for 15 hours and harvested DNA was separated on an ethidium bromide-stained agarose gel. The lane on the left is a 100-bp molecular weight marker.
LPS causes upregulation of the cytoprotectants A1 and A20.
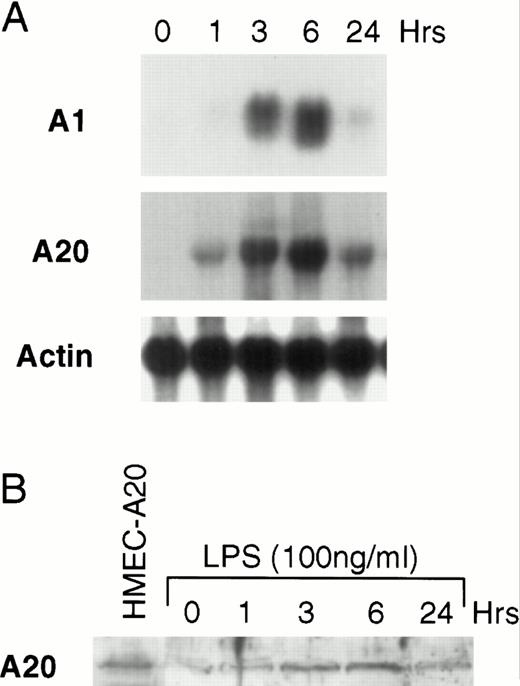
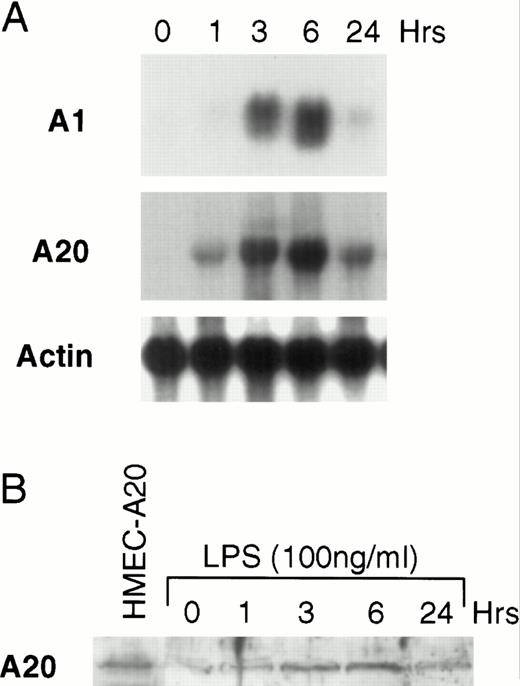
The finding described above suggests that LPS activates an apoptotic pathway as well as inducing the synthesis of cytoprotective proteins that prevent LPS-activated apoptosis. This finding is similar to that described for TNF.8,24-27 We have previously shown that TNF upregulates the Bcl-2 family member, A1, in endothelial cells.12 It has also been shown that the zinc-finger protein, A20, is inducible by TNF.14 28 To determine whether LPS can upregulate the mRNA of these two known cytoprotective proteins in microvascular endothelial cells, HMEC-1 cells were stimulated with LPS for various times and Northern blots were performed. Both A1 and A20 mRNA accumulates in HMEC-1 cells after LPS stimulation (Fig 2A). We repeated these experiments in human umbilical vein endothelial cells and found that LPS also induced A1 and A20 in these primary human endothelial cells (data not shown). Levels of A20 protein are also induced after LPS stimulation, and the kinetics of protein induction in HMEC-1 cells correspond to the mRNA accumulation (Fig 2B). A20 protein levels at 3 to 6 hours are similar to that seen in HMEC-1 cells engineered to constitutively express A20 (see below).
LPS induces A1 and A20 in HMEC-1 cells. (A) After treatment with LPS (100 ng/mL) for various times, total RNA from HMEC-1 cells was subjected to Northern analysis. (B) Protein lysates from HMEC-1 cells were also harvested at times after LPS stimulation as in (A) and analyzed by immunoblotting. The first lane shows HMEC-1 cells engineered to constitutively express A20 (HMEC-A20).
LPS induces A1 and A20 in HMEC-1 cells. (A) After treatment with LPS (100 ng/mL) for various times, total RNA from HMEC-1 cells was subjected to Northern analysis. (B) Protein lysates from HMEC-1 cells were also harvested at times after LPS stimulation as in (A) and analyzed by immunoblotting. The first lane shows HMEC-1 cells engineered to constitutively express A20 (HMEC-A20).
Overexpression of A1 or A20 inhibits LPS-mediated cell death.
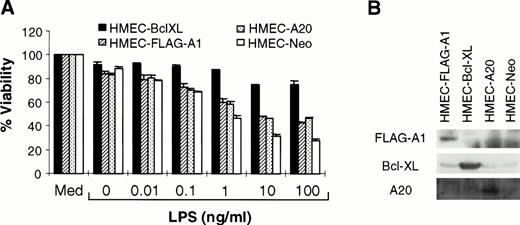
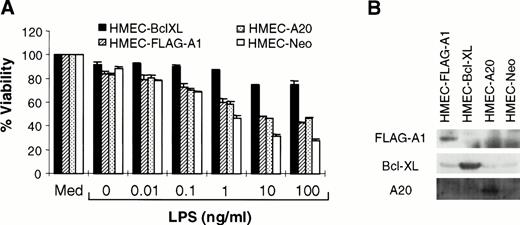
A1, a Bcl-2 homologue, protects against cell death in some but not all systems.13,29 Similarly, A20 attenuates TNF-initiated death, but not that induced by Fas ligation or reactive oxygen intermediates.14,30 Studies were performed to determine whether A1 or A20 could inhibit LPS-mediated apoptosis. HMEC-1 lines stably overexpressing FLAG-epitope–tagged A1 (HMEC-FLAG-A1) or A20 (HMEC-A20) were generated by retroviral-mediated transfer. Because the Bcl-2 family member, Bcl-XL, is also able to block various forms of cell death, HMEC-Bcl-XL cells were used as a positive control. Cells transduced with the empty vector (HMEC-Neo) were used as a negative control. Immunoblotting confirmed the expression of the specific protein in each of these cell lines (Fig 3B). Each of these cell lines was exposed to various concentrations of LPS in the presence of CHX. Both A1 and A20 were able to inhibit LPS-mediated cell death (Fig 3A). The partial protection conferred by these proteins has been noted before for TNF-initiated apoptosis.13,14 In the case of A1, the short half-life of the protein compared with Bcl-XL may provide one explanation for the incomplete protection.13Nevertheless, these experiments demonstrate that A1 and A20 serve to protect against LPS-initiated apoptosis.
A1 and A20 inhibit LPS-initiated endothelial apoptosis. (A) HMEC-1 cells stably transduced with various cDNA constructs, as indicated, were exposed to LPS and CHX (50 μg/mL). MTT assays were performed at 15 hours after treatment with LPS and CHX. Each data point is the proportion of viable cells with respect to cells treated with medium only (Med). Results are the means + SD of three experiments. (B) Each of the transduced cell lines was tested for expression of the relevant protein by immunoblotting.
A1 and A20 inhibit LPS-initiated endothelial apoptosis. (A) HMEC-1 cells stably transduced with various cDNA constructs, as indicated, were exposed to LPS and CHX (50 μg/mL). MTT assays were performed at 15 hours after treatment with LPS and CHX. Each data point is the proportion of viable cells with respect to cells treated with medium only (Med). Results are the means + SD of three experiments. (B) Each of the transduced cell lines was tested for expression of the relevant protein by immunoblotting.
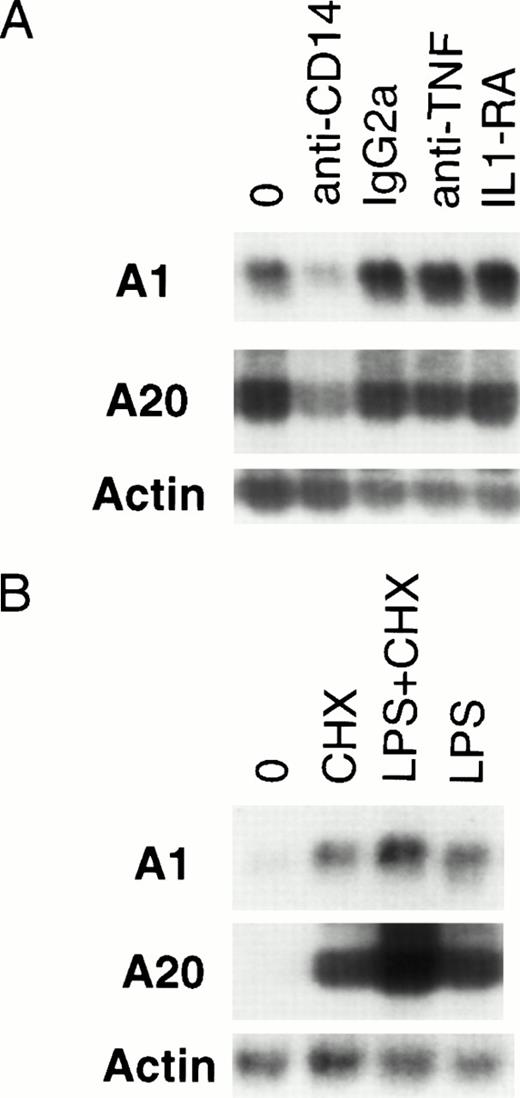
LPS induction of A1 and A20 is CD14-dependent.
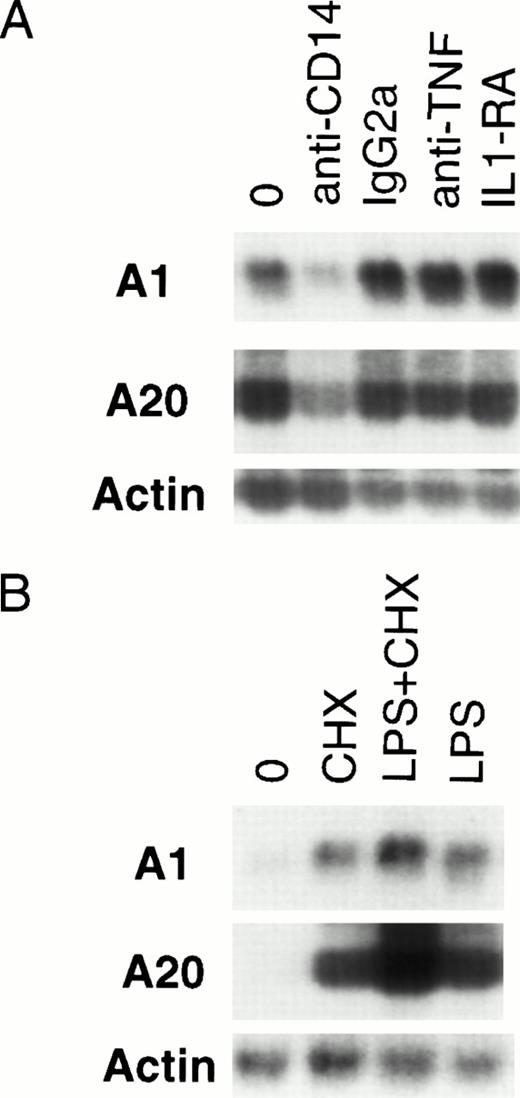
Optimal activation of endothelial cells by LPS requires the presence of serum.31 Although a cell-surface receptor for LPS has not been identified on endothelial or epithelial cells, many signaling functions are dependent on the presence of soluble CD14 contained in plasma.32,33 It has been suggested that many of the effects of LPS are secondary to TNF and IL-1, and various sequelae of Gram-negative sepsis have been attributed to TNF or IL-1.34-36 LPS has been shown to induce the expression of TNF and IL-1 in endothelial cells.37,38 Both TNF and IL-1 are capable of inducing A1 and A20 expression.12 28 Given the slightly delayed induction of A1 and A20 by LPS compared with TNF, it is conceivable that LPS-mediated induction is secondary to TNF or IL-1. To delineate the route of LPS-mediated upregulation of A1 and A20 in endothelial cells, HMEC-1 cells were pretreated with neutralizing antibodies (anti-CD14, 20 μg/mL; anti-TNF, 20 μg/mL) or IL-1 receptor antagonist (IL-1RA, 500 ng/mL) at concentrations sufficient to block signaling by these various pathways (data not shown). Only the anti-CD14 neutralizing antibody was capable of attenuating A1 and A20 expression (Fig 4A), suggesting that this induction is mediated by a CD14-dependent, but IL-1–independent and TNF-independent mechanism.
LPS-initiated accumulation of A1 and A20 mRNA is an early gene response dependent on CD14. (A) Northern analysis of HMEC-1 cells treated with neutralizing antibodies to CD14 and TNF, an IL-1 antagonist (IL-1RA), or an anti-CD14 isotype control (IgG2a) for 1 hour before stimulation with LPS (100 ng/mL) for 3 hours. (B) Northern analysis of HMEC-1 cells after 3 hours of treatment with LPS (100 ng/mL) only, CHX (50 μg/mL) only, or LPS and CHX.
LPS-initiated accumulation of A1 and A20 mRNA is an early gene response dependent on CD14. (A) Northern analysis of HMEC-1 cells treated with neutralizing antibodies to CD14 and TNF, an IL-1 antagonist (IL-1RA), or an anti-CD14 isotype control (IgG2a) for 1 hour before stimulation with LPS (100 ng/mL) for 3 hours. (B) Northern analysis of HMEC-1 cells after 3 hours of treatment with LPS (100 ng/mL) only, CHX (50 μg/mL) only, or LPS and CHX.
To clarify whether any intermediary proteins were required for A1 and A20 upregulation, HMEC-1 cells were treated with CHX to block protein synthesis before LPS stimulation for 3 hours. As seen in Fig 4B, CHX pretreatment did not block A1 or A20 induction. This finding in endothelial cells is consistent with A1 and A20 being early response genes to LPS stimulation.39 As described for other early response genes,39 treatment with CHX alone also resulted in accumulation of A1 and A20 mRNA.
LPS induction of A1 and A20 is NF-κB–dependent.
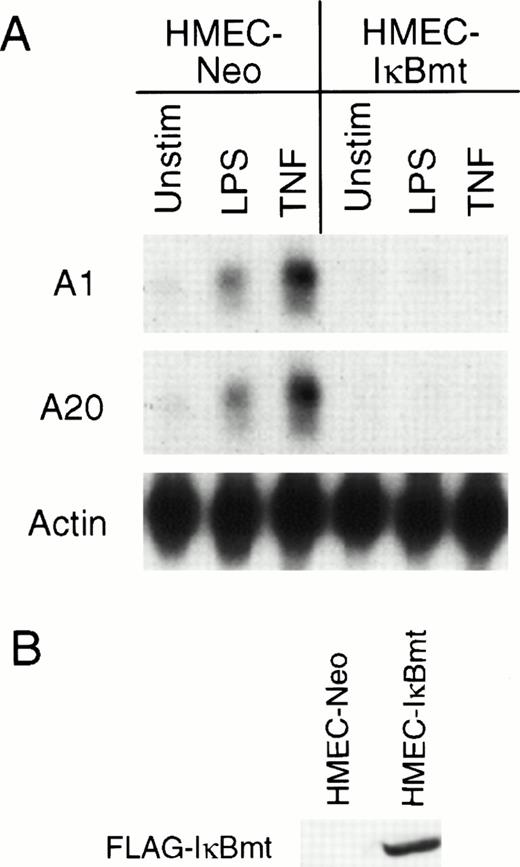
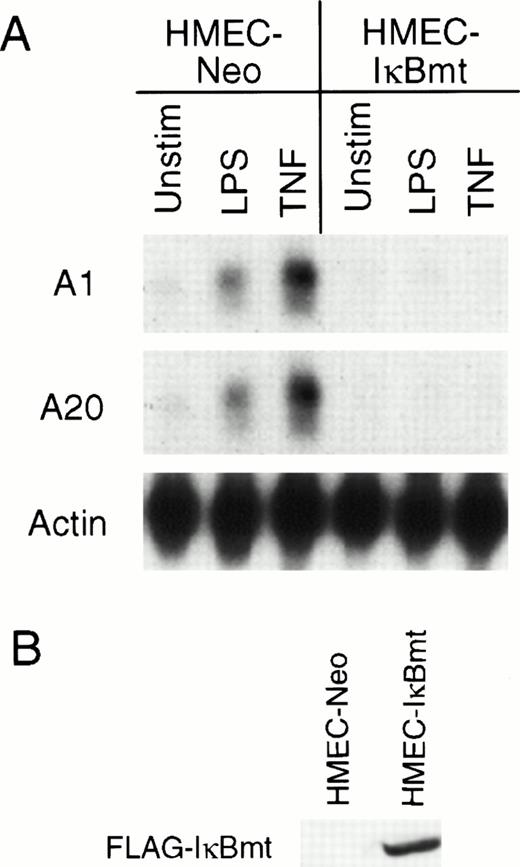
Recently, several groups have demonstrated that TNF initiates an antiapoptotic pathway by activation of the transcription factor NF-κB.25-27 NF-κB is normally sequestered in the cytoplasm by an inhibitor, IκB.40,41 After activation by TNF or other stimuli, IκB is phosphorylated on two serine residues, Ser32 and Ser36.42,43 This phosphorylation results in the ubiquitination and degradation of IκB and subsequent translocation of NF-κB to the nucleus.42,43 A mutant IκB with Ser32 and Ser36 mutated to alanine residues resists degradation, thereby preventing translocation of NF-κB to the nucleus.42,43 It was shown that inhibition of NF-κB by overexpression of this IκB mutant (IκBmt) sensitized normally resistant cells to TNF-initiated apoptosis.25-27 To determine whether the LPS induction of the cytoprotectants A1 and A20 was directed via NF-κB activation, HMEC-1 cells were transduced with the mutant IκB construct to generate HMEC-IκBmt cells. To confirm that HMEC-IκBmt cells were able to abrogate activation of NF-κB, these cells were tested for TNF-inducible expression of the cell adhesion molecule, vascular cell adhesion molecule-1 (VCAM-1), which is an NF-κB–dependent event, and were found to prevent this expression compared with the HMEC-Neo cells (data not shown). Figure 5A demonstrates that LPS-mediated induction of both A1 and A20 is blocked by overexpression of IκBmt. It has previously been shown that A20 induction by TNF is NF-κB–dependent in fibroblasts.44 Hence, the demonstration that TNF induction of A20 is also blocked by IκBmt confirms that NF-κB activation is blocked in the HMEC-IκBmt cells. This finding suggests that LPS signals upregulation of A1 and A20 in microvascular endothelial cells by an NF-κB–dependent mechanism.
Inhibition of NF-κB activation blocks LPS-mediated A1 and A20 induction. (A) HMEC-1 cells transduced with a FLAG-IκBmt construct (HMEC-IκBmt) or empty vector (HMEC-Neo) were treated with LPS or TNF for 3 hours and subjected to Northern analysis. (B) Transduced cell lines were immunoblotted with the FLAG (M2) antibody to confirm expression of the FLAG-IκBmt protein.
Inhibition of NF-κB activation blocks LPS-mediated A1 and A20 induction. (A) HMEC-1 cells transduced with a FLAG-IκBmt construct (HMEC-IκBmt) or empty vector (HMEC-Neo) were treated with LPS or TNF for 3 hours and subjected to Northern analysis. (B) Transduced cell lines were immunoblotted with the FLAG (M2) antibody to confirm expression of the FLAG-IκBmt protein.
DISCUSSION
Integrity of the endothelium is critical for modulating the inflammatory response.4 Endothelial injury plays a crucial role in the pathogenesis of septic shock due to Gram-negative bacteria.9 Endothelial cell death initiated by inflammatory mediators is species-dependent. In general, ovine and bovine endothelia are highly sensitive to LPS, whereas human cells are not.10,20,21 However, when RNA transcription or protein synthesis is inhibited, LPS does cause death of human endothelial cells, suggesting that LPS can induce parallel cell death and survival pathways.10
Our findings demonstrated that, similar to large vessel endothelium, the human dermal microvascular line, HMEC-1, only undergoes apoptosis in response to LPS in the presence of CHX. Thus, LPS must stimulate the expression of genes that provide protection against apoptosis. This is not a surprising finding given the critical importance of maintaining an intact vascular barrier that provides selective permeability to macromolecules. We have previously shown that human endothelial cells express several antiapoptotic members of the Bcl-2 family.17 It is likely that, under different physiologic or pathologic circumstances, different members of the Bcl-2 family play a key role. For instance, in ML-1 myeloid leukemia cells induced to undergo monocytic/macrophage differentiation, Mcl-1 may play a role in permitting survival.45 Similarly, A1 may also play an antiapoptotic role in differentiating hematopoietic cells.46 In endothelial cells, A1 may be the critical Bcl-2 homologue promoting viability during inflammation.8 13
Because LPS-initiated endothelial death only occurs in the presence of CHX, this model cannot directly be applied to in vivo findings during sepsis. However, there is evidence to suggest that the survival pathway may fail due to inhibition by other inflammatory mediators such as interferon-γ or that the death pathway overwhelms the survival pathway by synergism with other cytokines during sepsis.47,48 It has also been suggested that LPS-induced nitric oxide may synergize with IL-1 to render endothelial cells more susceptible to apoptosis.49 Others have reported that CHX upregulates Fas mRNA.50 However, Fas ligation does not kill endothelial cells,51 52 and we have not found the combination of LPS and Fas-ligation to cause endothelial death (A. Karsan, unpublished observations).
The situation in vivo is further complicated by the fact that LPS has functional effects on leukocytes that can then directly or indirectly affect the viability of endothelium.53 For instance, it has been shown that cytokine-activated neutrophils and LPS-activated mononuclear cells can cause endothelial damage.53,54 The apoptotic effect of LPS-treated monocytes has been attributed to membrane-bound TNF as well as an unidentified secreted factor.47,54 It has also been demonstrated in vivo that TNF and subsequent ceramide generation mediate the LPS-induced endothelial apoptosis.55
We were interested in determining the direct effects of LPS on HMEC-1. Because the initial findings suggested the stimulation of a cytoprotective pathway, we looked for induction of the cytoprotective molecules A1 and A20. mRNA for both proteins accumulated after LPS stimulation, although at slightly later times compared with TNF induction. In contrast to TNF, where peak induction of A1 is seen after 3 hours of exposure, the LPS-induced A1 expression is delayed, reaching a maximum at 6 hours or later.12 Similarly, A20 expression in fibroblasts after TNF exposure peaks at 1 hour,28whereas LPS-induced A20 expression is maximal at 3 to 6 hours in microvascular endothelial cells. Enforced expression of either A1 or A20 protected HMEC-1 cells from apoptosis induced by LPS and CHX. Thus, these molecules may account in part for the resistance of human endothelial cells to the direct toxic effects of LPS.
Several intracellular molecules have been implicated in transducing LPS signals. Activation of NF-κB, the Jak-STAT pathway, mitogen-activated protein kinases, and phosphatidylinositol 3-kinase have all been demonstrated to play a role in the intracellular signaling of LPS-mediated events.31,56,57 LPS complexed with a serum protein, LPS binding protein, signals through membrane-bound CD14 on monocytes and myeloid cells. In contrast, endothelial and epithelial cells, which are CD14− but still respond to LPS, require soluble CD14 present in serum to transduce LPS signals.31,33,58 It is still unclear how the LPS-CD14 complex actually transmits a signal across the cell membrane. Evidence has been presented to suggest the presence of a signaling transmembrane receptor recognizing the LPS-CD14 complex.59 Transmembrane signaling by LPS has also been shown to be mediated by CD11/CD18 integrins independently of CD14.60,61 However, others have postulated that LPS is internalized by a vesicular transport mechanism and mediates signals, at least partly, by structurally mimicking ceramide.62,63 In addition, many of the in vivo effects of LPS have been attributed to secretion of TNF and/or IL-1 by other cells.34-36 However, our results demonstrate that induction of A1 and A20 is independent of the expression of any secondary molecules, demonstrating that the cytoprotective activity is a direct effect of LPS stimulation. We also show that soluble CD14 is required for this activity. Previously, we have demonstrated that exogenous ceramide does not induce A1 mRNA, suggesting that the LPS-mediated A1 induction is likely not due to molecular mimicry of this lipid second messenger.13
Recently, it was demonstrated that inhibition of NF-κB by expressing an IκB mutant sensitizes cells to the apoptotic effects of TNF.25-27 This finding led to the proposal that TNF induces cytoprotective proteins by activation of NF-κB. To determine whether the LPS induction of A1 and A20 was also secondary to NF-κB activation, we overexpressed the mutant IκB in HMEC-1 cells. Inhibition of NF-κB by overexpression of the IκB mutant, in microvascular endothelial cells, inhibits expression of A1 and A20 by both TNF and LPS. Interestingly, concomitant expression of A20 with IκBmt was not able to reverse the sensitization of cells to TNF in one study.26 Furthermore, inhibition of NF-κB did not sensitize cells to Fas-mediated death.25 We have shown that LPS signals HMEC-1 death via an intracellular death-domain–containing protein, FADD (Choi et al22a). FADD signals death initiated by TNF receptor 1 as well as by Fas.8 It is interesting to note that, whereas A20 protects against both TNF and LPS-mediated death, it does not protect against Fas-mediated apoptosis.14 30 In other studies, we have shown that IκBmt overexpression does not sensitize endothelial cells to IL-1– or LPS-mediated apoptosis, but it does sensitize to TNF-initiated death (Zen et al, manuscript submitted). Thus, although there are several downstream molecules in common that are involved in pathways signaling apoptosis and protection against apoptosis, it is clear that many differences in these pathways remain to be elucidated.
Supported by grants from the Medical Research Council of Canada (CLN-1002-42547 and MT-14373) with funds from the British Columbia Lung Association. A.K. is a Clinician-Scientist of the Medical Research Council of Canada.
Address reprint requests to Aly Karsan, MD, UBC McDonald Research Laboratories, Room 292, St Paul’s Hospital, 1081 Burrard St, Vancouver, BC, Canada V6Z 1Y6; e-mail: akarsan@prl.pulmonary.ubc.ca.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.