Abstract
Progress in unraveling the biology of myeloma has suffered from lack of an in vitro or in vivo system for reproducible growth of myeloma cells and development of disease manifestations. The SCID-hu mouse harbors a human microenvironment in the form of human fetal bone. Myeloma cells from the bone marrow of 80% of patients readily grew in the human environment of SCID-hu mice. Engraftment of myeloma cells was followed by detectable human Ig levels in the murine blood. Myeloma-bearing mice had high levels of monotypic human Igs, high blood calcium levels, increased osteoclast activity, and severe resorption of the human bones. The human microenvironment was infiltrated with Epstein-Barr virus-negative monoclonal myeloma cells of the same clonality as the original myeloma cells. Active angiogenesis was apparent in areas of myeloma cell infiltration; the new endothelial cells were of human origin. We conclude that the SCID-hu mouse is a favorable host for studying the biology and therapy of myeloma and that a normal bone marrow environment can support the growth of myeloma cells.
© 1998 by The American Society of Hematology.
THERE HAS BEEN a recent explosion in data related to the biology of myeloma. However, most of these advances, potentially of major importance to our understanding of the development of myeloma and to its management, remain of uncertain importance due to the lack of in vitro and in vivo systems allowing reproducible growth of primary myeloma cells or the development of myeloma clinical manifestations. Our inability to positively identify the proliferative compartment in myeloma, to discern the role of circulating clonal B lymphocytes in maintaining the disease,1-5 to determine the role of pre-switch and isotypically diverse clonal cells in the development of myeloma,6,7 or to determine the importance of Kaposi’s sarcoma associated herpes virus (KSHV) in myelomagenesis8 9 is attributable primarily to the lack of a biological read out system.
Human myeloma cell lines readily grow in SCID mice.10-16However, success has been limited when primary myeloma cells were used,17 resulting in growth patterns more compatible with lymphoma than with myeloma. The difficulty in growing primary myeloma cells in SCID mice probably reflects the dependence of myeloma cells on environmental stimuli that cannot be replaced by the murine host.
We have inoculated fresh bone marrow cells obtained from patients with myeloma into SCID-hu mice.18-20 We report here that the SCID-hu mice provide a hospitable environment for reproducible growth of primary myeloma cells. Mice inoculated with bone marrow cells from patients with myeloma develop typical manifestations of the disease such as plasmacytosis, high levels of monoclonal Igs, and severe bone resorption.
MATERIALS AND METHODS
Myeloma cells.
Heparinized bone marrow aspirates were obtained from patients with active myeloma during scheduled clinic visits. Signed informed consents were obtained and are kept on record. Relevant patient information is provided in Table 1. The samples were separated using ficoll hypaque centrifugation (Histopaque; Pharmacia, Uppsala, Sweden). The proportion of myeloma cells in the light-density cell preparations (specific gravity ≤1.077 g/mL) was determined using CD38/CD45 flow cytometry.4,21 When indicated, myeloma cells were sorted on the basis of CD38/CD45 expression.4,21 The myeloma clone in each sample was characterized by CDRIII polymerase chain reaction (PCR).4 22
SCID-hu system.
CB.17/ICr-SCID mice were obtained from Harlan Sprague Dawley and were housed and monitored in our animal facility. All experimental procedures and protocols had been approved by the Institutional Animal Care and Use Committee. SCID-hu mice were generated as reported.23 Before inoculation of myeloma cells, the mice were exposed to 150 rad X-irradiation, using a model 143-45 Irradiator 137Cesium source, at a rate of 125 rads/min. Light-density bone marrow cells (1.5 to 10 × 106) were injected directly into the human bone in the SCID-hu mice in a final volume of 25 to 50 μL of phosphate-buffered saline (PBS). An increase in the levels of circulating monotypic human Ig (hIg) of the M protein isotype was used as an indicator of myeloma cell growth.
Determination of hIg levels.
Levels of human IgG, IgA, κ, and λ light chains were determined by enzyme-linked immunosorbent assay (ELISA). Antibodies were purchased from The Binding Site (San Diego, CA). Plates were coated with 50 μL/well of primary antihuman κ and λ (5 μg/mL) and antihuman IgA and IgG (10 μg/mL) and incubated overnight at 4°C. The plates were washed three times in PBS containing 0.5% (vol/vol) of Tween 20 and washed one more time with blocking buffer containing 4% bovine serum albumin (BSA) in PBS. Serial dilution of samples in PBS-containing 1% BSA (50 μL/well) were incubated at room temperature for 2 hours. Standards consisting of each purified Ig were added to the appropriate plates at concentrations ranging from 0.4 to 300 ng/mL. After washing three times with PBS/Tween, plates were incubated with 50 μL/well of biotinylated Ab (affinity-purified antihuman κ and λ light chains at 0.5 μg/mL and antihuman IgA and IgG at 0.2 μg/mL) for 1 hour. Fifty microliters per well of streptavidin-horseradish peroxidase were added to each well after washing and allowed to bind for 1 hour. After a final washing, 50 μL/well of OPD solution (DAKO, Carpinteria, CA) containing 3% H2O2 was added. Absorbance at 450 nm was determined on a Auto-Reader II ELISA reader (Ortho Diagnostic Systems, Raritan, NJ).
Methods of analysis.
Tissues and organs recovered from SCID-hu mice were processed as reported.24 Myeloma cells were identified morphologically by immunohistochemical staining for cytoplasmic Ig (cIg; DAKO immunoperoxidase kit) and their clonality was determined by in situ hybridization with patient-specific probes (ASO-ISH). Changes in bone remodeling were identified by x-radiography and by increased osteoclast activity as demonstrated by immunohistochemical staining for tartarate-resistant acid phosphatase (TRAP; Sigma, St Louis, MO). Immunohistochemical staining with a monoclonal antibody to CD34 (Cell Marque, Austin, TX) was used to demonstrate neo-vascularization as an indicator for the presence of a human microenvironment in areas of myeloma cell growth.
Determination of calcium.
Calcium levels were determined using a calcium determination kit (Sigma Diagnostics) according to the manufacturer’s recommendations.
In situ hybridization.
Clonality of the tumor cells grown in SCID mice was demonstrated by in situ hybridization using an adaptation of published methods.25 26 Antisense oligonucleotide sequences (24-32 bp) complementary to CDR III regions of the myeloma clone of each patient were biotinylated during synthesis (Life Technologies, Rockville, MD). Tissue sections were dewaxed in xylene, defatted in chloroform, and then rehydrated. The sections were treated with proteinase K (10 μg/mL in Tris-HCl buffer at 37°C for 1 hour). Hybridization mixture containing 50% formamide, 10% dextran, 4 × SSC (SSC is 150 mmol/L NaCL, 15 mmol/L trisodium citrate, pH 7), 25 mg/mL herring sperm DNA, and biotinylated probe (1 to 2 μg/mL) was applied to each section. The sections were covered with Parafilm and incubated in a humidified chamber overnight at 37°C. After hybridization, the slides were washed twice with 1 × SSC at room temperature and twice with 1 × SSC at 37°C to 42°C (depending on the size of the probe). Signals were visualized using an in situ hybridization kit (DAKO). Specificity was determined by using irrelevant patient probes and sections from different patients.
Screening for Epstein-Barr virus.
Myeloma cells recovered from SCID-hu mice were analyzed for the presence of EBV sequences by PCR.27 All samples were negative.
RESULTS
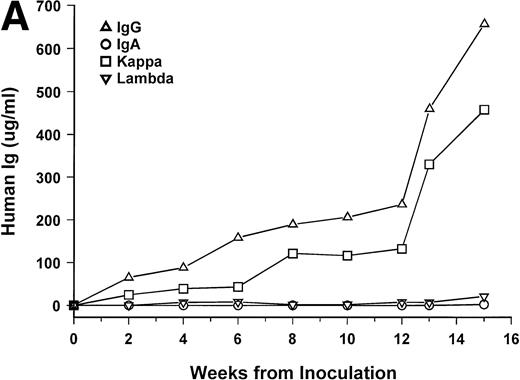
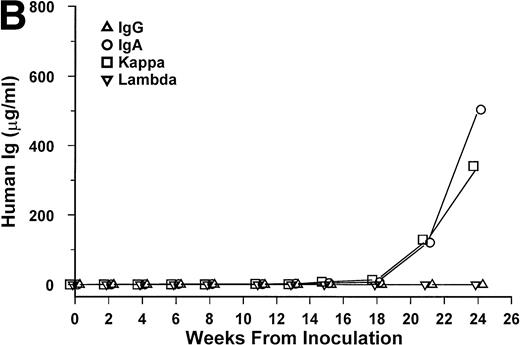
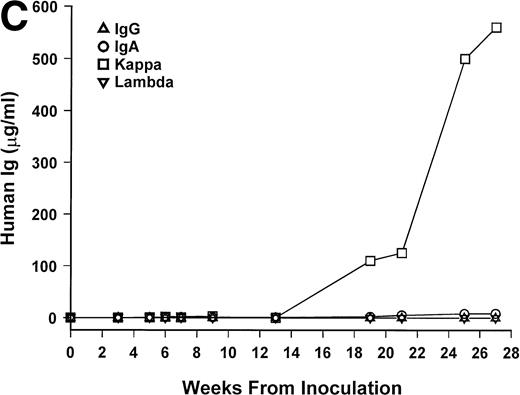
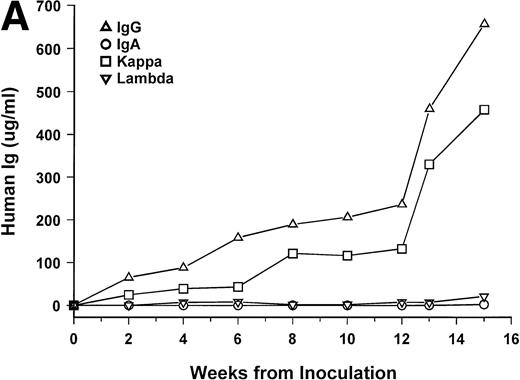
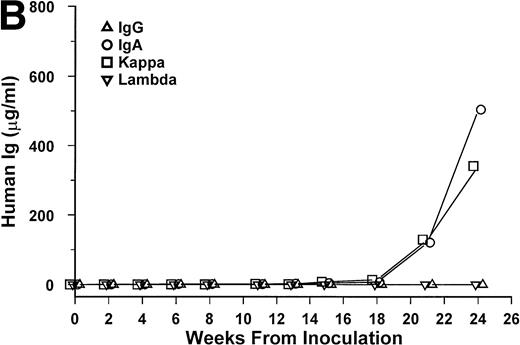
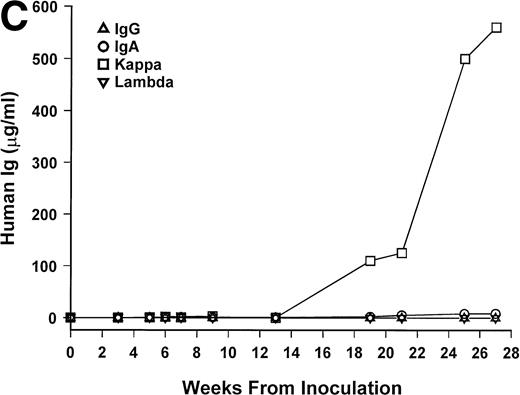
Bone marrow cells from 15 patients were studied in the SCID-hu system. Mice inoculated with cells from 12 patients had detectable circulating hIg 2 to 19 weeks after inoculation. Two mice had no detectable hIg even at 23 and 29 weeks, and one mouse, inoculated with cells from a patient with nonsecretory myeloma, also had no detectable circulating hIg (Table 1). In these 3 cases, myeloma growth could not be detected by histological and immunohistochemical examinations. With two exceptions, the circulating hIg was identical to the M protein isotype in terms of both the heavy and light Ig chains; for 1 patient with IgA λ myeloma, all 4 isotypes were expressed at 15 weeks (patient no. 10), and in 1 patient with nonsecretory myeloma, IgG κ was found at 3 weeks (patient no. 15). In this latter patient, the original myeloma cells contained IgG κ cytoplasmic Ig (data not shown). The kinetics of the increase of hIg levels varied among patients; examples are presented for 3 of these patients in Fig 1. Although the number of patients studied is too small to draw firm conclusions, there was no apparent correlation between the time to detection of hIg and the number of myeloma cells inoculated, marrow plasmacytosis, or any other patient characteristic, whether analyzed for the whole group or only for the 6 patients with IgG κ myeloma (Table 1).
hIg levels in SCID-hu mice. The mice were bled at the indicated time point after inoculation of myeloma cells, and the levels of Ig heavy and light chains were determined by ELISAs. Three examples are presented. (A) IgGκ myeloma (patient no. 1). (B) IgAκ myeloma (patient no. 2). (C) κ light chain myeloma (patient no. 6).
hIg levels in SCID-hu mice. The mice were bled at the indicated time point after inoculation of myeloma cells, and the levels of Ig heavy and light chains were determined by ELISAs. Three examples are presented. (A) IgGκ myeloma (patient no. 1). (B) IgAκ myeloma (patient no. 2). (C) κ light chain myeloma (patient no. 6).
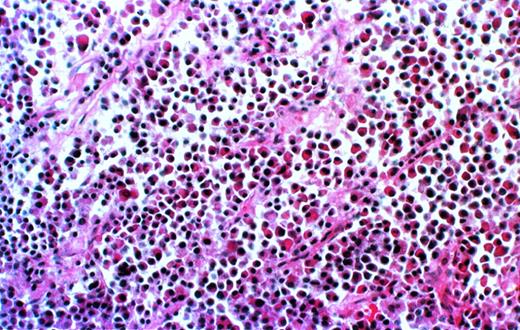
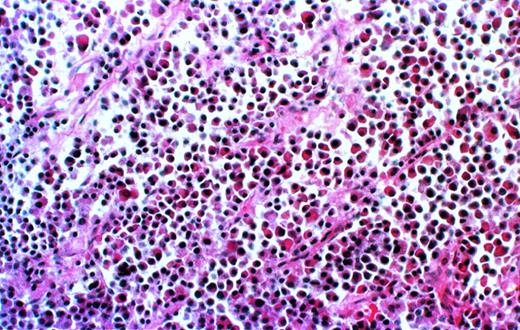
Further analysis is available only for the 8 patients for whom the experiments have been completed. Histological examination of decalcified sections of the human bone showed extensive infiltration of plasma cells (Fig 2). The cells uniformly contained monotypic clg (Fig 3), coexpressed Ig heavy and light chains, and reacted homogeneously and exclusively with the patient’s specific ASO (Fig4), indicating their clonal identity with the original patient’s myeloma cells. In all cases, myeloma plasma cells were found only in the human bone. Neither myeloma cells nor any other human cells were detected in any of the murine organs, bone marrow, or blood, as determined histologically, and by CD38/CD45 and HLA-ABC flow cytometry.
Myeloma cell infiltrate in the human bone of a SCID-hu mouse. Photomicrograph of a 5-μm section of decalcified human bone recovered from SCID-hu mouse (patient no. 4). Hematoxylin and eosin staining, 10× objective.
Myeloma cell infiltrate in the human bone of a SCID-hu mouse. Photomicrograph of a 5-μm section of decalcified human bone recovered from SCID-hu mouse (patient no. 4). Hematoxylin and eosin staining, 10× objective.
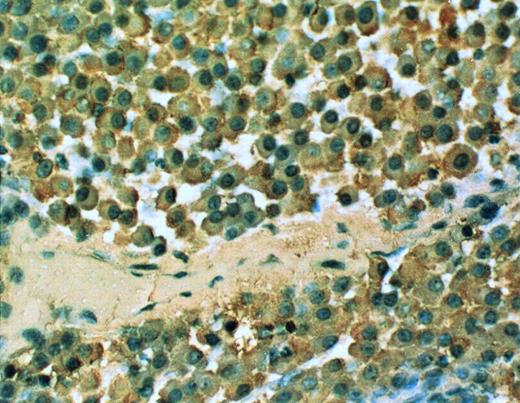
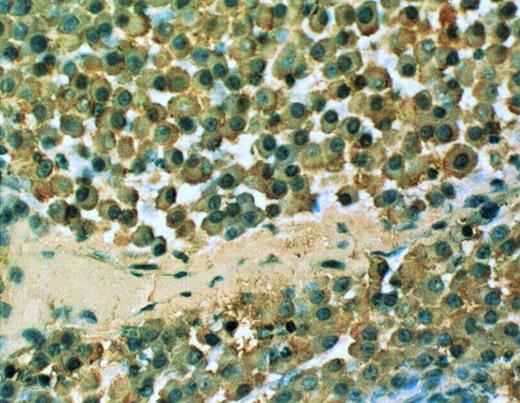
cIg expression in the SCID-hu mouse. 20× objective photomicrograph of a 5-μm section of decalcified human bone recovered from SCID-hu mouse reacted with monoclonal antibody to human κ light chain (patient no. 4).
cIg expression in the SCID-hu mouse. 20× objective photomicrograph of a 5-μm section of decalcified human bone recovered from SCID-hu mouse reacted with monoclonal antibody to human κ light chain (patient no. 4).
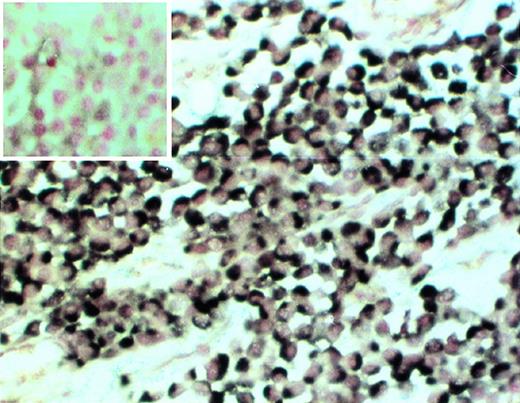
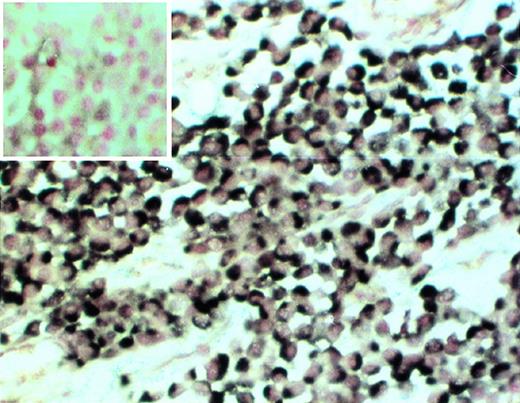
Clonality of myeloma cells in the SCID-hu mouse. Decalcified human bone section was hybridized with patient-specific ASO (patient no. 4). (Insert) Control section hybridized with the ASO probe to the myeloma clone from patient no. 2. 20× objective.
Clonality of myeloma cells in the SCID-hu mouse. Decalcified human bone section was hybridized with patient-specific ASO (patient no. 4). (Insert) Control section hybridized with the ASO probe to the myeloma clone from patient no. 2. 20× objective.
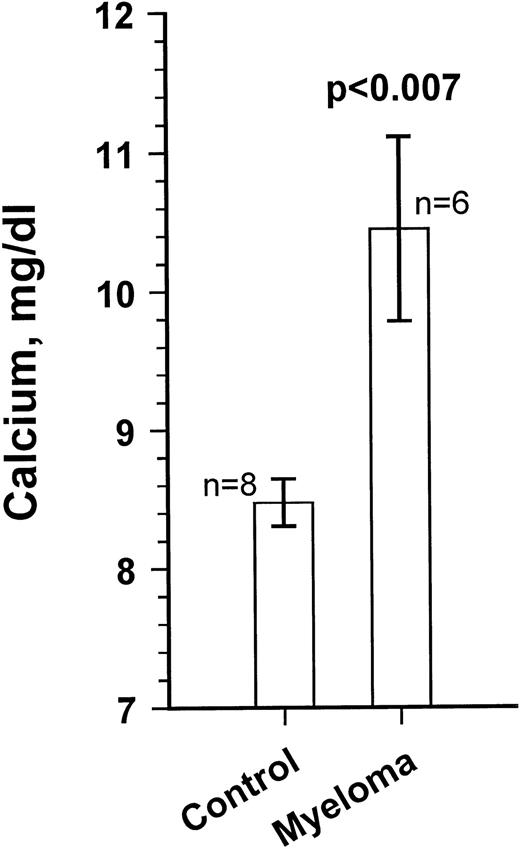
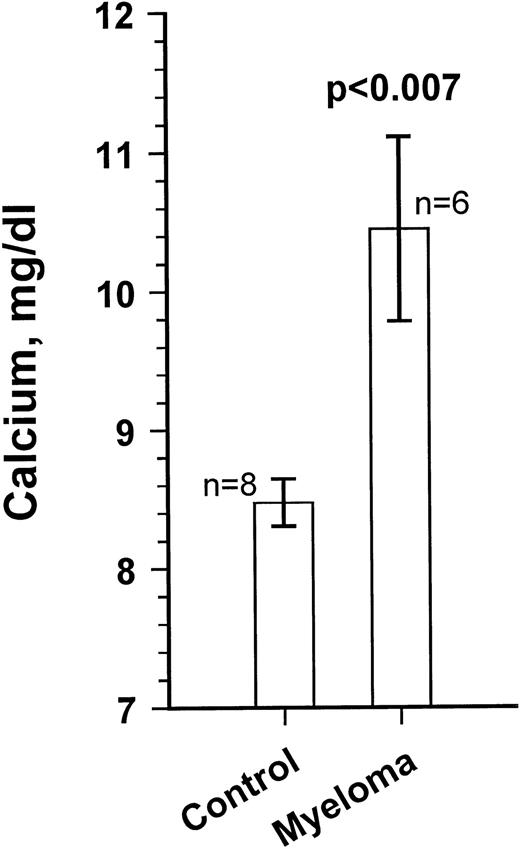
Growth of the myeloma cells was associated with increased blood Ca levels (summarized in Fig 5), suggesting anomalies in bone remodeling. Immunohistochemical examination of the kidneys showed varying amounts of light chain deposits (not shown). Decalcified bone sections stained for TRAP showed markedly increased osteoclast activity (Fig 6). Severe loss in human bone density was readily visible upon x-ray examination in all cases. Although density loss was severe in all mice, the degree of human bone resorption varied. An example depicting a moderate level of density loss is presented in Fig 7.
Blood calcium levels in a myeloma-bearing SCID-hu mouse. Ca levels were measured at the end of the experiments. Results for 8 age-matched control SCID-hu mice and the 6 myeloma-bearing mice are given. Ca levels for mice inoculated with cells from patients no. 5 and 8 were within control range. Results are presented as the mean ± SEM.
Blood calcium levels in a myeloma-bearing SCID-hu mouse. Ca levels were measured at the end of the experiments. Results for 8 age-matched control SCID-hu mice and the 6 myeloma-bearing mice are given. Ca levels for mice inoculated with cells from patients no. 5 and 8 were within control range. Results are presented as the mean ± SEM.
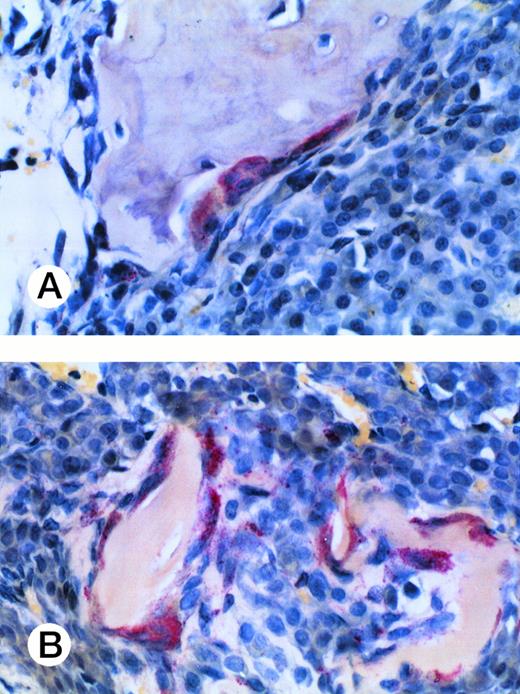
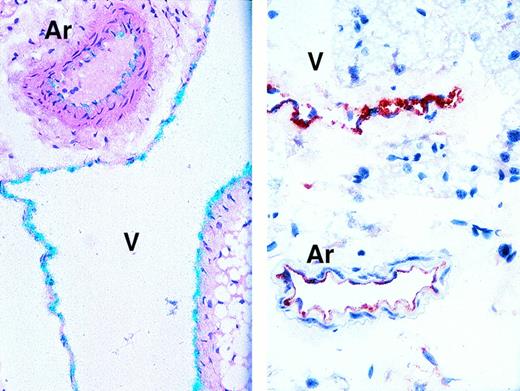
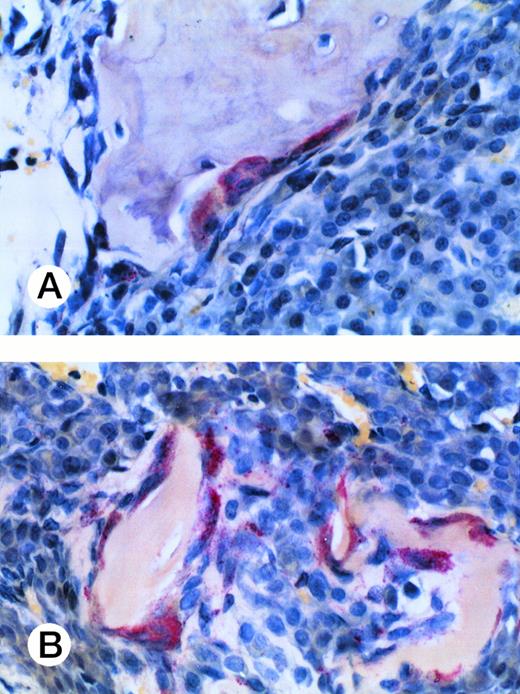
Osteoclast activity in myeloma-bearing SCID-hu mouse. Photomicrograph of a TRAP-stained decalcified human bone section. Multinucleated osteoclasts (cells with red cytoplasm) surround and excavate cancellous (A) and trabecular (B) bone. Patient no. 4, 20× objective.
Osteoclast activity in myeloma-bearing SCID-hu mouse. Photomicrograph of a TRAP-stained decalcified human bone section. Multinucleated osteoclasts (cells with red cytoplasm) surround and excavate cancellous (A) and trabecular (B) bone. Patient no. 4, 20× objective.
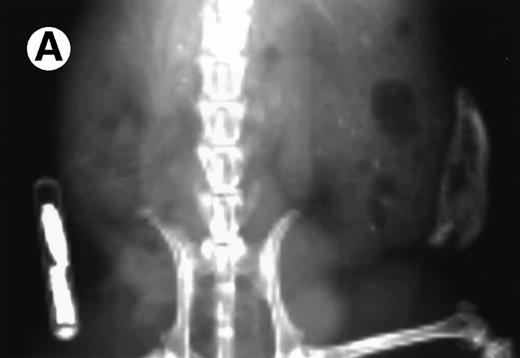
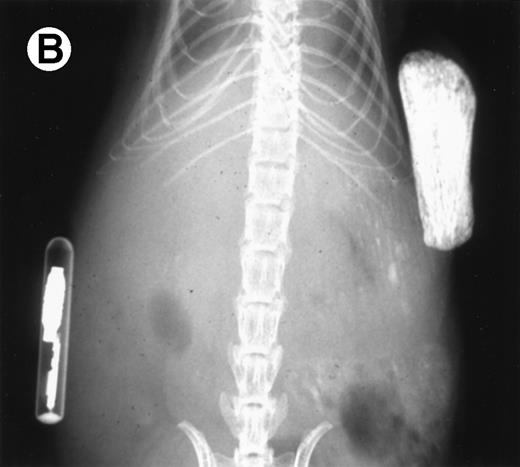
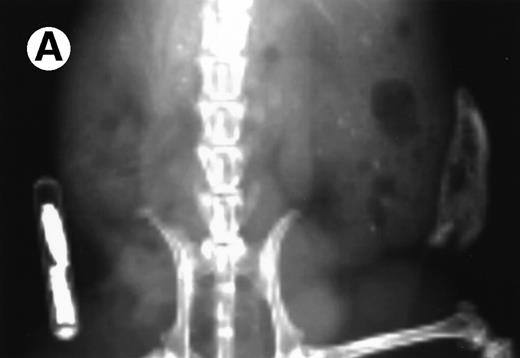
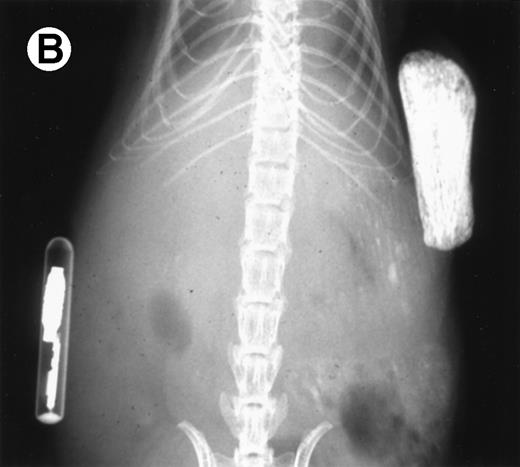
Loss in density of human bone in myeloma-bearing SCID-hu mouse as seen by x-radiography. Human bones are visible in the right-hand side. The highly contrasted devices visible in the bottom left corner are implant transponders used to identify the mice. (A) Myeloma-bearing mouse (patient no. 6): (B) Age-matched control SCID-hu mouse.
Loss in density of human bone in myeloma-bearing SCID-hu mouse as seen by x-radiography. Human bones are visible in the right-hand side. The highly contrasted devices visible in the bottom left corner are implant transponders used to identify the mice. (A) Myeloma-bearing mouse (patient no. 6): (B) Age-matched control SCID-hu mouse.
Expression of the endothelial cell antigen CD34 in a decalcified section of the human bone is demonstrated in Fig8. An active vascularization process is readily visible. In all cases, neo-vascularization was restricted to areas infiltrated with myeloma cells and was not visible in the human bones of control SCID-hu mice, suggesting that vascularization occurred in response to the presence of myeloma cells.
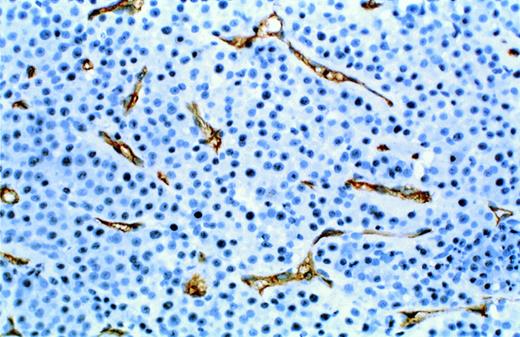
Neo-vascularization in myeloma bearing SCID-hu mouse. Decalcified human bone sections immunostained with a mouse monoclonal antibody to human CD34. Proliferative endothelial cells are stained brown. Patient no. 4, 10× objective.
Neo-vascularization in myeloma bearing SCID-hu mouse. Decalcified human bone sections immunostained with a mouse monoclonal antibody to human CD34. Proliferative endothelial cells are stained brown. Patient no. 4, 10× objective.
DISCUSSION
Primary myeloma cells, hitherto considered not capable of sustained proliferation when removed from the patient, readily grew in the human bone of SCID-hu mice. Interestingly, no other human hematopoietic cells were detectable in the murine bone marrow and blood. Growth of myeloma cells was restricted to the human environment, similar to reports with primary leukemic cells23 and very different from myeloma cell lines that readily grow in SCID mice and disseminate out of the human bone microenvironment in the SCID-hu system.28
Myeloma growth was accompanied by increased osteoclast activity and resorption of the human bone, a typical manifestation of myeloma.24 Increases in osteoclasts were also observed in the murine bones (femurs and vertebrae; data not shown). However, loss in density of murine bones was not discernible on x-radiograms.
As in other malignancies, vascularization is an important part of myeloma growth.29-31 In the SCID-hu system, newly formed blood vessels were made of human cells and were visible only in areas infiltrated with myeloma cells. These endothelial cells could originate from dormant stem cells in the implanted human bone or, alternatively, from the myeloma patients’ bone marrow. Because primary myeloma cells cannot grow in SCID mice (23 failed attempts, including some samples that grew in the SCID-hu system; data not shown), it appears that a normal human bone environment can support the growth of primary myeloma cells from bone marrow aspirates of patients with myeloma. Studies are underway to determine the source of the endothelial and other cells that constitute the human microenvironment in the SCID-hu mice and whether purified myeloma plasma cells will also engraft in this model.
The SCID-hu mouse provides a favorable host environment for reproducible growth of primary myeloma cells. This system is a suitable model for studying the biology of myeloma, its treatment, and manifestations.
Supported in part by Grant No. CA-55819 from the National Cancer Institute.
Address reprint requests to Joshua Epstein, DSc, MTRC, UAMS, 4301 W Markham, Slot #776, Little Rock, AR 72205; e-mail:jepstein@life.uams.edu.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.