Abstract
Sickle red blood cell (RBC) adhesion to the endothelium and to exposed, underlying subendothelial proteins is believed to contribute to vascular occlusion in sickle cell disease. Laminin, a major component of the subendothelium, supports significant adhesion of sickle, but not normal RBCs. The purpose of this study was to define the adhesive region for sickle RBCs within a human laminin preparation using a flow adhesion assay designed to mimic physiologic flow through postcapillary venules. Because sickle RBCs did not adhere to the common laminin contaminants entactin or collagen type IV, neither of these proteins are likely to contribute to the observed adhesion to laminin. Known adhesive regions of laminin neither supported nor inhibited sickle RBC adhesion to laminin, suggesting a mechanism of adhesion previously uncharacterized in other laminin adhesion studies. Moreover, sickle RBCs did not adhere to mouse EHS laminin or to human laminin-2 (merosin), eliminating the 1, 2, β1, and γ1 chains as mediators of sickle cell adhesion. The monoclonal antibody 4C7, which binds at or near the G-domain of the laminin 5 chain, significantly inhibited sickle RBC adhesion. These results suggest that an adhesive region for sickle RBCs is contained within the laminin 5 chain.
© 1998 by The American Society of Hematology.
THE HALLMARKS OF sickle cell disease are vascular occlusion and painful crises.1 Although crises vary among patients in severity, frequency, and duration, they are all periodic, acute episodes of pain believed to be caused by local disruptions of blood flow with possible tissue ischemia or infarction.1 The homozygous state for the sickle mutation (SS) generally has the most severe clinical course,2 which may ultimately lead to organ failure and death.3
The disruption of blood flow that leads to vascular occlusion may occur in large vessels4,5 but is most commonly associated with the microvasculature. Whereas erythrostasis due to the rigidity of polymerized S hemoglobin (Hb S) contributes to vaso-occlusion,6 erythrostasis alone is probably insufficient to explain the extent of vascular occlusion seen in patients.7,8 Sickle red blood cells (RBCs) have been shown to adhere to the endothelium in vitro9,10 and may contribute to vessel occlusion in vivo by delaying RBC travel time, leading to greater decreases in oxygen tension, increased cell sickling,11 12 and endothelial damage.
In addition to endothelial cell adhesion, sickle cells may also adhere to subendothelial proteins, because factors such as increased levels of tumor necrosis factor α (TNFα), fibrin/fibrinogen degradation products, and thrombin generation exist in sickle cell disease and are known to retract endothelial cells, causing subendothelial protein exposure in vitro.13-18 In fact, sickle cell patients have greater levels of circulating endothelial cells19-21 and extensive vascular damage upon postmortem examination.22
Once endothelial integrity has been compromised, extracellular matrix (ECM) components such as laminin, thrombospondin, fibronectin, von Willebrand factor, vitronectin, and collagen are potentially exposed to sickle RBCs. Abnormal adhesion between sickle RBCs and thrombospondin under static and flow conditions has been documented,23,24as well as even greater levels of adhesion to the ECM protein laminin.24 However, the mechanism of adhesion or the region of laminin that is adhesive to sickle cells is unknown.
Laminin is a multidomain, heterotrimeric protein composed of one each of the five known α, three known β, and two known γ chains.25 The chains complex in various combinations to form a characteristic cross-shape of three short arms and one long arm with a large globular domain at its C-terminus.26 Human placental laminin preparations likely contain a mixture of laminin isoforms, in addition to common contaminants entactin and collagen type IV.27
In this study we sought to better characterize the adhesion of sickle cells to laminin, to identify the component of the laminin preparation that is adhesive for sickle cells, and to identify the adhesive region within this component. We not only eliminate many likely adhesive sites, but also provide evidence that the laminin α5 chain, at or near the G-domain, contains an adhesive region for sickle cells, based on the inhibition studies with the 4C7 monoclonal antibody (MoAb). Because the laminin α5 chain is present in the subendothelium,25 this chain may contribute to vaso-occlusion suffered by sickle cell patients.
MATERIALS AND METHODS
Reagents.
Human placental laminin and human collagen type IV were obtained from GIBCO (Grand Island, NY). Recombinant human entactin was generously provided by R. Timpl (Max-Planck Institute, Martinsried, Germany). The antihuman laminin polyclonal antibody (pAb) was purchased from Upstate Biotechnology Inc (Lake Placid, NY). The MoAb 4C7 was purchased from GIBCO and Chemicon (Temecula, CA). Protein G Sepharose beads were obtained from Pharmacia Biotech (Piscataway, NJ). Synthetic laminin peptides of known adhesive regions of laminin were kindly provided by H. Kleinman (National Institutes of Health, Bethesda, MD). Hank’s Balanced Salts Solution (HBSS) was obtained from Sigma Chemical Co (St Louis, MO).
RBC preparation.
Sickle RBCs were obtained from homozygous sickle cell patients (Hb SS) during clinic visits to the UNC Comprehensive Sickle Cell Center. Normal RBCs (Hb AA) were obtained from healthy volunteers. All blood samples were drawn by venipuncture into 0.13 mol/L sodium citrate and processed immediately upon venipuncture by centrifuging at 150gfor 15 minutes to isolate blood cells from plasma and platelets. RBCs were then washed three times in CGS (1.29 mmol/L sodium citrate, 3.33 mmol/L glucose, 124 mmol/L NaCl, pH 7.2), then resuspended in phosphate-buffered saline (PBS; 137 mmol/L NaCl, 10.2 mmol/L NaH2PO4, 1.76 mmol/L KH2PO4, 2.68 mmol/L KCl), and packed by centrifugation at 800g for 10 minutes. A 1% hematocrit was then prepared by diluting 30 μL of packed RBCs per 1.5 mL of perfusion media (HBSS supplemented with 0.3% bovine serum albumin [BSA] and 20 mmol/L HEPES, pH 7.4).
For experiments shown in Table 1, RBC density fractions were prepared from unpacked RBCs using a modified arabinogalactan density gradient, using a high potassium isotonic buffer as previously modified23 from Sorette et al,28 an approach designed to minimize reticulocyte dehydration. RBCs from the top 20% and bottom 5% of the gradient were used. We found by methylene blue staining of RNA that reticulocytes made up 20% to 90% of the top fraction and less than 5% of the bottom fraction. Nucleated RBCs were not observed in Wrights stain of the isolated fractions. By trypan-blue exclusion, approximately 90% of the isolated RBCs were intact. Experiments were performed immediately after RBC isolation.
Antibody purification.
Protein G Sepharose beads were washed and resuspended in an equal volume of PBS to create a 50% slurry. This slurry (50 mL) was then added to the 4C7-ascites solution and inverted slowly at 4°C for 18 hours. After collecting the cleared ascites fluid, the beads were washed twice in PBS and then treated with 0.1 mol/L glycine • HCl, pH 2.7, to elute the 4C7 MoAb. Tris, pH 9.0, was added to neutralize the purified antibody, which was stored at 4°C until use.
Flow adhesion assay.
The flow adhesion system used for this study was designed by J. Moake and L. McIntire (Rice University, Houston, TX) to mimic blood flow through postcapillary venules, as described.23 The protein of interest (0.75 μg) was immobilized onto each of the two identical wells in the silicon gasket by incubating at 4°C overnight, thus allowing two separate conditions to be performed during each experiment.
A 1% hematocrit solution (1.5 mL) was made to flow over wells at a rate of 1.0 mL/min and constant shear stress of 1 dyne/cm2. This shear stress has been measured in postcapillary venules,29 in which some investigators think vaso-occlusion occurs29,30 and in which the greatest in vitro sickle cell adhesion has been measured.31-33 After a 6-minute wash period, the number of adhered cells from 4 representative areas of the well were counted from a 0.25-mm2 field, normalized, averaged, and presented as cells per square millimeter ± SD. Any white blood cells that may have adhered were not counted.
Inhibition assays.
Potential inhibitors of adhesive regions of laminin or the appropriate control were added to the wells containing the immobilized protein and incubated at 37°C for 2 hours. Wells were then washed with PBS before continuing the flow adhesion assay. Potential inhibitors of laminin receptors on RBCs were tested by adding the inhibitor to the 1% hematocrit solution and incubating at 37°C for at least 30 minutes before the flow adhesion assay.
RESULTS
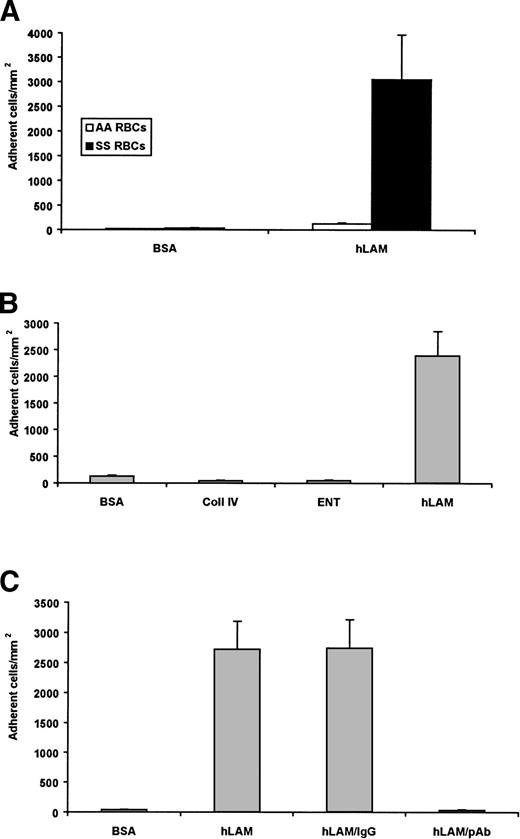
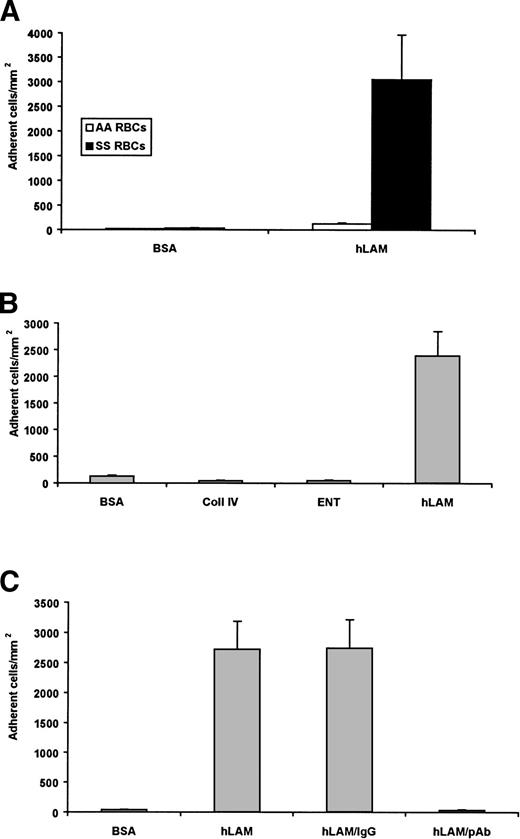
Using a microvessel flow adhesion system, we measured sickle RBC adhesion to the ECM protein laminin isolated from human placenta (hLAM). Laminin supported approximately 3,000 adherent sickle cells/mm2, but did not support the adhesion of normal RBCs (Fig 1A). These results suggest that laminin contains an adhesive site for RBCs that may be unique to the sickle cell disease state.
Sickle RBCs adhere to laminin. A 1% hematocrit of sickle (SS) RBCs or normal (AA) RBCs was flowed across immobilized protein (0.75 μg). Adherent cells from four representative regions were counted by microscopy, normalized to cells per square millimeter, and shown as the mean ± SD. (A) Significant adhesion of sickle, but not normal, RBCs was observed to human laminin (hLAM). Only background levels of adhesion were observed with sickle RBCs to the negative control BSA. Data from 10 sickle cell patients are shown. (B) Sickle RBCs were flowed across immobilized recombinant human entactin (ENT) or collagen IV (Coll IV). SS RBCs did not adhere to either entactin or collagen IV. BSA and hLAM are shown as negative and positive controls, respectively. Data from 3 patients are shown. (C) Immobilized hLAM was pretreated with an antilaminin polyclonal antibody (pAb) before the introduction of the SS 1% hematocrit. The antilaminin pAb significantly inhibited the adhesion of sickle RBCs to hLAM. Adhesion to laminin in the absence of an antibody (hLAM) and in the presence of a nonspecific, isotype-matched IgG antibody are shown as negative controls. Data shown represent experiments from 4 patients.
Sickle RBCs adhere to laminin. A 1% hematocrit of sickle (SS) RBCs or normal (AA) RBCs was flowed across immobilized protein (0.75 μg). Adherent cells from four representative regions were counted by microscopy, normalized to cells per square millimeter, and shown as the mean ± SD. (A) Significant adhesion of sickle, but not normal, RBCs was observed to human laminin (hLAM). Only background levels of adhesion were observed with sickle RBCs to the negative control BSA. Data from 10 sickle cell patients are shown. (B) Sickle RBCs were flowed across immobilized recombinant human entactin (ENT) or collagen IV (Coll IV). SS RBCs did not adhere to either entactin or collagen IV. BSA and hLAM are shown as negative and positive controls, respectively. Data from 3 patients are shown. (C) Immobilized hLAM was pretreated with an antilaminin polyclonal antibody (pAb) before the introduction of the SS 1% hematocrit. The antilaminin pAb significantly inhibited the adhesion of sickle RBCs to hLAM. Adhesion to laminin in the absence of an antibody (hLAM) and in the presence of a nonspecific, isotype-matched IgG antibody are shown as negative controls. Data shown represent experiments from 4 patients.
Human placental laminin preparations, regardless of source, are commonly contaminated with the proteins entactin and/or collagen type IV.27 To rule these proteins out as the mediators of sickle cell adhesion to human laminin, recombinant human entactin and collagen type IV were immobilized and tested for their ability to support sickle RBC adhesion. Neither protein supported the adhesion of the sickle cells beyond the background levels observed to the BSA control (Fig 1B). Therefore, it is unlikely that either of these contaminants is responsible for the observed adhesion of sickle RBCs. Furthermore, an antilaminin polyclonal antibody (hLAM pAb) was incubated on laminin-coated wells before the flow adhesion experiment. The hLAM pAb inhibited sickle RBC adhesion to laminin by 96% (Fig 1C), providing further evidence that laminin, not a contaminant of the preparation, is adhesive for sickle cells.
To further characterize the adhesion of SS RBCs to laminin, sulfated carbohydrates were tested as potential inhibitors. High molecular weight dextran sulfate (1 mg/mL) or fucoidan (1 mg/mL) was incubated in 1% SS RBC hematocrit for 1 hour at 37°C before the flow adhesion assay. High molecular weight dextran sulfate (HMW DS) and fucoidan inhibited the adhesion of SS RBCs to laminin by 87% and 80%, respectively (data not shown), suggesting that these sulfated carbohydrates may be useful in studies to isolate the SS RBC receptor and characterize its interaction with laminin.
To identify the most adhesive fraction of SS RBCs, the least dense (reticulocyte-enriched) or most dense (reticulocyte-depleted) fractions of SS RBCs were allowed to interact with immobilized laminin under flow conditions. The most dense SS RBC fraction was significantly more adherent to laminin than the least dense fraction (Table 1). However, because these fractions are not completely reticulocyte-depleted or enriched (see Materials and Methods), we cannot firmly conclude which age of SS RBCs is most adhesive. Interestingly, these results differ from our previous observations of SS RBC adhesion to thrombospondin, which supported higher levels of adhesion of least dense, reticulocyte-enriched RBCs.23 These results suggest that the laminin receptor on SS RBCs is more available in the denser fraction. Furthermore, only 10% of the SS RBCs adhering to laminin had visibly sickled morphologies (data not shown), suggesting that extensive distortion of the SS RBC membrane is not required to expose the laminin receptor on the SS RBC or for the adhesion to occur.
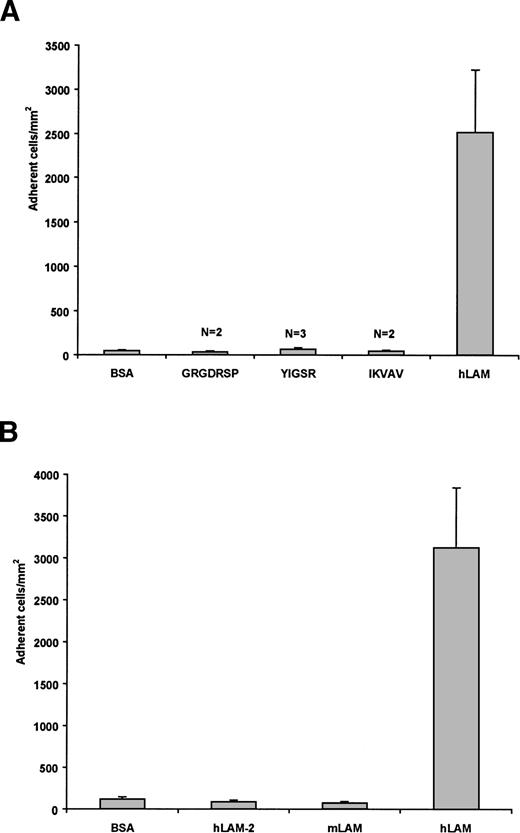
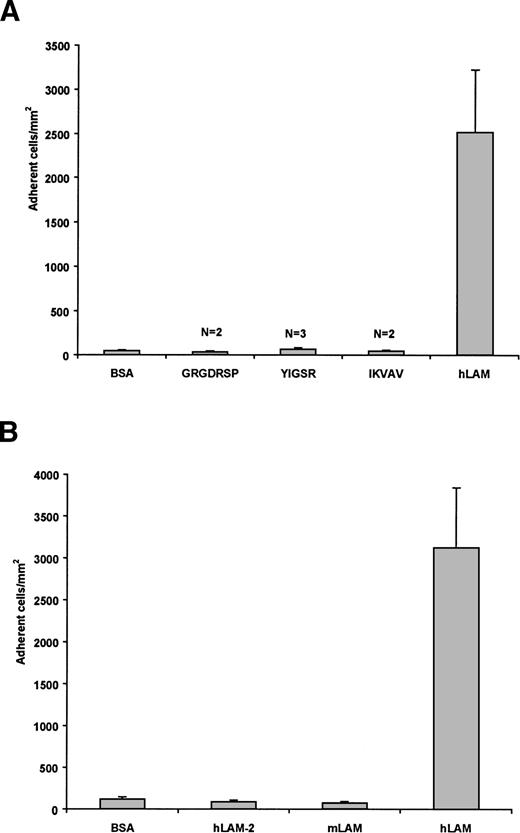
To determine if known adhesive sites of laminin mediate the adhesion of sickle cells, peptides representing these regions34 were tested for their activity with sickle RBCs in our flow adhesion system. These peptides interact with a variety of cell types and are known to retain their biological activity in peptide form in their respective adhesion studies35-37 and when immobilized onto plastic.38,39 The RGD sequence in the laminin α chain promotes cellular attachment via integrins, IKVAV of the α chain promotes neurite outgrowth, and YIGSR of the β chain inhibits tumor metastases.34 In our flow adhesion system, neither the GRGDSP, IKVAV, nor YIGSR peptides supported sickle cell adhesion beyond background levels (Fig 2A). These peptides (1 mmol/L) also did not inhibit the adhesion of sickle cells to laminin when incubated with sickle RBCs before the flow adhesion assay (data not shown). Results of this experiment suggest that none of these regions mediates the interaction between laminin and sickle RBCs. Moreover, laminin itself in solution did not inhibit sickle cell adhesion to immobilized laminin (data not shown), which suggests that adhesive sites of laminin differ in the immobilized and soluble forms.
Sickle RBCs do not adhere to known adhesive regions of laminin, murine EHS laminin, or human laminin-2. (A) Peptides representing known adhesive regions of laminin (GRGDSP, IKVAV, and YIGSR) were immobilized (1 mmol/L) and tested for their ability to support sickle RBC adhesion. These peptides did not support sickle RBC adhesion beyond nonspecific background levels. Adhesion to BSA and hLAM are shown as negative and positive controls, respectively. N = number of patients tested for each peptide. (B) SS RBCs were flowed across immobilized laminin-2 or mouse EHS laminin. Only background levels of sickle cells adhered to laminin-2 (hLAM-2) or mouse laminin (mLAM) compared with human placental laminin (hLAM). Both positive and negative controls are presented. Data shown represent the mean ± SD from six experiments involving 3 patients.
Sickle RBCs do not adhere to known adhesive regions of laminin, murine EHS laminin, or human laminin-2. (A) Peptides representing known adhesive regions of laminin (GRGDSP, IKVAV, and YIGSR) were immobilized (1 mmol/L) and tested for their ability to support sickle RBC adhesion. These peptides did not support sickle RBC adhesion beyond nonspecific background levels. Adhesion to BSA and hLAM are shown as negative and positive controls, respectively. N = number of patients tested for each peptide. (B) SS RBCs were flowed across immobilized laminin-2 or mouse EHS laminin. Only background levels of sickle cells adhered to laminin-2 (hLAM-2) or mouse laminin (mLAM) compared with human placental laminin (hLAM). Both positive and negative controls are presented. Data shown represent the mean ± SD from six experiments involving 3 patients.
Human placental laminin is likely a mixture of laminin heterotrimers, containing 1 each of the 5 known α chains, 3 β chains, and 2 γ chains. Laminin-2, or merosin, has the α2, β1, γ1 configuration. When tested for its ability to support sickle cell adhesion, only background levels of sickle RBCs adhered to the immobilized protein (Fig 2B). In inhibition assays, laminin-2 also did not inhibit the adhesion of sickle RBCs to immobilized human placental laminin (data not shown). The lack of interaction between sickle cells and laminin-2 suggests that the α2, β1, and γ1 chains are not involved in the adhesion of sickle RBCs. Similarly, sickle cells did not adhere to murine EHS laminin (Fig 2B). Although this may be due to disparate regions between the mouse and human versions of the proteins, this is less likely, because murine versions of the laminin molecules are highly homologous40 and often behave similarly.41,42 Murine EHS laminin has been characterized as containing the α1, β1, and γ1 chains,42 further decreasing the likelihood that the β1 or γ1 chains are adhesive and potentially eliminating the α1 chain as well. It should also be noted that, relative to results presented below, murine EHS laminin preparations lack the α5 chain.26
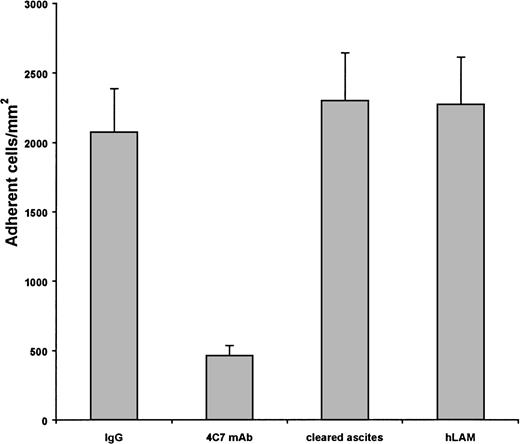
Because the greatest diversity of laminin is between its various α chains, the anti-α chain MoAb 4C7, which binds to the laminin α5 chain43-45 within or near the G-domain,41 was tested for its ability to inhibit sickle cell adhesion to laminin. Significantly less sickle cells adhered to human laminin in the presence of the antibody (79.6% inhibition), but not in the presence of an isotype-matched IgG2 control antibody (Fig 3). When the commercial antibody preparation was cleared of ascites, sickle RBC adhesion to laminin was still inhibited by approximately 60%, whereas the ascites fluid alone did not inhibit the adhesion (Fig 3). Finally, the 4C7 MoAb, relative to a control MoAb, reacted strongly with immobilized laminin in an enzyme-linked immunosorbent assay (data not shown), demonstrating that the 4C7 epitope is present in the human placental laminin preparation used in these studies. These results suggest that the laminin α5 chain contains an adhesive site for sickle RBCs at or near the G-domain and are consistent with a lack of sickle cell adhesion to the laminin-2 and murine EHS laminin preparations.
Sickle cell adhesion to laminin is inhibited by the 4C7 MoAb. Immobilized laminin was treated with the 4C7 antilaminin MoAb (4C7 MoAb) in ascites before the 1% hematocrit flow adhesion. Concentrations of the 4C7 IgG2 in ascites ranged from 15.0 to 37.5 μg/well. Adhesion of sickle RBCs to laminin was greatly reduced by the 4C7 MoAb, but not by an isotype-matched IgG2control antibody at amounts up to 37.5 μg/well or by ascites fluid isolated from the commercial 4C7 preparation. Adhesion to human laminin in the absence of antibody is presented as a negative control. Data shown represent 10 experiments involving 4 patients.
Sickle cell adhesion to laminin is inhibited by the 4C7 MoAb. Immobilized laminin was treated with the 4C7 antilaminin MoAb (4C7 MoAb) in ascites before the 1% hematocrit flow adhesion. Concentrations of the 4C7 IgG2 in ascites ranged from 15.0 to 37.5 μg/well. Adhesion of sickle RBCs to laminin was greatly reduced by the 4C7 MoAb, but not by an isotype-matched IgG2control antibody at amounts up to 37.5 μg/well or by ascites fluid isolated from the commercial 4C7 preparation. Adhesion to human laminin in the absence of antibody is presented as a negative control. Data shown represent 10 experiments involving 4 patients.
DISCUSSION
This study provides evidence that the laminin α5 chain contains an adhesive region for sickle RBCs. The α1, α2, β1, or γ1 chains of laminin are unlikely to account for the interaction with SS RBCs, because laminin-2 and EHS laminin did not support sickle cell adhesion. Furthermore, regions of laminin known to be adhesive to other cell types are not responsible for sickle cell adhesion, because peptides representing these regions were not active in our assay. Finally, the 4C7 MoAb, which recognizes the laminin α5 chain43-45 at or near the G-domain,41 significantly inhibited sickle cell adhesion to laminin. These results suggest that the laminin α5 chain contains an adhesive site within or near its G-domain to which sickle RBCs bind under flow conditions. This conclusion is consistent with a lack of adhesion to laminin preparations lacking the α5 chain, ie, laminin-2 and EHS laminin. The observed adhesion of sickle cells to laminin may be a contributing factor in vascular occlusion and the onset of the sickle cell crisis event.
Distinct topological features of the sickle RBC membrane and increased concentrations of certain plasma components combine to make sickle cells abnormally adherent to the endothelium.46 Sickle RBCs may also adhere to the subendothelium when subendothelial proteins are abnormally exposed. Endothelial damage22 and increased levels of circulating endothelial cells19-21 have both been documented in sickle cell disease. The ECM proteins thrombospondin and laminin are both adhesive for sickle RBCs,23,24 but laminin supports higher levels of sickle RBC adhesion than any other reported adhesogenic molecule. Our assay involves the examination of sickle cell adhesion to immobilized laminin, which is most likely to mimic the conformation of laminin in its biologically relevant form, ie, immobilized in the ECM. With the strong correlation between levels of sickle cell adhesion to the endothelium and clinical vaso-occlusive severity,47 the interaction between sickle cells and laminin may be an important determinant of crisis onset and severity.
Laminin-mediated adhesion of sickle RBCs appears to be the result of a receptor distinct from that which mediates SS RBC binding to thrombospondin, because the less dense, reticulocyte-enriched RBC population is associated with a higher level of adhesion to thrombospondin.23 In contrast, the denser, reticulocyte-depleted fraction of SS RBCs is more adhesive to laminin. This SS RBC population contains less deformable RBCs that are more likely to cause erythrostasis, possibly suggesting a greater likelihood of decreased flow rates and increased opportunity to interact with the subendothelium. Our experimental protocol precludes specific identification of the age of cells that actually adhere, preventing a firm conclusion of whether older cells are actually more adherent. However, these results do suggest that the laminin receptor is more readily available in the denser population of SS RBCs. An acidic glycolipid has been proposed as the SS RBC receptor for both thrombospondin and laminin.24 If so, our results suggest that density-related conformational differences in the SS RBC membrane make the lipid more or less adhesive for a given matrix protein. Alternatively, the denser SS RBC may feature a separate and distinct receptor for laminin. The protein B-CAM/LU of the lutheran blood group antigens is also a likely receptor candidate for the adhesion of SS RBCs to laminin, because it is uniquely adhesive to laminin in the sickle cell disease state.48 Whichever receptor is involved, our results with sulfated carbohydrates suggest that its ability to bind to laminin should be inhibited by HMW DS or fucoidan.
Having been studied widely for its interactions with various cell types, specific laminin regions have been identified as adhesive. Peptides representing known laminin adhesive regions were tested for their interaction with sickle RBCs. Although these peptides are reported to support and/or inhibit the adhesion of other cell types,35-37 we found that none had activity for sickle cells in our flow adhesion system. These results suggest that sickle RBC adhesion to laminin is mediated by a mechanism not previously described in other laminin adhesion studies. The adhesion also appears to be specific to the sickle cell disease state, because normal RBCs showed little affinity for immobilized laminin and did not adhere beyond background levels (Fig 1A). Similarly, RBCs with high reticulocyte counts do not adhere to laminin in the absence of the Hb S hemoglobinopathy.24
When the MoAb 4C7 was allowed to interact with the immobilized laminin before the introduction of sickle RBCs in the flow adhesion assay, 4C7 significantly inhibited sickle cell adhesion to laminin. Although the antibody’s epitope has not been precisely mapped, it has been shown to be present in a basal membrane component that is expressed in capillaries.49 Electron micrograph and immunohistochemical studies indicate that 4C7 likely recognizes the G-domain of the laminin α5 chain.41,43-45 The inhibition of sickle cell adhesion to laminin by 4C7 therefore suggests that the laminin α5 chain, within or near the G-domain, is the adhesive region of laminin for sickle cells. Because α5 forms both laminin-10 and laminin-11,25 it is likely that these two molecules are the laminin isoforms that are adhesive for sickle RBCs.
The laminin α5 chain is believed to have the widest expression of the α chains, with its transcripts being found in nearly all tissues,25 making it largely available for sickle cell interaction wherever the endothelium is damaged. The laminin α5 chain is also found in areas of regeneration43 and is likely to be abundant around sites of endothelial damage where higher concentrations of plasma proteins like fibronectin also exist and increase the adhesiveness of sickle cells.46 Furthermore, topological orientation of the laminin molecule in the basal lamina indicates that the G-domain is adjacent to the endothelium,50 making it potentially available for RBCs upon exposure of the ECM.
In our study, sickle cells did not adhere to laminin-2. This laminin isoform (α2, β1, γ1) has characteristically conserved α chain regions, with homology to the other α chains exceeding 80% in some domains. However, the α2 G-domain is only 20% homologous to the α5 G-domain,51 suggesting likely differences in properties and functions. The inability of laminin-2 to support sickle RBC adhesion provides additional evidence that the highly conserved, known adhesive regions of laminin are not active in sickle cell adhesion and suggest the role of a laminin domain that is not highly conserved among the isoforms. Of the known α chains, all have similarly low levels of homology among the G-domains. Therefore, adhesive interactions of the G-domains are likely to be chain-specific. This may explain why sickle cells did not adhere to the highly homologous murine laminin, which also does not contain the α5 chain.25
It is interesting to note that, although the G-domain has been described as responsible for most of the cell binding activity of laminin,52 few specific regions or sequences from this domain have been characterized for adhesion. This may be due to the high level of variability between α chain G-domains51 and the fact that the α3,53 α4,54 and α555 chains were only recently identified. Adding to the complexity is the possible existence of novel isoforms of individual α chains, as is the case with the α3 chain.25 Moreover, the widely used 4C7 antibody was until recently thought to interact with the α1 G-domain,43-45 therefore causing often conflicting data about α chain expression.
Presumably, each α chain has specific as well as overlapping functions. Unique phenotypes have linked α2 to a muscular dystrophy subset56 and α3 to epidermolysis bullosa.57This study suggests that α5 may play a role in sickle cell disease. The recent identification of laminin α5 in an endothelial cell line58 used widely in previous studies of sickle cell adhesion to the endothelium9,59 suggests that laminin could be involved in endothelial as well as subendothelial adhesion of sickle cells. Higher levels of α5 expression in the lungs and kidneys58 may correspond to the clinical features of acute chest syndrome and kidney complications that are common in sickle cell patients.2 Increased levels of α5 expression at the onset of sexual maturity58 could possibly explain why some patients do not experience painful crises until adolescence or early adulthood.60 Given the amount of adhesion in vitro in the absence of the many adhesogenic factors present in patients, sickle RBC adhesion to laminin may prove to be an important factor in the initiation of vaso-occlusion and the onset of crises. A therapeutic agent designed to inhibit sickle cell adhesion to laminin may aid in preventing vaso-occlusion, thus preserving health and improving the quality of life of sickle cell patients.
ACKNOWLEDGMENT
Many thanks to David Shock for his technical assistance and to Susan Jones, Wendy O’Kelly, B.J. Lee, Anne Criss, and the UNC Comprehensive Sickle Cell Center for their help recruiting patients and obtaining samples for this study.
Supported by National Institutes of Health Grants No. HL58939, HL45923, HL45100, and RR00046.
Address reprint requests to Leslie V. Parise, PhD, Department of Pharmacology, CB#7365, University of North Carolina, Chapel Hill, NC 27599-7365.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.