Abstract
B-lymphoproliferative disorder (BLPD) is a rare but severe complication of organ and bone marrow transplantation (BMT). Profound cytotoxic T-cell deficiency is thought to allow the outgrowth of Epstein-Barr virus–transformed B cells. When possible, reduction of immunosuppressive treatment or surgery for localized disease may cure BLPD. Therapeutic approaches using chemotherapy or antiviral drugs have limited effects on survival. Adoptive immunotherapy with donor T-cell infusions has given promising results in BMT recipients. We previously reported that administration of two monoclonal anti–B-cell antibodies (anti-CD21 and anti-CD24) could contribute to the control of oligoclonal BLPD. Here we report the long-term results of treatment with these monoclonal anti–B-cell antibodies for cases of severe BLPD. In an open multicenter trial, 58 patients in whom aggressive B-cell lymphoproliferative disorder developed after BMT (n = 27) or organ (n = 31) transplantation received 0.2 mg/kg/d of specific anti-CD21 and anti-CD24 murine monoclonal antibodies (MoAbs) for 10 days. The treatment was well tolerated. Thirty-six of the 59 episodes of BLPD in the 58 patients presented complete remission (61%). The relapse rate was low (3 of 36, 8%). Multivariate analysis identified the following risk factors for partial or no response to anti–B-cell MoAb therapy: multivisceral disease (P ≤ .005), central nervous system involvement (P ≤ .05), and late onset of BLPD (P ≤ .005). The overall long-term survival was 46% (median follow-up, 61 months); it was lower among BMT patients (35%) than organ transplant patients (55%). None of the patients who had received BMT for hematological malignancy survived for 1 year. Eight of these 11 patients presented monoclonal BLPD. Tumor burden was the only other variable that contributed significantly to poor survival. Thus, as assessed from this long-term study, the use of anti–B-cell MoAbs therefore appears to be a safe and relatively effective therapy for severe posttransplant BLPD.
© 1998 by The American Society of Hematology.
B-LYMPHOPROLIFERATIVE disorder (BLPD) is a severe complication of organ and bone marrow transplantation (BMT). Epstein-Barr virus (EBV) has been found in almost all investigated tumor cells from patients.1-7 Primary EBV infection leads to latently infected immortalized B cells expressing part of the viral genome with episomal EBV persistence.8 The control of these cells is dependent on cytotoxic T cells,9 the function of which is variably impaired after organ and BMT.10-12 BLPD is thought to be the result of a severe deficiency of cytotoxic T cells allowing the outgrowth of EBV-transformed B cells, as suggested by in vitro13,14 and in vivo data.5,14-16Proliferating B cells may express the panel of EBV latent genes EBNA 1 to 6, LMP1 and 2, LP, and two small RNA EBER1 and 2.15,17,18 BLPD occurs in 1% to 5% of kidney and liver transplant patients,1,19-23 4.9% to 15% of heart and heart-lung transplant patients,19,24-26 and 11% to 15% of intestinal transplant patients.27,28 In BM recipients, the incidence of BLPD is between 0.4% after HLA-matched noncomplicated transplants, and 24% after T-cell–depleted highly immunosuppressed transplants.15,22,29 The prognosis of BLPD is poor. Forty percent to 60% of organ transplant patients19,22,24,28,30and 90% of BMT recipients who develop a BLPD die despite reduction of immunosuppressive treatment.16,22,29,31 Treatment is still controversial: surgery may be lifesaving in cases of localized BLPD, but chemotherapy is of limited value in documented EBV-associated BLPD.22,32 Preliminary data have suggested improved survival with the use of interferon-α and intravenous infusion of high doses of Igs.33 Antiviral therapy (Acyclovir, Ganciclovir [Wellcome, London, UK]) has not been proven to be efficient alone, although rare cases of remissions have been reported.22,34,35 For BLPD occurring after BMT, infusion of donor T lymphocytes or EBV-specific donor cytotoxic T lymphocytes has brought significant improvement.10,36 As based on laboratory data found in severe combined immunodeficiency (SCID) mice,14 use of murine monoclonal anti–B-cell antibodies anti-CD24 and anti-CD2137 38 was shown to be partially efficient in 26 BM or organ transplant recipients with BLPD. Here we report the efficacy of this treatment among 58 organ and BMT recipients enrolled in an open multicenter study. We investigated the prognostic factors of BLPD and the long-term outcome of the patients.
PATIENTS AND METHODS
Patients
An open multicenter therapeutic study of the use of anti-CD21 and anti-CD24 monoclonal antibodies (MoAbs) in patients presenting with posttransplant BLPD was initiated in August 1985 and closed in July 1993. It involved 20 centers in four countries (Belgium, Canada, France, United States). The endpoint for data analysis was May 1995. Some of these cases were previously reported.38-40Eligibility criteria for organ or BMT patients were: (1) multivisceral B lymphoproliferation that was not responsive to immunosuppression treatment tapering within 8 days; and/or (2) rapidly progressive multiple lymphoproliferative lesions precluding surgical therapy; and/or (3) histologically invasive disease with nodal capsule disruption, and presence of atypical cells and necrosis.
Sixty-six patients met the eligibility criteria. Two patients died before initiation of therapy because of disease progression. No clinical or histological data are available for six additional cases. Therefore, 58 patients with severe posttransplant B-lymphoproliferative disease included in this study were evaluated. Patient characteristics are summarized in Table 1.
Twenty-seven patients received 28 allogeneic BMT either for hematological malignancies (n = 11: acute lymphoblastic leukemia, 7; chronic myeloblastic leukemia, 3; and acute myeloblastic leukemia, 1) or congenital immunodeficiency (ID) (n = 16: Wiskott-Aldrich syndrome, 8; SCID, 7; and combined ID, 1). Twenty-six transplanted marrow samples were T-cell depleted because of HLA antigen mismatch at one or more loci between donor and recipient or because the donor was unrelated. Patient 4 with Wiskott-Aldrich syndrome was transplanted twice at 18-month intervals with grafts from the same donor and suffered BLPD on each occasion (Table 1). Six BMT patients received highly aggressive immunosuppressive therapy for grade III or higher acute graft-versus-host disease. Highly aggressive immunosuppressive therapy was defined as the use of at least one of the following treatments: anti-thymocyte globulin (ATG), anti-CD3 antibodies (OKT3), methylprednisolone at a dose of 5 mg/kg/d for more than 1 week, or high-dose methylprednisolone bolus (1 g/1.73 m2).
Thirty-one patients received an organ transplant. They consisted of heart transplant (n = 12), kidney transplant (n = 9), lung transplant (n = 3), liver transplant (n = 3), heart + lung transplant (n = 1), kidney + pancreas transplant (n = 2), and cluster mesenteric transplant (stomach, small bowel, liver, and pancreas transplant in 1 patient). Highly aggressive immunosuppressive therapy was administered to 20 organ transplant recipients because of graft rejection episodes and to 11 organ transplant recipients as part of the rejection prevention regimen.
Because many of the participating bone marrow transplant centers were pediatric services, BMT recipients were much younger (median age, 2.2 years; range, 0.35 to 37 years) than organ transplanted patients (median age, 37 years; range, 0.3 to 67.5 years).
Immunological Investigations
The following B- and T-cell–specific MoAbs were used, as previously described,38 to characterize T and B lymphocytes in blood and in BM and organ tissue samples when available: anti-Ig heavy-chain and light-chain isotype,37 anti-CD19, CD24, CD21, and CD23 antibodies (Immunotech, Marseille, France), and anti-CD3, CD4, and CD8 antibodies (Becton Dickinson, San Diego, CA). Analyses were performed by indirect immunofluorescence cytofluorometry. Fresh cells were used for membrane immunofluorescence analysis and fixed cells for intracytoplasmic staining. Immunoperoxidase staining of biopsy sections was performed as previously described.41Serum Ig levels were measured by nephelometry and monoclonal Ig components by immunofixation.42 Anti-mouse Ig antibodies were detected with a direct enzyme-linked immunosorbent assay.
Virology
EBV-DNA was detected either in frozen material by Southern blotting using a randomly primed P32-labeled probe specific for theBamH1 W internal repeats of the virus and/or by in situ hybridization with EBV-specific probes43 and/or by polymerase chain reaction (PCR) analysis.44 Specific antibodies (IgG and IgM isotypes) against EBV (viral capsid antigen, early antigen, and Epstein-Barr nuclear antigen) in organ transplant recipients were detected by an enzyme-linked immunosorbent assay. Immunoperoxidase staining of biopsy sections was also performed for LMP1.41
Cytogenetic analyses were performed to assess proliferating B cells of donor or recipient origin (together with VNTR [variable number tandem repeats] system analysis and intra-genic β-globin haplotypes) and/or to detect cytogenetic abnormalities.
Clonality Studies
Clonality of proliferative B cells was assessed by membrane and intracytoplasmic indirect immunofluorescence with anti-Ig heavy-chain or light-chain antibodies or by immunoperoxidase staining. Ig gene rearrangement studies were performed by Southern blotting using a probe for the sequence encoding the heavy-chain joining region (JH). Clonal rearrangement was considered to be present if discrete bands were seen on blots prepared with at least two restriction enzymes. BLPD was considered to be monoclonal when a single light chain and a single heavy chain were present on the surface and in the cytoplasm of B lymphoblasts and/or when a single Ig rearrangement was observed in all pathological specimens analyzed, regardless of whether a single monoclonal serum component had been detected by immunofixation. BLPD was considered to be oligoclonal when several light chains and heavy-chain isotypes were present on B lymphoblasts, and/or when several distinct serum monoclonal Ig components were detected by immunofixation and/or when no unique Ig heavy-chain rearrangements were observed in the analyzed pathological specimens.
BLPD Diagnosis
Treatment Characteristics
MoAbs.
As previously described,38 two murine MoAbs, ALB9 (IgG1) specific for CD24, an antigen expressed by the B-cell lineage and granulocytes, and BL13 (IgG1) specific for CD21 (Immunotech, Marseille, France), respectively, were administered at the dose of 0.2 mg/kg/d for 10 days by intravenous 4- to 6-hour infusion. Four patients (nos. 4B, 7, 12, 13) received 0.4 to 0.8 mg/kg/d because of insufficient target saturation (as assessed by cytometry fluorescence analysis of murine antibody-coated blood B cells). Five patients (nos. 25, 26, 38, 56, 57) received anti-CD24 antibody only because anti-CD21 antibody was not available at the time of treatment. Patient 42 received 1/3 of the anti-CD21 dose and no anti-CD24 antibody because of chemotherapy-induced neutropenia. Three of 14 patients with central nervous system (CNS) involvement received intraventricular anti-CD21 injection through an Omaya device, as previously described.38 39 The treatment protocol was approved by the ethics committee of the Hôpital Necker-Enfants-Malades. Informed consent was obtained from all patients or parents.
Treatment Assessment
Complete remission was defined as complete clinical and radiological disappearance of tumors at all sites, disappearance of circulating B lymphoblasts, and the absence of new involved sites. Partial remission was defined as at least a 50% volume reduction of involved organs but was considered as a failure of therapy. Treatment tolerance was scored according to the World Health Organization recommendations.
Statistical Analysis
Qualitative and quantitative data were compared between groups by the Chi-square and Wilcoxon tests, respectively. Probabilities of survival and of complete remission were calculated by the Kaplan-Meier method and differences were assessed by the log-rank test.45Multivariate analysis based on the logistic regression46and Cox’s proportional hazards regression model47 was performed to select the characteristics that significantly contributed to remission and survival.
RESULTS
BLPD Characteristics
The characteristics of the BLPD for the 58 patients are summarized in Table 1. BLPD occurred significantly earlier after transplantation in BM recipients (median, 55 days after transplant; range, 21 to 120 days) than in organ recipients (median, 165 days; range, 37 to 1,980 days) (P ≤ .02). The patterns of organ involvement are summarized in Tables 1 and 2. The number of organs involved was significantly higher in BM recipients (median, 4 sites; range, 2 to 8) than in organ recipients (median, 3 sites; range, 1 to 8) (P ≤ .02). Monoclonal BLPD was diagnosed in 17 of 27 organ-transplanted patients and in 11 of 27 BM transplanted patients. BM patients transplanted for hematological malignancy (HM) were mainly monoclonal (8 of 11; 73%) and older (median, 7.6 years; range, 0.35 to 37) than those transplanted for ID (3 of 16 were monoclonal; age range, 0.5 to 5.4 years; median, 1.8). These differences were statistically significant (P = .015 for clonality and P = .003 for age). The majority of post-BMT BLPD were of donor origin (12 of 14 tested). In tested organ transplant patients, all were of recipient origin (3 of 3). Twenty of 23 BLPD in BMT recipients and 29 of 30 in organ transplant recipients were positive for the EBV genome.
Anti-CD21 and Anti-CD24 MoAb Therapy
The median time interval between BLPD diagnosis and therapy was 6.5 days in BMT patients (range, 1 to 33 days) and 37 days in organ transplant patients (range, 2 to 165 days). Immunosuppressive therapy was reduced for 38 cases of BLPD and assessed for at least 1 week before antibody treatment. The BLPD was unresponsive (Table 1). The effectiveness of immunosuppression tapering was assessed at least for 1 week before treatment. Rapidly progressive BLPD or histologically invasive disease was observed in the 21 other patients whose immunosuppression treatment was not modified or was modified for less than 1 week before treatment initiation.
Before anti–B-cell MoAb therapy, 7 of 28 BLPD events in BMT recipients occurred during Acyclovir administration, a treatment maintained throughout anti–B-cell antibody therapy. One organ transplanted patient received chemotherapy for 1 month before MoAb treatment. Patients 17 and 26 received interferon-α and high-dose Ig before anti–B-cell antibody therapy.
After anti–B-cell MoAb therapy, 5 patients (4 after organ transplantation [nos. 39, 47, 48, and 49] and patient 4Bafter BMT) received chemotherapy because of disease progression; 2 patients received palliative radiotherapy (nos. 20 and 49); and 1 BMT patient received peripheral blood mononuclear cells (1 × 105 CD3+ cells/kg) from the donor because of disease progression (no. 27).
One BMT patient (no. 26) received anti-CD19 and anti-CD37 chimeric antibodies to treat a BLPD relapse because anti-CD21 and anti-CD24 were not available at that time.
Tolerance
About one third of the patients (19 of 59 BLPD) experienced clinical side effects of anti-CD24 and anti-CD21 MoAb treatment, usually after the first infusion. Grade II fever and/or shivering appeared in 13 patients during the first infusion. Other clinical signs were: grade I pain in 2 patients, grade II transient hypotension in 2 patients, diarrhea and/or vomiting in 2 patients, and isolated grade I skin rash in 1 patient. Twenty-four (42% of the patients) suffered isolated neutropenia, usually during the 10 days of treatment and lasting until up to 5 days after the end of treatment. Transient neutropenia and thrombocytopenia occurred in 3 patients with the same kinetics as isolated neutropenia. One case of sepsis and two of bacteremia were observed in neutropenic patients, all with favorable outcome after antibiotic therapy. Anti-mouse Ig antibodies were detected in 6 of the 12 patients tested; 2 of 3 patients experienced transient hypotension during MoAb infusions. Circulating B cells were not detected during treatment in 29 of the 42 patients tested; they progressively reappeared within 15 days of the end of treatment.
Efficacy
Complete remission after treatment with anti-CD24 and anti-CD21 MoAbs was achieved in 36 of 59 BLPD cases (61%). The median time interval between the start of therapy and achieving complete remission was 15 days (range, 5 to 150 days). There was no significant difference in the complete remission rate according to the type of transplantation: the rate was 57% (16 of 28) for BLPD occurring after BMT and 64% (20 of 31) for organ transplant patients.
Predictive factors for no or partial remission (Table 3) were multivisceral disease (P ≤ .0001), monoclonality (P ≤ .05), BMT for hematological malignancies (P ≤ .01), CNS involvement (P ≤ .04), and late-onset BLPD (P ≤ .01). The median number of sites involved was 5 in partial or nonresponders (range, 1 to 8) versus 3 in complete responders (range, 1 to 5). Complete remission was achieved in 46% of the cases of monoclonal BLPD (13 of 28: 3 of 11 after BMT and 10 of 17 after organ transplantation) and in 80% of oligoclonal BLPD (21 of 26). Only 3 of 11 patients with hematological malignancy (28%) achieved complete remission versus 13 of 17 (76%) when BLPD occurred after BMT for congenital immunodeficiency (P≤ .01).
CNS involvement was also a poor prognostic factor. Complete remission was achieved in only 4 of 14 cases with CNS involvement (29%) versus 32 of 45 cases without CNS involvement (71%) (P ≤ .04). All cases of late-onset BLPD (more than 1 year after transplantation) were organ transplanted patients and late onset was associated with a low remission rate: 2 of 9 (22%) versus 18 of 22 after early onset BLPD (82%) in organ transplanted patients (P ≤ .01). The other factors analyzed did not influence the rate of complete remission; they include time interval between first symptoms of BLPD and initiation of anti–B-cell antibody treatment, occurrence of graft rejection episodes or graft-versus-host disease, number of circulating B cells, associated antiviral therapy, and interferon-α therapy. Multivariate analysis of the factors affecting the rate of complete remission showed a negative association with multivisceral disease (P ≤ .005, odds ratio = 2.5 per involved site), late-onset BLPD (P ≤ .005, odds ratio = 25), and CNS involvement (P ≤ .05, odds ratio = 6.7).
Four patients presented partial remission and all subsequently died of BLPD. Three of 36 initial responders relapsed (8%), including 2 BMT patients and 1 organ transplant patient (nos. 8, 26, and 36).
Survival
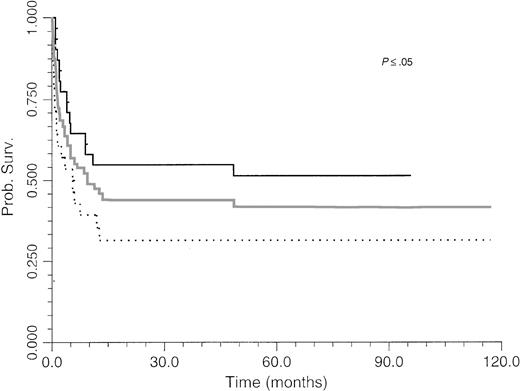
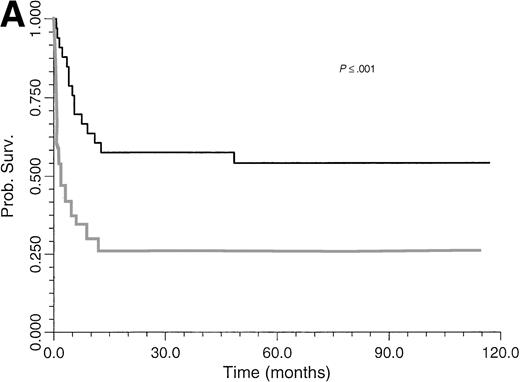
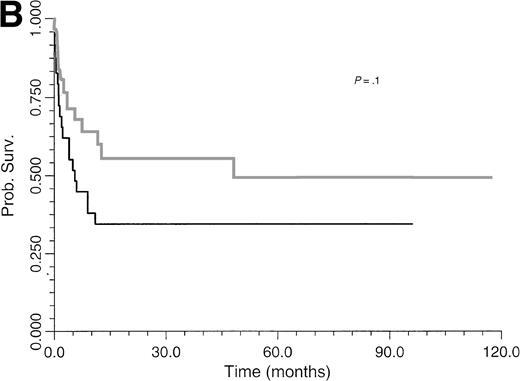
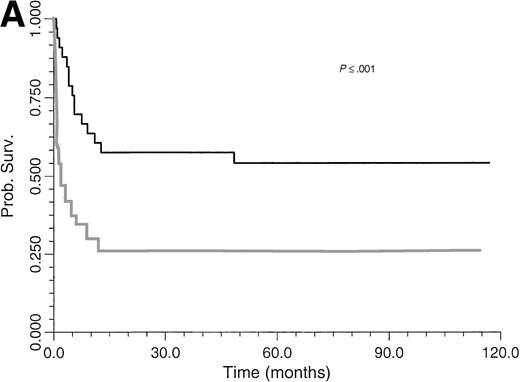
Survival of BLPD patients after BM or organ transplantation is shown in Fig 1. BLPD-related deaths were rapid after the onset of the first BLPD symptoms in BM recipients. The median time interval was 0.7 month (range, 0.2 to 12). BLPD-related death was also rapid after the onset of the first BLPD symptoms in organ-transplant patients: the median interval was 2.1 months (range, 1 to 9). The median follow-up of patients surviving BLPD is 61 months (range, 35.5 to 117 months). Details of the outcome for the 12 patients who died despite an initial complete remission are summarized in Table 1. Seven died from lethal opportunistic infections. One died from a preexisting grade IV graft-versus-host disease. One patient experienced a lethal acute recurrence of cardiac graft rejection. Only one patient died from a late BLPD relapse. One other patient died from a new malignancy. An analysis of the factors influencing survival is given in Table 4. The survival rate of BMT patients was significantly lower than that of organ transplant patients (P ≤ .05) (Fig 1). Survival (Table 4) was significantly better among patients with paucivisceral BLPD (<4 sites involved) than among patients with multivisceral BLPD (P ≤ .001) (Fig 2A). Among BMT patients, the prognosis was significantly worse for patients treated for hematological malignancy (no survivors at 1 year) than for patients transplanted for congenital immunodeficiency (P ≤ .005) (Table 4). In contrast to a previous report based on the first 26 patients treated, we found that survival was not significantly worse for patients with monoclonal BLPD than for patients with oligoclonal BLPD (P ≤ .1). Nevertheless, there was a tendency for better survival among patients with oligoclonal BLPD (Fig 2B), whereas monoclonal BLPD after BMT was associated with a very poor prognosis (1 of 11 survived). As expected, survival was also found to be significantly better among patients having achieved complete remission than patients with partial or no remission (Table 4) (P ≤ .0001, relative risk [RR] = 18). Prognostic analysis of the initial variables by Cox regression showed only a strong negative association between survival and multivisceral disease (P ≤ .001; RR = 1.4 per involved site).
Survival of BM and organ transplantation patients after treatment with anti-CD21 and anti-CD24 MoAbs for BLPD. Gray line, overall survival; black line, organ transplant patient survival; black dots, BMT patient survival. Survival was better among organ transplant patients than among BMT patients (P = .03).
Survival of BM and organ transplantation patients after treatment with anti-CD21 and anti-CD24 MoAbs for BLPD. Gray line, overall survival; black line, organ transplant patient survival; black dots, BMT patient survival. Survival was better among organ transplant patients than among BMT patients (P = .03).
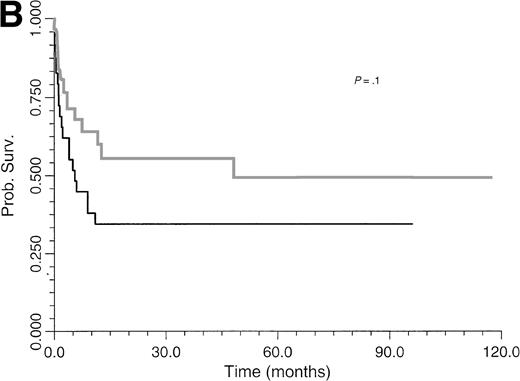
Comparative survival curves for BLPD patients treated with anti-CD21 and anti-CD24 MoAbs to two risk factors: number of involved sites (A) and clonality (B). (A) Black line, paucivisceral BLPD survival (<4 sites involved) (n = 33); gray line, multivisceral BLPD survival (4 sites or more involved) (n = 26). Survival was better among paucivisceral than multivisceral BLPD patients as assessed by both in univariate analysis (P = .0007) and in multivariate analysis (RR = 1.4 per involved organ, P = .0002). (B) Black line, monoclonal BLPD survival (n = 28); gray line, oligoclonal BLPD survival (n = 26). The difference between the two groups is not statistically significant (P = .10).
Comparative survival curves for BLPD patients treated with anti-CD21 and anti-CD24 MoAbs to two risk factors: number of involved sites (A) and clonality (B). (A) Black line, paucivisceral BLPD survival (<4 sites involved) (n = 33); gray line, multivisceral BLPD survival (4 sites or more involved) (n = 26). Survival was better among paucivisceral than multivisceral BLPD patients as assessed by both in univariate analysis (P = .0007) and in multivariate analysis (RR = 1.4 per involved organ, P = .0002). (B) Black line, monoclonal BLPD survival (n = 28); gray line, oligoclonal BLPD survival (n = 26). The difference between the two groups is not statistically significant (P = .10).
DISCUSSION
This study updates and completes the analysis of a previously reported38 cohort of transplanted patients who developed severe BLPD and were treated with monoclonal anti–B-cell antibodies to CD21 and CD24. Limited and transient adverse reactions were observed clinically in one third of the patients and biologically in two fifths of the patients. Survival and complete remission were strongly associated confirming the absence of deleterious long-term effects. In this series, the remission rate was high (57% in BMT patients and 64% in organ transplant patients). The relapse rate was very low (8%) and relapses occurred only when profound immunodeficiency persisted. The survival rate was 46% at 1 year and there were no BLPD-related deaths after 1 year, confirming our initial reports.37,38 Although no control study was performed, the survival rate appears higher than the 28.5% (28 survivors) among the 102 BLPD patients reported in the literature.15,19,22,24,29-31,48 Remission and survival rates for severely affected and susceptible patients treated with monoclonal anti–B-cell antibodies were much higher than those reported after treatment with chemotherapy (23% long-term survival rate) or antiviral drugs (Acyclovir, Ganciclovir, Foscarnet [Astra, Stockholm, Sweden]) (29% long-term survival rate).22,34,35,49,50 The 1-year remission and survival rates for cardiac or cardiopulmonary transplanted patients were 69% and 61%, respectively, much higher than the 8% to 40% previously reported.22,24,51 The factors associated with complete remission and survival in this series differ slightly from our previous preliminary report.38 Risk factors for no or partial response to anti–B-cell MoAb therapy were multivisceral disease, monoclonality, BMT for hematological malignancies, CNS involvement, and late-onset BLPD. Among these variables, only multivisceral disease, late-onset BLPD, and CNS involvement appear in multivariate analysis to contribute significantly to the nonresponder status. This is not surprising given the inaccessibility of the CNS to MoAbs injected intravenously.38,44 Late-onset BLPD, which is most often monoclonal, may possibly be caused by a different physiopathogenic mechanism. Secondary oncogenic events (such as bcl2rearrangements, c-myc, n-ras, and p53 mutations) and LMP1 deletions52-55 may be responsible for true lymphomas that could be responsive to chemotherapy: one third of the cases of late-onset BLPD responded to chemotherapy in our series.32
In this series, clonality does not appear as a major predictive factor for the response to therapy except among BMT patients. This might be due to intra-patient variability: the clonality of a single site does not reflect the clonality of the other involved sites (sampling artefact).7 The type of transplant, particularly BMT for hematological malignancies, was strongly predictive of survival and complete remission. However, it is not clear why patients with HM who underwent BMT developed monoclonal BLPD more frequently and whether monoclonality of BLPD and/or underlying conditions are associated with a poor prognosis despite anti–B-cell antibody treatment. Tumoral burden (number of involved sites) was the largest predictor of survival. Late-onset BLPD and CNS involvement did not appear to modify survival directly but was associated with a higher number of involved sites.
Anti–B-cell MoAbs might restore a “balance” between the outgrowth of EBV-infected B cells and T-cell immunosurveillance by diminishing the tumor burden. Therefore, we conclude that anti–B-cell MoAb therapy is a safe and effective treatment for severe posttransplant BLPD. For commercial reasons, ALB9 and BL13 are no longer available for therapeutic use. Given the long-term results reported in this study, it is hoped that similar antibodies will be made available for clinical studies. Any anti–B-cell MoAb that does not activate the complement system should be appropriate; eg, anti-CD19, -CD20, -CD21, -CD23, and -CD37 antibodies. If possible, a combination of anti–B-cell MoAbs should be used because of the variability of antigen expression in EBV-transformed B cells.17 The optimal administration regimen, to attain optimal target saturation, is not known. The usefulness of a loading dose of monoclonal anti–B-cell antibody is also not known. Additional anti–B-cell MoAbs initially developed to treat primary B lymphomas also could be used or are currently under investigation for posttransplant BLPD, ie, chimeric double Fc anti-CD19, anti-CD37, and anti-CD3856 and humanized anti-CD20 Rituxan antibody (Roche, Basel, Switzerland).57 Alternative therapeutic approaches are also being used. For example, administration of anti–interleukin-6 murine MoAb, which disrupts a possible autocrine growth loop,58,59 is currently being tested by a multicenter prospective investigation. In HLA-identical BMTs, donor peripheral blood leukocyte infusions have been used and gave a good remission rate (5 of 5 treated patients). They were complicated with two terminal, possibly toxic, respiratory failure syndromes and chronic graft-versus-host disease.10 In addition, this approach cannot be used for HLA-antigen–mismatched BMTs or organ transplants. Anti–EBV-specific donor cytotoxic T cells have been used to prevent or treat BLPD in T-cell–depleted BMT patients with high biological and clinical remission rates. However, this approach has not yet been used in organ transplant recipients.36,60 The procedure is effective but requires a 5- to 6-week period for cell preparation. BLPD prevention by the use of an anti-EBV vaccine (anti–GP350-220) and B-cell depletion is currently being investigated in BMT patients.61
In conclusion, anti–B-cell MoAb administration is an easy, safe, and relatively effective therapy for many patients with posttransplant BLPD. Further studies comparing its effectivness to other possible therapeutic strategies are warranted. We describe prognostic factors that could help delineate a group of patients with BLPD in whom anti–B-cell antibody administration may be an appropriate therapeutic strategy.
ACKNOWLEDGMENT
We thank the following clinicians and physicians who contributed to this study: C. Amerin, P. Bruneval, Hôpital Broussais, Paris, France; C. Bedos, Hôpital Bichat, Paris, France; E. Benz-Lemoine, Hôpital de Poitiers, France; Y. Blanloeil, Hôpital Laënnec, Nantes, France; F. Boulad, Memorial Sloan-Kettering Cancer Center, New York, NY; N. Brousse, J. Deblic, E. MacIntyre, P. Niaudet, Hôpital Necker-Enfants Malades, Paris, France; D. Durand, Hôpital de Toulouse, France; T. Facon, Hôpital Claude Huriez, Lille, France; E. Girodon, Hôpital Henri Mondor, Créteil, France; P. Hervé, Hôpital de Besançon, France; A. Lazarovits, University Hospital, London, Ontario, Canada; P. Lutz, Hôpital Central, Strasbourg, France; F. Mechinaud, Hôpital de la mère et de l’enfant, Nantes, France; J.S. Mercattelo, G. Soulier, Hôpital Debrousse, Lyon, France; J.F. Mornex, Hôpital L. Pradel, Lyon, France; M. Raphael, Hôpital Avicenne, Bobigny, France; Y. Redonnet, Hôpital de Rouen, France; F. Rosenberg, Hôpital St Vincent de Paul, Paris, France; E. Sokal, Hôpital UCL, Bruxelles, Belgium; and J.L. Stephan, Hôpital Nord, Saint Etienne, France. We also thank Marie-Christine Mourey for excellent secretarial assistance, and Jane Peake and Alex Edelman for help with the English.
Supported by grants from INSERM and la Ligue Parisienne contre le Cancer.
Address reprint requests to Malika Benkerrou, MD, Unitéd’Immunologie Hématologie Pédiatrique, Hôpital Necker Enfants Malades, 149 Rue de Sèvres, 75015 Paris, France.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.