To the Editor:
We read with interest the report of Peter et al1 in which the authors showed that the binding of abciximab (c7E3 Fab fragments), a new generation anti-thrombotic drug2,3 induced conformation changes in glycoprotein (GP) IIb-IIIa. According to these authors, these changes were such that when abciximab dissociated from the complex, a process facilitated in their experimentation by a large-scale dilution of the platelet suspensions, fibrinogen was able to bind without the normal requirement for platelet stimulation by an agonist such as adenosine diphosphate (ADP) or thrombin. It is now well known that in circulating platelets the level of protein tyrosine phosphorylation is in a basal state, and that platelet stimulation by ADP, thrombin, or collagen leads to a rapid increase in the tyrosine phosphorylation of a number of cytoplasmic proteins.4-6Such increases occur not only as a result of the interaction of the primary agonist with its receptor, but also following fibrinogen binding to GP IIb-IIIa complexes and during platelet aggregation. Tyrosine phosphorylation as a result of integrin occupancy has become an often-cited example of ‘outside-in’ signaling.7
We were interested in establishing whether abciximab binding itself led to increased tyrosine protein phosphorylation in platelets and whether the drug inhibited the tyrosine phosphorylation response induced after primary receptor activation. Washed platelets prepared as described elsewhere,8 were suspended at 2.5 × 108/mL in HEPES-buffered Tyrode’s buffer, pH 7.4, containing 2 mmol/L Ca2+,8 and incubated for 30 minutes at 37°C without stirring (except for an initial mixing) in the presence or absence of 10 μg/mL abciximab (Lilly-France, Saint-Cloud, France). That the surface pool of GP IIb-IIIa receptors is saturated by abciximab under these conditions has already been established in our laboratory.8 The suspensions were then incubated in a platelet aggregometer (PAP-4 model; Biodata Corp, Paris, France), with stirring in the presence or absence of 12.5 or 25 μmol/L thrombin receptor activating peptide (TRAP-14 mer; Neosystem, Strasbourg, France). The aggregometer tracings were recorded. TRAP-14 mer-induced platelet aggregation was inhibited by abciximab, although a small residual aggregation associated with a 10% to 20% light transmission change persisted due to the exposure of unblocked complexes from the internal pool.8,9 Samples were obtained at 30 seconds, 1 minute, and 5 minutes, and immediately solubilized in a buffer containing sodium dodecyl sulfate (SDS) and a cocktail of inhibitors of proteases and tyrosine phosphatases as reported previously from our laboratory.10 Proteins were separated by electrophoresis on 7% to 12% gradient gels and transferred to nitrocellulose membrane with phosphorylated tyrosine residues revealed using the monoclonal antibody PY99 (0.1 μg/mL; Santa Cruz Biotechnology, Santa Cruz, CA) and a chemiluminescence procedure.10
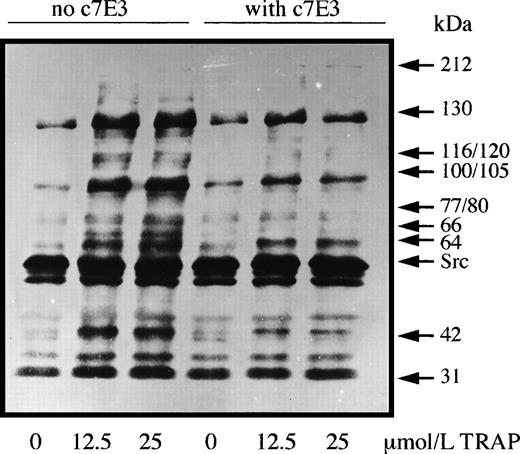
Significantly, at no time point did the protein tyrosine phosphorylation profiles of unstimulated platelets incubated with abciximab differ from those incubated without this antithrombotic drug. Typical results are shown in Fig 1; these represent experiments performed on platelets from three donors. As expected, TRAP-14 mer-induced platelet aggregation was associated with markedly increased levels of protein tyrosine phosphorylation. Increased labeling of bands at 60 (probably pp60c-src) and 31 kD was unaffected by the presence of c7E3 Fab, and the increased phosphorylation of these proteins is probably a direct result of activation through the PAR-1 receptor. Abciximab inhibited to varying extents the TRAP-induced phosphorylation of bands of 130, 116/120, 100/105, 77/80, 64, and 42 kD. Allowing for small differences in protein migration due to different experimental conditions (and taking into account the increased sensitivity of PY99 with respect to previously used antibodies), our results are similar to those obtained by Rosa et al5 for thrombin-activated platelets of patients with type I and type II Glanzmann thrombasthenia. These authors additionally showed that RGDS peptide and the intact anti-GP IIb-IIIa monoclonal antibody 10E5 also selectively blocked tyrosine-phosphorylation induced after GP IIb-IIIa engagement. Thus, c7E3 Fab fragments, as shown in our study, exert inhibitory effects on agonist-induced tyrosine phosphorylation probably linked to their inhibition of the platelet aggregation response. Furthermore, our results show that any conformation changes induced within the complex by abciximab do not lead to outside-in signaling linked to tyrosine phosphorylation. Therefore, abciximab does not mimic fibrinogen, further suggesting that cross-linking or immobilization of the occupied receptors is a necessary additional step for outside-in signaling by the integrin.7 It is also pertinent to emphasize that the trafficking of c7E3 Fab into and out of the platelet as recently shown by us8 is unlikely to be driven by an upregulation of protein tyrosine phosphorylation.
Washed platelets were preincubated with or without abciximab (10 μg/mL) for 30 minutes before being incubated with two concentrations of TRAP-14 mer under aggregating conditions. Samples were taken at the peak of the aggregation (2 minutes) and platelet proteins were separated by SDS-polyacrylamide gel electrophoresis before the detection of tyrosine-phosphate groups by Western blotting using the monoclonal antibody PY99.
Washed platelets were preincubated with or without abciximab (10 μg/mL) for 30 minutes before being incubated with two concentrations of TRAP-14 mer under aggregating conditions. Samples were taken at the peak of the aggregation (2 minutes) and platelet proteins were separated by SDS-polyacrylamide gel electrophoresis before the detection of tyrosine-phosphate groups by Western blotting using the monoclonal antibody PY99.